Abstract
Context:
Increased prevalence of type 2 diabetes mellitus and prediabetes worldwide is attributed in part to an unhealthy diet.
Objective:
To evaluate whether 12 weeks of high monounsaturated fatty acid (MUFA) or fiber-rich weight-maintenance diet lowers hepatic fat and improves glucose tolerance in people with prediabetes.
Design:
Subjects underwent a [6, 6-2H2]–labeled 75-g oral glucose tolerance test to estimate hepatic insulin sensitivity and liver fat fraction (LFF) using magnetic resonance spectroscopy before and after intervention.
Setting:
Mayo Clinic Clinical Research Trials Unit.
Participants:
43 subjects with prediabetes.
Intervention:
Subjects were randomized into three isocaloric weight-maintaining diets containing MUFA (olive oil), extra fiber, and standard US food (control-habitual diet).
Outcome Measures:
LFF, glucose tolerance, and indices of insulin action and secretion.
Results:
Body weight was maintained constant in all groups during the intervention. Glucose and hormonal concentrations were similar in all groups before, and unchanged after, 12 weeks of intervention. LFF was significantly lower after intervention in the MUFA group (P < 0.0003) but remained unchanged in the fiber (P = 0.25) and control groups (P = 0.45). After 12 weeks, LFF was significantly lower in the MUFA than in the control group (P = 0.01), but fiber and control groups did not differ (P = 0.41). Indices of insulin action and secretion were not significantly different between the MUFA and control groups after intervention (P ≥ 0.11), but within-group comparison showed higher hepatic (P = 0.01) and total insulin sensitivity (P < 0.04) with MUFA.
Conclusions:
Twelve weeks of a MUFA diet decreases hepatic fat and improves both hepatic and total insulin sensitivity.
We studied people with prediabetes and found that a 12-week diet supplemented with olive oil lowered liver fat measured with MRS and improved hepatic insulin sensitivity in the absence of weight loss.
The exponential increase in the prevalence of type 2 diabetes mellitus and prediabetes worldwide in recent years has been attributed, at least in part, to the modern lifestyle, and in particular, an unhealthy diet and lack of physical activity (1).
Prediabetes leads to overt type 2 diabetes in up to 50% of patients >5 to 10 years after diagnosis (2–4) and is associated with fatty liver (5), which has been implicated as a marker of insulin resistance. The latter is strongly linked to obesity, prediabetes, and type 2 diabetes.
Diet and nutrition play an important role in the primary prevention of prediabetes. Weight loss reduces liver fat (6–8) and is recommended in overweight patients with nonalcoholic fatty liver disease (NAFLD) and insulin resistance (9, 10). Weight loss, with calorie restriction and/or exercise (i.e., lifestyle changes), has also been shown to modestly improve glucose metabolism (6, 11–13). Prior studies using isocaloric diets, either low fat–high carbohydrate or high fat–low carbohydrate, have shown that liver fat content reduced in the former diet compared with the latter, with either no changes or very minimal changes to total and peripheral insulin sensitivity (14–19). However, the studies were of varying design and duration, some had a very small number of subjects, and some did not directly assess hepatic insulin sensitivity. Whether short-term dietary changes in the absence of weight loss can lower hepatic fat and improve hepatic insulin sensitivity is debatable.
There is increasing evidence in favor of high monounsaturated fatty acid (MUFA) and/or low calorie diets in managing people with type 2 diabetes regarding improving glycemic control and lowering low-density lipoprotein cholesterol levels (20, 21). High-fiber diets have been shown to improve carbohydrate and fat metabolism and prevent type 2 diabetes (22). Common dry beans (Phaseolus vulgaris L) are rich in dietary fiber and have up to 25% of their dry weight being comprised of protein (amino acids lysine and leucine) (23). Therefore, in this study we elected to use a combination of common dry beans and fiber bars as sources of fiber to mimic a healthy and acceptable diet.
The purpose of this study was to determine the effects of a weight-maintaining, short-term isocaloric dietary intervention with food rich in MUFA or fiber compared with a habitual diet (control) on hepatic fat content as a primary aim, and secondarily to explore whether changes in hepatic fat content impact carbohydrate metabolism in subjects with prediabetes.
Subjects and Methods
Subjects
After approval from the institutional review board, 55 subjects with prediabetes, as defined by current American Diabetes Association criteria, provided written consent to take part in this study. After screening and a run-in period, 43 (14 normal fasting glucose/impaired glucose tolerance; 8 impaired fasting glucose/normal glucose tolerance; 21 impaired fasting glucose/impaired glucose tolerance) of the 55 subjects were randomized to study. All participants were in good health, had a stable weight, and were not engaged in exercise programs. Metabolically neutral medications, such as thyroid medication, statins, and low-dose thiazides, were permitted. Individuals with a history of alcohol consumption above the American Diabetes Association guidelines (i.e., two drinks per day for men and one drink per day for women) or any chronic medical condition or any disorder that could potentially impact the outcome measures were excluded from participating in the study.
