Abstract
Context:
The P450 enzyme CYP24A1 is the principal inactivator of vitamin D metabolites. Biallelic loss-of-function mutations in CYP24A1 are associated with elevated serum levels of 1,25-dihydroxyvitamin D3 with consequent hypercalcemia and hypercalciuria and represent the most common form of idiopathic infantile hypercalcemia (IIH). Current management strategies for this condition include a low-calcium diet, reduced dietary vitamin D intake, and limited sunlight exposure. CYP3A4 is a P450 enzyme that inactivates many drugs and xenobiotics and may represent an alternative pathway for inactivation of vitamin D metabolites.
Objective:
Our goal was to determine if rifampin, a potent inducer of CYP3A4, can normalize mineral metabolism in patients with IIH due to mutations in CYP24A1.
Methods:
We treated two patients with IIH with daily rifampin (10 mg/kg/d, up to a maximum of 600 mg). Serum calcium, phosphorus, parathyroid hormone (PTH), liver, and adrenal function and vitamin D metabolites, as well as urinary calcium excretion, were monitored during treatment of up to 13 months.
Results:
Prior to treatment, both patients had hypercalcemia, hypercalciuria, and nephrocalcinosis with elevated serum 1,25-dihydroxyvitamin D3 and suppressed serum PTH. Daily treatment with rifampin was well tolerated and led to normalization or improvement in all clinical and biochemical parameters.
Conclusion:
These observations suggest that rifampin-induced overexpression of CYP3A4 provides an alternative pathway for inactivation of vitamin D metabolites in patients who lack CYP24A1 function.
Two patients with biallelic CYP24A1 mutations and intractable hypercalcemia were treated with rifampin for up to 13 months. CYP3A4 induction by rifampin resulted in normalization of mineral metabolism.
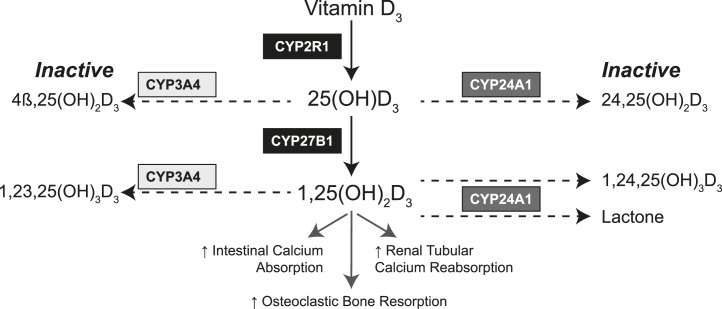
Idiopathic infantile hypercalcemia (IIH) (OMIM 143880) is an uncommon disorder of vitamin D metabolism that is characterized by hypersensitivity to vitamin D. It is associated with hypercalcemia and hypercalciuria, suppressed serum levels of parathyroid hormone (PTH), and elevated levels of vitamin D metabolites, particularly the active form of vitamin D, 1,25-dihydroxyvitamin D. Biallelic loss-of-function mutations of CYP24A1, the gene encoding a P450 24-hydroxylase enzyme that represents the principal pathway for inactivation of vitamin D metabolites (Fig. 1), cause the most common and severe form of IIH (1, 2).
Figure 1.
The activation and inactivation pathways of vitamin D, demonstrating the role of CYP24A1 in inactivating 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D] and the potential of CYP3A4 to provide an alternative inactivation pathway.
Although IIH is usually diagnosed in infants who present with severe hypercalcemia, failure to thrive, and nephrocalcinosis (3, 4), inactivating mutations of CYP24A1 have also been reported in adults with nephrocalcinosis and/or recurrent nephrolithiasis associated with hypercalciuria (5). Patients with CYP24A1 mutations have a life-long defect in vitamin D metabolism that increases risk for nephrocalcinosis, nephrolithiasis, and renal insufficiency.
There is no specific, long-term treatment for patients with vitamin D hypersensitivity due to CYP24A1 mutations, and conventional management consists of minimizing cutaneous vitamin D production through reduced exposure to sunlight and other sources of ultraviolet B radiation, a low-calcium diet, and avoidance of vitamin D–rich foods and supplements. These restrictions can reduce serum calcium levels to near normal but do not adequately address the risk of renal complications due to persistent hypercalciuria. Hence, the need for specific treatment has stimulated interest in pharmacological approaches to modify vitamin D metabolism.
