Abstract
Context:
Most cases of autosomal recessive hypoparathyroidism (HYPO) are caused by loss-of-function mutations in GCM2 or PTH.
Objective:
The objective of this study was to identify the underlying genetic basis for isolated HYPO in a kindred in which 3 of 10 siblings were affected.
Subjects:
We studied the parents and the three adult affected subjects, each of whom was diagnosed with HYPO in the first decade of life.
Methods:
We collected clinical and biochemical data and performed whole exome sequencing analysis on DNA from the three affected subjects after negative genetic testing for known causes of HYPO.
Results:
Whole exome sequencing followed by Sanger sequencing revealed that all three affected subjects were compound heterozygous for two previously reported mutations, c.967_979delCTGTCCCCTCCGC:p.(L323SfsX51) and c.995+(3_5)delGAGinsTAT, in AIRE, which encodes the autoimmune regulator protein that is defective in autoimmune polyglandular syndrome type 1 (APS-1). Each parent carries one mutation, and all of the children of the patients are either heterozygous for one mutation or wild type. The affected sister developed premature ovarian failure, but the two affected brothers have no other features of APS-1 despite elevated serum levels of anti–interferon-α antibodies.
Conclusions:
Our findings indicate that biallelic mutations in AIRE can cause isolated HYPO as well as syndromic APS-1. The presence of antibodies to interferon-α provides a highly sensitive indicator for loss of AIRE function and represents a useful marker for isolated HYPO due to AIRE mutations.
Loss-of-function mutations in AIRE encoding the autoimmune regulatory protein can cause isolated hypoparathyroidism as well as the more typical disorder type 1 autoimmune polyglandular syndrome.
Hypoparathyroidism (HYPO) is an uncommon endocrine disorder characterized by hypocalcemia and hyperphosphatemia due to insufficient secretion of biologically active parathyroid hormone (PTH). Although neck surgery is the most common cause of HYPO, HYPO may have a genetic basis with autosomal dominant, autosomal recessive, or X-linked recessive transmission (1, 2). These heritable forms of HYPO may manifest as an isolated endocrine disorder or may be only one component of a more complex syndrome [reviewed by Clarke et al. (2)]. Molecular genetic studies of patients with isolated HYPO have identified pathogenic mutations in an increasing number of associated genes, including PTH (3–5), CASR (6), SOX3 (7), GCM2 (8), and GNA11 (9–11), whereas mutations in GATA3 (HYPO, deafness, and renal dysplasia syndrome) (12), TBCE or FAM111A (Kenny-Caffey syndrome) (13, 14), AIRE [autoimmune polyendocrinopathy candidiasis ectodermal dystrophy or autoimmune polyglandular syndrome type 1 (APS-1) syndrome] (15, 16), TBX1 (DiGeorge sequence) (17), and CHD7 (CHARGE syndrome) (18) are associated with complex genetic syndromes in which HYPO is but one component.
The expanding role of gene-based diagnosis has greatly extended our appreciation of the highly variable manner in which many single-gene disorders can manifest clinical features and has highlighted the challenges of diagnosing complex disorders when a phenotype is restricted or partial. Here we describe the application of whole exome sequencing to identify the molecular basis for isolated HYPO in a large kindred that included three affected siblings who did not harbor mutations in the known genetic causes of isolated HYPO. Remarkably, we found that all affected subjects were compound heterozygotes for two previously described loss-of-function mutations in AIRE that have been identified in patients with typical APS-1 (OMIM #240300) (16, 19–21). The lack of other APS-1 features in two of the three patients with HYPO that we describe, despite over 50 years of follow-up for each subject, provides compelling evidence that AIRE mutations can also cause isolated HYPO.
