Abstract
The current study aimed to lay a theoretical foundation for further development of choline as an anti-hypoxia damage drug. Wild-type, 3- to 5-month-old male Sprague-Dawley rats, weighing 180–220 g, were used in this study. The rats were randomly divided into a normoxic control group (n=16) and a chronic intermittent hypoxia (CIH) group (n=16). The effects of CIH on acetylcholine (ACh)-mediated endothelium-dependent vasodilatation in the rat cerebral basilar arterioles and mesenteric arterioles, as well as the protective effects of choline on the arterioles damaged by hypoxia were observed. Moreover, the effects of choline on endothelial cell proliferation during hypoxia were observed, and choline's functional mechanism further explored. The ACh-mediated vasodilatation of rat cerebral basilar and mesenteric arterioles significantly reduced during hypoxia (P<0.01). Choline significantly increased dilation in the rat cerebral basilar (P<0.01) and mesenteric arterioles (P<0.05) damaged by CIH compared with those in the control group. In addition, under hypoxic conditions, choline significantly promoted the proliferation of rat aortic endothelial cells (P<0.05) and significantly reduced lactate dehydrogenase activity in the cell culture supernatant in vitro (P<0.05). Furthermore, the effect of choline could be related to its ability to significantly increase the secretion of vascular endothelial growth factor (P<0.01) and activation of α7 non-neuronal nicotinic acetylcholine receptors under hypoxia (P<0.01). This study demonstrated that choline could have protective effects against hypoxic injuries.
Keywords: chronic intermittent hypoxia, vasodilator effects, choline, vascular endothelium, cell proliferation
Introduction
Hypoxia is an environmental stress factor that induces damage to cells, tissues and organs, and is associated with numerous types of disease. Vascular endothelial cells (VECs), as target cells of hypoxic damage, are susceptible to hypoxia and serve a key function in regulating the activity of blood vessels, as well as in the occurrence and development of numerous hypoxia-related diseases and injuries (1). Under normal circumstances, VECs serve as a barrier between the tissues and blood lining in the endothelial layer of blood vessels (2). If the endothelial layer is damaged, VECs lose their original structure and function, directly causing defects in the endothelial layer, failure of intimal integrity and vascular dysfunction. This is the first step in the development of peripheral vascular injuries caused by hypoxia (3–5). However, it is important to recognize the endothelium as a critical secretory organ with both receptor and effector functions. It can sense a range of external physical and chemical stimuli, and synthesize and secrete important vasoactive substances, such as nitric oxide, endothelin-1 (ET-1) and vascular endothelial growth factor (VEGF) (6–9). These substances function in maintaining the vasomotor state, vascular permeability and a dynamic balance of the internal environment in a variety of diseases and injuries of many pathophysiological processes.
When exposed to hypoxic conditions, VEC function may be disrupted, causing an imbalance of the synthesis and secretion of various regulatory factors and vasoactive substances. This may eventually result in an increase in vascular permeability, a decrease in antioxidant capacity and a variety of hypoxia-related diseases and injuries (3–5). Therefore, the primary task in preventing and treating hypoxia-related disease is to protect VECs from damage and maintain their normal physiological function.
Choline is an essential water-soluble nutrient belonging to the B-complex family of vitamins (10,11). Adolph Strecker first identified it in ox bile in 1862 (12) and in 1932, it was recognized by Best, Hershey and Huntsman as an essential dietary nutrient (13). Since then, it has been found that choline plays a key role in many physiological processes such as signaling transduction, biosynthesis and integrity of cell membranes, DNA and histone methylation, and neurotransmitter acetylcholine (ACh) synthesis (14–16). It has also been found that choline metabolism changes under hypoxic exposure (17,18).
In the current study, the influences of chronic intermittent hypoxia [CIH; this refers to long-term, repetitive exposure to hypoxia, interspersed with periods in normoxic conditions (19,20)] on ACh-mediated endothelium-dependent vasodilatation function of the rat cerebral basilar arterioles and mesenteric arterioles were observed, as well as the protective effects of choline on the arterioles damaged by hypoxia. Furthermore, the effect of choline on cell proliferation, lactate dehydrogenase (LDH) release, and VEGF expression under hypoxic conditions in vitro was observed, as well as the influence of mecamylamine [(MLA; an antagonist of the α7 non-neuronal nicotinic acetylcholine receptor (α7 nAChR)] on choline effects. As one of the B-complex family of vitamins, choline serves a key role in a number of physiological processes such as signaling transduction, DNA and histone methylation, and neurotransmitter ACh synthesis. The aim of this study was to lay a theoretical foundation for further developing choline as an anti-hypoxia damage drug.
Materials and methods
Experimental animals and drugs
A total of 30 wild-type, 3- to 5-month-old male Sprague-Dawley (SD) rats, weighing 180–220 g, provided by the Experimental Animal Center of Beijing Academy of Military Medical Sciences (Beijing, China), were used in this study. The rats were housed individually at a temperature of 18–24°C and given ad libitum access to food and water. The care and use of animals in the experiments met the standards set out in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA) and closely complied with the Animal Care and Use Committee of the Tianjin Institute of Health and Environmental Medicine (Tianjin, China). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Tianjin Institute of Health and Environmental Medicine (permit no. 2013-D-3604). All efforts were made to lower the number of animals used and to reduce animal stress. Choline chloride (ChCl), ACh and ET-1 were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Animal grouping and treatment with CIH
The rats were randomly divided into a normoxic control group (no treatment, n=16) and a CIH group (treatment with CIH, n=16). Half the rats in each group were used to study arterial endothelial activity and the other half to study the selective dilatation effects of choline.
