Abstract
Context:
Lipodystrophy syndromes are rare disorders of deficient adipose tissue. Metreleptin, a human analog of leptin, improved metabolic abnormalities in mixed cohorts of children and adults with lipodystrophy and low leptin.
Objective:
Determine effects of metreleptin on diabetes, hyperlipidemia, nonalcoholic fatty liver disease (NAFLD), growth, and puberty in pediatric patients with lipodystrophy and low leptin.
Design:
Prospective, single-arm, open-label studies with continuous enrollment since 2000.
Setting:
National Institutes of Health, Bethesda, Maryland.
Patients:
Fifty-three patients aged 6 months to <18 years with lipodystrophy, leptin level <8 ng/mL (male patients) or <12 ng/mL (female patients), and ≥1 metabolic abnormality (diabetes, insulin resistance, or hypertriglyceridemia).
Intervention:
Subcutaneous metreleptin injections (0.04 to 0.19 mg/kg/d).
Main Outcome Measures:
Change in A1c, lipid, and transaminase levels after a mean ± standard deviation (SD) of 12 ± 0.2 months and 61 ± 39 months. Changes in liver histology, growth, and pubertal development throughout treatment.
Results:
After 12 months, the A1c level (mean ± SD) decreased from 8.3% ± 2.4% to 6.5% ± 1.8%, and median triglyceride level decreased from 374 mg/dL [geometric mean (25th,75th percentile), 190, 1065] to 189 mg/dL (112, 334; P < 0.0001), despite decreased glucose- and lipid-lowering medications. The median [geometric mean (25th,75th percentile)] alanine aminotransferase level decreased from 73 U/L (45, 126) to 41 U/L (25, 59; P = 0.001), and that of aspartate aminotransferase decreased from 51 U/L (29, 90) to 26 U/L (18, 42; P = 0.0002). These improvements were maintained over long-term treatment. In 17 patients who underwent paired biopsies, the NAFLD activity score (mean ± SD) decreased from 4.5 ± 2.0 to 3.4 ± 2.0 after 3.3 ± 3.2 years of metreleptin therapy (P = 0.03). There were no clinically significant changes in growth or puberty.
Conclusion:
Metreleptin lowered A1c and triglyceride levels, and improved biomarkers of NAFLD in pediatric patients with lipodystrophy. These improvements are likely to reduce the lifetime burden of disease.
In patients <18 years old with lipodystrophy, metreleptin treatment improved diabetes, hypertriglyceridemia, and NAFLD, without altering growth or pubertal development.
Lipodystrophy syndromes comprise a rare, heterogeneous group of disorders characterized by generalized or partial lack of adipose tissue and deficiency of leptin, an adipocyte-secreted hormone (1, 2). Lipodystrophy syndromes are inherited or acquired, and are classified into 4 major subtypes: acquired or congenital generalized lipodystrophy, and acquired or familial partial lipodystrophy (3, 4). An additional subtype is progeroid disorders, which are associated with either generalized or partial lipodystrophy. Most pediatric patients with congenital generalized lipodystrophy (CGL) present at birth or within the first year of life, whereas patients with acquired generalized and partial lipodystrophies may not present until early childhood or adolescence, or even in adulthood (3).
Patients with lipodystrophy have metabolic disturbances, including severe insulin resistance, diabetes, hypertriglyceridemia, and nonalcoholic steatohepatitis (NASH) (1, 5–8). These often lead to morbidity and increased mortality risk, even with use of conventional lipid-lowering or antihyperglycemic agents (9, 10). Metreleptin, a recombinant analog of leptin, has been shown to improve metabolic abnormalities in patients with generalized lipodystrophy (11–13) and in selected patients with partial lipodystrophy (13), leading to US Food and Drug Administration approval in 2014 of metreleptin for generalized lipodystrophy.
Previous analyses of metreleptin effects in patients with lipodystrophy have not distinguished between children and adults, although about half of patients in clinical trials were younger than 18 years (13). Because most patients with generalized lipodystrophy who will be considered for metreleptin treatment present in childhood, it is critical to understand effects of metreleptin specifically in pediatrics. In this study, we analyzed effects of 1 year and long-term metreleptin therapy on key metabolic comorbidities, including dysglycemia and dyslipidemia. Because metabolic complications of lipodystrophy often present or worsen during puberty, we compared metreleptin’s efficacy in younger children (<12 years old) vs adolescents (12 to 18 years old). In addition, because NASH can lead to cirrhosis and liver failure at young ages in patients with lipodystrophy (14), we sought to understand whether metreleptin can prevent advancement of liver disease, thus preventing late complications (e.g., portal hypertension, transplant, or death). We assessed this by studying effects of metreleptin on biomarkers of liver disease, including transaminases and pathologic markers of NASH. Finally, because leptin is permissive for puberty, we studied growth and pubertal development.
Methods
Study description
Patients enrolled in open-label studies of metreleptin at the National Institutes of Health, the design of which was described previously (11, 12). Patients with lipodystrophy (excluding HIV-associated lipodystrophy) were enrolled on a rolling basis if they were ≥6 months old, had low serum leptin level (<8 ng/mL in boys, <12 mg/mL in girls), and had ≥1 metabolic abnormality, including diabetes, insulin resistance (fasting insulin level ≥30 µIU/mL), or hypertriglyceridemia (fasting triglyceride levels >200 mg/dL). Studies were approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases (NCT00005905, NCT00025883, NCT01778556, NCT02262832, and NCT02262806). Consent was obtained from legal guardian(s) of pediatric patients, and assent from older children and adolescents.
During the first year of metreleptin use, doses of concomitant medications were not increased nor were new medications initiated, although doses could be reduced or medications discontinued. After 1 year, changes to concomitant medications were permitted at the treating physician’s discretion. Metreleptin was administered at home by subcutaneous injection once or twice daily per a weight-based protocol, with variation by age and sex, as described previously (15, 16); doses were titrated based on patient response.
