Abstract
The diagnostic criteria of osteoporosis were established based on bone mineral density (BMD). Therefore, BMD measured by dual energy X-ray absorptiometry has been recognized as the gold standard to diagnose osteoporosis. However, discrepancies between fracture risk and BMD have been recognized. Bone is composed of collagen scaffold reinforced by hydroxyapatite. Both protein scaffold and hydroxyapatite are involved in bone quality. BMD may indicate bone mineralization but potentially fail to assess the protein scaffold. Vitamin K contributes to bone mineralization and as a protein scaffold. A deficiency of vitamin K upregulates the level of serum undercarboxylated osteocalcin (ucOC), and serum ucOC correlates with fracture risk. However, direct association of ucOC and bone quality has not been demonstrated. For the present study, a total of 49 healthy young Japanese female college students underwent calcaneal; quantitative ultrasound sonometry (QUS) and determination of serological bone metabolic markers. QUS parameters were significantly correlated with serum 25-hydroxyvitamin D (25-OH-D) concentrations (P<0.05). A significant negative correlation was also identified between log transformed serum ucOC concentrations [Ln(ucOC)] and a QUS parameter, speed of sound (SOS) (P<0.05). Stepwise multiple regression analysis indicated that Ln(ucOC) was an independent determinant of SOS, and 25-OH-D was an independent determinant of the other two QUS parameters, transmission index (TI) and synthetic parameter osteo-sono-assessment index. As vitamin D is involved in bone mineralization, TI may reflect the mineralization. Correlation of vitamin K status, indicated by ucOC, with SOS may clarify the correlation between vitamin K status and bone quality, although the material factors that connect them have not been identified.
Keywords: vitamin K-dependent bone protein, vitamin K, osteoporosis, clinical laboratory techniques, bone matrix
Introduction
The diagnostic criteria of osteoporosis was established based on bone mineral density (BMD) by the World Health Organization in 1994 (1). Therefore, BMD measured by dual energy X-ray absorptiometry (DXA) has been recognized as the gold standard to diagnose osteoporosis. However, discrepancies between fracture risk and BMD have been recognized (2,3).
Bone is composed of protein scaffolds adhered and reinforced by hydroxyapatite (4). Bone quality is affected by vitamin D and other nutrients including vitamin K, calcium (Ca) and phosphorus (P). Vitamin D is involved in bone mineralization, maintaining the serum level of the major bone minerals, Ca and P (5). Vitamin K serves as a coenzyme for γ-carboxylation of glutamic residues in protein to produce the γ-carboxyglutamic (Gla) residue. Osteocalcin, the second most abundant protein in bone, is a Gla-containing protein (6). Therefore, the level of serum undercarboxylated osteocalcin (ucOC) is influenced by the status of vitamin K (7) and associated with low bone mass (8).
The association of vitamin D status, indicated by the concentration of serum 25-hydroxyvitamin D (25-OH-D) and BMD has been demonstrated (9). By contrast, no association between ucOC and BMD was reported, despite an association with fracture risk being observed (2,3).
Quantitative ultrasound sonometry (QUS) has been widely used to assess bone quality (10–12). However, a meta-analysis concluded it does not correspond sufficiently to the DXA diagnosis (13). An association of QUS parameters with bone turnover markers has been reported in Caucasian postmenopausal elderly women (14), German men (15) and the Framingham Offspring cohort (16). Therefore, QUS may serve a role in assessing bone quality reflected by vitamin K status.
The present study aimed to assess the correlation between QUS parameters and bone metabolic parameters including serum ucOC in healthy young Japanese females.
Patients and methods
Patients
A total of 49 healthy female college students were recruited from a dormitory of Juntendo University (Chiba, Japan) in April 2013 and their food and drink intake was not controlled by the study. Any subjects that were undergoing treatment for pre-existing diseases or conditions were not included in the present study. Patient ages and anthropometric characters were as follows: Age, 18.5±0.9 years; body height (BH), 160.9±6.0 cm; body weight (BW), 54.8±6.7 kg; and body mass index (BMI), 21.1±1.9 kg/m2.
The present study was conducted according to the guidelines provided by the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of Juntendo University Graduate School of Health and Sports Science (Chiba, Japan; Approval no. 25–1). Written informed consent was obtained from all patients.
