Abstract
Context:
Obesity, insulin resistance (IR), and diabetes are increasing in youth, especially in girls. IR is associated with muscle mitochondrial dysfunction in youth and adults with diabetes. However, it is unknown whether this relationship is present in youth prior to development of diabetes.
Objective:
Assess IR and mitochondrial function, including sex differences, in nondiabetic youth.
Design:
Cross-sectional study of youth in the Exploring Perinatal Outcomes among Children, Resistance to InSulin in Type 1 And Type 2 diabetes, and Androgens and Insulin Resistance Study cohorts.
Setting:
Academic medical university.
Participants:
Two hundred seventy-five youth, 13 to 19 years old [43% males: 17.1 (16.52, 17.63) years, body mass index z-score (BMI-Z) 0.36, 64.7% Tanner 5; 57% females: 17.2 (16.43, 17.67) years, BMI-Z 0.72, 78.9% Tanner 5].
Interventions:
Fasting laboratories, oral glucose tolerance test, and 31P magnetic resonance spectroscopy.
Main Outcome Measures:
IR [triglyceride:high-density lipoprotein (HDL) ratio, Matsuda index, and homeostasis model for insulin resistance (HOMA-IR)] and muscle mitochondrial function (adenosine 5′-diphosphate time constant and oxidative phosphorylation rate).
Results:
Compared with males, females were more insulin resistant, with higher triglyceride:HDL ratio [1.95 (1.30, 2.79) vs 1.69 (1.21, 2.23), P = 0.042], HOMA-IR [3.18 (2.42, 4.39) vs 2.76 (2.02, 4.08), P = 0.035], and fasting free fatty acids (FFAs) and lower Matsuda score [3.98 (2.71, 5.96) vs 5.39 (3.43, 7.57), P < 0.001]. After adjustment for the higher BMI and Tanner stage and lower physical activity levels seen in females, there were no sex differences in mitochondrial function nor in any IR measure except FFAs. We did not find an association between measures of IR and mitochondrial function.
Conclusions:
The greater IR seen in adolescent girls vs boys is mostly explained by differences in BMI and physical activity. Mitochondrial function does not appear to be related to IR in a large cohort of nondiabetic youth.
In a large cohort of adolescents, early insulin resistance assessed by oral glucose tolerance test is found only in girls and is not accompanied by postexercise muscle mitochondrial dysfunction measured with spectroscopy.
The physiology of insulin resistance (IR) in adults with type 2 diabetes (T2D) has been widely studied. Though the exact etiology of IR remains incompletely understood, previous studies suggest that altered mitochondrial function has a pivotal role (1, 2). Excess or ectopic lipids, including serum free fatty acids (FFAs), intramyocellular lipids (IMCLs), intrahepatic triglycerides (TGs), and lipid subspecies such as diacyglycerol, may relate to IR and are elevated in adults with T2D (3, 4). This relationship could be explained by a decrease in FFA oxidation by mitochondria, leading to increased IMCL and diacylglycerol, which could impair insulin signaling (5). In the nondiabetic but insulin-resistant adult offspring of patients with T2D, IR in skeletal muscle was closely associated with the dysregulation of IMCL metabolism, suggesting a possible inherited defect in mitochondrial oxidative phosphorylation as the underlying mechanism (1). Increased fat accumulation in muscle and liver tissue was also reported in the elderly, who in parallel also have significantly more IR than younger control adults (6). IR is also associated with mitochondrial changes after burn trauma, providing further support for the hypothesis that IR is associated with altered mitochondrial function across a wide variety of IR-inducing disease states (7).
The prevalence of T2D among adolescents has increased dramatically, carrying high rates of morbidity and early mortality (8). Youth with T2D are typically overweight or obese, and the increased prevalence of T2D is thought to be related to increases in pediatric obesity. IR is commonly present in obese adolescents prior to the onset of T2D and thus is likely the initiating step in the development of T2D (4, 5, 9). As in adults, we and others have documented increased IMCLs in youth with T2D and an association between aspects of IR and mitochondrial function in youth with T2D and type 1 diabetes (T1D) (8, 10–12). However, it is not known whether mitochondrial dysfunction precedes IR in youth, occurs secondary to IR, or occurs secondary to other components characteristic of diabetes, such as hyperglycemia. Furthermore, in T1D youth, the IR and muscle mitochondrial dysfunction occur despite low IMCLs, evidence that IR and mitochondrial dysfunction are not always IMCL mediated (11). Therefore, to better understand the progression to T2D in youth, it is important to first understand the relationship between IR and muscle mitochondrial function in youth without diabetes.
