Abstract
Context and Objective:
Compared with the general population, hemodialysis patients have a substantially higher risk of hypothyroidism, as defined by an elevated serum thyrotropin (TSH) level, and cardiovascular mortality. Whereas an elevated TSH is associated with cardiovascular disease and death in the general population, associations among dialysis patients have been inconsistent.
Design, Setting, Participants, and Main Outcome:
We examined 541 hemodialysis patients from 17 southern California dialysis centers in the prospective Hypothyroidism, Cardiovascular Health, and Survival study who underwent protocolized measurement of repeated serum TSH levels every 6 months from 2013 to 2015. Associations between TSH tertiles (<1.28, 1.28 to <2.14, and 2.14 to 86.7 mIU/L) and mortality were estimated using time-dependent Cox models with four adjustment levels. In sensitivity analyses, we excluded patients receiving thyroid hormone supplementation.
Results:
Compared with the lowest TSH tertile, the highest TSH tertile was associated with a 2.2- to 2.5-fold higher mortality risk in unadjusted, case-mix, expanded case-mix+laboratory, and expanded case-mix+laboratory+medication models [hazard ratios (95% confidence interval), 2.54 (1.32 to 4.89), 2.53 (1.30 to 4.93), 2.19 (1.11 to 4.32), and 2.28 (1.45 to 3.58), respectively]. We observed a consistent trend between higher TSH tertiles and numerically higher mortality risk across all models. Similar findings were observed in analyses excluding patients receiving thyroid hormone supplementation.
Conclusion:
In time-dependent analyses, TSH levels in the high-normal to high range were independently associated with higher death risk in hemodialysis patients. Further studies are indicated to determine whether normalization of TSH levels with thyroid hormone supplementation improves survival in this population.
In a prospective cohort of hemodialysis patients who underwent protocolized measurement of repeated serum thyrotropin (TSH) levels, higher TSH levels were associated with higher death risk.
Disorders of thyroid function are an exceedingly common endocrine complication in patients with chronic kidney disease, including those receiving dialysis (1–7). Data from the Third National Health and Nutrition Examination Survey have shown an increasing burden of hypothyroidism with incrementally impaired kidney function, such that participants with an estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2 had a nearly fivefold higher prevalence compared with those with an eGFR ≥90 mL/min/1.73 m2 (∼23% vs ∼5%, respectively) (2). In a study of 461,607 US veterans with stages 3 to 5 chronic kidney disease, it was also shown that a 10 mL/min/1.73 m2 lower eGFR was associated with an 18% higher risk of hypothyroidism, defined by elevated serum thyrotropin (TSH) levels and/or receipt of thyroid hormone supplementation, independent of sociodemographic and comorbidity characteristics (4). Although varying prevalence estimates have been reported in dialysis studies (3, 5–7), a greater burden of hypothyroidism has also been observed in these patients vs the general population (i.e., ∼13% to 25% vs ∼5%, respectively) (8).
In the general population, hypothyroidism has been identified as a risk factor for adverse cardiovascular sequelae via multiple pathways (e.g., systolic and diastolic dysfunction, endothelial dysfunction, dyslipidemia, accelerated atherosclerosis) (9–12). Although large population-based studies of hypothyroidism and mortality have shown mixed findings, it has been suggested that these associations may depend on underlying cardiovascular risk. For example, there has been a tendency toward positive associations in populations with high cardiovascular risk (i.e., recent cardiac events or atherosclerotic risk factors) (13–15), and Third National Health and Nutrition Examination Survey data have shown that hypothyroidism is associated with higher death risk in participants with congestive heart failure but not in those without (16). Given the exceedingly high prevalence of structural heart disease and cardiovascular mortality of patients undergoing dialysis (40% of deaths), thyroid status may have important implications on the cardiovascular health and survival of this population (17–19).
Indeed, an increasing number of studies have examined thyroid status, defined by serum TSH levels (20), as a novel risk factor for mortality in patients undergoing dialysis (3, 5, 6, 21). Among these, 3 retrospective studies using US regional clinical data and/or large national dialysis organization records (i.e., in which thyroid testing was conducted at the discretion of medical providers) have demonstrated a significant association between higher serum TSH levels (i.e., hyperthyotropinemia) and death risk in hemodialysis and peritoneal dialysis patients (3, 5, 6). However, in a secondary analysis of diabetic hemodialysis patients from the Die Deutsche Diabetes Dialyze Studie (4D Trial), in which thyroid testing was conducted among all patients, thyroid functional disease assessed at baseline was not associated with mortality (21). Thus, to better inform the field, we designed the prospective, multicenter Hypothyroidism, Cardiovascular Health, and Survival in Kidney Disease (HyCARDS) study, which recruited a well-characterized cohort of hemodialysis patients who underwent protocolized thyroid functional tests every 6 months, as well as rigorous collection of sociodemographic, comorbidity, other laboratory, medication, and outcomes data. In this study, we examined the association of baseline and repeated (longitudinal) measures of serum TSH with mortality in hemodialysis patients across southern California.