The current analysis included all metreleptin-treated patients as of 1 July 2015 who were <18 years old at initiation of metreleptin treatment and had both baseline and 1-year data available. One year was defined as the visit closest to 12 months from metreleptin initiation (range, 6 to 18 months). Of 56 patients <18 years old, 3 were excluded: one lacked baseline data, another died before follow-up data could be collected (death was due to pancreatitis associated with noncompliance with metreleptin), and the third did not yet have follow-up data. In addition, data were analyzed from the most recent visit at which the patient was receiving metreleptin.
Outcomes
Laboratory outcomes including glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), insulin, C-peptide, and hemoglobin A1c (A1c) levels were collected after an 8- to 12-hour fast and analyzed by standard methods (17). The baseline fasting leptin level was determined by radioimmunoassay (EMD Millipore, St. Charles, MO). Because prior studies have shown that most metreleptin-treated patients develop nonneutralizing antibodies that interfere with leptin assays, leptin levels during metreleptin treatment are not presented (18).
Z-scores for height based on age and sex were defined using 2000 Centers for Disease Control and Prevention data (19). Stature was categorized as short (below the fifth percentile), normal (5th to 95th percentile), or tall (>95th percentile). Bone ages were independently interpreted by 2 pediatric endocrinologists (R.J.B., K.I.R.) using the Greulich-Pyle method (20), and categorized as normal if within 2 standard deviations (SDs) of the mean for age and sex (20).
Growth completion before metreleptin treatment was defined as achieving 98% of predicted growth based on bone age (girls, ≥14 years; boys, ≥15.75 years) obtained 24 months before to 3 months after metreleptin initiation. If a bone age was not available (n = 26), determination of growth completion was based on chronologic age (girls, ≥15.0 years old; boys, ≥17.0 years old).
Tanner stages of breast development and testicular volumes were extracted from medical records. All available determinations of Tanner staging obtained after metreleptin initiation were analyzed in patients who had not completed puberty at metreleptin initiation. Pubertal status in girls was defined as precocious if breast development was at least Tanner stage II before age 8 years or at least two stages advanced for age, as delayed if at Tanner stage I at age 12 years or at least two stages delayed for age, and otherwise as normal. Pubertal status in boys was categorized as precocious if testicular volume was ≥4 mL before age 9 years or at least two stages advanced for age, as delayed if testicular volume was <4 mL at age 14 years or at least two stages delayed for age, and otherwise as normal.
Liver histology was assessed in 17 patients who underwent liver biopsy before and after at least 1 year of metreleptin treatment. If multiple biopsies were performed, the most recent biopsy specimen was used for analysis. Biopsy specimens were scored by a single pathologist (D.E.K.) using the NASH Clinical Research Network (CRN) scoring system (21). Outcomes included presence or absence of NASH, steatosis, 2 measures of inflammation (portal and parenchymal), ballooning score, and fibrosis stage. Overall disease activity was measured as the nonalcoholic fatty liver disease (NAFLD) activity score (NAS).
Treatment emergent (as assessed by investigators) adverse events and all deaths are reported in Results. The presence of neutralizing antibodies to leptin was assessed as previously reported (18).
Statistical analyses
The children subgroup was defined as <12 years of age. The adolescent subgroup was defined as ≥12 to <18 years of age.
Outcomes are reported as mean ± SD or median (25th, 75th percentile) based on normality of the data. Paired t tests or Wilcoxon signed-rank test were used to compare outcomes before vs after 12 months of metreleptin treatment or after long-term metreleptin treatment. Unpaired t tests and χ2 tests were used to compare children vs adolescents, and patients who did vs did not undergo liver biopsies. Kruskal-Wallis tests or analysis of covariance with post hoc Dunn or Tukey multiple comparisons tests were used to compare baseline characteristics and response to metreleptin among lipodystrophy subtypes. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
Baseline characteristics of participants are listed in Table 1. There were 53 patients (41 girls; 12 boys). Mean age was 12.8 ± 4.4 years (range, 1.7 to 18.0 years), including 19 children and 34 adolescents. The median endogenous leptin concentration was 1.1 ng/mL (0.8, 2.5). Thirty-two patients had CGL [16 had an AGPAT2 mutation (CGL1); 16 had a BSCL2 mutation (CGL2)], 8 had acquired generalized lipodystrophy (AGL), 8 had familial partial lipodystrophy (FPL; 3 with LMNA mutation, 2 with PPARγ mutation, 1 with PCYT1a mutation, and 2 unknown), and 5 had generalized lipodystrophy due to atypical progeria (all with LMNA mutation). There were no differences in baseline metabolic parameters among lipodystrophy subtypes (Supplemental Table 1 (17.8KB, docx) ), except for endogenous leptin (higher in FPL than AGL, CGL1, and CGL2).
Table 1.
Baseline Characteristics
| Parameter | Children (≤12 Years Old; n = 19) | Adolescents (>12 and <18 Years Old; n = 34) | All Pediatric Patients <18 Years Old (N = 53) |
|---|---|---|---|
| Demographics | |||
| Female sex, n (%) | 13 (68) | 28 (82) | 41 (77) |
| Age, y | 8.0 ± 3.5 | 15.5 ± 1.8 | 12.8 ± 4.4 |
| Lipodystrophy type, n (%): | |||
| Acquired generalized lipodystrophy | 5 (26) | 3 (9) | 8 (15) |
| Congenital generalized lipodystrophy | 10 (53) | 22 (65) | 32 (60) |
| Familial partial lipodystrophy | 1 (5) | 7 (21) | 8 (15) |
| Atypical progeria | 3 (16) | 2 (6) | 5 (9) |
| Metabolic parameters | |||
| Fasting leptin, median (25th,75th percentile), ng/mLa | 1.0 (0.5, 1.4) | 1.2 (0.8, 3.1) | 1.1 (0.8, 2.5) |
| Triglycerides, median (25th,75th percentile), mg/dLa | 228 (149, 374) | 556 (254, 2727) | 374 (190, 1065) |
| HDL, mg/dL | 29 ± 11 | 28 ± 8.3 | 29 ± 9.4 |
| LDL, mg/dLa | 74 ± 34 | 115 ± 56 | 96 ± 51 |
| Glucose, mg/dLa | 115 ± 43 | 210 ± 78 | 176 ± 81 |
| C-peptide, median (25th,75th percentile), ng/mLa | 7.0 (5.5, 8.9) | 4.4, (2.8, 6.2) | 5.7 (3.3, 7.3) |
| Hemoglobin A1c, %a | 6.1 ± 1.5 | 9.6 ± 1.9 | 8.3 ± 2.4 |
| ALT, median (25th,75th percentile), U/L | 90 (47, 225) | 65 (39, 102) | 73 (45, 126) |
| AST, median (25th,75th percentile), U/L | 64 (39, 126) | 52 (28, 69) | 51 (29, 90) |
All values are mean ± SD unless otherwise stated.