Study design
This was a randomized trial with two interventional groups consuming additional MUFA (n = 15) or fiber (n = 15) in their diet and a control group (n = 13), which represented the habitual diet consisting of high carbohydrate, low fat, and low fiber. The data analysts remained blinded to the study arms. After an overnight fast, subjects underwent a 3-hour labeled oral glucose tolerance test (OGTT) with 4% enriched [6,6-2H2] glucose to measure hepatic insulin sensitivity. Anthropometric measurements were recorded for all subjects. Body composition was measured using lunar dual-energy x-ray absorptiometry (version 11.0; GE Health care Technologies, Madison, WI). Hepatic fat content was measured using proton magnetic resonance spectrometry (MRS) by a radiologist blinded to the study. A Web-based, self-administered, graphical Food Frequency Questionnaire (Viocare Technology, Princeton, NJ) (24) was used to assess each subject’s nutritional intake over the previous 3 months. If a subject reported substantial MUFA or fiber-rich food in their diet at screening, then they were deemed ineligible for the study. Prior to the dietary intervention, subjects were instructed to follow a 1-week run-in period on their habitual diet without olive oil, dry beans, or other fiber-rich foods.
All participants were instructed to follow an isocaloric diet to maintain a constant body weight (within 2%) during the entire study. Subjects were required to visit the Clinical Research and Trials Unit (CRTU) every 2 weeks for weight measurement and submission of 3-day food records, and to receive the predefined dietary products (i.e., olive oil, dry beans, fiber bars). The control group was asked to continue their habitual intake of food groups, but they were required to come to the CRTU every 2 weeks as well to review their food records and be weighed. Food records were analyzed for macronutrient consumption using ESHA Food Processor software, Version 10.11 (ESHA Research, Salem, OR). Subjects randomized to the MUFA group were instructed to consume 28% of total energy intake from MUFA, with 50% of the MUFA coming from olive oil. Subjects in the fiber group were instructed to consume 20 g of dietary fiber per 1000 kcal, with a minimum of 1 cup of cooked beans per day and a maximum of 72 g of dietary fiber per day, with 40% of dietary fiber coming from beans. Bean and olive oil requirements were individually calculated by a dietician based on each subject’s daily calorie needs. Subjects were allowed to be off the diet, if necessary, for up to a maximum of 2 d/wk. Energy intake was assessed by the dietitians from the 3-day food records every 2 weeks and by weight measurements taken during CRTU visits. Extra virgin olive oil or assortment of cooked beans of the dry bean family were provided to subjects.
Dieticians provided feedback to all subjects by phone based on their 3-day food record analysis to ensure compliance with the diet intervention requirements. Subjects were also reminded at the time of their CRTU visits or during phone calls to not make any changes to their physical activity during the study period. Activity was evaluated using a general practice physical activity questionnaire at baseline, 8 weeks, and 12 weeks. After 8 and 12 weeks of the diet, all subjects underwent a second and third labeled OGTT and new anthropometric measurements.
Hepatic fat measurement
Liver fat content was measured using a single-voxel single-breath–hold proton stimulated-echo acquisition mode magnetic resonance spectroscopy technique (25). A 2- × 2- × 2.5-cm3 voxel was measured in segment VI of the liver, with repetition time set to 3 seconds to minimize T1-weighting effects, echo time set to 10 ms to maximize signal, and mixing time set to 5 ms to minimize J coupling effects. Spectra were acquired both with and without water suppression. Data were processed using LC-Model (LCMODEL Inc, Oakville, Ontario, Canada); liver fat fraction (LFF) was calculated from the sum of the three major lipid groups referenced to water: (Lip09 + Lip13 + Lip16)/water (25).
Genotyping
This study also offered the unique opportunity to evaluate whether single nucleotide polymorphisms (SNPs) influencing hepatic fat/NAFLD susceptibility, which were previously identified by large-scale genome-wide scans or candidate gene association studies, could be reliably predicted with a small sample size. These coding and noncoding variants are located in genes [including Patatin-like phospholipase domain-containing 3 (PNPLA3), Glucokinase regulator (GCKR), and Neurocan (NCAN)] with known physiologic involvement in carbohydrate and lipid metabolism in liver (26, 27). In addition, an SNP located in SREBF-1c, which is a master regulatory transcription factor involved in lipogenesis, and an SNP located in TCF7L2 gene, which has shown the strongest association with type 2 diabetes susceptibility, were selected for further analysis.
Targeted metabolomics
Targeted metabolomic analyses of fatty acids were done for triglyceride fatty acid composition and nonesterified fatty acids, including oleic acids, linoleic acids, palmitoleic acids, linolenic acids, eicosapentaenoic acids, docosahexaenoic acids, palmitic acids, arachidonic acids, myristic acids, and triacylglycerol (TAG), as previously established (28).
Analytic techniques
All blood samples were immediately placed on ice, centrifuged at 4°C, separated, and then stored at −20°C until analyses were performed. Sample analysis was carried out in the Mayo Mass Spectrometry or Immunochemical Core Laboratories. Plasma glucose was measured using a glucose analyzer (YSI Inc., Yellow Springs, OH), insulin (Access Ultrasensitive Immunoenzymatic; Beckman Coulter, Brea, CA), and C-peptide and glucagon using a radioimmunoassay (Linco Research, St. Louis, MO). Plasma [6, 6-2H2] glucose enrichment was measured using gas chromatography–mass spectrometry. Plasma samples were analyzed for fatty acid concentration (28).
Genomic DNA isolated from blood samples was used for genotyping on the Sequenom MassARRAY system (Sequenom Inc., San Diego, CA). Sequenom genotyping procedures were performed according to the manufacturer’s iPLEX application guide at the Center for Human Genomics and Personalized Medicine Core Laboratory (Wake Forest School of Medicine). Details of all selected SNPs are provided in Supplemental Table 1 (31KB, pdf) . A total of 16 SNPs were successfully genotyped in this study.