Bone quality
Calcaneal bone quality was assessed using the AOS-100NW ultrasound bone densitometry system (Hitachi, Ltd., Tokyo, Japan) according to the manufacturer's protocol. This quantitative ultrasonometry system measures two types of indices: The speed of sound (SOS) and the transmission index (TI). SOS is the ultrasound velocity that penetrates the calcaneus. Since this velocity depends on the density of the calcaneus, a higher SOS indicates a higher density. TI is an index related to the frequency-dependent attenuation of the ultrasound penetrating the heel. A higher TI indicates greater bone mass. A synthetic parameter, the osteo-sono-assessment index (OSI), is computed from SOS and TI according to the following equation: OSI=(SOS)2 × TI (17). It has been previously confirmed by DXA measurements that calcaneal OSI is associated with BMD in the distal radius (18).
Serological indices
Venous blood was collected from the cubital vein one morning in May 2013 prior to breakfast. According to the protocols provided by the manufacturers, serological indices were analyzed using the following reagents and apparatus: Serum 1,25-dihydroxyvitamin D (1,25-OH2-D) and 25-OH-D were measured by radioimmunoassay (RIA) using 1,25 (OH)2D RIA kits (Immunodiagnostic Systems Holdings PLC, Tyne and Wear, UK) and 25-hydroxyvitamin D 125I RIA Kits (DiaSorin S.p.A., Saluggia, Italy). Serum fibroblast growth factor-23 (FGF-23), N-telopeptide of type I collagen (NTX), and tartrate resistant acid phosphatase-5b (TRACP-5b) underwent ELISA using an FGF-23-ELISA kit (Kainos Laboratories, Inc., Tokyo, Japan), Osteomark® NTx serum ELISA (Alere, Inc., Waltham, MA, USA), and Osteolinks-TRACP-5b® test kit (DS Pharma Biomedical, Co., Ltd., Osaka, Japan) kits, respectively. Serum intact parathyroid hormone (PTH) and bone alkaline phosphatase (BAP) were analyzed using electrochemiluminescence immunoassay kits (Roche Diagnostics K.K., Tokyo, Japan; Beckman Coulter, Brea, CA, USA, respectively). Serum Ca and P were measured using a 7170 chemistry analyzer (Hitachi, Ltd.). Serum ucOC was assayed using a Picolumi® ucOC kit (Eisai, Co., Ltd., Tokyo, Japan). Analysis of samples was completed by a Japanese clinical analytical laboratory (SRL, Inc., Tokyo, Japan) and the references ranges used are provided in Table I.
Table I.
Serological parameters of the patients.
| Variable | Reference (range) | Mean ± SD | Range |
|---|---|---|---|
| 1,25-OH2-D (pg/ml) | 20.0–60.0a | 64.8±15.6 | 35.4–99.4 |
| 25-OH-D (ng/ml) | 7-41b | 23±5 | 13–34 |
| Ca (mg/dl) | 8.2–10.2c | 9.4±0.3 | 8.8–9.8 |
| P (mg/dl) | 2.4–4.4d | 4.2±0.4 | 3.4–4.4 |
| TRACP-5b (mU/dl) | 120-420e | 268±65 | 159–437 |
| NTX (nmol BCE/l) | 7.5–16.5e | 23.0±5.9 | 11.5–37.5 |
| BAP (ng/ml) | 2.9–14.9e | 14.8±5.0 | 6.4–28.4 |
| PTH (pg/ml) | 10-65a | 37±14 | 15–71 |
| ucOC (ng/ml) | <4.5e | 7.85±4.48 | 1.49–24.49 |
| FGF-23 (pg/ml) | – | 43±10 | 25–78 |
Provided by the manufacturer of the reagents or kit
ref (33)
ref (34)
provided by the clinical laboratory SRL (Tokyo, Japan) that assessed subjects
ref (32). SD, standard deviation; 1,25-OH2-D, 1,25-dihydroxyvitamin D; 25-OH-D, 25-hydroxyvitamin D; Ca, calcium; P, phosphorus; TRACP-5b, tartrate resistant acid phosphatase-5b; NTX, N-telopeptide of type I collagen; BAP, bone alkaline phosphatase; PTH, parathyroid hormone; ucOC, undercarboxylated osteocalcin; FGF-23, fibroblast growth factor-23.
Statistical analysis
Descriptive data are presented as means ± standard deviation, unless otherwise noted. The normality of the data was assessed by the Shapiro-Wilk test. Variables not normally distributed were natural log transformed.
Associations between two parameters were assessed using Pearson's correlation coefficient, with r>0.2 considered to indicate statistical significance. A stepwise multiple linear regression analysis was performed to explore determinants of QUS parameters, removing the variables if the probability of F≥0.1. The following plausible anthropometric and serological parameters were included in the model: Age, BW, BH, BMI, 1,25-OH2-D, 25-OH-D, Ca, P, TRACP-5b, NTX, PTH, and Log transformed concentrations of BAP, ucOC and FGF-23; denoted as Ln(BAP), Ln(ucOC) and Ln(FGF-23), respectively.