Materials and Methods
Source cohort
The HyCARDS study is an ongoing, prospective, multicenter observational study of incident/prevalent hemodialysis patients enrolled from 17 outpatient dialysis units in the South Bay-Los Angeles area and who are undergoing protocolized assessment of various thyroid functional markers, cardiovascular measures, and outcomes. The study population was recruited from a subcohort of patients from the Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) cohort (Clinicaltrials.gov no. NCT01415570) examining racial and ethnic differences in dietary factors and nutritional status in hemodialysis patients who had serum TSH measurement over the period of May 2013 to August 2015 (22). Patients were included if they were 18 to 85 years old, received thrice-weekly in-center hemodialysis for at least 4 consecutive weeks, and signed a local institutional review board-approved consent form. Patients were excluded if they were actively receiving peritoneal dialysis, had a life expectancy of less than 6 months (e.g., stage IV cancer), or were unable to provide consent without a proxy (e.g., dementia). The study was approved by the institutional review board of the University of California Irvine Medical Center.
Exposure ascertainment
The exposure of interest was thyroid status defined by serum TSH level. Serum TSH was measured from fresh serum samples obtained before dialysis during weekday hemodialysis treatments at the time of study entry and that chronologically coincided with routine blood tests conducted at outpatient dialysis facilities. Patients’ TSH levels were tested at the time of serum collection. Serum TSH was measured using second-generation chemiluminescent immunoassay tests (reference range, 0.5 to 5.0 mIU/L; Beckman Coulter, Chaska, MN) in the Clinical Pathology Laboratory of the University of California Irvine Medical Center. The coefficient of variation for interassay precision was 3.7%.
In primary analyses, we sought to examine the association between time-dependent thyroid function and all-cause mortality, in which thyroid status was time updated with repeated TSH measures (1) to ascertain the short-term association of thyroid function with death risk, and (2) to account for changes in thyroid function over time (23). Using this approach, patients who were found to have a change in TSH testing immediately crossed over to the new exposure category, with the reasoning that change in thyroid function had occurred during the prior exposure period; the minimum to maximum number of TSH measurements contributed by each patient ranged from 1 to 5. In secondary analyses, we examined the association between baseline thyroid function and all-cause mortality to ascertain the long-term association of thyroid function with death risk. As the “normal” reference range for TSH in dialysis patients remains undefined, we elected to categorize TSH levels into tertiles of observed baseline values, as follows: Tertiles 1, 2, and 3 corresponded to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 86.7 mIU/L, respectively.
Outcome ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after serum TSH measurement, and patients were censored for kidney transplantation, transfer to a nonaffiliated outpatient dialysis unit or peritoneal dialysis, or at end of the study (15 September 2015). Each semester, information regarding mortality, censoring events, and associated dates from the preceding 6 months was collected from event forms completed by the HyCARDS/MADRAD research coordinators and reviewed by 2 HyCARDS/MADRAD study nephrologists (C.M.R. and K.K.-Z.).
Sociodemographic, comorbidity, medication, laboratory, and body anthropometry data
Information on sociodemographics, comorbid conditions, medications, and dialysis treatment characteristics (i.e., vascular access type) were collected at study entry and every semester thereafter by HyCARDS/MADRAD research coordinators. Dialysis vintage was defined as the time between the date of study entry and the date of hemodialysis initiation. Routine dialysis laboratory measurements were performed by the outpatient dialysis laboratories on a monthly or quarterly basis using automated methods. Serum lipid tests (i.e., total cholesterol, triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels) were conducted every semester in the University of California Irvine Medical Center Clinical Pathology Laboratory.
At study entry and every semester thereafter, measurements of body composition surrogates were conducted while patients underwent routine hemodialysis treatments. These included body mass index, subcutaneous fat (determined from biceps and triceps skinfold), visceral fat (determined from waist circumference), lean muscle mass [determined from midarm circumference and midarm muscle circumference (MAMC)], and body fat percentage (measured by near-infrared [NIR] interactance). MAMC (measured in centimeters) was estimated using the following formula: MAMC = midarm circumference − 3.142 × triceps skinfold (24, 25). NIR interactance body fat (reported as a percentage) was measured by placing a Futrex NIR interactance sensor (portable 6100 model) on the nonvascular-access upper arm for several seconds, after inputting the required data (i.e., date of birth, sex, weight, and height) for each patient. This has been shown to be highly correlated with other body fat and nutritional metrics in hemodialysis patients (26–28).
Statistical analyses
Baseline characteristics between exposure groups were compared using χ2, analysis of variance, and Kruskal-Wallis tests, according to variable type. We first examined the relationship of relevant clinical characteristics with high serum TSH level at study entry (defined as the highest TSH tertile) using logistic regression. We then estimated the association between time-dependent and baseline TSH tertiles with all-cause mortality using time-dependent and fixed covariate Cox regression, respectively. In the time-dependent analytic approach, the follow-up time for each patient was divided into different time windows (i.e., approximately 6-month intervals) defined by their serial TSH measurements over time. For each time window, a separate Cox regression was carried out using the specific value of TSH at the start of the specific time window, and a weighted average of all time-window–specific results was calculated. The weighted average of a series of relatively short-term effects was presented as 1 hazard ratio as the result of the analysis (23). Logistic regression and Cox regression models were analyzed using 3 incremental levels of covariate adjustment:
-
(1)
unadjusted model: included serum TSH level as the primary exposure of interest;
-
(2)
case-mix analyses: adjusted for covariates in the unadjusted model, as well as age, sex, race, ethnicity, and diabetes; and
-
(3)
expanded case-mix+laboratory adjusted analyses: adjusted for covariates in the case-mix model, as well as dialysis vintage, vascular access, body mass index, and serum albumin levels ascertained at study entry.