Subjects and Methods
Patients
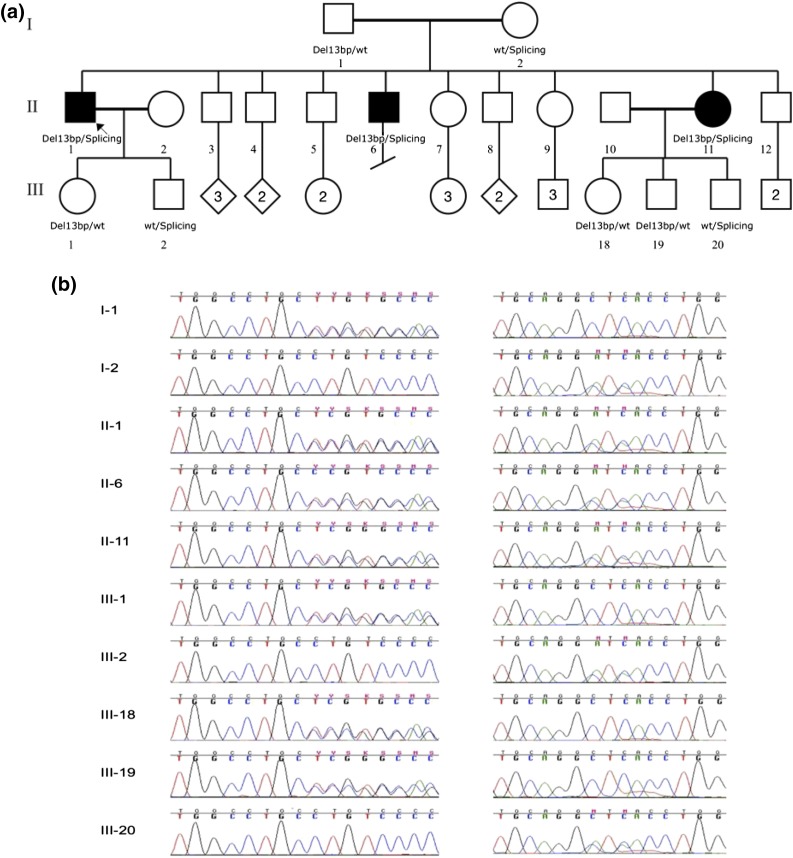
This study included two affected brothers (II-1 and II-6) and their affected sister (II-11) as well as their unaffected parents (I-1 and I-2) and children (III-1, III-2, III-18, III-19, and III-20) (Fig. 1), all of whom provided informed consent for participation in the study. The study was approved by the institutional review board at The Children’s Hospital of Philadelphia. We isolated genomic DNA from blood or saliva obtained from the study subjects. The affected subjects were diagnosed with HYPO at the ages of 2 (proband, II-1), 5 (brother, II-6), and 7 (sister, II-11) years and are now aged 63, 57, and 53 years, respectively (Table 1). At diagnosis, all subjects had low serum concentrations of calcium and PTH and elevated serum concentrations of phosphate. The earliest available laboratory data for II-1 (1995) showed serum intact PTH <12.7 pg/mL, ionized Ca 1.04 mmol/L (1.17 to 1.31), and phosphate 5.4 mg/dL (2.3 to 4.3); 1988 data for II-2 showed serum c-terminal PTH 51 pg/mL (50 to 340 pg/mL), calcium 7.9 mg/dL, and phosphate 4.7 mg/dL; and 1998 data for II-11 showed intact PTH <1 pg/mL, calcium 6.9 mg/dL, and phosphate 6.8 mg/dL while on treatment with ergocalciferol and calcium supplements, although compliance may have been poor. To date, the two brothers have had no clinical or biochemical features of additional endocrine, autoimmune, or developmental disorders, and all are in good health. The sister developed premature ovarian failure at age 33 years and was treated with estrogen/progestin hormone replacement therapy for 10 years. The proband has had temporal lobe seizures after developing acute meningitis as a child. The two brothers have been treated with high-dose ergocalciferol since diagnosis, and the sister was treated with calcitriol and more recently with recombinant human PTH 1-84. There is no history of renal stones.
Figure 1.
Pedigree of the studied family and AIRE chromatograms. (a) The pedigree is drawn using open circles to denote unaffected female subjects and the open squares to denote unaffected male subjects; the solid figures indicate affected subjects. AIRE genotypes are noted beneath the symbol for each subject from whom DNA was available for testing. (b) Chromatograms for each family member. The two mutations are very close; the splicing variant is ∼30 bp downstream of the 13-bp deletion. The reverse sequence is shown for the splicing variant. Left: 13-bp deletion; right: splicing mutation.
Table 1.