Animals from the CIH group were separately housed in cages, which were placed into a Plexiglas chamber (40×30×30 cm). At the beginning of hypoxia, nitrogen was pumped into the chamber to reduce the oxygen concentration. This was continuously measured using an oxygen concentration monitor (CYES-II, Shanghai Scientific Instruments, Shanghai, China) and was stabilized at a level of 10±0.5% (which mimics the hypoxic environment of 6,000 m above sea level) for 8 h (8:00 a.m. to 4:00 p.m.) via an exhaust valve controlled by an automatic computer system. The chamber contained a small hole that allowed the pressure inside to remain consistent with the external environment. Carbon dioxide and water vapor in the chamber were absorbed with soda lime and anhydrous calcium chloride, respectively. Intermittent hypoxia (8 h/day, 6 days/week) lasted for 12 weeks. The treatment of animals from the normoxic control group was the same as that of the CIH group except that they were not given CIH treatment. At the end of the CIH exposure duration, animals were administered 1–2% isoflurane through a face mask for anesthesia prior to sacrifice by decapitation and surgical procedures were performed.
Arterial tension measurement
After the rats were sacrificed by decollation, intestinal and brain tissues were rapidly removed and placed into culture dishes containing Krebs-Ringer nutrient solution (118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgSO4, 0.026 mM EDTA and 11.1 mM glucose) at 4°C. Under a light microscope, the rat basilar arteries (300-µm diameter) and the third-order branches of mesenteric arteries (250-µm diameter) were carefully dissected using microtweezers and cut into ~2-mm microarterial rings. The arterial rings were then mounted in a wire myograph (Model 610M; Danish Myo Technology AS, Aarhus, Denmark) as described in a previous study (21) and fixed into the 37°C thermostatic bath of a microvascular tension meter (Danish Myo Technology AS). When the tension index became stable, 6 nM ET-1 was added into basilar arteries and 40 µM potassium chloride into mesenteric arteries to induce a precontracted model. The arterial endothelial activity was evaluated by ACh with single or cumulative concentration, which is a classical tool used in medicine to assess whether the vascular endothelium is intact or impaired. In another experiment, ChCl was cumulatively mixed into the 37°C thermostatic bath in accordance with concentration-response relationships to observe the effects of the drug on the dilation of cerebral basilar or mesenteric arteries.
Analysis of the effects of acute hypoxic exposure
A total of 48 rats were used in the analysis of acute hypoxic exposure. As described above, the rats were housed individually under a 12-h light/dark cycle at a temperature of 18–24°C and relative humidity of 56±10% and provided ad libitum access to food and water under normoxic conditions. After the rats were anesthetized with 1–2% isoflurane, they were sacrificed by decapitation, the mesenteric arteries were dissected and cut into ~2-mm microarterial rings. The arterial rings were randomly divided into three groups: Normoxic control (no treatment, n=16), H1 (pretreatment with hypoxia for 1 h, n=16) and H3 (pretreatment with hypoxia for 3 h, n=16). All the arterial rings were fixed into the 37°C thermostatic bath of the microvascular tension meter and arterial tension measurements were conducted. This was carried out as described above, except that the arterial rings of the H1 and H3 groups were placed into a hypoxic incubator containing 1% O2, 92% N2 and 5% CO2, for 1 and 3 h, respectively, before they were fixed into the 37°C thermostatic bath of the microvascular tension meter.
Cell culture and treatment
A total of 5 wild-type, 3- to 5-month-old male Wistar rats, weighing 180–220 g, provided by the Experimental Animal Center of Beijing Academy of Military Medical Sciences (Beijing, China), were used for the RAECs acquisition. The rats were housed individually under a 12-h light/dark cycle at a temperature of 18–24°C and relative humidity of 56±10% and provided ad libitum access to food and water. The care and use of animals in the experiments met the standards set out in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA) and closely complied with the Animal Care and Use Committee of the Tianjin Institute of Health and Environmental Medicine (Tianjin, China). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Tianjin Institute of Health and Environmental Medicine (permit no. 2013-D-3806). Animals were administered 1–2% isoflurane through a face mask for anesthesia prior to sacrifice by cervical dislocation. The chest was opened under sterile conditions and the aortas were quickly removed and washed twice using D-Hanks solution (Beijing Leagene Biotechnology Co., Ltd., Beijing, China; pH=7.2) to wash away the blood. The connective tissues surrounding blood vessels were excluded, and the blood vessels were longitudinally cut open, and sliced into 1 mm2 sections, which were affixed to the bottom of a Petri dish. M199 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum (Hyclone; GE Healthcare, Logan, UT, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.) was slowly added to the Petri dish, once the chips were firmly affixed to the bottom. The Petri dish was transferred to an incubator at 37°C and 5% CO2 for 72 h. When a small amount of RAECs were visible, half the medium was replaced. The medium was completed replaced after 5 days, when a large number of cells had proliferated. Finally, Rat aortic endothelial cells (RAECs) were identified using an antibody against CD31 labeled with FITC (ab33858, 1:200; Abcam, Cambridge, UK). The cells from 4 to 10 generations were selected to be used in the experiments, and when cells at the logarithmic growth phase were digested into a single cell suspension and plated into 96-well plates with 102–104 cells/well, in an M199 medium supplemented with 20% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin in a 37°C, 5% CO2 incubator until they reached ~90% confluence. The cells were then randomly divided into two groups and all of them were cultured in the serum-free M199 medium. One group remained in the normoxic incubator containing 5% CO2 at 37°C, and the other was placed into a 37°C incubator containing 1% O2, 92% N2 and 5% CO2.