Calculations
Concentrations of glucose are expressed in millimolars, insulin in picomolars, C-peptide in nanomolars, and glucagon in picograms per milliliter. Concentrations of TAG and lipoproteins are expressed as milligrams per deciliter. Insulin sensitivity was measured using the oral minimal model, whereas β -cell responsivity indexes were estimated using the oral C-peptide minimal model, incorporating age-associated changes in C-peptide kinetics. The model assumes that insulin secretion is comprised of a static and dynamic component (29–31). The disposition index was calculated by multiplying the total insulin secretion by insulin sensitivity. All indices of insulin secretion and insulin sensitivity were calculated per lean body mass. Percent change in LFF was calculated as 100 × (LFFend − LFFbaseline)/LFFbaseline (20).
Statistical analyses
The sample size calculations for the study were designed to provide 80% power to detect a 30% difference in LFF between the two modified diets relative to the control group. This calculation was based on a two-sided t test of size 0.05 (i.e., no correction for multiple testing) and a standard deviation (SD) of the differences of 25%. The SD of 25% was derived from a recently published report (15). Accounting for potential dropout, we aimed to enroll 16 subjects per group to achieve at least 12 participants at week 12, the minimum number to achieve 80% power.
Sample distributions are described by mean and SD. The primary outcome LFF at 12 weeks was compared between the MUFA and control groups and between the fiber and control groups using analysis of covariance, including the baseline value as a covariate. Secondary outcomes were analyzed analogously using analysis of covariance, including the baseline values as covariates. Emphasis was given to the comparison at 12 weeks, with the comparison at 8 weeks being secondary. As part of secondary analyses, within-group changes from baseline to either 8 or 12 weeks were assessed using a paired t test, except for LFF, where we used a one-sample t test on the percent change in LFF. Statistical analysis was done using SAS version 9.4 (SAS Institute Inc., Cary, NC). A two-sided P value <0.05 was considered statistically significant for each outcome measure. To assess the robustness of the findings because of possible baseline imbalance, we repeated these analyses including model terms for sex and lean body mass, and the results remained qualitatively the same. To assess the robustness of the findings for insulin sensitivity and disposition index to the skewed distributions expected for these variables, we repeated the analysis using log-transformation, and the conclusions were unchanged.
Bivariate analysis using JMP version 10.0 (SAS Institute Inc, Cary, NC) was performed to compare the plasma TAG concentrations and fatty acids obtained by the metabolomics assay. Associations between genotypes of SNPs and LFF were tested using linear regression models (additive and dominant), implemented in PLINK v1.07 software (Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA), which included age and sex as covariates. Similar associations with triglyceride and hemoglobin A1c were also tested. All skewed variables were ln-transformed for this analysis. A P value <0.05 was considered to be statistically significant without correcting for multiple analyses based on strong prior hypotheses and a high correlation between tested traits. In a secondary analysis, case-control association tests were also performed. Participants were dichotomized to case and control based on established clinical cutoffs for liver fat (32). Individuals with an LFF <5% were considered to be a control (n = 11), and individuals with an LFF ≥5% were considered to be a case (n = 32). Comparisons of allele and genotype frequencies between cases and controls were performed using Pearson goodness-of-fit χ2 test and Cochran-Armitage trend test, respectively, implemented in the DeFinetti program (Institute of Human Genetics, Munich, Germany).
Results
There were 43 subjects who entered into trial. All 43 subjects (i.e., 15 subjects from the MUFA group, 15 subjects from the fiber group, 13 subjects from the control group) were included in the data analyses presented in Table 1 (demographics). However, MRS data were only available for 15 in the MUFA group, 13 in the fiber group, and 11 in the control group because of technical difficulties whereby subjects were unable to hold their breath appropriately.
Table 1.
Baseline Characteristics of Subjects Participating in the Study
| Characteristic |
Study Groups |
||
|---|---|---|---|
| MUFA (n = 15) | Fiber (n = 15) | Control (n = 13) | |
| Age, y | 62 ± 10 | 63 ± 13 | 60 ± 12 |
| Sex, male/female | 8/7 | 7/8 | 10/3 |
| BMI, kg/m2 | 30 ± 3.0 | 31 ± 3.0 | 32 ± 5.1 |
| Waist-hip ratio | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Lean body mass, kg | 51 ± 9 | 46 ± 9 | 58 ± 11 |
| Total body fat, % | 37.4 ± 6.3 | 41.7 ± 6.2 | 37.2 ± 8.3 |
| Fasting plasma glucose, mM | 5.8 ± 0.3 | 5.8 ± 0.5 | 5.8 ± 0.6 |
| 2-h glucose, mM | 9.8 ± 1.6 | 9.1 ± 1.7 | 9.0 ± 1.8 |
| HbA1c, % (mmol/mol) | 5.5 ± 0.4 (37 ± 4.4) | 5.6 ± 0.3 (38 ± 3.3) | 5.5 ± 0.3 (37 ± 3.3) |
Data are expressed as mean ± SD or as otherwise indicated.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c.
Subjects were weighed every 2 weeks and instructed by dieticians to maintain weight. Baseline, 8-week, and 12-week body mass index recorded for the MUFA group were 30 ± 3 vs 30 ± 3 vs 30 ± 3 kg/m2, for the fiber group were 31 ± 3 vs 30 ± 3 vs 31 ± 4 kg/m2, and for the control group were 32 ± 5 vs 32 ± 5 vs 32 ± 5 kg/m2, respectively. Physical activity questionnaire data were consistent with maintenance of activity in all subjects from each group throughout the 12-week study period.