Demographic and Immune Characteristics of the Patients With Isolated HYPO
| Patient 1 (II-1) | Patient 2 (II-6) | Patient 3 (II-11) | |
|---|---|---|---|
| Age at diagnosis of HYPO, y | 2 | 5 | 7 |
| Current age, y; sex | 63, M | 57, M | 53, F |
| Duration of follow-up, y | 61 | 52 | 46 |
| Thyroid | |||
| Thyroid peroxidase Ab | Negative | Negative | Negative |
| Thyroglobulin Ab | Negative | Negative | Negative |
| Pancreatic islet cell | |||
| GAD65 Ab (nl <1U/mL) | >30 | <1 U/mL | ND |
| Islet cell Ab titer (nl <1.25 U/mL) | 20 | Negative | ND |
| Adrenal | |||
| 21-hydroxylase Ab | Negative | Negative | ND |
| Intestine | |||
| Tissue transglutaminase IgA Ab (nl < 4 U/mL) | 1 | Negative | Negative |
| Parietal cell | |||
| Intrinsic factor Ab | Negative | Negative | ND |
| Parathyroid | |||
| NALP5 | Negative | Negative | ND |
| IFN-α | Positive | Positive | ND |
Abbreviations: F, female; M, male; ND, not done; nl, normal.
Immunological analyses
Serum from all three patients was tested for autoantibodies to thyroid peroxidase antigen, thyroglobulin, intrinsic factor, tissue transglutaminase, adrenal steroid 21-hydroxylase, islet cells, and glutamic acid decarboxylase 65 (GAD65) by standard techniques at commercial reference laboratories. Antibodies to interferon (IFN)-α and NACHT leucine–rich-repeat protein 5 (NALP5) were assayed by radioligand binding assay. In brief, IFN-α and NALP5 proteins were radiolabeled with 35S-methionine using the TNT system kit (Promega) and purified using Sephadex G-25 DNA–grade columns. Radiolabeled proteins were incubated with serum samples in duplicate and then immunoprecipitated with protein A/G beads. The radioactivity was measured using a liquid scintillation counter. Each assay also included sera from normal control subjects and patients with APS-1. An antibody to each antigen target (IFN-α, Abcam; NALP5, Santa Cruz Biotech) was used as the positive control, and the negative control was without antibody. The index was calculated as [counts per minute (CPM) of test serum – CPM of negative control] ÷ (CPM of positive control – CPM of negative control). An index >3 standard deviations above the mean value of the normal control sera was considered positive as it represents values outside of the 99.7% range.
Exome sequencing and bioinformatic analysis
We performed exome capture and sequencing as well as read processing, mapping to human genome reference hg19, variant calling, annotations, and filtering for rare variants affecting the coding sequence and/or consensus splice sites as previously described (11). Because the family pedigree suggested an autosomal recessive mode of inheritance, we only considered nonsynonymous, splice-altering variants, and frameshift variants that co-segregated with the disease in the family with a minor allele frequency ≤1% in public databases (i.e., 1000 Genomes Project and NHLBI ESP6500SI). Subsequent gene prioritization was performed on the basis of deleterious predication and biological and clinical relevance by referring to existing databases [i.e., Online Mendelian Inheritance in Man (OMIM) and Human Gene Mutation Database].
Results
Genetic analyses
Analysis of candidate genes CASR, PTH, SOX3, GCM2, and GNA11 was performed by Sanger sequencing of the exons and exon-intron boundaries but failed to identify any disease-causing mutations. Whole exome sequencing followed by confirmatory Sanger sequencing revealed compound heterozygous mutations, c.967_979delCTGTCCCCTCCGC:p.(L323SfsX51) and c.995+(3_5)delGAGinsTAT, in AIRE (MIM: 607358; NM_000383.3) in all three affected patients. Each parent carried one mutant allele, and all of the children of the three affected subjects were either heterozygous for one mutation or wild type (Fig. 1). The 13-base pair (bp) deletion has been observed in about half of reported patients with APS-1 from many different geoethnic origins (16, 20, 22), whereas the splicing mutation was found in only two Italian patients with the classic features of APS-1 (21). The concurrence of these two mutations in a single subject has not previously been described. In addition, the heterozygous carriers of one AIRE mutation had no evidence of endocrine or autoimmune disease in this kindred.