P<0.05 was considered to represent a statistically significant difference. The statistical analysis was performed using SPSS software, version 19 (IBM SPSS, Armonk, NY, USA).
Results
Serological markers
The mean 25-OH-D concentration (23.1 ng/ml) was above the vitamin D insufficiency borderline (20 ng/ml); however, 16 patients (33%) did not exceed the borderline. By contrast, the mean 1,25-OH2-D (64.77 pg/ml) was higher than the reference range (20.0–60.0 pg/ml). The mean ucOC (7.851 ng/ml) was higher than the cut-off level (<4.5 ng/ml) and only 8 subjects (16%) were below the cut-off level. The mean NTX [23.02 nmol bone collagen equivalent (BCE)/l] and BAP (14.78 ng/ml) exceeded the reference ranges of NTX (11.5–37.5 nmol BCE/l) and BAP (6.4–28.3 ng/ml), and 7 (14%) and 25 (51%) subjects were within the ranges for NTX and BAP, respectively. Other parameters were primarily distributed within the reference range. Blood biomarker concentrations are summarized in Table I.
QUS and anthropometric parameters
The mean SOS (1,600 m/sec), TI (1.223) and OSI (3.140×106) were higher than the means of reference values from matched age and gender Japanese individuals. Even the minimum values were within the mean ± 2x standard deviations of all parameters (Table II). Reference values were provided by the ultrasonometry equipment manufacturer (Hitachi, Ltd.).
Table II.
QUS parameters measured in subjects.
| Variable | Reference (mean ± SD) | Reference (range) | Mean ± SD |
|---|---|---|---|
| SOS (m/sec) | 1.600±28 | 1.539-1,651 | 1.564.4±21.7 |
| TI | 1.223±0.107 | 1.005–1.005 | 1.091±0.074 |
| OSI, ×106 | 3.140±0.370 | 2.417–4.417 | 2.698±0.298 |
Reference range indicates the distribution in healthy Japanese females provided by the clinical laboratory. SD, standard deviation; QUS, quantitative ultrasound sonometry; SOS, speed of sound; TI, transmission index; OSI, osteo-sono-assessment index.
All QUS parameters were significantly associated with BW and BMI, and TI exhibited a significant association with BH (Table III). The strongest correlation was identified between TI and BW (r=0.493). SOS was primarily associated with BMI (r=0.364). The synthetic variable OSI exhibited significant associations with BW (r=0.455) and BMI (r=0.410; Table III).
Table III.
Associations between QUS parameters with age, body height, body weight and body mass index of subjects analyzed by Pearson's correlation coefficient.
| Age | Body height | Body weight | Body mass index | ||
|---|---|---|---|---|---|
| SOS | r | 0.155 | 0.046 | 0.301a | 0.364b |
| P-value | 0.289 | 0.756 | 0.036 | 0.010 | |
| TI | r | 0.012 | 0.295a | 0.493b | 0.408b |
| P-value | 0.933 | 0.039 | <0.001 | 0.004 | |
| OSI | r | 0.059 | 0.233 | 0.455b | 0.410b |
| P-value | 0.687 | 0.107 | 0.001 | 0.003 |
P<0.05
P<0.01. QUS, quantitative ultrasound sonometry; SOS, speed of sound; r, Pearson's correlation coefficient; TI, transmission index; OSI, osteo-sono-assessment index.
Correlations between serological and QUS parameters
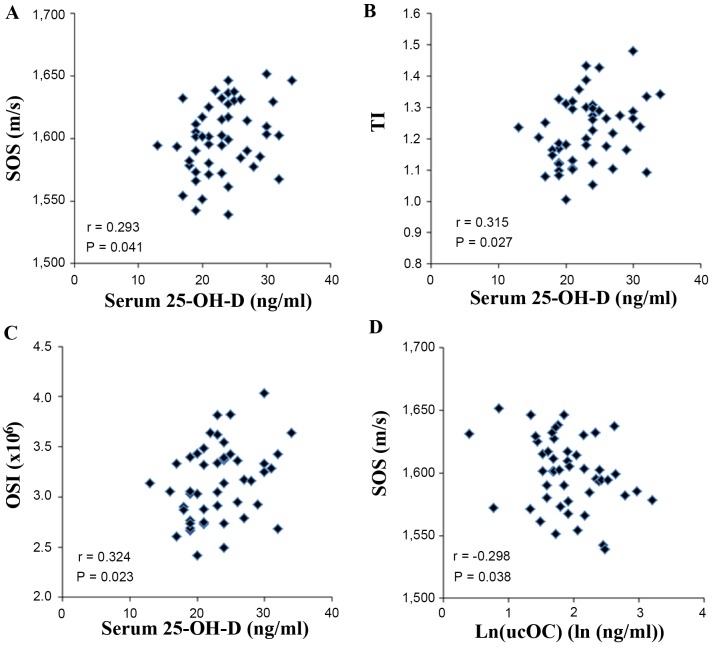
The association between serological and QUS parameters was assessed using the Pearson's correlation coefficient. The concentrations of BAP, ucOC and FGF-23 were natural log transformed to achieve a normal distribution (Table I). The level of 25-OH-D indicated a significant positive correlation (P<0.05) with all QUS parameters: SOS (r=0.293), TI (r=0.315) and OSI (r=0.324). Ln(ucOC) correlated negatively with SOS (r=−0.298; Fig. 1). The other serological parameters did not exhibit any significant correlations with QUS parameters (Table IV).