To account for thyroid hormone replacement, we also conducted sensitivity analyses in which we (1) incrementally adjusted for use of thyroid hormone supplementation use in expanded case-mix+laboratory+medication adjusted analyses (adjusted for covariates in the expanded case-mix+laboratory model, as well as baseline thyroid hormone supplementation use); and (2) excluded patients receiving thyroid hormone supplementation at study entry (n = 37). Given the wide range of TSH values of the highest TSH tertile, we also conducted sensitivity analyses in which we excluded outlier TSH levels above the ∼99.5th percentile of observed values (TSH > 16.0 mIU/L).
The proportional hazards assumption was checked graphically. Effect modification of TSH–mortality associations on the basis of age, sex, race, ethnicity, diabetes, dialysis vintage, vascular access, body mass index, and serum albumin level was explored through the addition of 2-way interaction terms with TSH (separately) using likelihood ratio testing. Missing data were handled using multiple imputation (with 10 imputed datasets). There were no missing values for age, sex, race, ethnicity, and diabetes. The remaining covariates ascertained at baseline had ≤1% missing values, except for vascular access (26%), body mass index (16%), and serum albumin (20%). Analyses and figures were generated using SAS version 9.4 (SAS Institute Inc., Cary, NC), Stata version 13.1 (Stata Corp., College Station, TX), and SigmaPlot Version 12.5 (Systat Software, San Jose, CA).
Results
Study population
Among 541 patients meeting eligibility criteria (Supplemental Fig. 1 (224.4KB, tif) ), the mean ± standard deviation (SD), median [interquartile range (IQR)], and minimum to maximum baseline TSH values were 2.21 ± 4.19, 1.60 (1.08, 2.45), and 0.01 to 86.7 mIU/L, respectively (Supplemental Fig. 2 (123.3KB, tif) ). Based on TSH levels and thyroid medication status ascertained at study entry, 3.3% (n = 18), 86.1% (n = 466), and 10.5% (n = 57) of patients were considered to be hyperthyroid (defined as having a TSH level <0.5 mIU/L), euthyroid (TSH level of 0.5 to 5.0 mIU/L), and hypothyroid (TSH level of >5.0 mIU/L and/or thyroid hormone supplementation use), respectively. Because serum thyroxine (T4) and triiodothyronine (T3) measurements were not available, the hyperthyroid category included patients with subclinical hyperthyroidism (low serum TSH and normal T4 and T3 levels) and overt hyperthyroidism (low serum TSH and elevated T4 and T3 levels). The hypothyroid category included patients with subclinical hypothyroidism (elevated serum TSH and normal T4 levels) and overt hypothyroidism (elevated serum TSH and low T4 levels).
Compared with patients in the lowest TSH tertile, those in the highest tertile were less likely to be black and more likely to be Hispanic (Table 1). In contrast, patients were similar in terms of sex, prevalence of diabetes, body mass index, dialysis vintage, vascular access type, serum albumin levels, and use of thyroid hormone supplementation (6% to 7% of patients across each tertile). Notably, no patients were receiving antithyroid medications at study entry.
Table 1.
Baseline Characteristics According to Baseline TSH Level Categorized as Tertiles
| Serum TSH Categories | |||||
|---|---|---|---|---|---|
| Overall (N = 541) | Tertile 1a (n = 181) | Tertile 2a (n = 179) | Tertile 3a (n = 181) | P | |
| Age, mean ± SD, y | 54 ± 15 | 54 ± 13 | 54 ± 15 | 55 ± 15 | 0.84 |
| Female sex, % | 45 | 44 | 43 | 48 | 0.60 |
| Black race, % | 30 | 40 | 28 | 23 | 0.001 |
| Hispanic ethnicity, % | 51 | 44 | 48 | 61 | 0.004 |
| Diabetes, % | 55 | 53 | 56 | 54 | 0.81 |
| Body mass index, mean ± SD, kg/m2 | 28 ± 7 | 28 ± 6 | 28 ± 7 | 28 ± 7 | 0.70 |
| Dialysis characteristics | |||||
| Vintage, mean ± SD, mo | 53 ± 46 | 51 ± 46 | 50 ± 44 | 59 ± 49 | 0.08 |
| Vascular access, % | |||||
| AVF/AVG | 80 | 79 | 81 | 81 | 0.93 |
| Catheter | 20 | 21 | 19 | 19 | |
| Laboratory tests | |||||
| Mean TSH, mean ± SD, mIU/L | 2.21 ± 4.19 | 0.83 ± 0.30 | 1.64 ± 0.25 | 4.16 ± 6.82 | N/A |
| Serum albumin, median (IQR), g/dL | 4.0 (3.8, 4.2) | 4.0 (3.8, 4.2) | 4.0 (3.8, 4.2) | 4.0 (3.7, 4.2) | 0.57 |
| Thyroid hormone supplementation use, % | 7 | 7 | 6 | 7 | 0.90 |
TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 86.7 mIU/L, respectively.
Clinical characteristics associated with thyrotropin level
In unadjusted, case-mix, and expanded case-mix+laboratory adjusted logistic regression analyses, patients of Hispanic ethnicity and longer dialysis vintage had a higher likelihood of having a high serum TSH level (defined as the highest TSH tertile) at study entry (Table 2). In unadjusted analyses, patients of black race were less likely to have a high TSH level, but these associations were somewhat attenuated with adjustment for case-mix and expanded case-mix+laboratory covariates. In case-mix and expanded case-mix+laboratory adjusted analyses, higher serum albumin level was inversely associated with likelihood of having a high serum TSH level.