Macronutrient composition analyses of the diet for all three groups at baseline and during the study are shown in Table 2. The average daily energy intake was not significantly different between the groups; however, by design, the MUFA group consumed higher amounts of fat in the form of MUFA than the control group (P < 0.001), whereas the fiber group consumed greater amounts of fiber than the control group (P < 0.001). There were no statistical differences in carbohydrate content between the MUFA and control groups (P = 0.09).
Table 2.
Isocaloric Diet Composition of Study Groups
| MUFA (n = 15) | Fiber (n = 15) | Control (n = 13) | ||
|---|---|---|---|---|
| Energy consumed, calories, kcal/d | Baseline | 2124 ± 873 | 1926 ± 622 | 2022 ± 792 |
| Study duration | 2064 ± 300 | 1889 ± 476 | 2006 ± 644 | |
| Proteins, % TEI | Baseline | 18 ± 3 | 16 ± 5 | 16 ± 3 |
| Study duration | 14 ± 3 | 17 ± 2 | 17 ± 3 | |
| Fat, % TEI | Baseline | 36 ± 5 | 36 ± 7 | 36 ± 8 |
| Study duration | 46 ± 3a | 28 ± 2 | 34 ± 5 | |
| MUFA, % TEI | Baseline | 14 ± 2 | 15 ± 5 | 14 ± 4 |
| Study duration | 22 ± 2a | 7 ± 1 | 8 ± 2 | |
| Saturated fat, % TEI | Baseline | 13 ± 3 | 13 ± 3 | 13 ± 3 |
| Study duration | 11 ± 2 | 9 ± 1 | 12 ± 2 | |
| PUFA, % TEI | Baseline | 7 ± 1 | 8 ± 2 | 7 ± 2 |
| Study duration | 5 ± 1 | 4 ± 1 | 4 ± 2 | |
| Carbohydrate, g | Baseline | 228 ± 104 | 219 ± 73 | 232 ± 87 |
| Study duration | 188 ± 41 | 256 ± 59 | 241 ± 108 | |
| Fiber, g/1000 kcal | Baseline | 11 ± 4 | 11 ± 3 | 11 ± 4 |
| Study duration | 8 ± 3 | 21 ± 4a | 10 ± 3 |
Data are expressed as mean ± SD.
Abbreviations: Baseline, prior to start of study; PUFA, polyunsaturated fatty acids; Study duration, mean dietary intake over the entire 12-week study; TEI, total energy intake.
P < 0.05 vs control.
Primary outcome
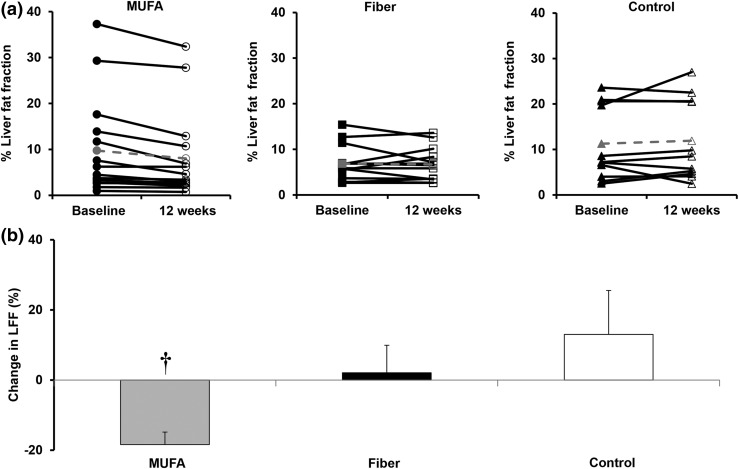
The LFF after 12 weeks of dietary supplementation in the three groups is shown in Fig. 1. LFF was significantly lower in the MUFA group (9.7% ± 2.8% vs 8.0% ± 2.5%; P < 0.0003), but remained unchanged in the fiber group (6.9% ± 1.1% vs 6.3% ± 1.0%; P = 0.25) and the control group (11.2% ± 2.5% vs 11.9% ± 2.7%; P = 0.45). For the MUFA group, LFF was reduced by −18% ± 3% compared with baseline (P = 0.0001) at 12 weeks. Changes from baseline in the fiber group of 2% ± 8% (P = 0.80) and control group of 13% ± 12% (P = 0.32) in LFF were not significant.
Figure 1.
(a) LFF at baseline (closed symbols) and after 12 weeks (open symbols) of dietary intervention in the MUFA group (left panel) (n = 15) before (●) and after (○) intervention, fiber group (middle panel) (n = 13) before (black square) and after (white square) intervention, and control group (right panel) (n = 11) before (black triangle) and after (white triangle) intervention. Control denotes habitual diet. Mean data are shown in gray dashed lines. (b) Percent change in LFF in MUFA (gray bar) (n = 15), fiber (black bar) (n = 13), and control (white bar) (n = 11) groups at baseline and after 12 weeks of dietary intervention. Control denotes habitual diet. †MUFA group vs control group at 12 weeks, P < 0.01.
Secondary outcome
Glucose, insulin, C-peptide, and glucagon concentrations during the 3-hour labeled OGTT at baseline and at 8 and 12 weeks after intervention are shown in Supplemental Fig. 1 (597.3KB, TIF) . Glucose, insulin, C-peptide, and glucagon concentrations during the OGTT were not significantly different at baseline or after intervention in the three study groups.