Biochemical and immunological studies
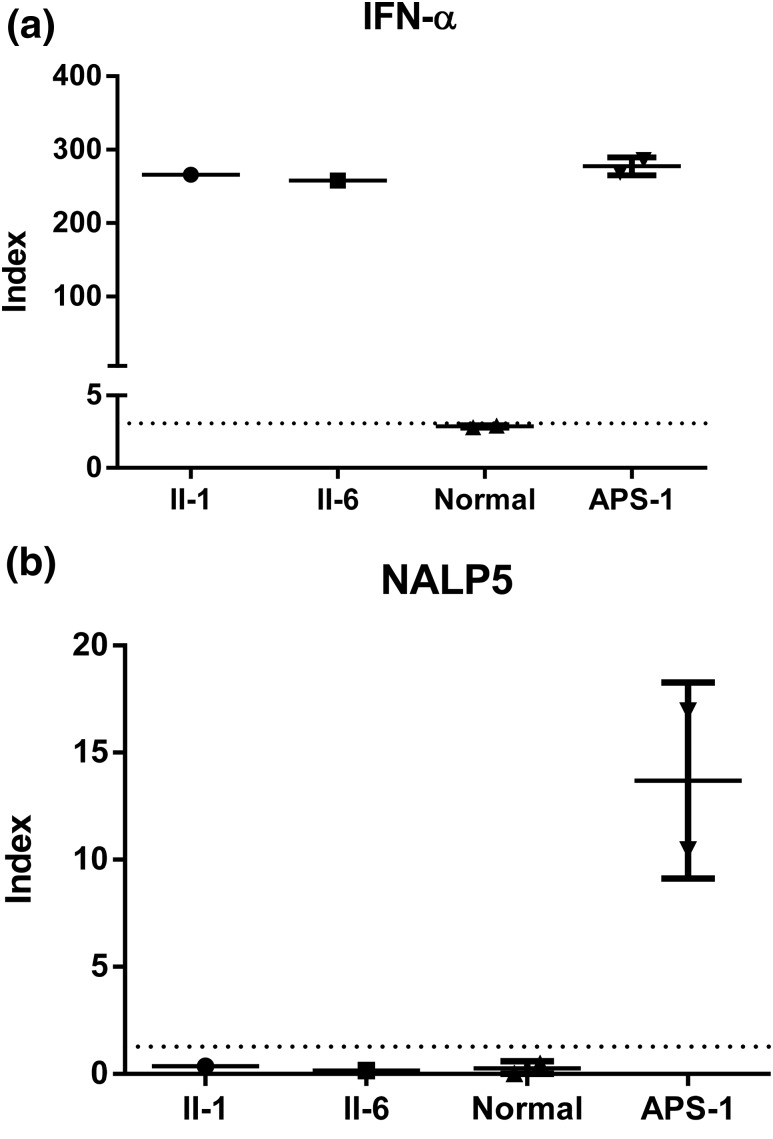
Although these patients harbored mutations in AIRE, which is known to predispose to autoimmunity, only one of the three siblings developed an additional APS-1–associated feature (i.e., premature ovarian failure). All three patients lacked clinical or biochemical signs of thyroid or adrenal insufficiency, mucocutaneous candidiasis, or ectodermal dysplasia. To determine whether these patients harbored subclinical autoimmune features, we also tested them for the presence of specific tissue autoantibodies. Conventional autoantibodies against thyroid and intestinal antigens were negative in all three siblings (Table 1). The two older brothers (II-1 and II-6) are also negative for assayed autoantibodies against adrenal and parietal cells. The proband (II-1) had positive antibodies to GAD65 and islet cells, whereas his brother (II-6) had negative results. Autoantibodies to type 1 IFNs (23, 24) and NALP5 (25) have been previously linked to HYPO and/or APS-1. Serum samples were available from only the two affected brothers and were positive for IFN-α antibodies but negative for the NALP5 antibody (Fig. 2).
Figure 2.
Analysis of IFN-α and NALP5 autoantibodies. Sera from patients (II-1 and II-6), normal control subjects, and patients with APS-1 were tested for the presence of autoantibodies to IFN-α (a) and NALP5 (b). Sera were tested in duplicate. Dotted line represents 3 standard deviations above the mean value of the normal control sera.