Other factors beyond obesity also influence insulin sensitivity in youth. IR develops during puberty, particularly in females. IR reaches a peak at Tanner stages 3 and 4 and then slowly resolves upon maturation (13). Differences in IR between males and females have also been noted to develop by midpuberty (13, 14), and most studies report higher prevalence rates of IR in girls (15, 16). However, studies relating mitochondrial function to this rise in IR during puberty are lacking. Another factor influencing insulin sensitivity in youth is physical activity, and there is evidence that physical activity could effectively ameliorate IR (17, 18). Physical activity levels will thus play a role in mediating various degrees of IR in adolescents.
Few studies have evaluated in vivo muscle mitochondrial function in relatively healthy youth. In a study of 22 nondiabetic, obese adolescent girls, mitochondrial function was related to IR, but this relationship was not mediated by IMCLs, subcutaneous adipose tissue, or visceral adipose tissue (19). Fleischman et al. found that in a cohort of 70 normal-weight and overweight children between the age of eight and 19 years, the more insulin-resistant children had a more prolonged phosphocreatine (PCr) recovery time constant, which is indicative of slower oxidative metabolism (20). These two relatively small studies assessing IR and mitochondrial function in adolescents suggest that changes in mitochondrial function may play a role in altering insulin sensitivity.
We have previously examined the relationship between insulin sensitivity as assessed by a hyperinsulinemic-euglycemic clamp and in vivo mitochondrial function in a diet- and exercise-controlled, nonphysiologic setting (9, 10, 12). However, the role of mitochondrial dysfunction in early puberty and sex-associated insulin sensitivity in youth with disease is unknown. Our goal was to determine the association between in vivo muscle mitochondrial function and estimated IR across a larger cohort of youth without diabetes, in a setting reflective of their day-to-day life. Additionally, we explored potential sex differences in IR and mitochondrial function and the impact of pubertal stage, body mass index (BMI), and physical activity.
Methods
Participants
Two hundred seventy-five participants were recruited from three cohorts of youth: (1) the Exploring Perinatal Outcomes among Children (EPOCH) study, a longitudinal study of the impact of maternal gestational diabetes on long-term health outcomes, with youth originally identified at ages six to 13 years from a community sample using a Kaiser database of youth with varied BMI and physical activity level, of whom 16% were exposed to gestational diabetes mellitus; (2) the Resistance to InSulin in Type 1 And Type 2 diabetes (RESISTANT) study; and (3) the Androgens and Insulin Resistance Study (AIRS), the latter two being cross-sectional cohorts that included nondiabetic sedentary youth of varied BMI, recruited from the local community and general pediatrics and obesity clinics at Children’s Hospital Colorado (21). All youth were between 12 and 19 years of age. Participants with diabetes or acute illness on day of study were excluded from the study. Tanner stage of the participant was determined by a pediatric endocrinologist in RESISTANT and AIRS cohorts and self-assessed in EPOCH. All studies were approved by the University of Colorado Anschutz Medical Campus Institutional Review Board and the Children’s Hospital of Colorado Scientific Advisory Review Committee. Parental informed consent and participant assent were obtained from all participants less than 18 years old, and participant consent was obtained from those aged 18 years and above.
Overall study design
All measures were a priori standardized across all three cohorts. A two-hour oral glucose tolerance test (OGTT; 1.75 g/kg up to a maximum of 75 g) was performed with blood sampling for fasting glucose, glycemic markers, FFAs, and insulin and then insulin and glucose at 30 and 120 minutes. A three-day pediatric activity recall questionnaire of physical activity levels for the three previous days was completed with study staff assistance to yield a single activity score (8, 22). Magnetic resonance imaging (MRI) imaging of the leg was performed for maximal cross sectional area and in-MRI exercise testing with 31phosphorus magnetic resonance spectroscopy (31P-MRS) to assess mitochondrial function.
Laboratory measures
Insulin was analyzed with radioimmunoassay (Millipore, Billerica, MA), and FFA (Wako Chemincals, Inc., Richmond, VA) were determined enzymatically. Measurements of total cholesterol, high-density lipoprotein (HDL) cholesterol, and TGs were performed enzymatically (Hitachi 917; Boehringer Mannheim Diagnostics, Indianapolis, IN). Low-density lipoprotein cholesterol was calculated by the Friedewald equation (23).
Magnetic resonance imaging and spectroscopy
Imaging acquisition
Imaging and spectroscopy were performed on a General Electric 3 Tesla magnet with HDx MRI (General Electric, Milwaukee, WI) running version 15M4 software equipped with the General Electric multinuclear hardware spectroscopy accessory and research software as well as a custom 1H/31P leg coil (Clinical MR Solutions, Brookfield, WI) (24). Cross-sectional area of the calf was measured as previously described (24).