Indices of insulin secretion and insulin action (hepatic and extrahepatic) during OGTT are shown in Table 3. Indices of insulin action and secretion, although numerically higher, did not significantly differ between the MUFA and control groups after intervention. However, within-group comparison of the MUFA group showed significant improvement in hepatic insulin sensitivity (P = 0.01) and total insulin sensitivity (P = 0.04) at 12 vs 8 weeks, whereas there were no differences between baseline and 8 weeks. On the other hand, in the fiber and control groups, indices of insulin action or secretion remained unchanged.
Table 3.
Indices of Insulin Sensitivity, Insulin Secretion, and Disposition Index at Baseline and After 8 and 12 Weeks of Dietary Intervention in the Study Groups
|
Study Groups |
MUFA vs Control P values | Fiber vs Control P Values | ||||
|---|---|---|---|---|---|---|
| MUFA (n = 15) | Fiber (n = 15) | Control (n = 13) | ||||
| Siliver (10−4 dL/kg/min per µU/mL) | Baseline | 6.1 ± 4.0 | 5.8 ± 3.0 | 6.4 ± 3.7 | ||
| 8 | 4.7 ± 2.7 | 6.1 ± 5.1 | 9.9 ± 8.9 | 0.16 | 0.59 | |
| 12 | 10.0 ± 9.4a | 7.2 ± 5.6 | 6.4 ± 5.5 | 0.39 | 0.72 | |
| Si* (10−4 dL/kg/min per µU/mL) | Baseline | 5.7 ± 7.0 | 5.3 ± 8.1 | 4.1 ± 3.4 | ||
| 8 | 4.4 ± 2.7 | 6.0 ± 6.8 | 4.3 ± 3.1 | 0.98 | 0.52 | |
| 12 | 9.7 ± 11.3 | 7.8 ± 14.8 | 7.4 ± 11.0 | 0.61 | 0.96 | |
| Sitotal (10−4 dL/kg/min per µU/mL) | Baseline | 11.8 ± 9.3 | 11.0 ± 9.4 | 10.5 ± 6.3 | ||
| 8 | 9.2 ± 3.7 | 12.1 ± 11.1 | 14.2 ± 11.1 | 0.22 | 0.81 | |
| 12 | 19.7 ± 18.3a | 15.0 ± 17.6 | 13.7 ± 16.2 | 0.63 | 0.98 | |
| Phibasal (10−9 min−1) | Baseline | 9.4 ± 2.2 | 9.9 ± 1.7 | 12.2 ± 6.0 | ||
| 8 | 10.6 ± 2.3 | 9.9 ± 2.6 | 11.8 ± 5.7 | 0.02 | 0.65 | |
| 12 | 9.6 ± 3.0 | 9.4 ± 2.0 | 12.1 ± 6.0 | 0.75 | 0.46 | |
| Phitotal (10−9 min−1) | Baseline | 63.8 ± 34.6 | 61.0 ± 13.4 | 61.8 ± 27.1 | ||
| 8 | 67.3 ± 27.5 | 57.8 ± 14.5 | 60.8 ± 23.6 | 0.30 | 0.59 | |
| 12 | 58.0 ± 18.7 | 56.4 ± 12.7 | 68.2 ± 35.4 | 0.20 | 0.11 | |
| DI total (10−14 dL/kg/min2 per pmol/L) | Baseline | 1394 ± 1497 | 1143 ± 1047 | 935 ± 488 | ||
| 8 | 1384 ± 993 | 1124 ± 1124 | 960 ± 508 | 0.38 | 0.75 | |
| 12 | 2026 ± 2215 | 1532 ± 2153 | 1024 ± 809 | 0.19 | 0.48 | |
Data are expressed as mean ± SD or as otherwise indicated. Estimate of difference between treatment group and control adjusting for baseline (P value). There were no statistical changes from baseline to week 12 observed in these indices for the three groups.
Abbreviations: DI total, total disposition index; Phibasal, basal insulin secretion; Phitotal, total insulin secretion; Si*, peripheral insulin sensitivity; Siliver, hepatic insulin sensitivity; Sitotal, total insulin sensitivity.
P < 0.05 vs 8 weeks.
Exploratory analyses
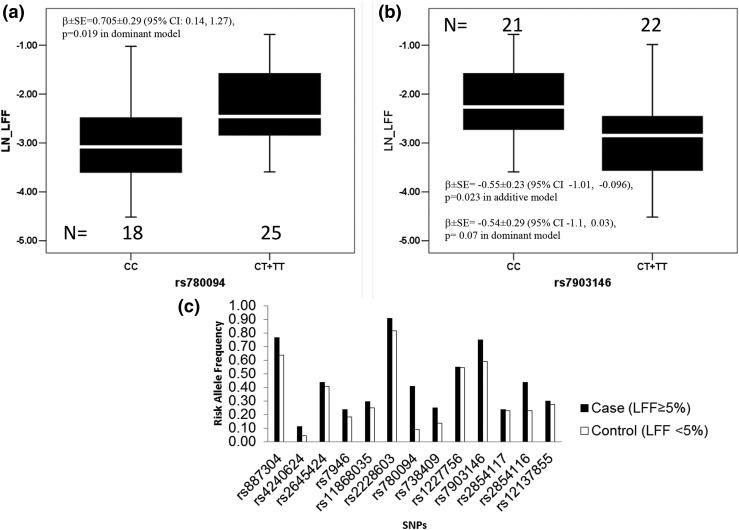
We also evaluated the role of genetic factors in determining the LFF and metabolic profile in this study, which have previously been established by large genome-wide scan studies (27). An intronic SNP of the GCKR gene (rs780094) was significantly associated with the MRS-derived LFF [β = 0.705 ± 0.29; 95% confidence interval [CI], 0.14 to 1.27; P = 0.019, under a dominant genetic model] (Fig. 2; Supplemental Table 1 (31KB, pdf) ). The minor allele T of this SNP was also associated with an increase in hemoglobin A1c (P = 0.021) and triglyceride (P = 0.023) levels. In addition, the frequency of the T allele of SNP rs780094 was significantly higher in individuals with an LFF ≥5% (frequency of T = 0.406) compared with participants with an LFF <5% (frequency of T= 0.091), indicting a strong effect of this SNP in increasing LFF (odds ratio, 6.8; 95% CI, 1.47 to 31.81; P < 0.006). Interestingly, the type 2 diabetes−associated allele (T) of SNP rs7903146 in TCF7L2 was significantly associated with a decrease in LFF (β = −0.553 ± 0.23; 95% CI, −1.01 to −0.096; P = 0.023, under additive genetic model) (Fig. 2; Supplemental Table 1 (31KB, pdf) ).