Figure 1.
Correlations between serological markers and QUS parameters. (A) Serum 25-OH-D and SOS, (B) serum 25-OH-D and SOS, (C) serum 25-OH-D and (D) Log transformed serum ucOC and SOS. P<0.05 represents a statistically significant difference. 25-OH-D, 25-hydroxyvitamin D; SOS, speed of sound; ucOC, undercarboxylated osteocalcin.
Table IV.
Correlations between QUS parameters and serological parameters.
| 1,25-OH-D | 25-OH-D | Ca | P | TRACP-5b | NTX | Ln(BAP) | PTH | Ln(ucOC) | Ln(FGF-23) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOS | r | −0.110 | 0.293a | 0.033 | −0.019 | −0.068 | −0.242 | −0.089 | −0.109 | −0.298a | 0.198 |
| P-value | 0.454 | 0.041 | 0.819 | 0.899 | 0.641 | 0.094 | 0.544 | 0.455 | 0.038 | 0.173 | |
| TI | r | −0.007 | 0.315a | 0.158 | −0.047 | 0.125 | −0.105 | 0.087 | −0.043 | −0.216 | 0.109 |
| P-value | 0.960 | 0.027 | 0.278 | 0.748 | 0.390 | 0.472 | 0.554 | 0.767 | 0.137 | 0.455 | |
| OSI | r | −0.041 | 0.324a | 0.129 | −0.047 | 0.069 | −0.145 | 0.034 | −0.060 | −0.253 | 0.141 |
| P-value | 0.779 | 0.023 | 0.379 | 0.750 | 0.636 | 0.320 | 0.817 | 0.681 | 0.080 | 0.334 |
P<0.05. r, Pearson's correlation coefficient; QUS, quantitative ultrasound sonometry; SOS, speed of sound; TI, transmission index; OSI, osteo-sono-assessment index; 1,25-OH-D, 1,25-hydroxyvitamin D; 25-OH-D, 25-hydroxyvitamin D; Ca, calcium; P, phosphorus; TRACP-5b, tartrate resistant acid phosphatase-5b; NTX, N-telopeptide of type I collagen; Ln(BAP), log transformed serum concentration of bone alkaline phosphatase; PTH, parathyroid hormone; Ln(ucOC), log transformed serum concentration of undercarboxylated osteocalcin; Ln(FGF-23), long transformed serum concentration of fibroblast growth factor-23.
Stepwise regression analysis to examine determinants of QUS parameters
From the correlation analysis, anthropometric parameters, the level of 25-OH-D and Ln(ucOC) were considered to be associated with QUS parameters, although the correlation coefficients were weak (0.2<r<0.4; Table IV). However, the correlation with anthropometric parameters may conceal the associations between serological and QUS parameters. Therefore, stepwise multiple linear regression analysis was performed to examine the determinants for QUS parameters. In the model, in addition to anthropometric parameters, 25-OH-D and Ln(ucOC), the following parameters were included: Bone formation marker Ln(BAP), bone absorption markers (NTX and TRAP-5b), and markers reflecting Ca and P status [Ca, P, 1,25-OH2-D, PTH and Ln(FGF-23)].
The regression analysis provided well-fitted predictive equations (P<0.003). This indicated that Ln(ucOC) and BMI were independent determinants of SOS, whereas 25-OH-D and BW were independent determinants of TI and OSI. For OSI, Ln(ucOC) was also selected in the final model, but the probability was not significant (P=0.100; Table V).
Table V.