Table 2.
Clinical Characteristics Associated With the Highest TSH Tertile, Using Logistic Regression With 3 Levels of Adjustment (vs Lowest and Middle Tertilesa Combined)
| Minimally Adjusted | Case-Mix Adjustedb | Expanded Case-Mix+Laboratory Adjustedb OR (95% CI) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Age, ∆10 y | 1.04 (0.92–1.17) | 1.09 (0.95–1.25) | 1.07 (0.93–1.22) |
| Female sex | 1.20 (0.84–1.71) | 1.24 (0.86–1.78) | 1.13 (0.78–1.65) |
| Black (vs non-black) | 0.57 (0.38–0.86) | 0.86 (0.46–1.31) | 0.67 (0.39–1.15) |
| Hispanic (vs non-Hispanic) | 1.81 (1.26–2.60) | 1.69 (1.06–2.70) | 1.62 (1.00–2.61) |
| Diabetes | 0.98 (0.68–1.40) | 0.83 (0.56–1.23) | 0.84 (0.55–1.28) |
| Body mass index, ∆5 kg/m2 | 1.07 (0.92–1.23) | 1.09 (0.94–1.27) | 1.07 (0.92–1.26) |
| Dialysis vintage, ∆6 mo | 1.03 (1.00–1.05) | 1.03 (1.00–1.05) | 1.03 (1.01–1.06) |
| Vascular access | 0.76 (0.27–2.10) | 0.51 (0.17–1.51) | 1.02 (0.41–2.52) |
| AVF/AVG (vs catheter) | 1.32 (0.48–3.68) | 1.96 (0.66–5.79) | 0.98 (0.40–2.42) |
| Thyroid hormone supplementation use | 1.08 (0.54–2.18) | 1.05 (0.51–2.16) | 1.03 (0.50–2.14) |
| Laboratory tests | |||
| Serum albumin level, ∆0.5g/dL | 0.77 (0.59–1.01) | 0.75 (0.56–1.00) | 0.74 (0.56–1.00) |
| nPCR, ∆0.2 g/kg/d | 1.00 (0.87–1.15) | 0.94 (0.81–1.09) | 0.94 (0.81–1.10) |
| Serum creatinine level, ∆1 mg/dL | 0.98 (0.91–1.04) | 1.01 (0.93–1.09) | 1.01 (0.93–1.10) |
| spKt/V, ∆0.2 | 1.06 (0.94–1.19) | 0.98 (0.86–1.12) | 0.98 (0.86–1.13) |
| Calcium, ∆1 mg/dL | 1.09 (0.80–1.48) | 1.06 (0.77–1.47) | 1.04 (0.75–1.44) |
| Phosphate, ∆1 mg/dL | 0.96 (0.86–1.09) | 0.96 (0.84–1.09) | 0.97 (0.85–1.10) |
| PTH, ∆25 pg/mL | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) |
| Hemoglobin, ∆1 g/dL | 0.92 (0.76–1.11) | 0.91 (0.75–1.11) | 0.93 (0.76–1.13) |
| Ferritin, ∆25 ng/mL | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) |
| Iron saturation, ∆5% | 1.06 (0.98–1.15) | 1.05 (0.97–1.14) | 1.07 (0.98–1.16) |
| Platelet count, ∆50 ×109/L | 0.90 (0.78–1.04) | 0.91 (0.79–1.06) | 0.92 (0.80–1.07) |
| Mean platelet volume, ∆1 fL | 1.11 (0.91–1.36) | 1.14 (0.92–1.40) | 1.15 (0.93–1.43) |
| WBC count, × 109/L | 0.95 (0.86–1.05) | 0.95 (0.86–1.05) | 0.95 (0.86–1.06) |
| Total cholesterol, ∆50 mg/dL | 0.94 (0.75–1.17) | 0.90 (0.72–1.13) | 0.94 (0.75–1.19) |
| HDL, ∆10 mg/dL | 1.08 (0.97–1.20) | 1.08 (0.96–1.21) | 1.11 (0.98–1.25) |
| LDL, ∆25 mg/dL | 0.92 (0.80–1.07) | 0.90 (0.77–1.04) | 0.91 (0.78–1.06) |
| Triglycerides, ∆50 mg/dL | 0.99 (0.92–1.07) | 0.98 (0.90–1.06) | 0.99 (0.91–1.07) |
| Body anthropometry | |||
| Waist circumference, ∆5 cm | 1.02 (0.93–1.11) | 1.03 (0.93–1.13) | 0.99 (0.85–1.14) |
| Biceps skinfold, ∆10 mm | 1.12 (0.86–1.46) | 1.10 (0.83–1.47) | 0.88 (0.61–1.25) |
| Triceps skinfold, ∆10 mm | 1.11 (0.87–1.40) | 1.10 (0.85–1.43) | 0.91 (0.66–1.26) |
| Near infrared body fat, ∆10 mm | 1.35 (1.04–1.75) | 1.57 (1.09–2.27) | 1.78 (0.93–3.41) |
Boldface type indicates statistically significant associations.
Abbreviations: ∆, delta (increment); HDL, high-density lipoprotein; LDL, low-density lipoprotein; nPCR, normalized protein catabolic rate; PTH, parathyroid hormone; spKt/V, single-pool quantifier of hemodialysis and peritoneal dialysis treatment adequacy, where K = dialyzer clearance of urea, t = dialysis time, and V = volume of distribution of urea; WBC, white blood cell.
TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 86.7mIU/L, respectively.
Case-mix analyses adjusted for age, sex, race, ethnicity, and diabetes.
Expanded case-mix+laboratory adjusted analyses adjusted for covariates in case-mix model, plus vintage, vascular access, body mass index, and serum albumin level.
Serum thyrotropin level and all-cause mortality
Patients contributed a total of 817 patient-years of follow-up, during which time 71 all-cause deaths occurred. The median (IQR) at-risk time was 1.60 (1.08, 2.09) years. In time-dependent analyses, the highest TSH tertile was associated with higher mortality risk in comparison with the lowest TSH tertile in unadjusted models (hazard ratio [HR] [95% confidence interval (CI)], 2.54 [1.32 to 4.89], P = 0.005; Fig. 1; Supplemental Table 1 (24.8KB, docx) ). The association between the highest TSH tertile and higher mortality remained statistically significant with incremental adjustment for case-mix and expanded case-mix+laboratory covariates. The middle TSH tertile was associated with numerically higher risk but did not reach statistical significance in unadjusted, case-mix, and expanded case-mix+laboratory models. In expanded case-mix+laboratory+medication analyses that incrementally adjusted for thyroid hormone supplementation use, we observed a robust relationship between the highest TSH tertile and higher mortality risk [adjusted HR (95% CI) 2.28 (1.45 to 3.58); P < 0.001].
Figure 1.
Association of time-dependent TSH tertiles with all-cause mortality. Case-mix analyses adjusted for age, sex, race, ethnicity, and diabetes. Expanded case-mix+laboratory analyses adjusted for covariates in the case-mix model, plus dialysis vintage, vascular access, body mass index, and serum albumin level. Expanded case-mix+laboratory+medication analyses adjusted for covariates in the expanded case-mix+laboratory model, plus thyroid hormone supplementation use. TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 86.7 mIU/L, respectively.
In secondary analyses of baseline TSH, compared with the lowest TSH tertile, the middle and highest TSH tertiles were associated with numerically higher risk but did not reach statistical significance in unadjusted, case-mix, expanded case-mix+laboratory, and expanded case-mix+laboratory+medication models (the reference was the lowest TSH tertile; Supplemental Table 1 (24.8KB, docx) ).
Sensitivity analyses excluding patients on thyroid hormone supplementation use and outlier thyrotropin levels
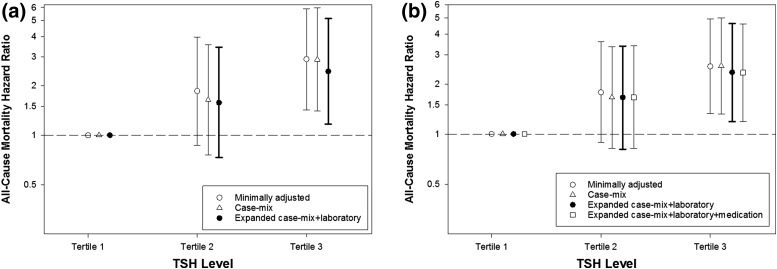
In sensitivity analyses that excluded patients receiving thyroid hormone supplementation, we observed a persistent association between the highest time-dependent TSH tertile and higher mortality risk in unadjusted, case-mix, and expanded case-mix+laboratory adjusted models [Fig. 2(a); Supplemental Table 2 (24.8KB, docx) ). In analyses that excluded outlier TSH values, we similarly observed a persistent association between the highest time-dependent TSH tertile and higher death risk across all 3 multivariable models [Fig. 2(b); Supplemental Table 3 (24.8KB, docx) ). We did not observe a significant association between baseline TSH tertile and mortality risk in sensitivity analyses that excluded patients receiving thyroid hormone supplementation (Supplemental Table 2 (24.8KB, docx) ) or outlier TSH values (Supplemental Table 3 (24.8KB, docx) ).
Figure 2.
Sensitivity analyses of the association between time-dependent TSH tertiles and (a) all-cause mortality with medication exclusion (n = 504) and (b) with removal of TSH outliers [defined as TSH values greater than the ~99.5th percentile of observed values (TSH level >16.0 mIU/L; n = 538)]. Case-mix analyses adjusted for age, sex, race, ethnicity, and diabetes. Expanded case-mix+laboratory analyses adjusted for covariates in case-mix model, plus dialysis vintage, vascular access, body mass index, and serum albumin level. (b) Expanded case-mix+laboratory+medication analyses adjusted for covariates in the expanded case-mix+laboratory model, plus thyroid hormone supplementation use. (a) TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 31.3 mIU/L, respectively. (b) TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.27, 1.27 to <2.13, and 2.13 to 15.2 mIU/L, respectively.