Spectroscopy
31P-MRS performed at 51.70 MHz with the 1H/31P coil was used to assess rates of mitochondrial phosphorylation. Auto-shimming with 1H was performed, and then a 31P scan was performed for resting baseline relaxed measurements. The 31P exercise scan was then performed under partially saturated conditions (relaxation time, 1000 ms; flip angle, 135; 2048 points) (11).
31P-MRS exercise protocol
Strength testing was done on a custom-built, MR-compatible plantar flexion device with force measurement capability to determine maximal volitional contraction (MVC). The 31P-MRS exercise protocol consisted of measurements during rest for 90 seconds, isometric plantar flexion exercise for 90 seconds at 70% MVC, and then seven minutes of rest (25). Force was recorded as previously described (5, 26).
Spectroscopy analysis
Time domain fitting was performed by jMRUi (27, 28) utilizing prior knowledge files (29) to determine peak positions and areas of interest (PCr, inorganic free phosphate, β-adenosine triphosphate [ATP; three peaks], α-ATP [two peaks], γ-ATP [two peaks], and phosphomonoester). The fully relaxed spectra were used to correct exercise spectra for saturation. The jMRUi data were used to calculate metabolic variables as previously described (30). Calculations included the rates of oxidative phosphorylation (OxPhos) following exercise, creatine kinase reaction, initial PCr synthesis, anaerobic glycolysis, and QMAX, the apparent mitochondrial capacity, i.e., maximal oxidative ATP production rate, as previously reported (10, 24). Regression analyses were used to calculate adenosine 5′-diphosphate (ADP), PCr, and inorganic free phosphate time constants (Sigmaplot; Systat Software, Inc, San Jose, CA).
Calculations and statistics
The homeostasis model for insulin resistance (HOMA-IR) was calculated as: (fasting glucose × fasting insulin)/405. Matsuda index was calculated as 10,000/[square root (fasting glucose × fasting insulin × mean glucose × mean insulin)].
The distribution of all variables was examined, and results were presented as mean ± standard deviation of the mean or median (25%, 75%) as appropriate. Group comparisons were made using Fisher’s exact test, t test, or Kruskal-Wallis for continuous variables. The associations between HOMA-IR, Matsuda index, and TG:HDL ratio based on OGTT values and the two primary outcomes from the 70% exercise were examined (ADP time constant and OxPhos) using multiple regression. The group was then stratified by sex to further compare IR and mitochondrial function between males and females using linear models with and without adjustment for potential confounders [BMI z-score (BMI-Z), Tanner stage, and physical activity level score]. P values < 0.05 were considered statistically significant. Statistical analyses were performed with SigmaStat Software version 11.2 (Systat Software, Inc, San Jose, CA) and SAS Software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Participant characteristics
A total of 275 participants across the three cohorts were included in this analysis: 119 males and 156 females (213 EPOCH, 37 AIRS, and 24 RESISTANT). Unadjusted descriptive statistics by sex are shown in Table 1. When stratified by sex, males and females were of similar age, although females had a higher BMI-Z (P = 0.01) and a higher percentage of participants in Tanner 5 stage (78.9% vs 74.7%). Females also had significantly lower habitual levels of physical activity than males (Table 1; P = 0.012) as assessed by the mean total metabolic equivalents. The sex differences in activity level remained significant even after adjusting for BMI-Z and Tanner stage (P = 0.001).
Table 1.
Unadjusted Descriptive Characteristics of Study Participants by Sex
| Characteristics | Males (n = 119) | Females (n = 156) |
|---|---|---|
| Age (years) | 17.1 (16.52, 17.63) | 17.2 (16.43, 17.67) |
| BMI-Z | 0.36 ± 1.20 | 0.72 ± 1.07b |
| Waist (cm) | 79.1 (73.00, 89.50) | 82.5 (74.45, 92.45) |
| Tanner 2 (n) | 2 | 0 |
| Tanner 3 (n) | 1 | 5 |
| Tanner 4 (n) | 39 | 28 |
| Tanner 5 (n) | 77 | 123 |
| Tanner 5 (%) | 64.71 | 78.85 |
| Laboratory measures | ||
| Fasting glucose (mg/dL) | 90.0 (86.00, 93.00) | 86.0 (82.00, 91.00)c |
| Two-hour glucose (mg/dL) | 88.0 (77.00, 103.0) | 100 (85.00, 119.0)c |
| Fasting insulin (IU/mL) | 12.0 (9.00, 17.00) | 14.0 (11.00, 19.00)b |
| Two-hour insulin (IU/mL) | 36.0 (20.50, 55.00) | 72.5 (45.00, 117.0)c |
| Total cholesterol (mg/dL) | 134 (121.0, 153.0) | 140 (122.0, 168.0) |
| TG (mg/dL) | 76.0 (55.00, 99.00) | 85.0 (61.00, 122.0)b |
| HDL (mg/dL) | 44.0 (39.00, 49.00) | 45.0 (38.00, 53.00) |
| TG:HDL | 1.69 (1.21, 2.23) | 1.95 (1.30, 2.79)a |
| Low-density lipoprotein (mg/dL) | 75.2 (62.60, 91.60) | 82.0 (64.00, 107.0)a |
| FFA baseline (mmol/L) | 364 (264.0, 526.0) | 458 (343.5, 616.8)b |
| Mean total metabolic equivalents | 67.3 (57.50, 82.00) | 62.7 (56.67, 71.00)b |
| Mitochondrial controls | ||
| Muscle area (mm2) | 4238 (3575, 5100) | 3644 (3205, 41,965)c |
| Average force (kg) | 27.7 (24.05, 31.37) | 28.4 (24.98, 31.47) |
Values are mean ± standard deviation, median (25th percentile, 75th percentile), or n (%) as indicated.