Figure 2.
Distribution of LFF in genotypic groups (a and b) observed are shown in the upper panel. A bar graph (c) shows the risk allele frequency in subjects with <5% (control) and >5% (case) LFF on the bottom panel. Sixteen SNPs were analyzed, and 13 were polymorphic in this study. The box represents the interquartile range, which contains 50% of the values. The whiskers are lines that extend the box to the highest and lowest values, excluding outliers. A line across the box indicates the median. CC, homozygous for the major allele; CT + TT, heterozygous and homozygous for the minor allele; LN_LFF, natural logarithm of liver fat fraction; SE, standard error.
The lipid panel at baseline, 8 weeks, and 12 weeks after intervention are shown in Supplemental Fig. 1 (597.3KB, TIF) . Of interest, plasma triglyceride concentrations were numerically lower, but not statistically significant (P = 0.06), in the MUFA group after 12 weeks of intervention compared with the control group.
Targeted metabolite analyses after 12 weeks of intervention showed no substantial statistical difference in MUFA vs control or fiber vs control diets. Indirect indices of de novo lipogenesis (DNL) [i.e., serum TAG palmitic/linoleic acid ratio, steroyl-CoA desaturase activity (SCD-1) serum TAG palmitoleic/palmitic acid ratio] were measured.
Ratio of palmitic to linoleic acid concentration (DNL) did not change after the MUFA diet (0.69 ± 0.13 vs baseline, 0.73 ± 0.16; P = 0.5) or the fiber diet (0.75 ± 0.01 vs baseline, 0.76 ± 0.10; P = 0.7). The SCD-1 activity, measured as the ratio of palmitoleic to palmitic acid concentration, did not change after the MUFA diet (0.09 ± 0.02 vs baseline, 0.09 ± 0.02; P = 0.8) or the fiber diet (0.09 ± 0.03 vs baseline, 0.09 ± 0.03; P = 0.7).
Discussion
In patients with liver steatosis, a nonpharmacologic treatment approach has focused mainly on weight loss strategies and physical exercise (9). However, the present data show that in the absence of weight loss and vigorous physical activity, a 12-week diet rich with MUFA significantly decreased liver fat content in prediabetes subjects. Supplementing 28% of total energy intake with MUFA reduced LFF by approximately 17% after only 12 weeks of consumption of olive oil (Fig. 1).
In this study, all diets were isocaloric, and the subjects in all groups maintained a constant body weight. Furthermore, subjects were monitored to ensure that they maintained constant physical activity throughout the study. Therefore, the reduction of liver fat cannot be explained by weight loss or exercise, but rather by dietary changes alone. These data extend those observations by Bozzetto et al. (19, 20) by showing that an isocaloric diet enriched in MUFA results in a reduction in hepatic fat content (by increasing fat oxidation) independent of an aerobic training program in people with prediabetes and in people with type 2 diabetes.
It has been shown that lifestyle-induced weight loss improves insulin action in prediabetes subjects, but has no effect on reduction of postprandial hyperinsulinemia (30). Therefore, we wanted to explore whether changes in liver fat content would be accompanied by an improvement in hepatic insulin action in the absence of changes in weight.
Insulin action and secretion indices and disposition index (insulin secretion adjusted for the prevailing degree of insulin resistance) are presented in Table 3. An MUFA diet resulted in a numerical increase in all three parameters from baseline compared with the other diets assessed, but the differences were not statistically significant, likely because of the small sample size and relatively short duration of the study.
The fiber supplemented group did not show substantial changes in liver fat content (Fig. 1). Although fiber supplementation has shown to enhance weight loss and improve both carbohydrate and fat metabolism (22), our study provides evidence that in the absence of weight loss, high-fiber diets do not improve insulin sensitivity or secretion and do not significantly reduce liver fat in humans with prediabetes.
Our control diet was a typical Midwestern diet, which is typically high in fat (mainly saturated) and carbohydrates with low fiber content (Table 2). The control group subjects continued to eat their habitual diet. Although there is no standardized macronutrient diet composition for managing type 2 diabetes or prediabetes (33), many dietary interventions focus mainly on carbohydrate/calorie restriction. Our study did not find any marked improvement in glucose tolerance or insulin sensitivity and secretion after the control diet over the short duration of this trial, perhaps because this diet closely mimicked the diet that these subjects typically ate. A recent, excellent review has been published by Yki-Järvinen (34), highlighting the various types of diets and their effects on various metabolic parameters in people with nonalcoholic fatty liver disease. The current study directly measured hepatic insulin sensitivity in conjunction with a dietary intervention. Hepatic and total insulin sensitivity were significantly improved after he MUFA diet at 12 weeks (P < 0.05), whereas there were no significant changes observed in either insulin action or secretion indices with the other two diets.