Recent reports have demonstrated short-term benefits of treatment with ketoconazole (6) and fluconazole (7), imidazole derivatives that can partially inhibit CYP27B1, the enzyme that generates 1,25-dihydroxyvitamin D3 from substrate 25-hydroxyvitamin D3. Induction of CYP3A4 (EC 1.14.13.97), an enzyme that is expressed in the liver, small intestine, and other tissues and that metabolizes many xenobiotics, steroids, and drugs, represents an alternative therapeutic strategy to reduce serum levels of vitamin D metabolites. Indeed, the phenomenon of drug-induced vitamin D deficiency and osteomalacia is a well-recognized complication of many medications that induce CYP3A4 (8) and establishes CYP3A4 as an alternative degradative pathway to CYP24A1 (Fig. 1). Our group recently identified a recurrent gain-of-function mutation in CYP3A4 that results in enhanced bioinactivation of 1,25-dihydroxyvitamin D3, which provides further support for such a therapeutic strategy (9). Here we describe the use of rifampin, a potent inducer of CYP3A4 (8), to normalize mineral metabolism in two patients with IIH due to mutations in CYP24A1.
Patients and Methods
Clinical
Two patients with CYP24A1 mutations, hypercalcemia, and/or hypercalciuria initiated treatment with rifampin as compassionate rescue therapy. Both patients consented or assented to the off-label use of rifampin. Rifampin dosages in these patients were consistent with standard antituberculous treatment (10 mg/kg, up to a maximum of 600 mg daily), at which rifampin has a very low risk of hepatotoxicity (10) or increased catabolism of adrenal hormones (11). Liver and adrenal function were monitored throughout treatment. In addition to routine clinical studies, biochemical and molecular studies were performed under a protocol that was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia. Chart review was performed in accordance with the policies and procedures of the Institutional Review Board as a retrospective review of a limited case series.
Molecular analyses
Genomic DNA was isolated from peripheral blood mononuclear cells of the patients and their first-degree relatives. CYP24A1 mutations were confirmed by Sanger sequencing after the variants were initially identified by whole-exome sequence analysis of the two probands (12).
Biochemical analyses
We measured urine and serum calcium, phosphorus, creatinine, and alkaline phosphatase by standard methods. Measurements of vitamin D3 and vitamin D metabolites were performed by liquid chromatography tandem mass spectrometry, with an ultrahigh-resolution chromatographic separation procedure (13) used to enable complete separation of 1,25-dihydroxyvitamin D3 from 4β,25-dihydroxyvitamin D3, the principal product of CYP3A4 oxidation of 25-hydroxyvitamin D3 (8). Thus, we used the serum concentration of 4β,25-dihydroxyvitamin D3 and the ratio of 4β,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 as biomarkers of CYP3A4 activity. Intact PTH was measured using the Immulite 2000 PTH assay (Diagnostics Product Corp., Los Angeles, CA). Reference ranges of vitamin D metabolites were determined in a subset of 23 healthy young subjects living in Seattle, WA, as described elsewhere (8).
Results
Patients
Case 1
An 18-year-old man presented with unexplained hypercalcemia, hypercalciuria, and recurrent episodes of hematuria. Serum levels of PTH were suppressed; analyses of serum concentrations of vitamin D metabolites revealed low or undetectable levels of 24,25-dihydroxyvitamin D, elevated or inappropriately normal concentrations of 1,25-dihydroxyvitamin D3, and an elevated level of 25-hydroxyvitamin D, likely the result of extensive sunbathing without use of an ultraviolet B–blocking sunscreen and unrestrained consumption of vitamin D–fortified milk prior to elucidation of his genetic diagnosis (Table 1).
Table 1.