P < 0.05–0.01.
P < 0.01–0.001.
P < 0.001.
IR data
There was no significant difference in waist circumference between the male and female participants before or after adjusting for BMI-Z, Tanner stage, and physical activity. There were significant differences in OGTT measures between males and females before adjustments. Males had significantly higher fasting glucose concentrations (Table 1; P < 0.001), but their 2-hour glucose (P < 0.001), fasting insulin (P = 0.004), and 2-hour insulin concentrations (P < 0.001) were significantly lower than female participants. Females were more insulin resistant, as assessed by significantly higher HOMA-IR (P = 0.042), TG:HDL ratio (P = 0.049), and fasting FFAs (P = 0.002) and lower Matsuda index (P < 0.001) than males, before adjusting for BMI-Z, age, and physical activity. After adjusting for BMI-Z and Tanner stage, only differences in Matsuda index and FFAs between sexes remained significant (P = 0.004). When adjusting additionally for physical activity, all differences in IR measures became insignificant except for FFAs (P = 0.015).
31P-MRS data
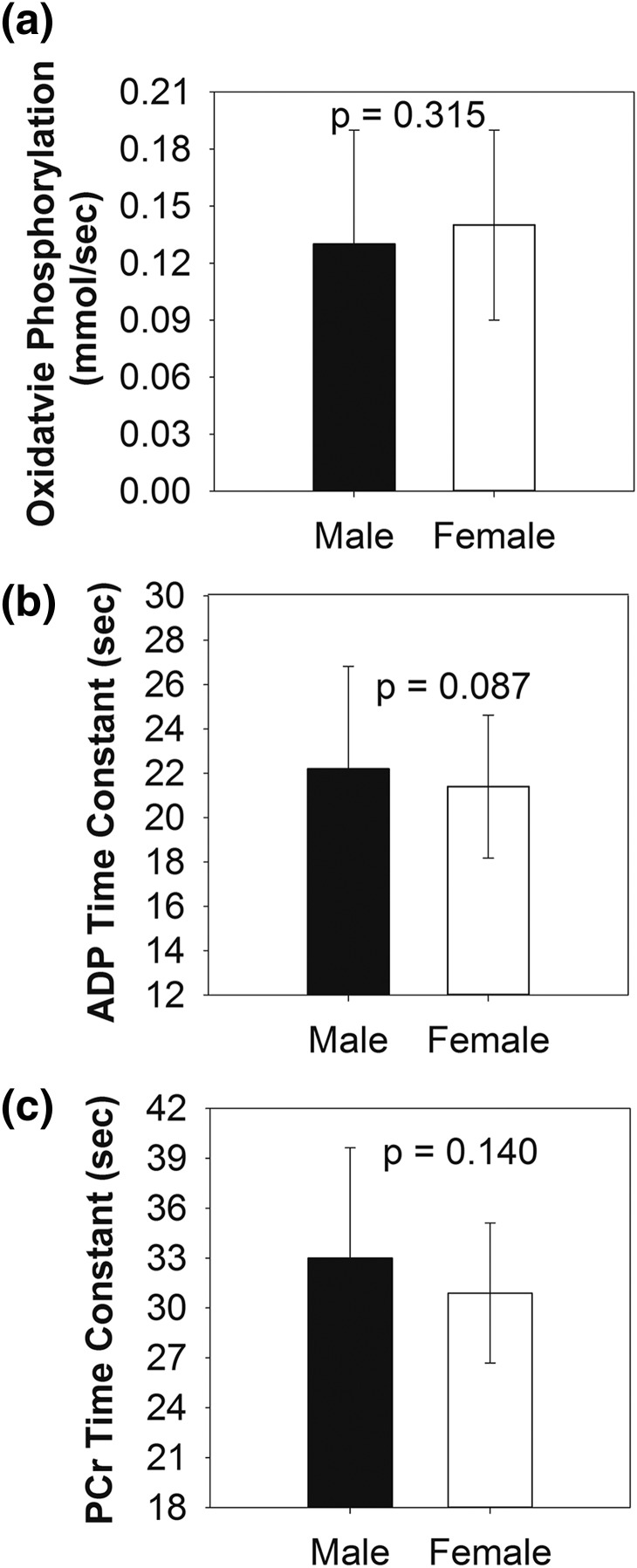
Group means by sex of calculated data from 31P-MRS for 70% exercise, adjusted for BMI-Z, Tanner stage, and physical activity are shown in Fig. 1. The rate of OxPhos did not differ between males and females either before (P = 0.128) or after adjustment [Fig. 1(a); P = 0.315], nor did the ADP time constant before (P = 0.299) or after adjusting for BMI-Z, Tanner stage, and physical activity [Fig. 1(b); P = 0.087]. PCr time constant also did not significantly differ between sexes before (P = 0.258) or after adjusting for BMI-Z, Tanner stage, and physical activity [Fig. 1(c); P = 0.160].