P < 0.05 for children vs adolescents.
Most patients (72%; 32% of children, 94% of adolescents) had diabetes, based on fasting glucose or A1c levels, or medical history. Compared with children, adolescents had greater severity of metabolic disease, as evidenced by higher A1c, triglyceride, and LDL levels, and lower C-peptide level, with no difference in HDL (Table 1).
Changes with metreleptin
At 12 months of treatment
Mean treatment duration was 12 ± 0.2 months. The average dose of metreleptin was 0.082 ± 0.028 mg/kg/d (range, 0.04 to 0.19 mg/kg/d; absolute dose, 4.1 ± 2.2 mg/d). Doses were nonsignificantly higher in adolescents (0.087 ± 0.030 mg/kg/d) vs children (0.073 ± 0.024 mg/kg/d; P = 0.07). Patients with FPL used higher metreleptin doses compared with those with AGL, atypical progeria, or CGL2 (Supplemental Table 2 (17.8KB, docx) ).
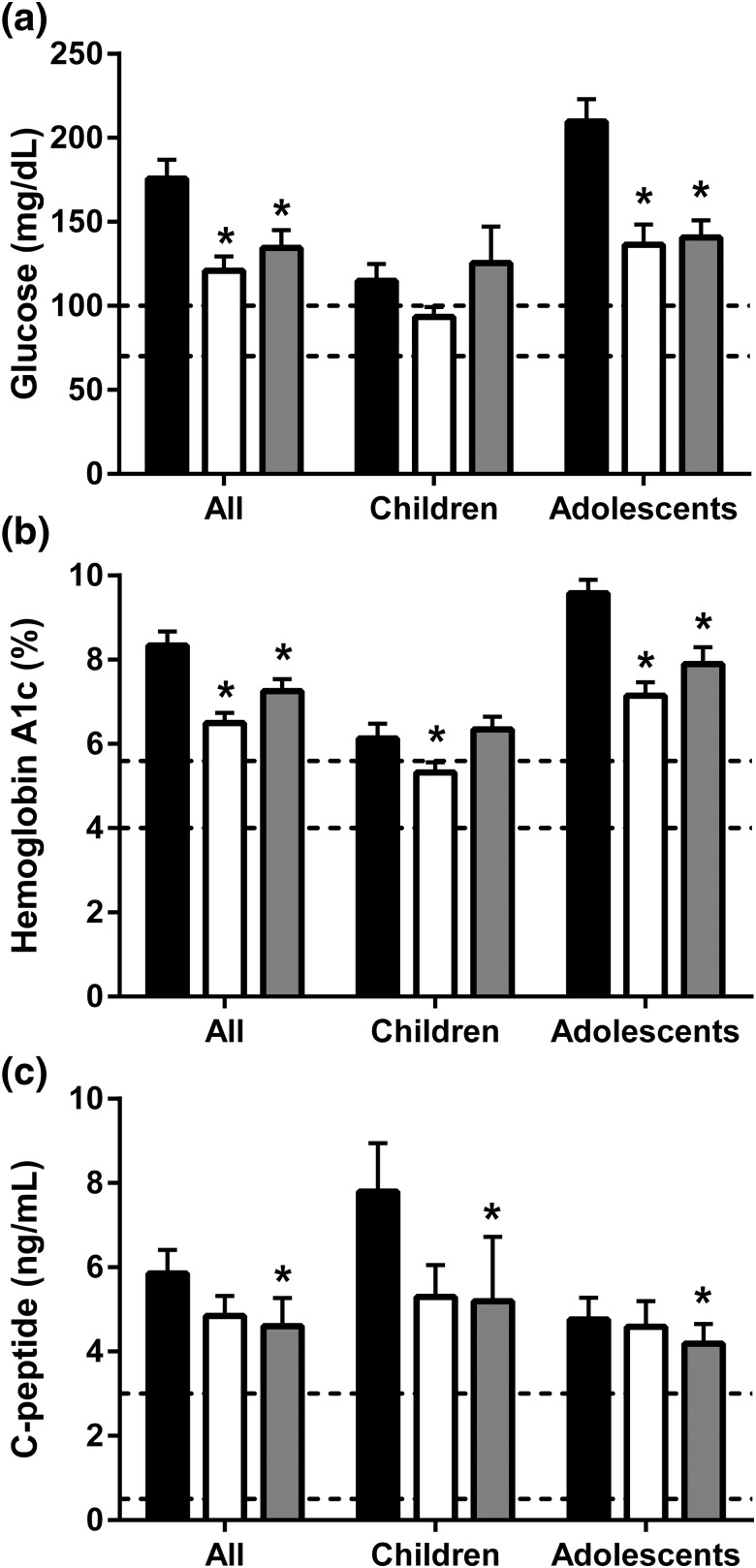
Glycemic outcomes are shown in Fig. 1. In the entire cohort, the mean fasting glucose level decreased from 176 ± 81 mg/dL to 121 ± 61 mg/dL (P < 0.0001). In children, glucose level did not significantly decline (115 ± 43 mg/dL at baseline; 94 ± 26 mg/dL at 12 months; P = 0.14). In adolescents, glucose decreased from 210 ± 78 mg/dL to 136 ± 70 mg/dL (P < 0.0001). The mean A1c level decreased from 8.3% ± 2.4% to 6.5% ± 1.8% in the entire cohort (P < 0.0001), from 6.1% ± 1.5% to 5.3% ± 1.1% in children (P = 0.02), and from 9.6% ± 1.9% to 7.1% ± 1.8% in adolescents (P < 0.0001). The mean C-peptide level decreased from 5.9 ± 4.0 ng/mL to 4.8 ± 3.4 ng/mL in the entire cohort (P = 0.03), but changes were not statistically significant in the child or adolescent subgroups. The percentage of patients requiring insulin decreased from 45% before metreleptin treatment to 23% after 1 year of treatment (P = 0.023). Among those who used insulin at baseline, the mean insulin dose decreased from 385 ± 789 U/d to 138 ± 403 U/d (P = 0.008). The mean number of diabetes medications patients used (including insulin and oral hypoglycemic agents) decreased from 1.0 ± 0.7 to 0.8 ± 0.7 (P = 0.03).