Baseline Characteristics of Both Patients and Response to Treatment
| Reference Range | Case 1 | Case 2 | |||||
|---|---|---|---|---|---|---|---|
| Sex, age | Male, 18 y | Female, 8 y | |||||
| Weight | 68.3 kg | 22.5 kg | |||||
| Presentation | Hematuria, nephrocalcinosis | Polyuria, nephrocalcinosis, hypercalcemia | |||||
| Rifampin dose | 600 mg daily | 300 mg daily | |||||
| Baseline | 1 mo | 10 mo | Baseline | 11 mo (on treatment) | 13 mo (off treatment × 2 mo) | ||
| Albumin adjusted calcium, mg/dL | 8.9–10.4 | 10.5 | 9.6 | 9.6 | 10.7 | 10 | 11.5 |
| Phosphorus, mg/dL | 2.5–4.5 | 3.6 | 3.1 | 4.1 | 4.2 | 3.6 | 4.5 |
| Alkaline phosphatase, U/L | 184–415 (Case 1) | 82 | 114 | 84 | 242 | 467 | 345 |
| 0.2–0.73 (Case 2) | |||||||
| Intact PTH, pg/mL | 9–69 | 2 | 13 | 16 | 3 | 31 | 3 |
| Creatinine, mg/dL | 0.3–0.8 (Case 1) | 1.4 | 1.2 | 1.1 | 0.59 | 0.54 | 0.65 |
| 0.2–0.73 (Case 2) | |||||||
| 25(OH)D3, ng/mL | 28 ± 27 | 102 | 83 | 65 | 23 | 16 | 35 |
| 1,25-dihydroxyvitamin D3, pg/mL | 56 ± 27 | 99 | 53 | 59 | 55 | 58 | 76 |
| 24,25-dihydroxyvitamin D3, ng/mL | 1.6 ± 1 | Undetectablea | 0.2 | 0.1 | Undetectable | 0.1 | 0.1 |
| 25-hydroxyvitamin D3/24,25-dihydroxyvitamin D3, ng/ng | 22 ± 13 | N/Ab | 415 | 650 | N/A | 160 | 350 |
| 4β,25-dihydroxyvitamin D3, pg/mLc | 53 ± 34 | 275 | 594 | 528 | N/A | 82 | 56.7 |
| 4β,25-dihydroxyvitamin D3/25-hydroxyvitamin D3, (10−3)c | 2.1 ± 0·7 | 2.7 | 7.2 | 5.4 | N/A | 3.7 | 1.3 |
Test was performed, but the concentration was below the detectable limits of the assay.
Not tested.
4β,25-dihydroxyvitamin D3 and the 4β,25-dihydroxyvitamin D3/25-hydroxyvitamin D3 ratio are biomarkers of CYP3A4 induction.
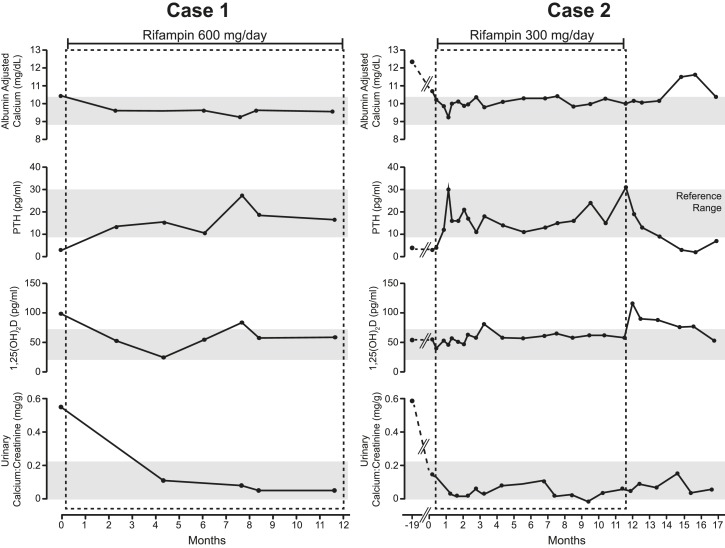
The proband carries two CYP24A1 mutations, a novel maternal deletion (c.1421delT; p.L474WfsX102) that is predicted to be pathogenic and a previously reported paternal missense mutation (c.443T>C; p.L148P) (5) that reduces CYP24A1 activity by 30% to 50% (14). Ultrasonography revealed extensive bilateral nephrocalcinosis and multiple renal parenchymal cysts (maximum size, 2 cm × 2.9 cm); the patient had reduced renal function with a serum creatinine concentration of 1.4 mg/dL. Within 1 month of treatment with 600 mg rifampin daily, there was marked improvement in mineral metabolism, with reductions in serum levels of 1,25-dihydroxyvitamin D3 and calcium and increasing PTH into the normal range (Table 1; Fig. 2). Urinary calcium excretion declined, and the serum creatinine improved to 1.1 mg/dL. Both heterozygous parents were clinically unaffected.