Figure 1.
The mean rates of (a) oxidative phosphorylation and (b) ADP and (c) PCr time constants following calf exercise are shown for males and females. Results are adjusted for BMI-Z, Tanner stage, and physical activity level.
Correlations
In the entire cohort, there were no significant associations between mitochondrial function and IR measures either in unadjusted analyses or in analyses adjusted for BMI-Z, Tanner stage, and physical activity (Fig. 2; Table 2). After stratifying by sex, in unadjusted and adjusted analyses, mitochondrial function and IR measures were not related in females. However, the unadjusted analyses indicated that in males, OxPhos was associated with HOMA-IR (P = 0.042, β = 0.009) and TG:HDL ratio (P = 0.004, β = 0.021). When adjusting only for BMI-Z and Tanner stage, OxPhos was still associated with HOMA-IR (P = 0.042, β = 0.009) and TG:HDL (P = 0.003, β = 0.023) in males. In models adjusted for BMI-Z, Tanner stage, and physical activity, only the TG:HDL ratio correlated with the PCr time constant (Table 2; P = 0.044, β = 2.276).
Figure 2.
Mean values from three different measures of IR: (a) HOMA-IR, (b) Matsuda index, and (c) TG:HDL ratio are shown for males and females. Results are adjusted for BMI-Z, Tanner stage, and physical activity level.
Table 2.
Correlations Between Estimated IR Variables and Mitochondrial Function Parameters, Overall and by Sex
| Variable | Entire Cohort (n = 275): Regression Coefficient | Females (n = 156): Regression Coefficient | Males (n = 119): Regression Coefficient |
|---|---|---|---|
| Outcome: oxidative phosphorylation | |||
| HOMA-IR | 0.000 | −0.004 | 0.005 |
| Matsuda index | −0.001 | −0.001 | −0.002 |
| TG:HDL | −0.009 | −0.020 | 0.009 |
| Outcome: ADP time constant | |||
| HOMA-IR | −0.115 | −0.139 | 0.146 |
| Matsuda index | −0.112 | −0.031 | −0.246 |
| TG:HDL | 0.760 | 0.916 | 1.410 |
| Outcome: PCr time constant | |||
| HOMA-IR | −0.226 | −0.260 | −0.071 |
| Matsuda index | −0.248 | −0.204 | −0.259 |
| TG:HDL | 1.186 | 0.167 | 2.276a |
Results of multiple linear regression models for the entire cohort and groups stratified by sex, with mitochondrial function measures as outcomes and IR surrogate indices as the predictor, adjusted for age, BMI-Z, and physical activity.
P < 0.05–0.01.
Discussion
We examined the relationship between IR and mitochondrial function as well as the sex differences in these variables in healthy adolescents without diabetes and with varied BMI and physical activity levels. We found that overall, muscle mitochondrial function did not relate to IR assessed by OGTT or fasting measures when adjusted for BMI, Tanner stage, and physical activity level. When divided by sex, there was still no relationship between mitochondrial function and IR in girls. In boys, HDL:TG related to mitochondrial function, whereas HOMA-IR and the Matsuda index did not. Therefore, in this large multiethnic cohort, there was overall no evidence that the development of IR in healthy youth is preceded by or related to muscle mitochondrial function, and in boys, it is rather influenced by physical activity.
The relationship between IR and in vivo muscle mitochondrial function in youth varies with disease state. We have previously shown that in youth with T1D, T2D, or polycystic ovarian syndrome (PCOS), diseases with significant IR, magnetic resonance spectroscopy–assessed mitochondrial dysfunction was present, and hyperinsulinemic euglycemic clamp measures of IR did correlate with measures of mitochondrial function. In PCOS, we found that IR was related to postexercise oxidative phosphorylation (9) and that IR in youth with T1D was similarly related to ADP time constant and oxidative phosphorylation (10). We documented that IR in youth with T2D is profound, as compared with BMI-similar youth without diabetes and normal-weight controls (8, 12). In this cohort, we found that mitochondrial function was impaired in the youth, with T2D relative to normal-weight controls, and that IMCLs were higher. However, clamp assessed IR related to the ADP time constant and FFA concentrations during hyperinsulinemia, but had no relation to IMCLs (12).