Figure 1.
Mean ± standard error of the mean for (a) glucose, (b) hemoglobin A1c, and (c) C-peptide levels at baseline (black bars) and after 1 year (white bars) and ∼5 years (gray bars) of metreleptin treatment. Normal ranges are indicated by dashed lines (glucose, 70–100 mg/dL; hemoglobin A1c, 4%–5.7%; C-peptide, 0.5–3 mg/dL). *Statistically significant changes relative to baseline.
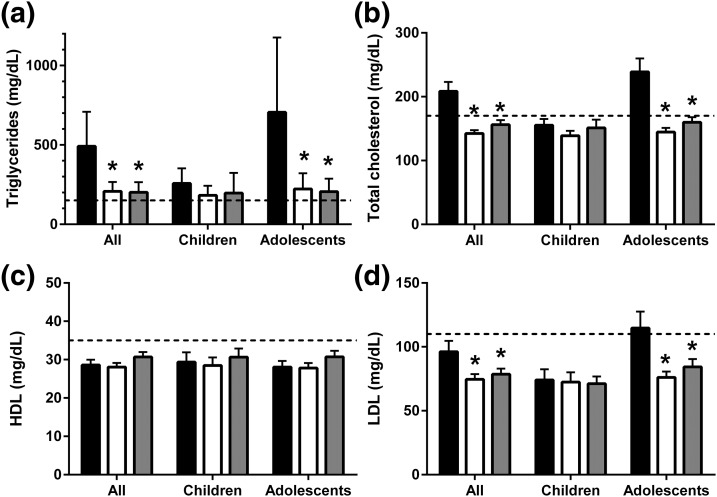
Lipid outcomes are shown in Fig. 2. The mean LDL concentration decreased from 96 ± 51 mg/dL to 75 ± 28 mg/dL in the entire cohort (P = 0.0005), from 115 ± 56 mg/dL to 76 ± 25 mg/dL in adolescents (P < 0.0001), and did not change in children (from 74 ± 34 mg/dL to 72 ± 32 mg/dL; P = 0.4). The mean HDL level remained low at baseline (29 ± 9 mg/dL) and at 12 months (28 ± 8 mg/dL; P = 0.9). Median triglyceride levels decreased from 374 mg/dL (190, 1065) to 189 mg/dL (112, 334) in the entire cohort (P < 0.0001), from 556 mg/dL (254, 2727) to 226 mg/dL (107, 393) in adolescents (P < 0.0001), and did not significantly change in children [from 228 mg/dL (149, 374) to 177 mg/dL (122, 273); P = 0.2]. The mean number of lipid-lowering medications decreased from 0.7 ± 0.9 to 0.3 ± 0.5 (P < 0.0001).
Figure 2.
Mean ± standard error of the mean of lipid levels (geometric mean and 95% confidence intervals for triglyceride levels) at baseline (black bars) and after 1 year (white bars) and ~5 years (gray bars) of metreleptin treatment. (a) Triglyceride, (b) total cholesterol, (c) HDL, and (d) LDL levels. Upper limits for low cardiovascular risk in children for levels of triglycerides (150 mg/dL), total cholesterol (170 mg/dL), and LDL (110 mg/dL), and the lower limit for low cardiovascular risk for HDL level (35 mg/dL) are indicated by dashed lines. *, statistically significant changes relative to baseline.
The median (25th, 75th percentile) ALT level decreased from 73 U/L (45, 126) to 41 U/L (25, 59) in the entire cohort (P = 0.001), from 65 U/L (39, 102) to 29 U/L (23, 48) in adolescents (P < 0.0001), and did not change in children [from 90 U/L (47, 225) to 67 U/L (39, 126); P = 0.8]. The median AST level decreased from 51 U/L (29, 90) to 26 U/L (18, 42) in the entire cohort (P = 0.0002), from to 52 U/L (28, 69) to 22 U/L (17, 34) in adolescents (P < 0.0001), and did not change in children [from 51 U/L (32, 99) to 40 U/L (24, 64); P = 0.3].
There were no statistically significant differences in metabolic responses to metreleptin based on lipodystrophy subtype (Supplemental Table 2 (17.8KB, docx) ).
At long-term follow-up
Long-term follow-up data for metreleptin treatment was available for 47 patients [mean duration, 61 ± 39 months (range, 14 to 170 months); mean age, 17.5 ± 5.6 years (range, 5.8 to 31.4)]. The mean meter leptin dose was higher at long-term follow-up (0.11 ± 0.04 mg/kg/d). Improvements in glucose, A1c, C-peptide, triglyceride, and total cholesterol levels were maintained over long-term treatment in the entire cohort (P < 0.05 for all; Fig. 1). ALT and AST reductions were maintained [39 U/L (22,72) and 27 U/L (21,43), respectively]. Only changes in LDL were no longer statistically significant.