Figure 2.
Serum calcium, PTH, 1,25-dihydroxyvitamin D3 [1,25(OH)2D], and urinary calcium/creatinine concentrations before and during rifampin treatment in Case 1 and Case 2. Note that Case 2 had been treated with pamidronate, calcitonin, low-calcium diet, and reduced vitamin D intake prior to starting rifampin treatment. Case 2 also had a period of observation after discontinuation of rifampin treatment after month 11, with rebound hypercalcemia and suppression of PTH.
Case 2
An 8-year-old girl presented 2 years previously with poor growth, hypercalcemia (albumin-adjusted calcium concentration of 12.8 mg/dL), and hypercalciuria (urinary calcium/creatinine ratio of 0.59 mG/mG; normal <0.22 mG/mG). Serum PTH was suppressed at 4 pg/mL, and serum 1,25-dihydroxyvitamin D3 was inappropriately normal at 49 pg/mL. She had been treated with intravenous pamidronate and was receiving subcutaneous calcitonin, as well as a low-calcium and low–vitamin D diet, immediately before beginning rifampin (Table 1).
Case 2 was compound heterozygous for two previously reported CYP24A1 mutations, a three–base pair deletion in exon 2 (c.428_430delAAG:p.Glu143del) inherited from her mother and a paternal missense mutation (c.1186C>T: p.R396W) that encodes a CYP24A1 enzyme with no activity (2). Her older brother and sister are heterozygous for the paternal mutation, and a second sister is wild type for both CYP24A1 alleles. All heterozygous carriers were clinically and biochemically unaffected.
Case 2 was treated with rifampin (300 mg daily). Serum levels of albumin-adjusted calcium declined from 10.7 to 9.8 mg/dL, with a corresponding increase in previously suppressed intact PTH concentration from 3 to 31 pg/mL (Table 1; Fig. 2). Her polyuria resolved, and her linear growth improved. One month after discontinuation of rifampin, her serum and urinary calcium levels increased, her 1,25-dihydroxyvitamin D3 concentration rose from 58 to 90 pg/mL, and the serum concentration of intact PTH was once again suppressed (Fig. 2).
Both patients had very low or undetectable serum levels of 24,25-dihydroxyvitamin D and extremely high ratios of 25-hydroxyvitamin D/24,25-dihydroxyvitamin D, consistent with an absence of CYP24A1 activity (Table 1). The ratio of serum 4β,25-dihydroxyvitamin D3, an inactive metabolite of 25-hydroxyvitamin D generated by CYP3A4 (13, 15), to substrate 25-hydroxyvitamin D is a marker of CYP3A4 activity and induction (8), and ratios remained elevated in both cases during rifampin treatment. Moreover, this ratio decreased in case 2 after discontinuation of rifampin (Table 1). Both patients tolerated rifampin without developing hepatic dysfunction, adrenal insufficiency, or symptoms that could be attributed to side effects of therapy.
Discussion
Normal vitamin D homeostasis requires biochemical equipoise between production and inactivation of 1,25-dihydroxyvitamin D, with CYP24A1 playing a critical role in the defense against hypercalcemia and hypercalciuria through the inactivation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D. Both patients described in this report had biallelic loss-of-function mutations in CYP24A1; nevertheless, both patients had detectable circulating 24,25-dihydroxyvitamin D, which may be due to residual CYP24A1 activity or modest 24-hydroxylase activity of other P450 enzymes. Here we have demonstrated that rifampin is a potential treatment of this condition. The likely mechanism of action is through overexpression of CYP3A4, thereby providing an alternative enzymatic pathway for inactivating vitamin D metabolites in patients with CYP24A1 mutations.