Discussion
Mutations in AIRE are typically associated with APS-1, a highly complex syndrome that is diagnosed by the presence of two or more of the three major features, which usually appear in a characteristic temporal sequence: mucocutaneous candidiasis, HYPO, and Addison’s disease. In addition, patients commonly manifest other features that indicate APS-1 is a generalized disturbance in autoimmune regulation, including hepatitis, keratitis, periodic rashes with fever, intestinal malabsorption, severe obstipation, alopecia, vitiligo, hypogonadism, hypothyroidism/hyperthyroidism, type 1 diabetes, pernicious anemia, dental enamel hypoplasia, or nail pitting (15, 16, 20–22, 26–28). AIRE encodes a transcription factor that functions as an important regulator in thymic epithelial cells. AIRE induces expression of important “self” identity proteins in the thymus (i.e., “tissue-restricted antigens”), and T cells that respond to those proteins are eliminated in the thymus through apoptosis, hence avoiding autoimmune disorders (20, 29). The loss of AIRE is associated with autoimmune attack against many tissues and the generation of many autoantibodies, including type 1 IFN–specific autoantibodies (24). Autoimmune regulator (Aire)-deficient mice and humans have circulating autoantibodies against a multitude of organs and multiorgan autoinflammatory infiltrates. Recent studies have shown that B lymphocytes, the source of these circulating autoantibodies, are required for Aire-deficient mice to develop fulminant infiltrates. Remarkably, although the autoantibodies are not directly pathogenic, the B cells play a critical early role in T cell priming or expansion (30). Hence, it is reasonable to hypothesize that the lack of AIRE results in survival of autoreactive T cells that could react with a parathyroid protein and destroy the parathyroid glands. The primary target antigen in the parathyroid remains unknown; however, it is unlikely to be NALP5 (see later).
More than 100 different mutations of AIRE gene have been identified in patients with APS-1 (31); in most cases inheritance is autosomal recessive, but in some patients an autosomal dominant mode of inheritance has also been described (32). Most AIRE mutations are located at four mutational hotspots: in exon 2, where a putative homodimerization domain is localized; in exon 6, between the SAND domain and the first PHD-finger motif; in exon 8, in the coding region for the first PHD-finger; and in exon 10, between the first and the second PHD-finger (20). Two mutations in exon 6 and 8 are the most frequent in North America and in most of Europe: R257X in exon 6 changes an arginine codon to a stop codon at amino acid position 257, which encodes a truncated, nonfunctional protein of 256 amino acids. The second common mutation is the 13-bp deletion in exon 8 present in the kindred we describe, which causes a frameshift that generates a truncated protein without any PHD fingers. The second AIRE variant that we identified in our kindred is a complex mutation, c.995+(3_5)delGAGinsTAT (IVS8 + (3_5)delGAGinsTAT), that is predicted to damage the splice donor site, which would either eliminate exons encoding the PHD fingers from a transcript or more likely lead to a premature termination codon and nonsense-mediated decay. This variant has been previously reported in two subjects with complete APS-1 who also carried a second mutation (21). Hence, both of the mutations that we have identified in affected members of this kindred are pathogenic and have been shown to produce the complete form of APS-1 in other subjects.
The initial manifestation of APS-1 usually occurs in childhood, and expression of the other main features usually follows within the first two decades of life. APS-1–associated diseases continue to appear at least until the fifth decade of life. It has been suggested that the earlier the first component appears, the more likely it is that multiple manifestations of APS-1 will develop (27). Thus, it is uncertain why two of the three patients that we described have such a limited manifestation of APS-1. One speculation would be that the genetic background in these patients presented here affords some protection against the other components of APS1 (e.g., the notable HLA haplotype, DRB1*15:01-DQA1*01:02-DQB1*06:02, which protects from type 1 diabetes development). However, our exome sequencing revealed that both brothers have DRB*01:01-DQB1*05:01-DPB1*10:01 and DRB*13:01-DQB1*06:03-DPB1*03:01 haplotypes, whereas the sister has DRB*01:01-DQB1*05:01-DPB1*10:01 and DRB*11:04-DQB1*03:01-DPB1*129:01 haplotypes, neither of which has been implicated in association with protection of type 1 diabetes in white subjects. Another speculation is based on the notion that environmental triggers contribute to the development of specific autoimmunities. Accumulated evidence indicates that close interplay between environmental factors and genetics is responsible for the loss of immunological tolerance and autoimmunities (33, 34) (e.g., a search on PubMed using the term “association between virus and type 1 diabetes” produces more than 200 publications). Similarly, both genetic and environmental factors have been implicated as the basis for the variable Aire-deficient phenotype in mice, in which targets and severity vary with the genetic background, implicating “modifier” genes (35). Finally, the phenotype and pattern of disease patients with APS1 may reflect stochastic effects. Overall, it is possible that organ-specific pathology results from a threshold of reactivity in the antigen receptor repertoires of T and B lymphocytes. In this respect, the near-constant development of HYPO in APS1 would suggest that parathyroid involvement occurs at a very low threshold and represents a very mild expression of the disease. As next-generation sequencing leads to identification of additional families with isolated features of APS1, it will eventually become possible to isolate these variable underlying mechanisms.