Subgroup analyses
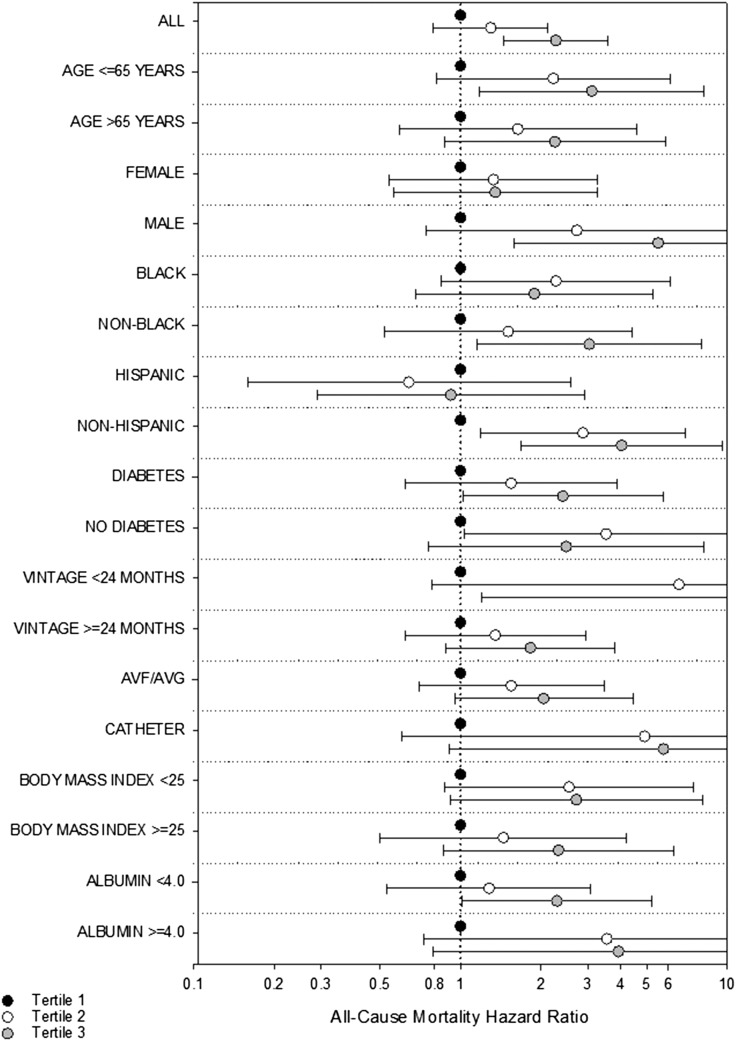
We did not detect effect modification on the basis of age, sex, race, ethnicity, underlying diabetes, dialysis vintage, vascular access type, body mass index, or serum albumin level (P for interaction = 0.76, 0.08, 0.22, 0.14, 0.59, 0.50, 0.86, 0.93, and 0.44, respectively; Fig. 3; Supplemental Table 4 (24.8KB, docx) ). In all subgroups, the nominal HR for the highest TSH tertile was >1 except among Hispanic patients; nominal associations were statistically significant in the following subgroups: age ≤65 years, male, non-black, non-Hispanic, diabetic, dialysis vintage <24 months, and those whose serum albumin level was <4 g/dL.
Figure 3.
Subgroup analyses of the association between time-dependent TSH tertiles with all-cause mortality adjusted for expanded case-mix+laboratory covariates. Expanded case-mix+laboratory analyses adjusted for age, sex, race, ethnicity, diabetes, dialysis vintage, vascular access, body mass index, and serum albumin level. TSH tertiles 1, 2, and 3 correspond to TSH levels of <1.28, 1.28 to <2.14, and 2.14 to 86.7 mIU/L, respectively. AVF, arteriovenous fistula; AVG, arteriovenous graft.
Discussion
In this prospective, multicenter cohort of 541 hemodialysis patients who underwent protocolized serum TSH testing every 6 months, we found that time-dependent TSH levels in the highest tertile were associated with a greater than twofold higher mortality risk independent of case-mix and laboratory covariates. This strong association between the highest serum TSH tertile and death risk was robust across multiple sensitivity analyses that accounted for thyroid medication use and excluded patients with outlier TSH values.
An increasing body of evidence has demonstrated that underlying thyroid status is associated with survival in dialysis patients (3, 5, 6). Among 2,715 prevalent dialysis patients who underwent TSH testing within 2 tertiary care centers in Boston, MA, those with hypothyroidism at baseline (12.9% of the cohort) had a higher mortality risk compared with euthyroid patients independent of sociodemographic and comorbidity status (3). In a subsequent study of 8840 incident hemodialysis patients receiving care from a large national dialysis organization in the United States, time-dependent and baseline hypothyroidism were each associated with higher death risk in case-mix adjusted analyses (5). Most recently, in a study of 1,484 national peritoneal dialysis patients who underwent repeated TSH measures over time, time-dependent TSH levels in the subclinical and overt hypothyroid range were associated with 1.63- and 3.11-fold higher death risk, respectively (6).
To our knowledge, this is the first prospective study that has examined the association between time-dependent thyroid status defined by repeated TSH measures and mortality risk in hemodialysis patients. It should be noted that the cited studies examined data collected for clinical purposes, in which thyroid functional testing was conducted at the discretion of medical providers, with potential implications upon generalizability. As an important distinction, in the current study, we collected repeated serum TSH measures at protocolized, uniform (i.e., every 6 months) intervals in all patients. There has been 1 study that uniformly examined thyroid function, defined by serum TSH, T4, and T3 levels, in 1,000 diabetic hemodialysis patients from the 4D Trial, albeit at a single point in time (i.e., baseline) only (21). Although adjusted analyses did not demonstrate a significant association between subclinical hypothyroidism or hyperthyroidism (assessed separately or in conjunction with the corresponding overt thyroid disorder) with adverse cardiovascular events, sudden cardiac death, or all-cause mortality, when follow-up time was parsed into short- vs longer-term intervals (>1 to 4 years), there was a trend toward an association between subclinical hyperthyroidism and sudden cardiac death over short-term follow-up only (≤1 year). In our study, we similarly observed a significant relationship between thyroid status and mortality over short-term follow-up (i.e., time-dependent TSH analyses), whereas a null association was observed over long-term follow-up (i.e., baseline TSH analyses). Although our findings suggest that higher serum TSH levels carry short-term risk (23), the lack of an observed association in baseline analyses may have been due to an attenuation in thyroid status as a risk factor over longer-term follow-up, change in thyroid function over time, and/or limited sample size to detect significant associations.