Stepwise regression analysis to explore the determinants for QUS parameters.
| Dependent variable | Independent variable | Std coefficient | P-values (Coefficient) | P-values (Model) |
|---|---|---|---|---|
| SOS | BMI | 0.363 | 0.008b | 0.003b |
| Ln(ucOC) | −0.297 | 0.027a | ||
| TI | BW | 0.488 | <0.001b | <0.001b |
| 25-OH-D | 0.308 | 0.014a | ||
| OSI | BW | 0.457 | <0.001b | <0.001b |
| 25-OH-D | 0.272 | 0.033a | ||
| Ln(ucOC) | −0.207 | 0.100 |
P<0.05
P<0.01. Std coefficient, standardized coefficient; P (Coefficient), P-value of the coefficient; P (Model), P-value of the model; CI, confidence index; SOS, speed of sound; TI, transmission index; OSI, osteo-sono-assessment index; BMI, body mass index; Ln(ucOC), log transformed serum concentration of undercarboxylated osteocalcin; BW, body weight; 25-OH-D, 25-hydroxyvitamin D.
Discussion
The present study examined bone metabolic markers and QUS parameters in Japanese females. Overall, 33% of the subjects exhibited vitamin D deficiency (25-H-D<20 ng/ml) and 84% were suspected to have vitamin K insufficiency according to their levels of ucOC (>4.5 ng/ml). The bone quality was assessed by QUS, and typically was above the reference range of an age- and gender-matched Japanese population. All QUS parameters indicated positive correlations with BW and BMI. Significant but moderately weak correlations were also identified for SOS with ucOC, and for TI and OSI with 25-OH-D. Stepwise multiple regression analysis demonstrated that Ln(ucOC) and BMI were independent determinants of SOS, while 25-OH-D and BW were independent determinants of TI and OSI.
Serum 25-OH-D correlated with TI and OSI, but not with SOS. Sohl et al (19) reported that 25-OH-D was associated with BMD and another QUS parameter broadband ultrasound attenuation (BUA), but not with SOS, in elderly Dutch subjects with a low-to-normal BMI (<25 kg/m2). Tanabe et al (20) demonstrated the effect of genetic variation. A significant correlation between QUS parameters and serum 25-OH-D was observed in FF type but not in ff type vitamin D receptor polymorphism in young Japanese adults. Since the incidence rate of type was small (12%) in the Japanese population (20), these correlations may be observed. However, the polymorphism was not assessed in the present subjects.
Bone is composed of collagen scaffold strengthened by calcium hydroxyapatite (4). Since serum 25-OH-D indicates vitamin D status, it affects bone mineralization via serum calcium concentration. Therefore, the association of 25-OH-D and BMD was demonstrated in subjects of the US National Health and Nutrition Examination Surveys (9). The association of 25-OH-D and TI and OSI was thus considered to be indicative of BMD.
On stepwise multiple regression analysis, Ln(ucOC) and BMI were demonstrated to be independent determinants of SOS, and 25-OH-D and BW were independent determinants of TI and OSI. The associations of anthropometric parameters with QUS parameters were generally in accordance with Brunner et al (11). The major finding of the present study was the clarification of the independent association between ucOC and SOS.
An association between serum ucOC and hip fracture has been reported (8,21,22). Tsugawa et al (2) reported the negative association of vertebrate fracture incident with the serum phylloquinone (vitamin K1) level. Stratified by the serum vitamin K1 level, vertebral fracture incidents were higher in the low vitamin K1 group; however, no significant difference was observed in BMD (2). Specifically, it was observed that the correlation coefficient between log transformed serum ucOC and fracture incidents was positive, though not significant [P=0.088; (2)]. Shiraki et al (3) also documented that fracture risk was associated with serum ucOC but not with BMD in osteoporotic patients. A meta-analysis indicated that vitamin K antagonists increased the fracture risk, but did not affect BMD (23). Therefore, vitamin K status should be associated with fracture risk, independent of BMD.
Vitamin K contributes to bone quality via two mechanisms. First, it serves as a coenzyme to mature Gla proteins. Bone Gla proteins hold calcium ions to strengthen the bone. This mechanism may contribute to bone mineralization (24). Second, it serves as a ligand for the steroid and xenobiotic receptor (SXR), to stimulate the expression of ‘tsukushin’ (TSK) (25). TSK is an extracellular protein involved in collagen assembly. SXR also stimulates matrilin-2 (MATN2), a matrix protein participating in the formation of fibrillar or filamentous structures. TSK and MATN2 are associated with collagens to promote collagen-accumulation in osteoblastic cells (26). This mechanism may affect the scaffold structure of bone.