With rifampin treatment, both patients described in this study experienced biochemical and clinical improvement, with normalization of serum and urine concentrations of calcium and relief of symptoms of hypercalcemia. In addition, serum concentrations of intact PTH increased to the normal range, signaling re-establishment of normal mineral homeostasis. CYP3A4 oxidizes 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D (Fig. 1) to the inactive metabolites 1,23,25-trihydroxyvitamin D and 4β,25-dihydroxyvitamin D, respectively. Although serum levels of 1,23,25 trihydroxyvitamin D were not measured in this study, we found significant increases in the serum concentrations of 4β,25-dihydroxyvitamin D, which provide evidence for CYP3A4 induction as the mechanism for this clinical improvement. Further proof of the therapeutic effect of rifampin was provided by subject 2, who- decided to stop treatment after 11 months and experienced a recurrence of hypercalcemia and hypercalciuria within 1 month of discontinuation of rifampin.
In both cases, serum concentrations of 25-hydroxyvitamin D declined with treatment. In case 2, 1,25-dihydroxyvitamin D concentrations were inappropriately normal or elevated prior to treatment with rifampin despite hypercalcemia and suppressed serum PTH. With treatment, the serum concentration of 1,25-dihydroxyvitamin D did not decrease significantly but was now associated with normal serum levels of calcium and PTH, indicating that 1,25-dihydroxyvitamin D homeostasis was now physiological. These observations are consistent with the notion that intracellular levels rather than serum levels of 1,25-dihydroxyvitamin D are the primary determinant of vitamin D action. Accordingly, the most significant improvement was a marked reduction in urinary excretion of calcium, presumably due to decreased vitamin D–dependent absorption of calcium from the intestine. CYP3A4 is highly expressed in the small intestine and liver (16) and is induced in both of these tissues by rifampin (17). Hence, it is possible that the therapeutic effect of rifampin is less dependent upon induction of CYP3A4 in the liver than in the enterocytes, where induction of CYP3A4 would lead to increased local degradation of 1,25-dihydroxyvitamin D3, thereby directly reducing calcium transport across the apical cell membrane.
The current treatment recommendations for patients with IIH due to CYP24A1 mutations consist of limiting sunlight exposure and avoiding dietary vitamin D, reflecting the effect of the mutation on both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the pathophysiology of this disease. Hence, an approach that focuses on restoring vitamin D homeostasis to normal, not merely blocking conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, would be desirable for optimal therapeutic benefit. In both patients reported here, rifampin therapy achieved that goal, but it is likely that long-term treatment with rifampin will be required to prevent ongoing hypercalcemia and complications of hypercalciuria.
Rifampin is among the most powerful inducers of CYP3A4 and has an extensive history of protracted use for a variety of chronic infections with an excellent safety profile. In 157 adolescents treated with rifampin at similar doses to these cases, 2.5% discontinued treatment due to alanine aminotransferase above two times the upper limit of normal (18). Short periods of higher doses, even in combination with other antituberculous medications, are well tolerated (19). Rifampin can increase hydrocortisone metabolism and, when used at higher doses than used here, has been reported to cause adrenal crisis in patients with underlying adrenal insufficiency (11), but clinically significant disturbances in the metabolism of other steroid hormones in otherwise healthy patients have not been described. Serum levels of adrenocorticotropic hormone and cortisol, measured in the morning, remained normal in our subjects during the study, indicating that rifampin treatment had not critically impaired production of glucocorticoid steroids. We did not perform adrenocorticotropic hormone stimulation tests, however, due to the absence of concerning clinical or biochemical features. Nevertheless, the specific dosage of rifampin that is necessary to induce CYP3A4 expression to optimal therapeutic benefit is unknown. It is also possible that other related antibiotics would have similar effects in this condition because rifabutin has been reported to be associated with osteomalacia (20, 21).