Individuals with prediabetes are frequently overweight or obese with a sedentary activity level (1). We think this study has great translational potential in this population. A therapeutic approach based on MUFA supplementation seems like an easier goal to achieve than weight loss or increase in physical activity. Furthermore, exercise with MUFA supplementation has not been shown to provide an added benefit compared with MUFA supplementation alone in decreasing liver fat content in patients with type 2 diabetes (20). We believe it is clinically relevant to study if dietary interventions (high fiber or MUFA) that have been purported to be beneficial are relevant in improving metabolic health when compared with the average diet consumed by most people in the United States. It was not our intention to compare the MUFA diet with the fiber diet. We did not note any substantial differences in the outcomes when we compared MUFA with fiber, but of interest, significantly lower triglyceride concentrations in the MUFA group (Supplemental Fig. 2 (306.8KB, tif) ) were observed compared with the fiber group (P = 0.0082) after 12 weeks of dietary intervention.
Despite the small sample size and short intervention duration, this study showed that MUFA is somewhat beneficial in lowering triglycerides, which is a biomarker for hepatic fat content (35). Consistent with observations by Bozzetto et al. (19), we did not find any substantial differences in DNL and SCD-1 activity. Lack of activity of these two processes suggests that the slightly lower amount of carbohydrates consumed by subjects in the MUFA group did not affect the primary end point (i.e., lowering of liver fat) because both these indices have been linked to higher carbohydrate intake (36). The rs780094 SNP in the GCKR gene was associated with liver fat accumulation and increased triglyceride concentrations in this study, which confirms the importance of genetic variants in conveying susceptibility to fatty liver in people with prediabetes.
As with any study, our study had limitations. A 12-week dietary intervention is relatively brief; therefore, it is entirely possible that greater changes may have been seen with a longer duration of intervention. Moreover, some subjects had difficulty maintaining breath holds as instructed during the liver MRS scans, resulting in data loss.
The isocaloric diet provided in this study resulted in slight differences in the macronutrient content. The MUFA group consumed slightly, but not significantly, lower amounts of carbohydrates (~15 g per meal; P = 0.09) and (by design) higher amounts of fat in the form of MUFA (P < 0.001) than the control group, whereas the fiber group (by design) consumed greater amounts of fiber than the control group (P < 0.001). All three groups consumed matched amounts of protein. Therefore, it is possible that the reduction in liver fat observed with the MUFA diet is not solely caused by the consumption of an increased amount of MUFA alone, but could perhaps also be because of the slightly lower amount of carbohydrates consumed by this group. However, we consider this to be unlikely because of the very small and nonsignificant differences in carbohydrates between groups as previously shown by Yki-Järvinen (34). Additionally, if we had substantially reduced the carbohydrate intake (i.e., the so-called low-carb diet), then most certainly this would have resulted in weight loss that would have confounded comparison between groups.
In conclusion, we believe this is a pragmatic study and shows that a short-term diet rich in MUFA can be beneficial in lowering liver fat independently of weight loss or physical activity in people with prediabetes. Genomic analyses of a relevant SNP in the GCKR gene confirmed its importance in hepatic fat accumulation through increased hepatic triglyceride accumulation. Further studies with a larger number of subjects and longer duration of intervention will be required to determine whether a MUFA-rich diet in the prediabetes population may prevent or slow down the progression of NAFLD to nonalcoholic steatohepatitis in people with prediabetes to overt type 2 diabetes.
Acknowledgments
We thank Dr. Robert Rizza (Professor of Medicine at Mayo Clinic) for his guidance and suggestions. We also thank the research participants. We also thank the staff of the Mayo Center for Clinical and Translational Science (CCaTS), the Clinical Research and Trials Unit, the CCaTS Immunochemical Core Laboratory, and the CCaTS Metabolomics Core facility (Mai Persson). We thank Barbara Norby (research nurse), Cheryl Shonkwiler (research nurse), and Pamela Reich (research assistant) for technical assistance and Brent McConahey (laboratory technician) for technical assistance and graphic design. All persons mentioned are at the Endocrine Research Unit, Mayo Clinic, Rochester, Minnesota.
Acknowledgments
This study was supported by National Institutes of Health Grants R01 DK29953 (to R.B.) and R01 DK090111 (to S.K.D.) and Grant UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health; the Dry Beans Health Research Foundation; and the Mayo Metabolomics Core Unit Grant U24DK100469.
Acknowledgments
Author contributions: I.E. contributed to the design, conduct of the study, data analyses, and writing of the manuscript; S.D., M.S., and R.V. analyzed data; S.N. and H.O. assisted with the dietary component and analyses of data; C.C. and A.B. contributed to interpretation of the data and manuscript review; S.K.D. analyzed the genomic data; W.K.K. performed the statistical analyses on the data; J.P. analyzed liver fat data; and R.B. contributed to the design, data analyses, interpretation, and manuscript writing, and has primary responsibility for final content. All authors read and approved the final manuscript.