The association of AIRE mutations with isolated HYPO is quite unusual, and the kindred we describe is unique due to the ages of the affected subjects and the duration of medical surveillance. Previous studies have described young patients with apparent isolated HYPO in association with AIRE mutations. Cervato et al. (36) described two unrelated children, aged 4 and 5 years, with biallelic AIRE mutations who lacked features of other APS-1 components but were similar to our patient were both positive for IFN antibody (i.e., IFN-ω). Eyal et al. (37) described two siblings with HYPO and biallelic AIRE mutations, but only the brother, aged 15 years, lacked additional clinical features of APS-1. Furthermore, Sahoo et al. (38) described three patients with HYPO and biallelic AIRE mutations; the youngest died of uncertain causes at age 7 years; a second adult patient subsequently developed premature ovarian failure; and a third, aged 39, had been followed for only 2 years. Similarly, isolated HYPO has been described in two unrelated girls, aged 14 and 5 years, with dominant negative AIRE mutations and apparently isolated HYPO (39). The young age of these patients and/or relatively brief duration of follow-up makes it difficult to predict whether they will develop additional features of APS-1 because components of APS-1 continue to manifest until the fifth decade of life (26–28). The strength of our study is the long duration of follow-up in our cases. Specifically, two of the three adult patients that we describe in this kindred are unique in that they all manifest a similar phenotype of isolated HYPO and have failed to develop any other clinical features of APS-1 with over 50 years of medical surveillance each: the proband (II-1) in our study is 63 years old, and his brother (II-6) is 57 years old. Although the sister (II-11) had developed premature ovarian failure, it is unlikely that at age 53 years she will develop additional features of APS-1. Indeed, results from the biggest cohort of APS-1 indicate that by age 50 years 100% of AIRE-associated patients have candidiasis, 88% have HYPO, and 84% have adrenal insufficiency. Moreover, about one-third have alopecia, vitiligo, or pernicious anemia, and about one-quarter have severe obstipation, chronic diarrhea, hepatitis, or keratoconjunctivitis (26). The weakness of our study is the small number of subjects that we report. Nevertheless, AIRE mutations may be present in up to 10% of patients with isolated HYPO (38).
Finally, we note that serum from the two available affected patients in this family have IFN-α antibodies despite lacking other features of APS-1. To date, all patients with APS-1 who have been analyzed are positive for high-titer IgG auto-antibodies against type 1 IFNs, and these antibodies are not present in heterozygous carriers of AIRE mutations or in people with other endocrine disorders (22, 23). In the absence of thymoma or myasthenia gravis, IFN-α and IFN-ω autoantibodies appear to have nearly 100% sensitivity and specificity for AIRE mutations (40). By contrast, antibodies to NALP5, previously proposed as a useful indicator of parathyroid autoimmunity in APS-1, were absent in our patients, suggesting that this autoantibody is not a very sensitive marker for HYPO (41, 42). By contrast, our data confirm an association between IFN antibodies and isolated HYPO due to biallelic mutations in AIRE and suggest that testing patients with unexplained HYPO for type 1 IFN antibodies can be a useful preliminary screen prior to proceeding to genetic analyses.
In summary, this report refines the phenotypic spectrum of clinical and endocrine abnormalities associated with mutations in AIRE and extends the number of candidate genes that can cause isolated HYPO.
Acknowledgments
The authors thank the family involved in this study for their participation; Fengxiang Wang, James Snyder, and Harsh Kanwar, who helped in the DNA sample extraction and handling; Cuiping Hou and Tiancheng Wang, who provided technical assistance; and Dr. Karen Winer (National Institutes of Health), who provided laboratory data for subject II-1.
Acknowledgments
This work was supported by Grant R01DK079970 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.A.L.), a grant from the Institutional Development Fund to Center for Applied Genomics from The Children’s Hospital of Philadelphia (to H.H.), and an Adele and Daniel Kubert donation (to H.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APS-1
- autoimmune polyglandular syndrome type 1
- bp
- base pair
- CPM
- counts per minute
- GAD65
- glutamic acid decarboxylase 65
- HYPO
- hypoparathyroidism
- IFN
- interferon
- NALP5
- NACHT leucine–rich-repeat protein 5
- PTH
- parathyroid hormone.