Another notable finding of our study was the observation that both high-normal and high TSH levels in the highest TSH tertile (defined as TSH level ≥2.14 mIU/L) predicted higher death risk in hemodialysis patients. In the general population, some expert groups have recommended that the upper limit of normal of the euthyroid reference range should be reduced from 4.0 to 5.0 mIU/L to 2.5 to 3.0 mIU/L, whereas others have advised using reference ranges based on age and race/ethnicity (29–32). Although the optimal TSH range in dialysis patients remains uncertain, our observations corroborate findings from a national study of hemodialysis patients showing that higher TSH levels even within the normal range (i.e., >3.0 mIU/L) were incrementally associated with higher death risk (5). Although our multivariable-adjusted analyses showed that elevated TSH levels were associated with adverse outcomes independent of age, it is important to note there is a shift toward higher TSH concentrations with increasing age in the general population (33). Thus, it is possible that a sizeable proportion of our hemodialysis patients with TSH levels in the highest tertile may be considered euthyroid based on general population thresholds. Studies are needed to confirm the “normal” TSH range in hemodialysis patients and whether treatment of high-normal and hypothyroid TSH levels to a low-normal target (<2.5 to 3.0 mIU/L) improves survival in this population.
We elected to focus on TSH-based ascertainment of thyroid function as a more sensitive and specific metric of thyroid functional status (20). Although T3 and T4 levels may be used to distinguish severity of thyroid disease (i.e., subclinical vs overt thyroid functional disease) and other etiologies of thyroid functional test abnormalities (i.e., nonthyroidal illness), in patients with end-stage renal disease (1) T3 levels may be confounded by underlying illness (i.e., the peripheral conversion of T4 to T3 is highly sensitive to mild illness, malnutrition, and inflammation) (19, 34, 35), and (2) routinely used free-T4 assays measuring the minute fraction of bioactive T4 (e.g., free-T4 analog assay or free-T4 index) are hormone-protein binding dependent and may not be accurate in dialysis patients (i.e., conditions in which circulating substances such as uremic toxins impair hormone-protein binding, routinely used-free T4 assays may result in spurious levels) (19, 35, 36).
Although TSH levels are a comparatively more robust marker of thyroid functional status, we are unable to confirm a causal relationship between TSH levels and mortality in this observational study. For example, some TSH aberrations have been reported in kidney disease, such as impaired glycosylation and function, blunted response to thyrotropin-releasing hormone, altered clearance, decreased pulsatility, and increased half-life (19, 35), and we cannot exclude confounding of the TSH–mortality association on this basis. TSH levels may also be altered in illness states. However, although TSH levels are suppressed in the setting of severe illness (34, 37), our study population was restricted to ambulatory hemodialysis patients presenting for their routine thrice-weekly dialysis treatments, rendering the inclusion of patients with severe illness states low.
Our study has a number of strengths, including its prospective examination of a well-defined hemodialysis cohort, with detailed collection of longitudinal data on sociodemographics, laboratory tests, and medications; rigorous outcome adjudication procedures; protocoled measurement of serum TSH samples among all patients in the outpatient setting who were uniformly tested in a single laboratory; comprehensive adjustment for confounders of the thyroid status–mortality association; and robust findings across multiple sensitivity analyses accounting for thyroid medication use and outlier TSH levels. However, several limitations of our study bear mention. First, although the “normal” TSH reference in dialysis patients remained undefined, categorization of TSH levels into tertiles may have combined patients with heterogenous etiologies of TSH aberrations (i.e., the lowest TSH tertile may have included patients with nonthyroidal illness as well as those with hyperthyroidism and euthyroidism based on general population thresholds). Second, our study was not specifically designed to examine the relationship between hyperthyroidism (subclinical and overall) and outcomes in the general population. Recent data in the general population suggest that subclinical hyperthyroidism may have important implications upon cardiovascular health and survival (9, 38); therefore, future studies examining this relationship in patients with kidney disease are needed. Third, our study had a modest sample size, which may have resulted in limited power to detect significant associations, particularly in the baseline and subgroup analyses. Fourth, we lacked information on cause-specific mortality to gain greater insight into mechanistic pathways by which thyroid status impacts mortality in hemodialysis patients. Fifth, although we had reliable information regarding the patients’ thyroid medication status at the time of study entry, we were not able to accurately determine patients’ longitudinal receipt of thyroid-modulating therapy over time. Last, it is possible that our findings may not be generalizable to geographic regions with distinct sociodemographic distributions and practice patterns.
In summary, our study shows that TSH levels in the high-normal to high range are associated with short-term mortality risk in a prospective cohort of hemodialysis patients. Future studies are needed to confirm findings, determine the optimal target TSH range in dialysis patients, and define the underlying mechanisms by which high-normal and high TSH levels negatively impact survival in this population.