In adolescents (mean age at follow-up, 20.4 ± 4.0 years), statistically significant improvements were maintained in fasting glucose, A1c, total cholesterol, triglyceride, and ALT levels. In children (mean age at follow-up, 13.2 ± 5.0 years), there were statistically significant reductions in C-peptide, ALT, and AST levels. At long-term follow-up, patients who started metreleptin therapy as children had lower A1c levels compared with individuals who start the treatment in adolescence (P = 0.006), whereas other metabolic parameters were indistinguishable (i.e., all lipid, glucose, C-peptide, ALT, and AST levels).
Evaluation of liver biopsy specimens
Seventeen patients underwent liver biopsies both before and after a mean of 3.3 ± 3.2 years of metreleptin treatment (Supplemental Table 3 (17.8KB, docx) ). Compared with patients who did not undergo paired biopsies, the biopsy cohort was more likely to have acquired lipodystrophy (41% vs 6%; P = 0.003), had higher mean ALT (167 ± 169 mg/dL vs 83 ± 70 mg/dL; P = 0.007) and AST levels (98 ± 88 mg/dL vs 58 ± 60 mg/dL; P = 0.04), and lower A1c level (7.2% ± 2.3% vs 8.9% ± 2.3%; P = 0.02). There were no between-group differences for other baseline characteristics, including age, sex, endogenous leptin level, or triglyceride levels.
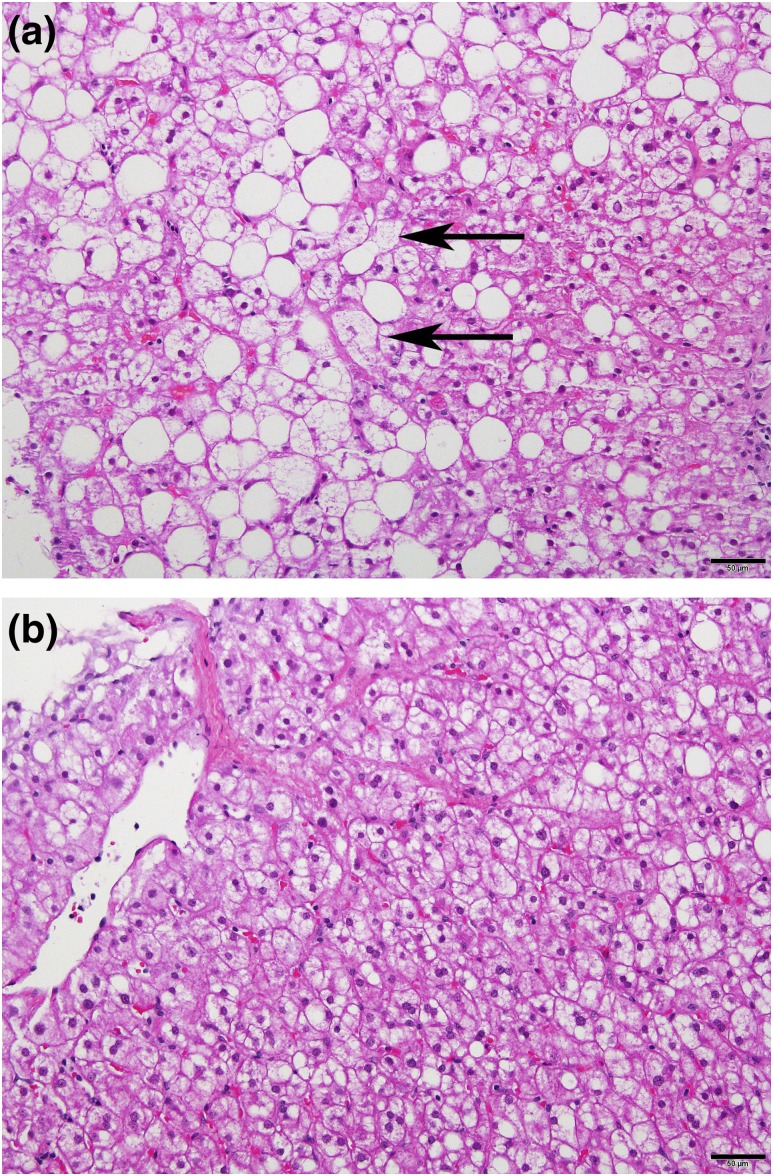
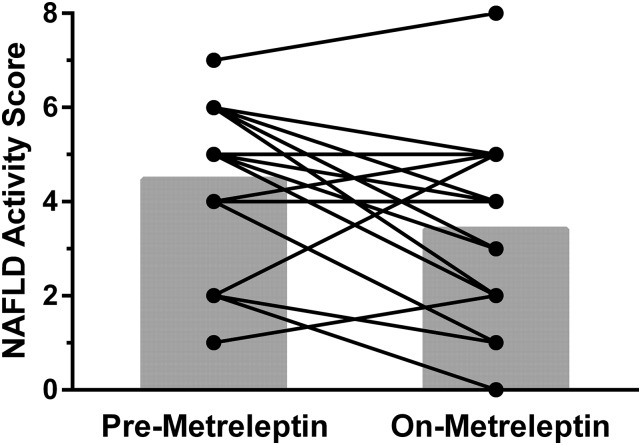
Figure 3 shows liver histologic findings in a patient before and during metreleptin treatment. Fifteen of 17 patients met criteria for NASH before starting metreleptin treatment and 10 of 17 met criteria for NASH after metreleptin treatment (P = 0.1). The mean NAS decreased from 4.5 ± 2.0 before to 3.4 ± 2.0 after metreleptin treatment (P = 0.03). Figure 4 shows the NAS before and after metreleptin therapy for each patient. The NAS improved in 11 of 17, worsened in 4, and was unchanged in 2. Of the 4 patients with worsened NAS, 3 had concomitant factors that might have led to worsened liver disease: 2 had autoimmune hepatitis, and 1 was noncompliant with metreleptin treatment.
Figure 3.