Analysis of the proliferation of RAECs
The proliferation and the survival rates of RAECs cultured in normoxic or hypoxic conditions were measured by MTS assay (ab197010; Abcam) in accordance with the manufacturer's instructions. In brief, cells at the logarithmic growth phase were digested into a single cell suspension and plated into 96-well plates with 102–104 cells/well, 100 µl per well. Cells were cultured in 37°C normoxic or hypoxic incubator for 48 h. 20 µl MTS solution was added per well, and incubated at 37°C for 2 h. Finally, the 96-well plates were measured under 490 nm wavelength, and the cell growth curve was drawn.
LDH assay to determine the degree of cell damage
LDH is an important glycolytic enzyme widely present in the cytoplasm, so determining its activity levels in the cell culture supernatant indicates the degree of cell damage (22). The activity of extracellular LDH was measured using an LDH Detection kit (Sigma-Aldrich; Merck KGaA). In brief, RAECs at the logarithmic growth phase were digested and plated into 96-well plates with 102–104 cells/well, in an M199 medium supplemented with 20% fetal bovine serum in the 37°C normoxic or hypoxic incubator. Cells were cultured until they adhered to the bottom, then the medium was replaced with a serum-free medium. Once the cells were treated under the normoxic or hypoxic conditions as described previously, they were collected with 1% bovine serum albumin analysis solution. Following this, 200 µl analysis solution containing 104 cells was added to 96-well plates and incubated for 2 h at 37°C in a normoxic or hypoxic incubator. Cells were then centrifuged for 10 min at 250 × g, and 100 µl supernatant per well was immediately transferred to new 96-well plates. To each well, 100 µl reaction mixture was added and incubated 30 min at room temperature. Finally, the absorbance of all samples was measured under 490 nm wavelength.
Determination of VEGF levels in cultured supernatant
After RAECs were treated with normoxic or hypoxic conditions as described above, the serum-free culture supernatant was collected (as described in LDH assay to determine the degree of cell damage), and the levels of VEGF in the supernatant were measured using commercially available ELISA kits (Cell Signaling Technology, Inc., Danvers, MA, USA), which contain the Wash Solution, Detection Antibody, Streptavidin solution and Stop Solution, according to a standard enzyme immunoassay procedure. In brief, when the serum-free cell culture supernatant was collected, 100 µl of each standard and sample was immediately added into appropriate wells. The plates was covered and incubated for 2.5 h at room temperature with gentle agitation. The solution was discarded and washed 4 times with 1X Wash Solution. Following this, 100 µl of 1X prepared Detection Antibody was added to each well and the plates were incubated for 1 h at room temperature with gentle agitation. The wash procedure was repeated and 100 µl of prepared Streptavidin solution was added to each well followed by 45 min incubation at room temperature and the wash procedure. TMB One-Step Substrate Reagent (100 µl) was added to each well. Finally, following 30 min incubation at room temperature in the dark with gentle agitation, 50 µl Stop Solution was added, and the absorbance of all samples was measured under 450 nm wavelength immediately.
Statistical analysis
All data are presented as the mean ± standard error. Results were analyzed using the Student's t-test and analysis of variance with SPSS version14.0 software (SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of CIH on the endothelial activity of rat cerebral basilar and mesenteric arterioles
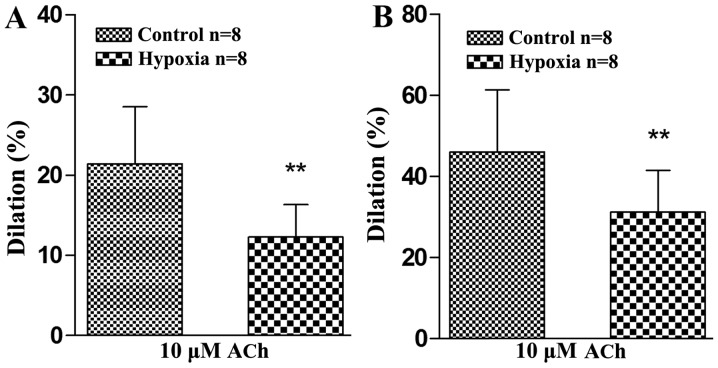
The endothelial activity of cerebral basilar and mesenteric arterioles of rats exposed to normoxic or CIH conditions were studied using 10 µM ACh. The results showed that the dilation of the normoxic control group was 21.41±7.18% when it was treated with 10 µM ACh, and that of the CIH group was 12.27±4.09% (Fig. 1A). The relaxation rate of the cerebral basilar arterioles was significantly lower in the CIH group compared with the normoxic control group (P<0.01), which suggested that the rat brain VECs had been injured by CIH treatment, and their endothelium-dependent relaxation response to ACh weakened.