The bone hydroxyapatite accounts for 60% of human bone, and its increase strengthens but decreases the flexibility of the bone. The cross-linking of the collagen scaffold also affects bone strength (4). Therefore, mineralization and scaffold structure contribute to bone quality. Bone mineralization is measured by DXA, as BMD, but BMD may not represent quality of the scaffold structure. This may account for the absence of an association of BMD with fracture risk (2,3).
The association of SOS with BMD has been controversial: Hans et al (12) supported the association, but Brooke-Wavell et al (10) did not. The results of the present study clarify the association of SOS with ucOC in young Japanese females, at the age where the highest BMD is identified, independent of anthropometric and other serological bone markers.
According to the QUS parameters, the subjects in the current study had an improved bone quality than the same age/sex population of Japanese individuals. All subjects were physically active college students, as the majority of them belonged to a college sports club, though they were not initially selected based on physical activity. This may account for the improved bone quality of this group, since physical activity positively correlates with bone status (27,28).
The vitamin D status of the subjects was also improved, compared with the same age/sex population of Japanese individuals (29,30). Nakamura et al (29) indicated that the level of 25-OH-D in Japanese females aged 19–24 years were 13.7±4.8 ng/ml. Ohta et al (30) reported that the 25-OH-D level in 274 Japanese women aged 19–25 years was 18.7±4.8 ng/ml. As with bone quality, the physical activity of the subjects may contribute to the vitamin D status (27,28), though it is unknown whether subjects in the Ohta et al study were more physically active.
The mean NTX and BAP values were higher than the reference ranges. Both parameters are indicators of bone turnover, which is high in childhood and decreases with age; they remain high at the age of the current subjects' (31). By contrast, the reference ranges were established in adult women aged 30–44 years (32), which may explain the discrepancy.
The correlation of QUS parameters with anthropometric parameters was in accordance with a previous study by Brunner et al (11), where positive correlations were identified between SOS, BMI and BW in elderly German women using an Achilles + Solo ultrasound bone densitometer (GE Lunar Corporation, Madison, WI, USA). Correlations were also identified in BUA and the synthetic parameter stiffness index calculated from SOS and BUA.
The limitations in this study were as follows: The major limitation was the absence of DXA assessment. The discrepancy of BMD with SOS and ucOC should be assessed in the same population at the same time. Second, the study subjects were a specific population, having almost the same ages and the sample size was small. Although the majority of the results were in accordance with previous studies, further study on a variety of subjects should be warranted to generalize the results. Third, bone quality is influenced by ethnicity, sex, age, nutritional status, physical activity, sun exposure and genetic background. In the current study, ethnicity, sex and age were identical; Ca, P, vitamin D and vitamin K were assessed by serological indices, while physical activity, sun exposure, and genetic polymorphism were not assessed. These factors should be monitored and controlled in a future study. Finally, although correlation of serum ucOC with SOS was demonstrated, serum ucOC is biological and SOS is physical, and material factors to connect them were not identified. Therefore, further studies are warranted.
In conclusion, serum 25-OH-D and ucOC were identified to have an association with calcaneal QUA parameters, TI and SOS, respectively, independent from other bone turnover indices and anthropometric parameters. As vitamin D is involved in bone mineralization, TI may reflect the level of mineralization. Vitamin K status, indicated by ucOC, was demonstrated to correlate with SOS. This may facilitate future studies to clarify the presence of a correlation between vitamin K status and bone quality, although the factors that connect them have not yet been identified.
Acknowledgements
The authors of the present study would like to thank all subjects who participated in the study. A part of this study was presented at a local meeting of the Japanese Society of Physical Fitness and Sports Medicine in March 2014 in Tokyo, Japan. The present study was funded in part by the Japan Dairy Association (J-milk; Tokyo, Japan) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) - Supported Program for the Strategic Research Foundation at Private Universities (grant no. S1101008; Tokyo, Japan).