The published results of mitochondrial studies from small cohorts of youth without diabetes or metabolic diseases are mixed, likely due to methodologic variation and failure to control for physical activity. Fleischman et al. measured muscle mitochondrial function following 30% MVC aerobic exercise perturbation in the vastus lateralis, which is a mixed fiber-type muscle, not predominantly an oxidative fiber type like the soleus, and this can influence results (20). Similar to our findings, they found that postexercise PCr recovery in 74 youth without diabetes did not differ between normal-weight and obese youth. They did find that PCr recovery was associated with HOMA-IR when dividing the cohort into HOMA-IR quartiles, but the multivariate analysis was not adjusted for physical activity status (20). In a mixed BMI cohort of 48 youth, PCr recovery following a short bout of aerobic exercise was not associated with HOMA-IR, although it was associated with BMI z-score (31). Again, physical activity was not factored into those analyses, although the authors collected historical and quantitative (bicycle exercise testing) exercise data. Finally, in 19 obese adolescent girls, HOMA-IR was associated with PCr recovery time in the calf following 30% MVC contraction, but not with IMCLs, subcutaneous adipose tissue, or visceral adipose tissue (19). Again, data on physical activity was not presented in this study, and these girls are all >95th percentile for BMI and much more similar to our at-risk PCOS and T2D youth, where we did find changes in mitochondrial function.
In contrast to previous studies from smaller cohorts mentioned earlier, we found in a larger cohort that in youth without diabetes, IR was not related to postexercise muscle mitochondrial function. There are many factors that can contribute to alterations in postexercise mitochondrial function and IR, such as activity/exercise level, diet, and sex hormones (32, 33). Our overall results are likely different from those previously discussed from other groups as we controlled our analyses for physical activity and had a more heterogeneous study population. The failure to correct for physical activity by other investigators is important, especially in light of our results in boys, which were significant in multiple measures until we accounted for activity. Further, in our PCOS and diabetes studies, where a relationship between IR and mitochondrial function was observed, subjects had a controlled diet for three days preceding metabolic measurements, were inactive for three days prior to the study, and overall were sedentary, performing less than three hours of activity a week. In the current study, we did not control participants’ diet or activity level, as we sought to study everyday conditions. Rather, we used a questionnaire to assess participants’ daily activity level to control for activity. Although a questionnaire is not as good as objective activity data such as an accelerometer, it typically is systematically biased in the direction of overestimation of activity and thus still provides reasonable data for large cohorts of this size. This allowed us to provide a realistic representation of many type of adolescents who have various exercise levels and dietary habits.
Measuring IR can be challenging, and many different methods can be used. Whereas the hyperinsulinemic euglycemic clamp is the gold standard to assess insulin sensitivity and we have used this in youth with disease, it is expensive and time consuming and has an increased subject risk that is often considered unethical in healthy youth. We thus assessed IR using three clinical indicators to maximize the opportunity to relate IR to mitochondrial measures: HOMA-IR reflecting a fasting condition, Matsuda index reflecting an oral glucose challenge, and TG:HDL ratio reflecting IR over time. None of these measures of IR were associated with mitochondrial function in our overall cohort or in girls. Whereas an OGTT was performed in all of the other three published cohorts of youth from other investigators, data describing the relationship with mitochondria and IR indicators other than HOMA-IR were also not presented. Both TG and HDL concentrations are effected by sex steroids, and thus of the three IR measures, the TG:HDL ratio is most likely to be altered (34). Further, testosterone improves muscle function, perhaps through increased mitochondrial function (34, 35). This influence on both measurements may contribute to the association between TG:HDL and PCr time constant we saw in just the boys. Future studies assessing sex steroid levels are needed to test this hypothesis.
Several studies have previously demonstrated that adolescent females are more insulin resistant than males, especially in later puberty (13, 14). Our findings of a worse Matsuda index, HOMA-IR, TG:HDL ratio, and fasting FFAs are consistent with these studies. Moran et al. found that in nonobese adolescents, the sex differences in IR were explained by differences in adiposity as measured by skinfold thickness (14). Similarly, we found that sex differences in IR were explained by BMI and physical activity level. Adolescent females engage less in physical activity than boys and become more sedentary throughout adolescence (36, 37). In a longitudinal study evaluating the physical activity level of 405 adolescents over a period of two years, both sexes showed a decline in physical activity, but the unfavorable changes in sedentary behavior were more severe in females (36). Our cross-sectional physical activity measures support these studies, as the females in our cohort were significantly less physically active than similarly aged males. Finally, sex differences are not uniformly found in well-matched adult cohorts, nor do they reveal that females have better insulin sensitivity, indicating that changes in IR may not be sex hormone driven (38). Thus, the mild IR seen in females was likely caused by decreased physical activity, as well as by their higher BMI, rather than by sex-induced differences in mitochondrial function per se.