Figure 1.
Effects of chronic intermittent hypoxia on endothelium-dependent vasodilatation induced by ACh in rat cerebral basilar and mesenteric arterioles. (A) Vasodilator response of cerebral basilar arteries to 10 µM ACh, after being precontracted with 6 nM endothelin 1. (B) Vasodilator response of mesenteric arteries to 10 µM ACh, after being precontracted with 40 µM potassium chloride. **P<0.01 vs. control. ACh, acetylcholine.
Likewise, vascular endothelial damage caused by CIH was also found in the mesenteric arterioles (Fig. 1B). The dilation of the normoxic control group was 46.03±15.34% after treatment with 10 µM ACh, and that of the CIH group was 31.13±10.37%. The relaxation rate of the mesenteric arterioles was significantly lower in the CIH group compared with the normoxic control group (P<0.01). This suggested that CIH exposure caused rat systemic peripheral resistance vessel endothelium injuries and weakened the endothelium-dependent relaxation response to ACh.
Vasodilator effects of choline on rat cerebral basilar arterioles and mesenteric arterioles treated with CIH
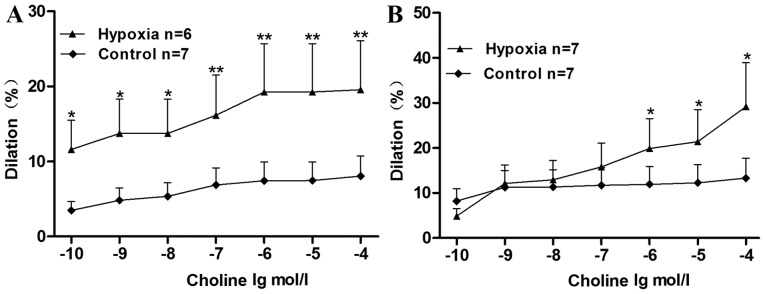
The maximum relaxation rates of cerebral basilar arterioles in the normoxic control and CIH groups after treatment with choline were 8.06±2.68 and 19.55±6.51%, respectively (P<0.01; Fig. 2A). The relaxation rate was significantly higher in the hypoxia group compared with the control group (P<0.05 for choline concentrations 10–10, 10–9 and 10–8 mol/l, P<0.01 for choline concentrations 10–7, 10–6, 10–5 and 10–4 mol/l). This indicated that the vasodilator effect of choline on the cerebral basilar arterioles was stronger in the CIH group than in the control group, suggesting that choline could have protective effects against cerebral ischemia induced by hypoxia and improve cerebral circulation.
Figure 2.
Vasodilator effects of choline on chronic intermittent hypoxia-treated rat cerebral basilar and mesenteric arterioles. (A) Vasodilator effects of choline (of varying concentrations between 10−10 and10−6 mol/l) on endothelium-intact cerebral basilar aorta rings, precontracted with 6 nM endothelin 1. (B) Vasodilator effects of choline (of varying concentrations between 10−10 and 10−6 mol/l) on endothelium-intact mesenteric aorta rings, precontracted with 40 µM potassium chloride. *P<0.05, **P<0.01 vs. control.
The vasodilator effects of choline on the mesenteric arterioles showed the same trend. The maximum relaxation rates of the normoxic control and the CIH groups after treatment with choline were 13.32±4.44 and 29.18±9.72%, respectively (P<0.05; Fig. 2B). The relaxation rate was significantly higher in the hypoxia group compared with the control group at choline concentrations of 10−6, 10−5 and 10−4 mol/l (P<0.05). This indicated that the vasodilator effect of choline on the mesenteric arterioles was greater in the CIH group than in the normoxic control group, suggesting that choline could have vasodilator effects against peripheral resistance vascular injuries induced by hypoxia.
The vasodilator effect of choline on normal arterioles was weaker than that on arterioles injured by hypoxia. The selectivity may contribute to the function of choline, currently used for treating stroke, hypertension and other acute and chronic diseases caused by hypoxia, with the advantage of less adverse reactions to the normal human. Therefore, it suggests that choline may be a potential treatment for these diseases.
Effects of acute hypoxic exposure and choline on the endothelial activity of rat isolated mesenteric arterioles
In the aforementioned experiments, it was found that the endothelial activity of rat cerebral basilar and mesenteric arterioles was affected by CIH, and choline showed protective effects against vascular endothelium damage caused by CIH. The current study subsequently investigated whether these effects were due to CIH treatment specifically, or hypoxia treatment in general.