Glossary
Abbreviations
- BMD
bone mineral density
- DXA
dual energy X-ray absorptiometry
- Gla
γ-carboxyglutamic residue
- ucOC
undercarboxylated osteocalcin
- 25-OH-D
25-hydroxyvitamin D
- QUS
quantitative ultrasound sonometry
- BH
body height
- BW
body weight
- BMI
body mass index
- SOS
speed of sound
- TI
transmission index
- OSI
osteo-sono-assessment index
- 1,25-OH2-D
1,25-dihydroxyvitamin D
- RIA
radioimmunoassay
- FGF-23
fibroblast growth factor-23
- NTX
N-telopeptide of type I collagen
- TRACP-5b
tartrate resistant acid phosphatase-5b
- PTH
parathyroid hormone
- BAP
bone alkaline phosphatase
- Ca
calcium
- P
phosphorus
- TSK
tsukushin
- SXR
steroid and xenobiotic receptor
- MATN2
matrilin-2
References
- 1.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 2.Tsugawa N, Shiraki M, Suhara Y, Kamao M, Ozaki R, Tanaka K, Okano T. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26:79–85. doi: 10.1007/s00774-007-0790-8. [DOI] [PubMed] [Google Scholar]
- 3.Shiraki M, Yamazaki Y, Shiraki Y, Hosoi T, Tsugawa N, Okano T. High level of serum undercarboxylated osteocalcin in patients with incident fractures during bisphosphonate treatment. J Bone Miner Metab. 2010;28:578–584. doi: 10.1007/s00774-010-0167-2. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29:621–631. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Cashman KD. Diet, Nutrition, and bone health. J Nutr. 2007;137:2507S–2512S. doi: 10.1093/jn/137.11.2507S. (11 Suppl) [DOI] [PubMed] [Google Scholar]
- 7.Sokoll LJ, Booth SL, O'Brien ME, Davidson KW, Tsaioun KI, Sadowski JA. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr. 1997;65:779–784. doi: 10.1093/ajcn/65.3.779. [DOI] [PubMed] [Google Scholar]
- 8.Vergnaud P, Garnero P, Meunier PJ, Bréart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: The EPIDOS study. J Clin Endocrinol Metab. 1997;82:719–724. doi: 10.1210/jcem.82.3.3805. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National health and nutrition examination survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooke-Wavell K, Khan AS, Taylor R, Masud T. Lower calcaneal bone mineral density and broadband ultrasonic attenuation, but not speed of sound, in South Asian than white European women. Ann Hum Biol. 2008;35:386–393. doi: 10.1080/03014460802089817. [DOI] [PubMed] [Google Scholar]
- 11.Brunner C, Pons-Kühnemann J, Neuhäuser-Berthold M. Impact of age, anthropometric data and body composition on calcaneal bone characteristics, as measured by quantitative ultrasound (QUS) in an older German population. Ultrasound Med Biol. 2011;37:1984–1992. doi: 10.1016/j.ultrasmedbio.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Hans D, Wu C, Njeh CF, Zhao S, Augat P, Newitt D, Link T, Lu Y, Majumdar S, Genant HK. Ultrasound velocity of trabecular cubes reflects mainly bone density and elasticity. Calcif Tissue Int. 1999;64:18–23. doi: 10.1007/s002239900572. [DOI] [PubMed] [Google Scholar]
- 13.Nayak S, Olkin I, Liu H, Grabe M, Gould MK, Allen IE, Owens DK, Bravata DM. Meta-analysis: Accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144:832–841. doi: 10.7326/0003-4819-144-11-200606060-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lappa V, Dontas IA, Trovas G, Constantelou E, Galanos A, Lyritis GP. Quantitative ultrasound is better correlated with bone mineral density and biochemical bone markers in elderly women. Clin Rheumatol. 2007;26:1067–1073. doi: 10.1007/s10067-006-0448-2. [DOI] [PubMed] [Google Scholar]
- 15.Kyvernitakis I, Saeger U, Ziller V, Bauer T, Seker-Pektas B, Hadji P. The effect of age, sex hormones, and bone turnover markers on calcaneal quantitative ultrasonometry in healthy German men. J Clin Densitom. 2013;16:320–328. doi: 10.1016/j.jocd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.McLean RR, Booth SL, Kiel DP, Broe KE, Gagnon DR, Tucker KL, Cupples LA, Hannan MT. Association of dietary and biochemical measures of vitamin K with quantitative ultrasound of the heel in men and women. Osteoporos Int. 2006;17:600–607. doi: 10.1007/s00198-005-0022-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda-Futami E, Hans D, Njeh CF, Fuerst T, Fan B, Li J, He YQ, Genant HK. An evaluation of a new gel-coupled ultrasound device for the quantitative assessment of bone. Br J Radiol. 1999;72:691–700. doi: 10.1259/bjr.72.859.10624327. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki M, Harata S, Kumazawa Y, Mita R, Kida K, Tsuge M. Bone mineral density and osteo sono assessment index in adolescents. J Orthop Sci. 2000;5:185–191. doi: 10.1007/s007760050149. [DOI] [PubMed] [Google Scholar]