Our study has several strengths and weaknesses. We have a large cohort, with subjects from three separate studies with identical measures pooled for analysis. This large cohort allows for a diverse sample, with varied BMI, pubertal stage, activity level, and diet. However, this is also a weakness of the study, as early subtle changes in mitochondrial function may be undetectable with this type of variability. We also did not use the gold standard assessment of insulin sensitivity due to the large sample size, although we did use three surrogate measures of IR, representing three different physiologic settings. Our male cohort was earlier in Tanner stage than our female group; however, the IR of puberty peaks at Tanner 3, and thus this difference of pubertal stage between the sexes would be expected to influence lower insulin sensitivity in the boys, not the girls. Finally, unlike our diabetes and PCOS studies, due to the larger sample size, we were unable to provide a controlled diet or objectively measure physical activity level. Instead, we used a self-reported questionnaire to assess participants’ daily activity level, which is well validated in youth. However, by not artificially controlling the diet or activity, the results reflect the participants’ day-to-day metabolism.
Conclusion
In a large and diverse cohort of adolescents without diabetes, postexercise muscle mitochondrial function did not relate to IR, before or after adjusting for potential confounders such as BMI, Tanner stage, and physical activity level. The development of early, mild IR in youth without diabetes is not related to muscle mitochondrial dysfunction, a finding that differs from that in adults with IR and from youth with more severe IR. The lack of association between IR and mitochondrial function observed in relatively healthy youth support the hypothesis that initial mechanisms of IR in youth are distinct from those in adults. Understanding the factors contributing to the development of IR in youth has implications for future studies of diabetes prevention in the pediatric population. In addition, we found that differences in IR in girls relate to BMI and physical activity, arguing for interventions aimed at increasing physical activity in adolescent females.
Acknowledgments
We thank the volunteers and their families for participating.
Acknowledgments
This work was supported by NIH/NCRR Grant K23 RR020038-05, American Diabetes Association Career Development Award 7-11-CD-08, and Juvenile Diabetes Research Foundation Grants 11-2010-343 and 1R56DK088971 (to K.J.N.); NIH NIDDK Grant T32 DK063687, NIH Building Interdisciplinary Research Careers in Women's Health Grant K12HD057022, NIH NIDDK Grant K23 DK107871, American Heart Association Grant 13CRP 14120015, University of Colorado/NIH Grant TL1 RR025778, and Grant 2015212 from the Doris Duke Charitable Foundation (to M.C.-G.); the Bryn Mawr College LILAC internship program (to N.C.); and NIH NIDDK EPOCH Grant R01DK068001 (to D.D). Support was provided also by NIH/NCATS Colorado CTSA Grant UL1 TR001082.
Author contributions: M.C.-G. designed the study, researched data, and wrote the manuscript. N.C. analyzed the data and wrote the manuscript. L.P. performed all statistical analysis and edited the manuscript. M.S.B., B.R.N., and B.R. researched data and edited the manuscript. K.J.N. designed the study, researched data, contributed to discussion, and wrote the manuscript. D.D. designed the study, researched data, contributed to discussion, and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 31P-MRS
- 31phosphorus magnetic resonance spectroscopy
- ADP
- adenosine 5′-diphosphate
- AIRS
- Androgens and Insulin Resistance Study
- ATP
- adenosine triphosphate
- BMI
- body mass index
- BMI-Z
- body mass index z-score
- EPOCH
- Exploring Perinatal Outcomes among Children
- FFA
- free fatty acid
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model for insulin resistance
- IMCL
- intramyocellular lipid
- IR
- insulin resistance
- MRI
- magnetic resonance imaging
- MVC
- maximal volitional contraction
- OGTT
- oral glucose tolerance test
- OxPhos
- oxidative phosphorylation
- PCOS
- polycystic ovarian syndrome
- PCr
- phosphocreatine
- RESISTANT
- Resistance to InSulin in Type 1 And Type 2 diabetes
- T1D
- type 1 diabetes
- T2D
- type 2 diabetes
- TG
- triglyceride.