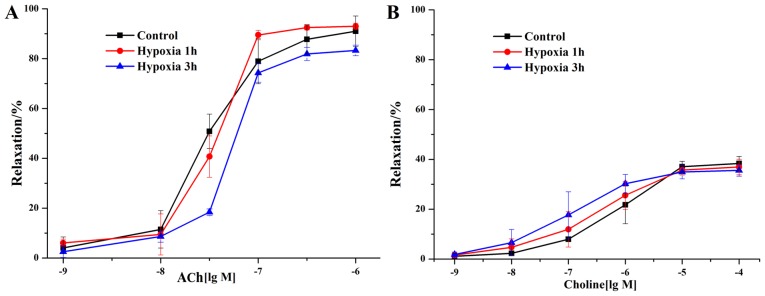
Healthy rat isolated mesenteric arteries were exposed to acute hypoxic conditions for 1 h (H1 group) or 3 h (H3 group) to observe the effects of acute hypoxic exposure on their vascular endothelial activity. To investigate the effect of acute hypoxic exposure on the endothelial activity of rat isolated mesenteric arterioles, then the arterioles was treated with 10 µM ACh. After treatment with ACh, the vascular endothelium-dependent relaxation of the H1 group was found to have no significant change compared with the control group (Fig. 3A). The relaxation response slightly decreased in the H3 group compared with the control group, but no significant difference was noted. The ACh half maximal effective concentration (EC50) values of the control, H1 and H3 groups were 3.049×10−8, 3.721×10−8 and 5.490×10−8 M, respectively.
Figure 3.
Effects of acute hypoxic exposure and choline on the endothelial activity of rat isolated mesenteric arterioles. (A) Effects of acute hypoxic exposure on the ACh-mediated endothelial activity of rat mesenteric arterioles. Healthy, normal rat isolated arteries were exposed to acute hypoxic conditions for 1 or 3 h to observe the effects of acute hypoxic exposure on their vascular endothelial activity. (B) Effects of choline on the endothelial activity of rat mesenteric arterioles under acute hypoxic exposure. ACh, acetylcholine.
Furthermore, the vasodilator effect of choline on the control, H1 and H3 groups was investigated. The response was found to be greater for the H1 group than for the control group, and the strongest effect was found in the H3 group. However, the differences between groups were not significant (Fig. 3B). The choline EC50 values of the control group, H1 group and H3 group were 7.995×10−7, 3.899×10−7 and 1.176×10−7 M, respectively. The results suggested that the vasodilator effects of choline on the mesenteric arterioles under acute hypoxic conditions gradually increased with time, but no significant differences were identified.
Effects of choline on promoting cell proliferation and protecting against cell damage under normoxic conditions
The effects of choline on promoting endothelial cell proliferation under hypoxic conditions and protecting endothelial cells against the damage caused by hypoxia were observed.
Normoxic conditions
RAECs were treated with choline for 24 h under normoxic conditions. A choline dosage of 1,000 µM was found to significantly increase the proliferation of endothelial cells compared with the control (P<0.01; Fig. 4A), but the LDH content in the supernatant did not increase significantly at any choline concentration tested (Fig. 4B). The same results and trends were found in the cells treated with choline under normoxic conditions for 48 h, although a significant increase in endothelial cell proliferation was observed from concentrations of 0.1 to 1,000 µM (P<0.01; Fig. 4C and D).
Figure 4.
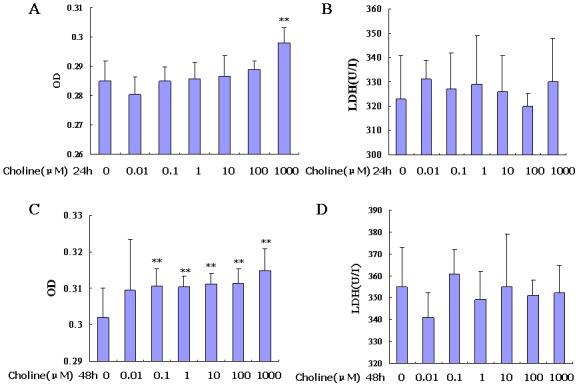
Effects of choline on promoting cell proliferation and protecting against damage under normoxic conditions. (A and B) show MTS assays and LDH activity levels, respectively, when RAECs were treated with choline for 24 h under normoxic conditions. (C and D) show, MTS assays and LDH activity levels, respectively, when RAECs were treated with choline for 48 h under normoxic conditions. **P<0.01 vs. control. RAEC, rat aortic endothelial cell; LDH, lactate dehydrogenase; OD, optical density.
Hypoxic conditions
The effects of choline on promoting cell proliferation and protecting against damage under hypoxic conditions were determined (Fig. 5). Under hypoxic conditions, choline with a concentration from 0.1 to 10 µM was able to significantly increase proliferation of RAECs, when choline treatment was for 24 (P<0.05; Fig. 5A and C). Choline treatment for both 24 and 48 h significantly reduced LDH activity in the cell supernatant, compared with the control (P<0.05; Fig. 5B and D).
Figure 5.

Effects of choline on promoting cell proliferation and protecting against damage under hypoxic conditions. (A and B) MTS assays and LDH activity levels, respectively, when RAECs were treated with choline for 24 h under hypoxic conditions. (C and D) MTS assays and LDH activity levels, respectively, when RAECs were treated with choline for 48 h under hypoxic conditions. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. hypoxia. RAEC, rat aortic endothelial cell; LDH, lactate dehydrogenase.