The imidazoles fluconazole and ketoconazole have also been reported as a potential treatment of patients with elevated serum concentrations of 1,25-dihydroxyvitamin D, including patients with CYP24A1 mutations, due to their ability to inhibit CYP27B1 and thereby reduce production of 1,25-dihydroxyvitamin D3 (22). Nguyen et al. (6) used ketoconazole at 3–9 mg/kg/d to treat 10 of 20 infants with IIH, many of whom likely had unidentified CYP24A1 mutations, for several months. Serum calcium and urinary calcium excretion decreased to normal in treated and untreated children, although normalization of serum calcium was achieved earlier in treated children. By contrast, the time required to reach the upper normal limit for calcium/creatinine ratio was not significantly different in the two groups. Of concern is that one of the nine treated infants developed a marked reduction in serum cortisol that required cessation of ketoconazole treatment (6). A similar approach was reported as effective by Tebben et al. (23) in a single adult patient. However, ketoconazole requires administration three times daily and has a less favorable side effect profile, which can include hepatotoxicity, gastrointestinal complaints, adrenal insufficiency, and hypogonadism (24). Although the incidence of fluconazole-related hepatotoxity is lower than that associated with ketoconazole, severe jaundice, fatal acute hepatic necrosis, and even death have been reported (25). Finally, it is worth noting that both fluconazole and ketoconazole can also inhibit CYP3A4 (26), an undesirable effect of treatment. In one other infant with biallelic CYP24A1 mutations that we have managed, severe hypercalcemia was induced by fluconazole that had been prescribed for an infection. Once fluconazole was discontinued, the infant’s serum and urinary calcium levels normalized. Taken together with the salutatory effect of rifampin that we show here, it is possible that the reduced penetrance of CYP24A1 mutations in some affected individuals may reflect the coexistence of CYP3A4 alleles that are characterized as “high metabolizing” (27) and that act as important modifiers that ameliorate the severity of the biochemical defect in vitamin D homeostasis.
The effects of loss-of-function CYP24A1 mutations remain challenging to treat, with ongoing risk of hypercalcemia, hypercalciuria, and nephrocalcinosis. The potential for renal damage is significant, as evidenced by the increased serum creatinine in case 1 and the presence of multiple small renal cysts in both of our patients. These renal cysts have previously been reported in other patients with CYP24A1 mutations (28) and likely reflect blockage of small renal collecting ducts by microcalcification. Our results suggest that rifampin is an alternative treatment to consider in patients with recalcitrant hypercalcemia and/or hypercalciuria due to excessive 1,25-dihydroxyvitamin D, but confirmation of this proposal should be explored with further studies. In the meantime, it would seem prudent to avoid the use of medications (29) and foods (e.g., starfruit, pomegranate, and white grapefruit) that can inhibit CYP3A4 in patients with CYP24A1 mutations.
Acknowledgments
Acknowledgments
This work was supported in part by Grant R01 DK079970 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.A.L.); by Grant R01GM063666 from the National Institutes of General Medical Sciences (to K.E.T.); by Grant UL1TR000003 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health; and by a PhD grant from the National Children’s Research Centre, Dublin, Ireland (to C.P.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- IIH
- idiopathic infantile hypercalcemia
- PTH
- parathyroid hormone.
References
- 1.Dauber A, Nguyen TT, Sochett E, Cole DE, Horst R, Abrams SA, Carpenter TO, Hirschhorn JN. Genetic defect in CYP24A1, the vitamin D 24-hydroxylase gene, in a patient with severe infantile hypercalcemia. J Clin Endocrinol Metab. 2012;97(2):E268–E274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(5):410–421. [DOI] [PubMed] [Google Scholar]
- 3.Lightwood R, Stapleton T. Idiopathic hypercalcaemia in infants. Lancet. 1953;265(6779):255–256. [DOI] [PubMed] [Google Scholar]
- 4.Creery RD, Neill DW. Idiopathic hypercalcaemia in infants with failure to thrive. Lancet. 1954;267(6829):110–114. [DOI] [PubMed] [Google Scholar]
- 5.Nesterova G, Malicdan MC, Yasuda K, Sakaki T, Vilboux T, Ciccone C, Horst R, Huang Y, Golas G, Introne W, Huizing M, Adams D, Boerkoel CF, Collins MT, Gahl WA. 1,25-(OH)2D-24 hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol. 2013;8(4):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen M, Boutignon H, Mallet E, Linglart A, Guillozo H, Jehan F, Garabedian M. Infantile hypercalcemia and hypercalciuria: new insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. J Pediatr. 2010;157(2):296–302. [DOI] [PubMed] [Google Scholar]