Clinical trial registry: ClinicalTrials.gov no. NCT01729078 (registered 9 November 2012).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- CRTU
- Clinical Research and Trials Unit
- DNL
- de novo lipogenesis
- GCKR
- Glucokinase regulator
- LFF
- liver fat fraction
- MRS
- magnetic resonance spectrometry
- MUFA
- monounsaturated fatty acid
- NAFLD
- nonalcoholic fatty liver disease
- OGTT
- oral glucose tolerance test
- SCD-1
- steroyl-CoA desaturase activity
- SD
- standard deviation
- SNP
- single nucleotide polymorphism
- TAG
- triacylglycerol.
References
- 1.Gregg E, Imperatore G, Venkat Narayan K. Epidemiology of type 1 and type 2 diabetes. In: Umpierrez G, ed. Therapy for Diabetes Mellitus and Related Disorders. 6th ed Alexandria, VA: American Diabetes Association; 2014:77–96. [Google Scholar]
- 2.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R; Baltimore Longitudinal Study of Aging . The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–1484. [DOI] [PubMed] [Google Scholar]
- 3.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319(23):1500–1506. [DOI] [PubMed] [Google Scholar]
- 4.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CDA, Nijpels G, Bouter LM, Heine RJ. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285(16):2109–2113. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35(4):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007;30(5):1075–1080. [DOI] [PubMed] [Google Scholar]
- 7.Papandreou D, Andreou E. Role of diet on non-alcoholic fatty liver disease: an updated narrative review. World J Hepatol. 2015;7(3):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–384. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association . The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811–826. [DOI] [PubMed] [Google Scholar]
- 11.Barnard ND, Scialli AR, Turner-McGrievy G, Lanou AJ, Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;118(9):991–997. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Jia W, Guo Z. Reducing liver fat by low carbohydrate caloric restriction targets hepatic glucose production in non-diabetic obese adults with non-alcoholic fatty liver disease. J Clin Med. 2014;3(3):1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255–266. [DOI] [PubMed] [Google Scholar]
- 14.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlström H, Risérus U. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95(5):1003–1012. [DOI] [PubMed] [Google Scholar]
- 15.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O’Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–143. [DOI] [PubMed] [Google Scholar]
- 16.Westerbacka J, Lammi K, Häkkinen AM, Rissanen A, Salminen I, Aro A, Yki-Järvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90(5):2804–2809. [DOI] [PubMed] [Google Scholar]
- 17.van Herpen NA, Schrauwen-Hinderling VB, Schaart G, Mensink RP, Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J Clin Endocrinol Metab. 2011;96(4):E691–E695. [DOI] [PubMed] [Google Scholar]
- 18.Utzschneider KM, Bayer-Carter JL, Arbuckle MD, Tidwell JM, Richards TL, Craft S. Beneficial effect of a weight-stable, low-fat/low-saturated fat/low-glycaemic index diet to reduce liver fat in older subjects. Br J Nutr. 2013;109(6):1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzetto L, Costabile G, Luongo D, Naviglio D, Cicala V, Piantadosi C, Patti L, Cipriano P, Annuzzi G, Rivellese AA. Reduction in liver fat by dietary MUFA in type 2 diabetes is helped by enhanced hepatic fat oxidation. Diabetologia. 2016;59(12):2697–2701. [DOI] [PubMed] [Google Scholar]
- 20.Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, Mazzarella R, Longobardo M, Mancini M, Vigorito C, Riccardi G, Rivellese AA. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35(7):1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser A, Abel R, Lawlor DA, Fraser D, Elhayany A. A modified Mediterranean diet is associated with the greatest reduction in alanine aminotransferase levels in obese type 2 diabetes patients: results of a quasi-randomised controlled trial. Diabetologia. 2008;51(9):1616–1622. [DOI] [PubMed] [Google Scholar]
- 22.Kaline K, Bornstein SR, Bergmann A, Hauner H, Schwarz PEH. The importance and effect of dietary fiber in diabetes prevention with particular consideration of whole grain products. Horm Metab Res. 2007;39(9):687–693. [DOI] [PubMed] [Google Scholar]
- 23.Vigue JT, Marsh HV Jr, Pellett PL. Amino acid scores of dry bean proteins fractionated by deae cellulose. Agron J. 1978;70(6):1107–1108. [Google Scholar]
- 24.Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, Paskett ED. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. 2014;114(4):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dongiovanni P, Anstee QM, Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19(29):5219–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O’Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS, Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB; NASH CRN; GIANT Consortium; MAGIC Investigators; GOLD Consortium . Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7(3):e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson XM, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51(9):2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women [published correction appears in Diabetes 2007;56(5):1486]. Diabetes. 2007;56(3):753–766. [DOI] [PubMed] [Google Scholar]
- 30.Man CD, Toffolo G, Basu R, Rizza RA, Cobelli C. Use of labeled oral minimal model to ]measure hepatic insulin sensitivity. Am J Physiol Endocrinol Metab. 2008;295(5):E1152–E1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visentin R, Dalla Man C, Basu R, Basu A, Rizza RA, Cobelli C. Hepatic insulin sensitivity in healthy and prediabetic subjects: from a dual- to a single-tracer oral minimal model. Am J Physiol Endocrinol Metab. 2015;309(2):E161–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, Lin G, Brenner D, Gamst A, Ehman R, Sirlin C. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60(6):1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinneen SF, Maldonado D III, Leibson CL, Klee GG, Li H, Melton LJ III, Rizza RA. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care. 1998;21(9):1408–1413. [DOI] [PubMed] [Google Scholar]
- 34.Yki-Järvinen H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients. 2015;7(11):9127–9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JJ, Lambert JE, Hovhannisyan Y, Ramos-Roman MA, Trombold JR, Wagner DA, Parks EJ. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87(4):817–823. [DOI] [PubMed] [Google Scholar]