Mechanisms of choline in promoting endothelial cell proliferation
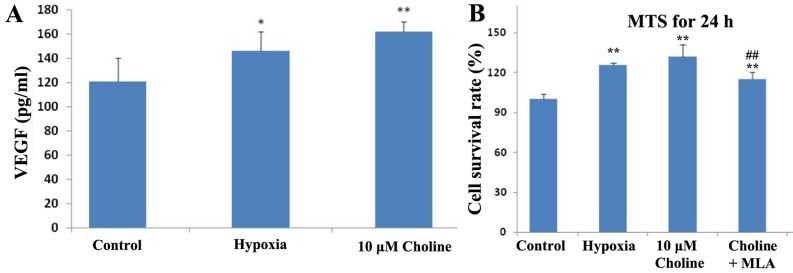
The mechanisms by which choline promotes endothelial cell proliferation were explored. First, the effect of choline on VEGF secretion of RAECs under hypoxic conditions was investigated. It was found that the quantity of VEGF in the endothelial cell culture supernatant significantly increased under hypoxic conditions for 24 h (P<0.05; Fig. 6A). When the RAECs were incubated with 10 µM choline, the quantity of VEGF in the supernatant increased further (P<0.01; Fig. 6A). Though there was no significant difference between the choline-treated RAECs and the hypoxic group (P>0.05; Fig. 6A), but there was a slight increase. This indicated that hypoxia promoted VEGF secretion from endothelial cells, and choline further increased the VEGF secretion of endothelial cells under hypoxic conditions, suggesting that the mechanism of choline stimulating endothelial cell proliferation might be related to promoting VEGF secretion.
Figure 6.
Investigating mechanisms of choline in promoting endothelial cell proliferation. (A) Effects of choline on VEGF secretion by RAECs under hypoxic conditions. (B) Influence of MLA on the choline effects of promoting endothelial cell proliferation in RAECs. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. Choline group. VEGF, vascular endothelial growth factor; RAEC, rat aortic endothelial cell; MLA, mecamylamine.
The effect of MLA, an antagonist of α7 nAChR, on choline was also observed. It was found that the effect of choline on promoting rat artery endothelial cell proliferation under hypoxic conditions was significantly inhibited by MLA (P<0.01; Fig. 6B). This suggested that choline may increase cell proliferation by activating α7 nAChR.
Discussion
Hypoxia exposure is continuous when exposed to high altitude; however, it is more common for exposure to be intermittent when it is associated with certain disease states. CIH, in particular, has been the focus of numerous clinical investigations (23–25) and animal model studies (26–29). It has been demonstrated to have epidemiological associations with mortality and morbidity in many acute and chronic diseases, including neurocognitive dysfunction (30–32), numerous cardiovascular disorders (33–35), respiratory diseases (19,36,37) and metabolic disorders (38,39). For example, obstructive sleep apnea (one of the pathological appearances of hypoxia), together with obesity, alcoholism, stress and high cholesterol, is an independent risk factor of cardiovascular and cerebrovascular disease, and closely related to their occurrence (34).
The current study aimed to provide a theoretical foundation for improving the human ability to tolerate hypoxia and effectively protecting against the damage of CIH to the human body. The effect of CIH on the ACh-mediated endothelium-dependent vasodilatation function of rat cerebral basilar and mesenteric arterioles was observed through isolated vascular ring perfusion and resistance vessel tension research platforms (21). The vascular endothelial diastolic function is an important indicator of endothelial function (40). The integrity of endothelial cell structure and function are important for maintaining the normal physiological activity of blood vessels, and once damaged, will become an influential factor in the pathogenesis of hypoxia-related diseases. In 1973, Ross and Glomset first proposed the endothelial damage response hypothesis, and since then vascular endothelial function has become a popular research focus in the study of numerous diseases (41).
In the current study, CIH was found to inhibit endothelium-dependent relaxation of rat cerebral basilar arterioles and mesenteric arterioles, which suggested that CIH caused damage to the vascular endothelium. The difference in relaxation rates between rat cerebral basilar arterioles and mesenteric arterioles might be caused by the differences of their vascular injuries or their hypoxic sensitivities.
By contrast, results from the current study showed that acute hypoxic exposure had no obvious influence on the rat mesenteric arteriole endothelium-dependent relaxation rate. This suggested that vascular endothelium may only be damaged by long-term hypoxia. Although there was no significant difference between the groups that were exposed to hypoxia for 1 or 3 h, it was speculated that with a prolonged time of hypoxia, the endothelium-dependent relaxation response would have been significantly damaged. However, the vessels were exposed to the external environment directly in the acute hypoxia experiment, which would have inevitably affected the activity of the blood vessels. This prevented further investigation by exposing the arterioles to hypoxic conditions for any longer time periods in this experiment.
As described above, choline, also known as vitamin B4, is an essential nutrient (10,11) which influences important physiological processes such as cell proliferation, differentiation, migration and apoptosis (42), and also participates in physiological functions such as signaling transduction, formation and integrity of cell membranes and ACh synthesis (14). In addition, choline metabolite betaine acts as an alternate methyl donor to folate, providing one methyl group to form methionine from homocysteine; thus, it is a major methyl donor needed for DNA and histone methylation (15,16).