References
- 1.Shoback DM, Bilezikian JP, Costa AG, Dempster D, Dralle H, Khan AA, Peacock M, Raffaelli M, Silva BC, Thakker RV, Vokes T, Bouillon R. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101(6):2300–2312. [DOI] [PubMed] [Google Scholar]
- 2.Clarke BL, Brown EM, Collins MT, Jüppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab. 2016;101(6):2284–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore). 1986;65(2):73–81. [PubMed] [Google Scholar]
- 4.Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990;86(4):1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Mannstadt M, Guo J, Kim SM, Yi HS, Khatri A, Dean T, Okazaki M, Gardella TJ, Juppner H. A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG. Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet. 1994;8(3):303–307. [DOI] [PubMed] [Google Scholar]
- 7.Bowl MR, Nesbit MA, Harding B, Levy E, Jefferson A, Volpi E, Rizzoti K, Lovell-Badge R, Schlessinger D, Whyte MP, Thakker RV. An interstitial deletion-insertion involving chromosomes 2p25.3 and Xq27.1, near SOX3, causes X-linked recessive hypoparathyroidism. J Clin Invest. 2005;115(10):2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. 2001;108(8):1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannstadt M, Harris M, Bravenboer B, Chitturi S, Dreijerink KM, Lambright DG, Lim ET, Daly MJ, Gabriel S, Jüppner H. Germline mutations affecting Gα11 in hypoparathyroidism. N Engl J Med. 2013;368(26):2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesbit MA, Hannan FM, Howles SA, Babinsky VN, Head RA, Cranston T, Rust N, Hobbs MR, Heath H III, Thakker RV. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368(26):2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Opas EE, Tuluc F, Metzger DL, Hou C, Hakonarson H, Levine MA. Autosomal dominant hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J Clin Endocrinol Metab. 2014;99(9):E1774–E1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406(6794):419–422. [DOI] [PubMed] [Google Scholar]
- 13.Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, Mortier G, Gregory S, Sharony R, Kambouris M, Sakati N, Meyer BF, Al Aqeel AI, Al Humaidan AK, Al Zanhrani F, Al Swaid A, Al Othman J, Diaz GA, Weiner R, Khan KT, Gordon R, Gelb BD; HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium . Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32(3):448–452. [DOI] [PubMed] [Google Scholar]
- 14.Unger S, Górna MW, Le Béchec A, Do Vale-Pereira S, Bedeschi MF, Geiberger S, Grigelioniene G, Horemuzova E, Lalatta F, Lausch E, Magnani C, Nampoothiri S, Nishimura G, Petrella D, Rojas-Ringeling F, Utsunomiya A, Zabel B, Pradervand S, Harshman K, Campos-Xavier B, Bonafé L, Superti-Furga G, Stevenson B, Superti-Furga A. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013;92(6):990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. [DOI] [PubMed] [Google Scholar]
- 16.Finnish-German APECED Consortium An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17(4):399–403. [DOI] [PubMed] [Google Scholar]
- 17.Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, Goldmuntz E, McDonald-McGinn DM, Zackai EH, Emanuel BS, Driscoll DA, Budarf ML. Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet. 2001;38(12):E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–957. [DOI] [PubMed] [Google Scholar]
- 19.Tóth B, Wolff AS, Halász Z, Tar A, Szüts P, Ilyés I, Erdos M, Szegedi G, Husebye ES, Zeher M, Maródi L. Novel sequence variation of AIRE and detection of interferon-omega antibodies in early infancy. Clin Endocrinol (Oxf). 2010;72(5):641–647. [DOI] [PubMed] [Google Scholar]
- 20.Björses P, Halonen M, Palvimo JJ, Kolmer M, Aaltonen J, Ellonen P, Perheentupa J, Ulmanen I, Peltonen L. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66(2):378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazza C, Buzi F, Ortolani F, Vitali A, Notarangelo LD, Weber G, Bacchetta R, Soresina A, Lougaris V, Greggio NA, Taddio A, Pasic S, de Vroede M, Pac M, Kilic SS, Ozden S, Rusconi R, Martino S, Capalbo D, Salerno M, Pignata C, Radetti G, Maggiore G, Plebani A, Notarangelo LD, Badolato R. Clinical heterogeneity and diagnostic delay of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome. Clin Immunol. 2011;139(1):6–11. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AS, Erichsen MM, Meager A, Magitta NF, Myhre AG, Bollerslev J, Fougner KJ, Lima K, Knappskog PM, Husebye ES. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J Clin Endocrinol Metab. 2007;92(2):595–603. [DOI] [PubMed] [Google Scholar]