References
- 1.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007;4(5):e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJ, Nicolay K, Prompers JJ. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J. 2008;22(11):3947–3955. [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):2237–2238. [DOI] [PubMed] [Google Scholar]
- 5.Cree-Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Curr Diab Rep. 2012;13(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cree MG, Fram RY, Barr D, Chinkes D, Wolfe RR, Herndon DN. Insulin resistance, secretion and breakdown are increased 9 months following severe burn injury. Burns. 2009;35(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, Pyle L, Reusch JE, Nadeau KJ. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308(9):E726–E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2014;64(2):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree-Green M, Gupta A, Coe GV, Baumgartner AD, Pyle L, Reusch JE, Brown MS, Newcomer BR, Nadeau KJ. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications. 2017;31(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran A, Jacobs DR Jr, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. [DOI] [PubMed] [Google Scholar]
- 15.Budak N, Oztürk A, Mazicioglu M, Yazici C, Bayram F, Kurtoglu S. Decreased high-density lipoprotein cholesterol and insulin resistance were the most common criteria in 12- to 19-year-old adolescents. Eur J Nutr. 2009;49(4):219–225. [DOI] [PubMed] [Google Scholar]
- 16.van der Aa MP, Fazeli Farsani S, Knibbe CA, de Boer A, van der Vorst MM. Population-based studies on the epidemiology of insulin resistance in children. J Diabetes Res. 2015:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaibi GQ, Roberts CK, Goran MI. Exercise and insulin resistance in youth. Exerc Sport Sci Rev. 2008;36(1):5–11. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo NS, Ruiz JR, Oja L, Veidebaum T, Sjöström M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr. 2008;87(3):586–592. [DOI] [PubMed] [Google Scholar]
- 19.Slattery MJ, Bredella MA, Thakur H, Torriani M, Misra M. Insulin resistance and impaired mitochondrial function in obese adolescent girls. Metab Syndr Relat Disord. 2014;12(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab. 2009;94(12):4923–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JE, Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: A RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications. 2016;30(6):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29(1):138–143. [DOI] [PubMed] [Google Scholar]
- 23.Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, Chiarelli F, Espe Lwpes Ispad Appes Apeg Slep J; ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; Insulin Resistance in Children Consensus Conference Group . Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cree-Green M, Newcomer BR, Brown M, Hull A, West AD, Singel D, Reusch JE, McFann K, Regensteiner JG, Nadeau KJ. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med Sci Sports Exerc. 2014;46(10):2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirikul B, Hunter GR, Larson-Meyer DE, Desmond R, Newcomer BR. Relationship between metabolic function and skeletal muscle fatigue during a 90 s maximal isometric contraction. Appl Physiol Nutr Metab. 2007;32(3):394–399. [DOI] [PubMed] [Google Scholar]
- 26.Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier RL. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 2000;13(1):14–27. [DOI] [PubMed] [Google Scholar]
- 27. Van Den Boogaart A. MRUI Manual v96.3. A User’s Guide to the Magnetic Resonance User Interface Software Package. Delft, Netherlands: Delft Technical University Press; 1997. [Google Scholar]
- 28.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14(1):26–30. [DOI] [PubMed] [Google Scholar]
- 29.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisová S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol (1985). 1999;87(6):2068–2072. [DOI] [PubMed] [Google Scholar]
- 30.Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20(3):336–346. [DOI] [PubMed] [Google Scholar]
- 31.Wells GD, Banks L, Caterini JE, Thompson S, Noseworthy MD, Rayner T, Syme C, McCrindle BW, Hamilton J. The association among skeletal muscle phosphocreatine recovery, adiposity, and insulin resistance in children [published online ahead of print February 24, 2016]. Pediatr Obes. doi: 10.1111/ijpo.12123. [DOI] [PubMed] [Google Scholar]
- 32.Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab. 2015;100(4):1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varlamov O. Western-style diet, sex steroids and metabolism [published online ahead of print June 3, 2016]. Biochim Biophys Acta. doi: 10.1016/j.bbadis.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Kloner RA, Carson C III, Dobs A, Kopecky S, Mohler ER III. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545–557. [DOI] [PubMed] [Google Scholar]
- 35.Guo W, Wong S, Li M, Liang W, Liesa M, Serra C, Jasuja R, Bartke A, Kirkland JL, Shirihai O, Bhasin S. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS One. 2012;7(12):e51180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basterfield L, Adamson AJ, Frary JK, Parkinson KN, Pearce MS, Reilly JJ; Gateshead Millennium Study Core Team . Longitudinal study of physical activity and sedentary behavior in children. Pediatrics. 2010;127(1):e24–e30. [DOI] [PubMed] [Google Scholar]
- 37.Nader PR, Bradley RH, Houts RM, McRitchie SL, O’Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300(3):295–305. [DOI] [PubMed] [Google Scholar]
- 38.Lundsgaard AM, Kiens B. Gender differences in skeletal muscle substrate metabolism: molecular mechanisms and insulin sensitivity. Front Endocrinol (Lausanne). 2014;5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]