Representative liver histology micrographs from an 11-year-old patient with CGL due to AGPAT2 mutation. The micrographs were taken before and after 1 year of metreleptin treatment, respectively. (a) Micrograph of pretreatment biopsy specimen showing steatohepatitis with ballooning (arrows) and moderate steatosis. (b) Micrograph of posttreatment biopsy specimen with reduction in steatosis and no ballooning. Scale bar = 50 μm.
Figure 4.
NASs before and after a mean of 3.3 ± 3.2 years of metreleptin treatment in 17 patients who underwent liver biopsies. The NAS is a combination of scoring for steatosis, inflammation, and ballooning, and ranges from 0 to 8. Mean NAS ± SD (gray bars) decreased from 4.5 ± 2.0 before to 3.4 ± 2.0 after metreleptin therapy (P = 0.03).
The NASH CRN ballooning score decreased from 1.1 ± 2.4 to 0.5 ± 1.2 (P = 0.01). The NASH CRN steatosis score was unchanged (1.9 ± 2.0 to 1.4 ± 1.9; P = 0.08). The NASH CRN lobular inflammation score was unchanged (1.5 ± 2.3 to 1.5 ± 1.1; P = 0.8), as were the NASH CRN portal inflammation score (1.2 ± 0.6 to 0.9 ± 0.6; P = 0.1) and fibrosis stage (from 2.5 ± 1.1 to 2.7 ± 1.2; P = 0.56).
Growth
At metreleptin initiation, 25 of 53 patients had completed growth; 28 were growing. Four patients had short stature, 34 had normal stature, and 15 had tall stature. After 1 year of metreleptin therapy in the 28 growing patients, height Z-scores significantly decreased from 0.9 ± 1.9 to 0.6 ± 1.7 (P = 0.0006).
Puberty
The pubertal status of each patient before and during metreleptin treatment is listed in Table 2. Thirty-one patients had normal pubertal development for age and sex both before and during metreleptin treatment. Of 11 patients in whom pubertal staging was not available before initiating metreleptin therapy, 5 (3 girls, 2 boys) were in normally timed puberty during treatment, 3 (2 girls, 1 boy) completed puberty normally, and there were no data for 3 (1 girl, 2 boys) before or during treatment. One female patient who was appropriately at Tanner III at age 13.6 years at metreleptin initiation did not progress further during treatment (last follow-up age, 18.9 years). Another girl was appropriately prepubertal (age, 7.9 years) at metreleptin initiation and progressed rapidly through puberty during treatment (Tanner V at 10.9 years). Her bone age at metreleptin initiation was advanced (10.2 years).
Table 2.
Pubertal Status Prior to and During Metreleptin Treatment
| Pubertal Category | Prior to Metreleptin | Receiving Metreleptin |
|---|---|---|
| Precocious puberty | 76, 31M, 48M | 67,a 76b |
| Normal prepubertal | 30, 59, 65, 66, 67, 68M, 69, 84, 50M | 65, 66, 69, 84, 68M, |
| Normal in puberty | 13, 27, 33, 43, 44, 60, 62, 77M, 82, 94, 95, 99, 108M | 10, 30, 41, 59, 62, 70, 34M, 48M, 77M, 79M, 108M |
| Normal puberty complete | 1,c 2, 4, 5, 22, 40, 42, 53, 75, 78, 81, 83, 88 | 1,c 2, 4, 5, 13, 22, 27, 33, 37,40, 42, 44, 49, 53, 75, 60, 64, 78, 81, 83, 88, 93, 94, 95, 99, 101, 24M, 31M, 50M, 54M |
| Delayed puberty | 9, 24M, 49, 64, 93 | 9, 43d |
| No data | 10, 37, 41, 45, 70, 101, 79M, 11M, 28M, 34M, 54M | 45, 82, 11M, 28M |
Each patient’s study number is listed in the appropriate category before and during metreleptin treatment. All available data during metreleptin treatment (duration, ≤15 years) were analyzed.
Abbreviation: M, male patient.
Accelerated progression of puberty while receiving metreleptin treatment: Tanner V at age 10.9 years.
Started gonadotropin-releasing hormone agonist after initiating metreleptin treatment.
Mammary aplasia before and after leptin but menstruating and otherwise pubertal.
Tanner III at initiation of metreleptin (age, 13.6 years) and Tanner III at last follow-up (age, 18.9 years).
Three patients (2 boys, 1 girl) had precocious puberty before metreleptin treatment. The female patient (age, 3.0 years) began receiving treatment with gonadotropin releasing hormone analog shortly after metreleptin initiation; she showed no further pubertal progression and maintained good metabolic control (observation period, 3 years). The 2 boys (ages, 8.2 and 9.1 years) progressed normally through puberty during metreleptin treatment (observation periods, 0.5 and 8.0 years, respectively).
Five patients (1 boy, 4 girls) had delayed puberty at metreleptin initiation. Four of these completed puberty subsequently while receiving metreleptin treatment. One girl was prepubertal at age 13.8 years when metreleptin was started and remained prepubertal during 1.3 years of treatment. She died of hepatorenal failure 7 months after metreleptin discontinuation.
Bone age
Twenty-seven of 53 patients had bone ages obtained before or close to metreleptin initiation (24 months before to 3 months after metreleptin initiation). No patient had delayed bone age, 16 (59%) had normal bone age, and 11 (41%) had advanced bone age. Eighteen patients had not completed growth and, thus, a change in bone age was expected during follow-up. At metreleptin initiation, average bone age was 9.9 ± 4.0 years at chronologic age 8.1 ± 3.8 years; at follow-up, bone age was 11.1 ± 3.5 years at chronologic age 9.6 ± 3.9 years. As bone age advanced by 1.2 ± 1.3 years during metreleptin treatment of 1.5 ± 0.5 years’ duration, metreleptin did not advance bone maturation.
Adverse events
Twenty-seven treatment emergent adverse events occurred in 22 patients, including decreased appetite and/or weight loss (n = 13); hypoglycemia (n = 4); fatigue (n = 3); in vitro neutralizing antibody activity to leptin (n = 2), as previously reported (18); hair loss (n = 2); nausea (n = 1); menorrhagia (n = 1); and anaplastic large cell lymphoma (n = 1), as previously reported (22).