- 23.Levin M. Anti-interferon auto-antibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3(7):e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meager A, Visvalingam K, Peterson P, Möll K, Murumägi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y, Willcox N. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3(7):e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alimohammadi M, Björklund P, Hallgren A, Pöntynen N, Szinnai G, Shikama N, Keller MP, Ekwall O, Kinkel SA, Husebye ES, Gustafsson J, Rorsman F, Peltonen L, Betterle C, Perheentupa J, Akerström G, Westin G, Scott HS, Holländer GA, Kämpe O. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358(10):1018–1028. [DOI] [PubMed] [Google Scholar]
- 26.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843–2850. [DOI] [PubMed] [Google Scholar]
- 27.Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322(26):1829–1836. [DOI] [PubMed] [Google Scholar]
- 28.Betterle C, Greggio NA, Volpato M. Clinical review 93: autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83(4):1049–1055. [DOI] [PubMed] [Google Scholar]
- 29.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140(1):123–135. [DOI] [PubMed] [Google Scholar]
- 30.Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: a therapeutic approach for APECED patients. Proc Natl Acad Sci USA. 2008;105(35):13009–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Martino L, Capalbo D, Improda N, Lorello P, Ungaro C, Di Mase R, Cirillo E, Pignata C, Salerno M. Novel findings into AIRE genetics and functioning: clinical implications. Front Pediatr. 2016;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cetani F, Barbesino G, Borsari S, Pardi E, Cianferotti L, Pinchera A, Marcocci C. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2001;86(10):4747–4752. [DOI] [PubMed] [Google Scholar]
- 33.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39(4):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selmi C. Autoimmunity in 2011. Clin Rev Allergy Immunol. 2012;43(1-2):194–206. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202(6):805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervato S, Morlin L, Albergoni MP, Masiero S, Greggio N, Meossi C, Chen S, del Pilar Larosa M, Furmaniak J, Rees Smith B, Alimohammadi M, Kämpe O, Valenzise M, Betterle C. AIRE gene mutations and autoantibodies to interferon omega in patients with chronic hypoparathyroidism without APECED. Clin Endocrinol (Oxf). 2010;73(5):630–636. [DOI] [PubMed] [Google Scholar]
- 37.Eyal O, Oren A, Jüppner H, Somech R, De Bellis A, Mannstadt M, Szalat A, Bleiberg M, Weisman Y, Weintrob N. Hypoparathyroidism and central diabetes insipidus: in search of the link. Eur J Pediatr. 2014;173(12):1731–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahoo SK, Zaidi G, Srivastava R, Sarangi AN, Bharti N, Eriksson D, Bensing S, Kämpe O, Aggarwal A, Aggarwal R, Bhatia E. Identification of autoimmune polyendocrine syndrome type 1 in patients with isolated hypoparathyroidism. Clin Endocrinol (Oxf). 2016;85(4):544–550. [DOI] [PubMed] [Google Scholar]
- 39.Oftedal BE, Hellesen A, Erichsen MM, Bratland E, Vardi A, Perheentupa J, Kemp EH, Fiskerstrand T, Viken MK, Weetman AP, Fleishman SJ, Banka S, Newman WG, Sewell WA, Sozaeva LS, Zayats T, Haugarvoll K, Orlova EM, Haavik J, Johansson S, Knappskog PM, Løvås K, Wolff AS, Abramson J, Husebye ES. Dominant mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity. 2015;42(6):1185–1196. [DOI] [PubMed] [Google Scholar]
- 40.Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, Willcox N. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132(1):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomar N, Kaushal E, Das M, Gupta N, Betterle C, Goswami R. Prevalence and significance of NALP5 autoantibodies in patients with idiopathic hypoparathyroidism. J Clin Endocrinol Metab. 2012;97(4):1219–1226. [DOI] [PubMed] [Google Scholar]
- 42.Kemp EH, Habibullah M, Kluger N, Ranki A, Sandhu HK, Krohn KJ, Weetman AP. Prevalence and clinical associations of calcium-sensing receptor and NALP5 autoantibodies in Finnish APECED patients. J Clin Endocrinol Metab. 2014;99(3):1064–1071. [DOI] [PubMed] [Google Scholar]