- 19.Sohl E, de Jongh RT, Swart KM, Enneman AW, van Wijngaarden JP, van Dijk SC, Ham AC, van der Zwaluw NL, Brouwer-Brolsma EM, van der Velde N, et al. The association between vitamin D status and parameters for bone density and quality is modified by body mass index. Calcif Tissue Int. 2015;96:113–122. doi: 10.1007/s00223-014-9943-7. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe R, Kawamura Y, Tsugawa N, Haraikawa M, Sogabe N, Okano T, Hosoi T, Goseki-Sone M. Effects of Fok-I polymorphism in vitamin D receptor gene on serum 25-hydroxyvitamin D, bone-specific alkaline phosphatase and calcaneal quantitative ultrasound parameters in young adults. Asia Pac J Clin Nutr. 2015;24:329–335. doi: 10.6133/apjcn.2015.24.2.01. [DOI] [PubMed] [Google Scholar]
- 21.Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res. 1994;9:1591–1595. doi: 10.1002/jbmr.5650091012. [DOI] [PubMed] [Google Scholar]
- 22.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993;91:1769–1774. doi: 10.1172/JCI116387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veronese N, Bano G, Bertozzo G, Granziera S, Solmi M, Manzato E, Sergi G, Cohen AT, Correll CU. Vitamin k antagonists' use and fracture risk: Results from a systematic review and meta-analysis. J Thromb Haemost. 2015;13:1665–1675. doi: 10.1111/jth.13052. [DOI] [PubMed] [Google Scholar]
- 24.Bügel S. Vitamin K and bone health in adult humans. Vitam Horm. 2008;78:393–416. doi: 10.1016/S0083-6729(07)00016-7. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281:16927–16934. doi: 10.1074/jbc.M600896200. [DOI] [PubMed] [Google Scholar]
- 26.Horie-Inoue K, Inoue S. Steroid and xenobiotic receptor mediates a novel vitamin K2 signaling pathway in osteoblastic cells. J Bone Miner Metab. 2008;26:9–12. doi: 10.1007/s00774-007-0792-6. [DOI] [PubMed] [Google Scholar]
- 27.Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: A comparative study between impact-loading and non-impact-loading sports. Osteoporos Int. 1998;8:152–158. doi: 10.1007/BF02672512. [DOI] [PubMed] [Google Scholar]
- 28.Creighton DL, Morgan AL, Boardley D, Brolinson PG. Weight-bearing exercise and markers of bone turnover in female athletes. J Appl Physiol. 2001;90:565–570. doi: 10.1152/jappl.2001.90.2.565. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Nashimoto M, Tsuchiya Y, Obata A, Miyanishi K, Yamamoto M. Vitamin D insufficiency in Japanese female college students: A preliminary report. Int J Vitam Nutr Res. 2001;71:302–335. doi: 10.1024/0300-9831.71.5.302. [DOI] [PubMed] [Google Scholar]
- 30.Ohta H, Kuroda T, Onoe Y, Orito S, Ohara M, Kume M, Harada A, Tsugawa N, Okano T, Sasaki S. The impact of lifestyle factors on serum 25-hydroxyvitamin D levels: A cross-sectional study in Japanese women aged 19–25 years. J Bone Miner Metab. 2009;27:682–688. doi: 10.1007/s00774-009-0095-1. [DOI] [PubMed] [Google Scholar]
- 31.Orito S, Kuroda T, Onoe Y, Sato Y, Ohta H. Age-related distribution of bone and skeletal parameters in 1,322 Japanese young women. J Bone Miner Metab. 2009;27:698–704. doi: 10.1007/s00774-009-0094-2. [DOI] [PubMed] [Google Scholar]
- 32.Nishizawa Y, Ohta H, Miura M, Inaba M, Ichimura S, Shiraki M, Takada J, Chaki O, Hagino H, Fujiwara S, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition) J Bone Miner Metab. 2013;31:1–15. doi: 10.1007/s00774-012-0392-y. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Okano T, Shida S, Okada K, Suginohara T, Nakao H, Kuroda E, Kodama S, Matsuo T. Variation of 25-hydroxyvitamin-D3 and 25-hydroxy-vitamin-D2 levels in human-plasma obtained from 758 Japanese healthy-subjects. J Nutr Sci Vitaminol (Tokyo) 1983;29:271–281. doi: 10.3177/jnsv.29.271. [DOI] [PubMed] [Google Scholar]
- 34.Takei I, Oguchi S, Ishibashi M, Ishida K, Sekiguchi H, Kikuchi H, Watanabe K, Sasaki A, Imamura C, Kohka K, Gonaikawa S, Aoki Y, Kano S, Kobayashi T, Miyazaki N. Reference ranges of endocrine, lipid and chemical laboratory tests at Keio University affiliated hospitals. Keio Igaku. 2003;80:105–110. [Google Scholar]