Choline is essential for human beings to maintain their normal physiological function. When dietary choline is deficient, most adult men and postmenopausal women present with organ dysfunction, which manifests in conditions such as fatty liver or muscle damage. A choline-deficient diet during pregnancy may influence fetal cognitive function and visuospatial memory (43–45). It has been reported, however, that hypoxia can reduce the cellular uptake of choline (17). Thus, supplementing choline under hypoxic conditions may effectively prevent a series of problems caused by the lack of choline. In a previous study, it was found that choline increased the intracellular Ca2+ concentration, proliferation and tube formation of endothelial cells; furthermore, in the rat model of acute myocardial infarction, choline similarly enhanced the capillary density in ischemic tissues (46). In the current study, the vasodilator effect of choline was observed in the cerebral basilar arterioles and mesenteric arterioles of rats subjected to CIH using ChCl, a product form of choline. ChCl was chosen instead of native choline in this study because it has better stability, it is more easily absorbed into the tissues and causes less irritation to tissues owing to its neutrality in solution (47). In this study, choline caused a significant increase in the dilation of rat cerebral basilar arterioles and mesenteric arterioles damaged by CIH, compared with those in the normoxic control group. Notably, the vasodilatation effect of choline on the normal arterioles was weaker than that on the arterioles injured by CIH. In the acute hypoxic exposure experiments on isolated arterioles, the rat mesenteric vascular endothelium-dependent relaxation response induced by choline gradually decreased with the prolonged hypoxia exposure, but no significant differences existed between the groups treated with hypoxia for 1 or 3 h and the control group. As described above, the acute hypoxia slightly reduced the relaxation rate of the vascular endothelium as the hypoxia exposure time increased, but this reduction was not significant.
The current study found that hypoxic exposure significantly increased levels of LDH in the cell culture supernatants, which suggested hypoxia-induced endothelial cell damage. The results further showed that under both normoxic and hypoxic conditions, choline significantly increased the proliferation of RAECs. This suggested that choline only played a protective role to the endothelial cells subjected to hypoxic injuries, but had no negative effects on normal endothelium. Thus, it is proposed that choline could be developed to treat chronic diseases caused by hypoxia, with low side effects.
The mechanism by which choline protects the endothelial function has not yet been reported. Mehta et al proposed that choline reduces the levels of reactive oxygen species (ROS), thereby reducing damage to the endothelium (48). Hypoxia could induce increased levels of ROS, which would decrease vascular activity and cause damage to vascular endothelial cells. In the current study, treatment with 10 µM choline significantly increased the secretion of VEGF in endothelial cells under hypoxic conditions. VEGF promotes endothelial cell proliferation, and the neoformative endothelial cells could supplement or replace the injured or dead endothelial cells caused by hypoxia. Thus, choline may protect the endothelium against hypoxic injuries by ensuring the continuity of the endothelial cell structure and function. Finally, the current study investigated whether choline protects against hypoxic damage via α7 nAChR. MLA, an antagonist of α7 nAChR, blocked the increased cell proliferation effect of choline under hypoxic conditions, indicating that choline may be activating the target α7 nAChR. However, the exact mechanisms of how choline protects endothelial function require further study.
The current study found that ACh-mediated vasodilatation of rat cerebral basilar arterioles and mesenteric arterioles was significantly reduced in the CIH group compared with the normoxic control group. This indicated that CIH could cause rat endothelial injuries, but the acute hypoxic exposure of isolated arterioles had no significant effect on ACh-mediated vasodilatation. Furthermore, it was found that choline dilated the rat cerebral basilar arterioles and mesenteric arterioles damaged by CIH significantly more than those in the normoxic control group, but had no significant dilative effects on isolated arterioles exposed to acute hypoxia. In addition, under hypoxic conditions, choline promoted the proliferation of RAECs and significantly reduced the level of LDH in the cell culture supernatant in vitro. Meanwhile, the effect of choline might be related to its ability to significantly increase the secretion of VEGF and activate α7 nAChR under hypoxia. This study demonstrated that choline could have protective effects against hypoxic injuries.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (grant nos. 81171870 and 31470061), the Natural Science Foundation of Tianjin (grant no. 11JCYBJC14700) and the Innovation Platform Special Program of Tianjin Science and Technology Innovation System (grant no. 14JCZDJC32700).