Two patients died years after metreleptin discontinuation for noncompliance, 1 of end-stage renal disease and the other of unknown cause. Three patients with cirrhosis prior to metreleptin treatment died of complications of cirrhosis after 4, 6, and 12 years of receiving metreleptin.
Discussion
This is, to our knowledge, the largest report of the efficacy of metreleptin in pediatric patients with lipodystrophy. Metreleptin treatment was effective at improving metabolic abnormalities associated with lipodystrophy and low baseline leptin levels, both in the short term (1 year) and long term (mean, 5 years). Many of these changes have the potential to substantially improve patients’ quality of life: Mean A1c level at 1 year was <7%, thus reducing risk of microvascular complications in patients facing a lifetime with diabetes. Moreover, half of patients who required insulin before metreleptin therapy discontinued insulin entirely at 1 year, thus dramatically reducing the burdens of diabetes management. These glycemic improvements with minimal changes in C-peptide level are consistent with prior studies demonstrating that metreleptin improves insulin sensitivity (23, 24). More than one-quarter of patients had severe elevation of triglyceride levels (>1000 mg/dL) at baseline sufficient to place them at risk for pancreatitis, whereas only 4 patients (7.5%) had triglyceride levels >1000 mg/dL after 1 year.
Adolescents had greater severity of metabolic disease before metreleptin treatment, supporting the idea that, in the absence of leptin replacement, complications of lipodystrophy tend to worsen with age. Because of the milder metabolic derangements in children, and smaller sample size, most improvements with metreleptin observed in adolescents were not statistically significant in children. However, metreleptin may be protecting children from progression of metabolic disease, particularly as they enter puberty, when insulin resistance worsens (25). Metabolic parameters were comparable in children and adolescents at long-term follow-up, suggesting that metreleptin therapy maintained a similar level of control in both groups, regardless of initial disease severity.
Patients with lipodystrophy typically have NAFLD before leptin replacement, and those with AGL may have concomitant autoimmune hepatitis (14, 26). Particularly in patients with BSCL2 mutations, NAFLD can lead to cirrhosis, portal hypertension, and early death or need for transplantation (14, 27–29). In the current study, ALT and AST levels decreased with metreleptin, suggesting decreased hepatocellular injury. The improvement in liver enzyme levels mirrors findings from a cohort of adult and pediatric patients from our group (11). Interestingly, in the current study, children younger than 12 years of age had nonsignificantly higher ALT and AST levels at baseline compared with adolescents but did not significantly improve with metreleptin treatment. A few children (3 of 19) and adolescents (2 of 34) had autoimmune hepatitis, which is associated with greater elevation in transaminase levels than is NAFLD and is not expected to improve with metreleptin. In addition, a greater proportion of children (8 of 19) than adolescents (8 of 34) had BSCL2 mutations. These differences in underlying liver disease, as well as smaller patient numbers, may have contributed to the lack of effect of metreleptin on transaminases in children.
Metreleptin also improved biopsy-specimen measures of NAFLD, as shown by reductions in the NAS. Statistically significant reductions in ballooning injury were observed, with nonsignificant reductions in steatosis and portal inflammation. Similar to a prior analysis by our group (including adults and children) (14), no change in fibrosis score was seen. Because pediatric patients who underwent liver biopsies did so for clinical reasons, it is not surprising that patients with paired biopsies differed from those who did not undergo biopsies. Patients who underwent biopsies were more likely to have acquired forms of lipodystrophy and to have higher transaminase and lower A1c levels. Although histologic improvements observed with metreleptin may have limited generalizability due to differences between the biopsy and nonbiopsy cohorts, our findings support a beneficial effect of metreleptin on NAFLD histology in pediatric patients with more severe baseline liver disease. Moreover, our study was biased against showing improvements in NAS, as we included patients with autoimmune hepatitis [who were excluded from prior analyses (14)], a condition which should not be altered by metreleptin, and because follow-up biopsies during metreleptin treatment were more likely to be performed at times when other markers of liver disease (e.g., ALT, AST) were worsening. Further investigation is needed to determine if metreleptin treatment in pediatric patients with lipodystrophy will alter the natural history of NAFLD, reducing the incidence of cirrhosis and its complications.
Given the role of leptin in reproduction and puberty (30), our data are reassuring in demonstrating that metreleptin treatment may help to normalize delayed pubertal progression but does not cause precocious puberty. Most patients had advanced bone age before initiating metreleptin treatment, but bone age progression was normal during treatment, further supporting the idea that metreleptin did not accelerate or trigger puberty. For the two patients who had delayed puberty during metreleptin treatment, poor pubertal progression appeared to be related to concurrent serious illness.
We previously demonstrated that patients with CGL are tall, with advanced bone age (31). The current study showed growth deceleration during metreleptin treatment. This likely represents partial normalization of rapid growth before initiating metreleptin therapy, because mean height remained within the upper part of the normal range. However, growth deceleration could occur for other reasons, including earlier than average puberty leading to earlier growth spurt and, hence, crossing of growth percentiles downward at the typical age of puberty; improvement in insulin resistance leading to less hyperinsulinemia driving excessive growth; or metreleptin-induced weight loss causing growth failure, which did not appear to be the case for most patients.
This study was limited by the lack of a placebo control arm, etiologic heterogeneity of lipodystrophy, and selection bias for patients with hypoleptinemia with metabolic abnormalities. Moreover, the sample size of pediatric patients with this rare condition was small, limiting statistical power. A larger sample size may help provide better information on the efficacy of metreleptin in both age groups, though it should be pointed out that no worsening of glycemia, triglyceride, or liver enzyme levels were observed in children or adolescents, as would be expected in the natural history of lipodystrophy (32). We found no differences in metreleptin response based on lipodystrophy subtype, but sample sizes were small.