Acknowledgments
Portions of these data have been presented in an abstract at the 2016 National Kidney Foundation Spring Clinical Meeting, April 27 to 1 May 2016, Boston, MA, and in an oral abstract at the 18th International Congress on Nutrition and Metabolism in Renal Disease, April 19 to 23, 2016, Okinawa, Japan.
Acknowledgments
This work was supported by research grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, including Grants K23-DK102903 (C.M.R.), K24-DK091419 (K.K.Z.), R01-DK092232 (D.V.N.), UL1-TR001414 (D.V.N.), and philanthropic support from Dr. Joseph Lee.
Clinical trial registry: ClinicalTrials.gov no. NCT01415570 (registered 10 August 2011).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- HR
- hazard ratio
- HyCARDS
- Hypothyroidism, Cardiovascular Health, and Survival
- MADRAD
- Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease
- T3
- triiodothyronine
- T4
- serum thyroxine
- TSH
- serum thyrotropin.
References
- 1.Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67(3):1047–1052. [DOI] [PubMed] [Google Scholar]
- 3.Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2013;8(4):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee CM, Kalantar-Zadeh K, Streja E, Carrero JJ, Ma JZ, Lu JL, Kovesdy CP. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30(2):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee CM, Kim S, Gillen DL, Oztan T, Wang J, Mehrotra R, Kuttykrishnan S, Nguyen DV, Brunelli SM, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J Clin Endocrinol Metab. 2015;100(4):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee CM, Ravel VA, Streja E, Mehrotra R, Kim S, Wang J, Nguyen DV, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Thyroid functional disease and mortality in a national peritoneal dialysis cohort. J Clin Endocrinol Metab. 2016;101(11):4054–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shantha GP, Kumar AA, Bhise V, Khanna R, Sivagnanam K, Subramanian KK. Prevalence of subclinical hypothyroidism in patients with end-stage renal disease and the role of serum albumin: a cross-sectional study from South India. Cardiorenal Med. 2011;1(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 9.Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N; Thyroid Studies Collaboration . Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270–278. [DOI] [PubMed] [Google Scholar]
- 11.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–509. [DOI] [PubMed] [Google Scholar]
- 12.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J; Thyroid Studies Collaboration . Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, L’Abbate A, Pingitore A. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167(14):1526–1532. [DOI] [PubMed] [Google Scholar]
- 14.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011;21(8):837–843. [DOI] [PubMed] [Google Scholar]
- 15.Molinaro S, Iervasi G, Lorenzoni V, Coceani M, Landi P, Srebot V, Mariani F, L’Abbate A, Pingitore A. Persistence of mortality risk in patients with acute cardiac diseases and mild thyroid dysfunction. Am J Med Sci. 2012;343(1):65–70. [DOI] [PubMed] [Google Scholar]
- 16.Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. Subclinical hypothyroidism and survival: the effects of heart failure and race. J Clin Endocrinol Metab. 2013;98(6):2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Renal Data System. USRDS 2014 Annual Data Report: Volume 2 – End-Stage Renal Disease (ESRD) in the United States. Bethesda, MD; US Renal Data System; 2014.
- 18.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–192. [DOI] [PubMed] [Google Scholar]
- 19.Rhee CM, Brent GA, Kovesdy CP, Soldin OP, Nguyen D, Budoff MJ, Brunelli SM, Kalantar-Zadeh K. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol Dial Transplant. 2015;30(5):724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladenson PW. Diagnosis of hypothyroidism, In: Braverman LE, Cooper DS, eds. Werner and Ingbar’s The Thyroid. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013:606–611.
- 21.Drechsler C, Schneider A, Gutjahr-Lengsfeld L, Kroiss M, Carrero JJ, Krane V, Allolio B, Wanner C, Fassnacht M. Thyroid function, cardiovascular events, and mortality in diabetic hemodialysis patients. Am J Kidney Dis. 2014;63(6):988–996. [DOI] [PubMed] [Google Scholar]
- 22.Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–997. [DOI] [PubMed] [Google Scholar]
- 24.Weber J, Kelley J. Assessing nutrition. In: Nieginski E, ed. Health Assessment in Nursing. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2003:165.
- 25.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, Benner D, Kopple JD, Kalantar-Zadeh K. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55(5):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant. 1999;14(1):169–175. [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83(2):202–210. [DOI] [PubMed] [Google Scholar]
- 29.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126. [DOI] [PubMed] [Google Scholar]
- 30.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–469. [PubMed] [Google Scholar]
- 31.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95(2):496–502. [DOI] [PubMed] [Google Scholar]
- 32.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90(9):5483–5488. [DOI] [PubMed] [Google Scholar]
- 33.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. [DOI] [PubMed] [Google Scholar]
- 34.Langton JE, Brent GA. Nonthyroidal illness syndrome: evaluation of thyroid function in sick patients. Endocrinol Metab Clin North Am. 2002;31(1):159–172. [DOI] [PubMed] [Google Scholar]
- 35.Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soldin OP. Measuring serum thyroid-stimulating hormone, thyroid hormones, thyroid-directed antibodies, and transport proteins. In: Braverman LE, Cooper DS, eds. Werner and Ingbar’s The Thyroid. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013:279–297.
- 37.Wiersinga WM, Van den Berghe G. Nonthyroidal illness syndrome. In: Braverman LE, Cooper DS, eds. Werner and Ingbar’s The Thyroid. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013:203–216.
- 38.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Åsvold BO, Sgarbi JA, Völzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N; Thyroid Studies Collaboration . Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]