Glossary
Abbreviations
- ACh
acetylcholine
- α7 nAChR
α7 non-neuronal nicotinic acetylcholine receptor
- CIH
chronic intermittent hypoxia
- ET-1
endothelin-1
- LDH
lactate dehydrogenase
- MLA
mecamylamine
- RAECs
rat aortic endothelial cells
- SD rats
Sprague-Dawley rats
- VECs
vascular endothelial cells
- VEGF
vascular endothelial growth factor
References
- 1.Cheng F, Lan J, Xia W, Tu C, Chen B, Li S, Pan W. Folic acid attenuates vascular endothelial cell injury caused by Hypoxia via the inhibition of Erk1/2/Nox4/Ros pathway. Cell Biochemistry and Biophysics. 2016;74:205–211. doi: 10.1007/s12013-016-0723-z. [DOI] [PubMed] [Google Scholar]
- 2.Negro R. Endothelial effects of antihypertensive treatment: Focus on irbesartan. Vasc Health Risk Manag. 2008;4:89–101. doi: 10.2147/VHRM.S1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venditti P, Pamplona R, Portero-Otin M, De Rosa R, Di Meo S. Effect of experimental and cold exposure induced hyperthyroidism on H2O2 production and susceptibility to oxidative stress of rat liver mitochondria. Arch Biochem Biophys. 2006;447:11–22. doi: 10.1016/j.abb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Xiong M, Cheng GQ, Ma SM, Yang Y, Shao XM, Zhou WH. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem Int. 2011;58:625–633. doi: 10.1016/j.neuint.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Fischer S, Renz D, Wiesnet M, Schaper W, Karliczek GF. Hypothermia abolishes hypoxia-induced hyperpermeability in brain microvessel endothelial cells. Brain Res Mol Brain Res. 1999;74:135–144. doi: 10.1016/S0169-328X(99)00272-7. [DOI] [PubMed] [Google Scholar]
- 6.Valbuena G, Walker DH. The endothelium as a target for infections. Annu Rev Pathol. 2006;1:171–198. doi: 10.1146/annurev.pathol.1.110304.100031. [DOI] [PubMed] [Google Scholar]
- 7.Michiels C, Arnould T, Remacle J. Endothelial cell responses to hypoxia: Initiation of a cascade of cellular interactions. Biochim Biophys Acta. 2000;1497:1–10. doi: 10.1016/S0167-4889(00)00041-0. [DOI] [PubMed] [Google Scholar]
- 8.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson I, Shibuya M, Wennström S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476–485. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Pitkin R, Allen L, Bailey L, Bernfield M. Dietary Reference Intakes for Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, Pantothenic acid, biotin and choline. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 11.Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- 12.McCollum EV. A History of Nutrition: The Sequence of Ideas in Nutrition Investigations. Houghton Mifflin; Boston, MA: 1957. [Google Scholar]
- 13.Best CH, Hershey JM, Huntsman ME. The effect of lecithine on fat deposition in the liver of the normal rat. J Physiol. 1932;75:56–66. doi: 10.1113/jphysiol.1932.sp002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Zeisel S. Guide To Nutritional Supplements. Elsevier; 2005. Choline and Phosphatidylcholine; pp. 108–113. [Google Scholar]
- 15.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nutr.14.1.269. [DOI] [PubMed] [Google Scholar]
- 16.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 17.Hara T, Bansal A, DeGrado TR. Effect of hypoxia on the uptake of [methyl-3H]choline, [1-14C] acetate and [18F]FDG in cultured prostate cancer cells. Nucl Med Biol. 2006;33:977–984. doi: 10.1016/j.nucmedbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wu JQ, Yang JJ, Wei JY, Gao WN, Guo CJ. Metabolomic study on vitamins B1, B2, and pp supplementation to improve serum metabolic profiles in mice under acute hypoxia based on 1h nmr analysis. Biomed Environ Sci. 2010;23:312–318. doi: 10.1016/S0895-3988(10)60069-4. [DOI] [PubMed] [Google Scholar]
- 19.Prabhakar NR. Oxygen sensing during intermittent hypoxia: Cellular and molecular mechanisms. J Appl Physiol (1985) 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer JA. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol (1985) 2001;90:1593–1599. doi: 10.1152/jappl.2001.90.4.1593. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Cheng KT, Wong CO, O'Neil RG, Birnbaumer L, Ambudkar IS, Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danz Brookins ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 24.Gozal D, O'Brien L, Row BW. Consequences of snoring and sleep disordered breathing in children. Pediatr Pulmonol Suppl. 2004;26:166–168. doi: 10.1002/ppul.70094. [DOI] [PubMed] [Google Scholar]
- 25.Bålfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150:1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 28.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004;385:217–221. doi: 10.1515/BC.2004.015. [DOI] [PubMed] [Google Scholar]
- 30.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161–166. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 31.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 33.Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med. 2000;162:2091–2096. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 34.Nieto F, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. ASsociation of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 35.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 38.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 39.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, Ewy GA, Howard BV, Punjabi NM. Sleep Heart Health Study: Diabetes and sleep disturbances: Findings from the sleep heart health study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 40.Pagnin E, Giacon B, Zaghetto F, Vianello D, Bonfante L, Huber W, Antonello A, Semplicini A, Calò L. Arterial hypertension and oxidative stress induced by cyclosporin. Effect of carvedilol. Ann Ital Med Int. 2001;16:101–105. (In Italian) [PubMed] [Google Scholar]
- 41.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 42.Thompson C. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 43.Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. NeuroReport. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 44.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 45.Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/S0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 46.Li X-W, Wang H. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sci. 2006;78:1863–1870. doi: 10.1016/j.lfs.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Riedesel CC, Hines HM. Studies on the absorption of choline chloride. J Am Pharm Assoc Am Pharm Assoc. 1953;42:579–581. doi: 10.1002/jps.3030420917. [DOI] [PubMed] [Google Scholar]
- 48.Mehta AK, Arora N, Gaur SN, Singh BP. Choline supplementation reduces oxidative stress in mouse model of allergic airway disease. Eur J Clin Invest. 2009;39:934–941. doi: 10.1111/j.1365-2362.2009.02190.x. [DOI] [PubMed] [Google Scholar]