In conclusion, metreleptin was effective in treating metabolic abnormalities in pediatric patients with lipodystrophy, including diabetes and hypertriglyceridemia, and improved biomarkers of NAFLD. The observed improvements are likely to lead to improved quality of life with reduced burden of disease. Longer follow-up is needed to determine the impact of metreleptin on life expectancy.
Acknowledgments
We gratefully acknowledge the services of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Clinical Core Laboratory and the companies that have donated metreleptin for these studies since 2000, including Amgen, Amylin Pharmaceuticals, Bristol Myers Squibb, Astra Zeneca, and Aegerion Pharmaceuticals.
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Cancer Institute.
Clinical trial registry: ClinicalTrials.gov nos. NCT00005905 (registered 9 June 2000), NCT00025883 (registered 27 October 2001), NCT01778556 (registered 26 January 2013), NCT02262832 (registered 10 October 2014), NCT02262806 (registered 9 October 2014).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A1C
- hemoglobin A1C
- AGL
- acquired generalized lipodystrophy
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- CGL
- congenital generalized lipodystrophy
- CGL2
- congenital generalized lipodystrophy with BSCL2 mutation
- CRN
- Clinical Research Network
- FPL
- familial partial lipodystrophy
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- NAFLD
- nonalcoholic fatty liver disease
- NAS
- nonalcoholic fatty liver disease activity score
- NASH
- nonalcoholic steatohepatitis.
References
- 1.Garg A. Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16(2):324–333. [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.4158/endp.19.1.v767575m65p5mr06. Handelsman Y, Oral EA, Bloomgarden ZT, Brown RJ, Chan JL, Einhorn D, Garber AJ, Garg A, Garvey WT, Grunberger G, Henry RR, Lavin N, Tapiador CD, Weyer C, American Association of Clinical Endocrinologists. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract. 2013;19(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rother KI, Brown RJ. Novel forms of lipodystrophy: why should we care? Diabetes Care. 2013;36(8):2142–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–4847. [DOI] [PubMed] [Google Scholar]
- 6.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–1782. [DOI] [PubMed] [Google Scholar]
- 7.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18–34. [DOI] [PubMed] [Google Scholar]
- 8.Van Maldergem L, Magré J, Khallouf TE, Gedde-Dahl T Jr, Delépine M, Trygstad O, Seemanova E, Stephenson T, Albott CS, Bonnici F, Panz VR, Medina JL, Bogalho P, Huet F, Savasta S, Verloes A, Robert JJ, Loret H, De Kerdanet M, Tubiana-Rufi N, Mégarbané A, Maassen J, Polak M, Lacombe D, Kahn CR, Silveira EL, D’Abronzo FH, Grigorescu F, Lathrop M, Capeau J, O’Rahilly S. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39(10):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally M, Mannon RB, Javor ED, Swanson SJ, Hale DA, Gorden P, Kirk AD. Successful renal transplantation in a patient with congenital generalized lipodystrophy: a case report. Am J Transplant. 2004;4(3):447–449. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Chong AY, Cochran EK, Kleiner DE, Haller MJ, Schatz DA, Gorden P. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. doi: 10.4158/EP11229.OR. Chan JL, Lutz K, Cochran E, Huang W, Peters Y, Weyer C, Gorden P. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27–35. [DOI] [PubMed] [Google Scholar]
- 13.Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002. [DOI] [PubMed] [Google Scholar]
- 16.Meehan CA, Cochran E, Kassai A, Brown RJ, Gorden P. Metreleptin for injection to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. Expert Rev Clin Pharmacol. 2016;9(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. [DOI] [PubMed] [Google Scholar]
- 18.Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, Brown RJ. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. [DOI] [PubMed] [Google Scholar]
- 20. Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 22.Brown RJ, Chan JL, Jaffe ES, Cochran E, DePaoli AM, Gautier JF, Goujard C, Vigouroux C, Gorden P. Lymphoma in acquired generalized lipodystrophy. Leuk Lymphoma. 2015; 57(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–541. [DOI] [PubMed] [Google Scholar]
- 24.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamran F, Rother KI, Cochran E, Safar Zadeh E, Gorden P, Brown RJ. Consequences of stopping and restarting leptin in an adolescent with lipodystrophy. Horm Res Paediatr. 2012;78(5-6):320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753–760. [DOI] [PubMed] [Google Scholar]
- 27.Casey SP, Lokan J, Testro A, Farquharson S, Connelly A, Proietto J, Angus PW. Post-liver transplant leptin results in resolution of severe recurrence of lipodystrophy-associated nonalcoholic steatohepatitis. Am J Transplant. 2013;13(11):3031–3034. [DOI] [PubMed] [Google Scholar]
- 28.Cauble MS, Gilroy R, Sorrell MF, Mailliard ME, Sudan DL, Anderson JC, Wisecarver JL, Balakrishnan S, Larsen JL. Lipoatrophic diabetes and end-stage liver disease secondary to nonalcoholic steatohepatitis with recurrence after liver transplantation. Transplantation. 2001;71(7):892–895. [DOI] [PubMed] [Google Scholar]
- 29.Arky RA, McCully KS. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1-1975. N Engl J Med. 1975;292(1):35–41. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Garrido MA, Tena-Sempere M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm Behav. 2013;64(2):187–194. [DOI] [PubMed] [Google Scholar]
- 31.Christensen JD, Lungu AO, Cochran E, Collins MT, Gafni RI, Reynolds JC, Rother KI, Gorden P, Brown RJ. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab. 2014;99(8):E1493–E1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinci B, Onay H, Demir T, Ozen S, Kayserili H, Akinci G, Nur B, Tuysuz B, Nuri Ozbek M, Gungor A, Yildirim Simsir I, Altay C, Demir L, Simsek E, Atmaca M, Topaloglu H, Bilen H, Atmaca H, Atik T, Cavdar U, Altunoglu U, Aslanger A, Mihci E, Secil M, Saygili F, Comlekci A, Garg A. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab. 2016;101(7):2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]