- 7.Sayers J, Hynes AM, Srivastava S, Dowen F, Quinton R, Datta HK, Sayer JA. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clin Kidney J. 2015;8(4):453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, Dickmann LJ, Nelson SD, Baillie TA, Hebert MF, Blough D, Davis CL, Thummel KE. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012;81(4):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roizen J, Li D, O’Lear L, Javaid MK, Shaw N, Ebeling P, Nguyen H, Rodda C, Thummel KE, Hakonarson H, Levine MA Vitamin D deficiency due to a recurrent gain-of-function mutation in CYP3A4 causes a novel form of vitamin D dependent rickets. Paper presented at: ASBMR Symposium on Bone-omics: Translating Genomic Discoveries into Clinical Applications; September 15, 2016; Atlanta, GA. [Google Scholar]
- 10.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR; ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee . An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–952. [DOI] [PubMed] [Google Scholar]
- 11.Kyriazopoulou V, Parparousi O, Vagenakis AG. Rifampicin-induced adrenal crisis in addisonian patients receiving corticosteroid replacement therapy. J Clin Endocrinol Metab. 1984;59(6):1204–1206. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Tian L, Hou C, Kim CE, Hakonarson H, Levine MA. Association of mutations in SLC12A1 encoding the NKCC2 cotransporter with neonatal primary hyperparathyroidism. J Clin Endocrinol Metab. 2016;101(5):2196–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Lin YS, Dickmann LJ, Poulton EJ, Eaton DL, Lampe JW, Shen DD, Davis CL, Shuhart MC, Thummel KE. Enhancement of hepatic 4-hydroxylation of 25-hydroxyvitamin D3 through CYP3A4 induction in vitro and in vivo: implications for drug-induced osteomalacia. J Bone Miner Res. 2013;28(5):1101–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann M, Prosser DE, Jones G. Bioengineering anabolic vitamin D-25-hydroxylase activity into the human vitamin D catabolic enzyme, cytochrome P450 CYP24A1, by a V391L mutation. J Biol Chem. 2011;286(33):28729–28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Senn T, Kalhorn T, Zheng XE, Zheng S, Davis CL, Hebert MF, Lin YS, Thummel KE. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4β,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, Kalhorn TF, Watkins PB, Schuetz EG, Thummel KE. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006;69(1):56–65. [DOI] [PubMed] [Google Scholar]
- 17.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90(5):1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarino ME, Ridzon R, Weismuller PC, Elcock M, Maxwell RM, Meador J, Smith PJ, Carson ML, Geiter LJ. Rifampin preventive therapy for tuberculosis infection: experience with 157 adolescents. Am J Respir Crit Care Med. 1997;155(5):1735–1738. [DOI] [PubMed] [Google Scholar]
- 19.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, Phillips PP, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE, Pan AC; PanACEA Consortium . A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191(9):1058–1065. [DOI] [PubMed] [Google Scholar]
- 20.Bolland MJ, Grey A, Horne AM, Thomas MG. Osteomalacia in an HIV-infected man receiving rifabutin, a cytochrome P450 enzyme inducer: a case report. Ann Clin Microbiol Antimicrob. 2008;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pentikis HS, Connolly M, Trapnell CB, Forbes WP, Bettenhausen DK. The effect of multiple-dose, oral rifaximin on the pharmacokinetics of intravenous and oral midazolam in healthy volunteers. Pharmacotherapy. 2007;27(10):1361–1369. [DOI] [PubMed] [Google Scholar]
- 22.Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab. 1992;75(6):1446–1452. [DOI] [PubMed] [Google Scholar]
- 23.Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97(3):E423–E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, Bihan H, Delemer B, Raverot G, Motte E, Philippon M, Morange I, Conte-Devolx B, Quinquis L, Martinie M, Vezzosi D, Le Bras M, Baudry C, Christin-Maitre S, Goichot B, Chanson P, Young J, Chabre O, Tabarin A, Bertherat J, Brue T. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014;99(5):1623–1630. [DOI] [PubMed] [Google Scholar]
- 25.Wingard JR, Leather H. Hepatotoxicity associated with antifungal therapy after bone marrow transplantation. Clin Infect Dis. 2005;41(3):308–310. [DOI] [PubMed] [Google Scholar]
- 26.Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28(9):1805–1808. [DOI] [PubMed] [Google Scholar]
- 27.Zanger UM, Klein K, Richter T, Toscano C, Zukunft J. Impact of genetic polymorphism in relation to other factors on expression and function of human drug-metabolizing p450s. Toxicol Mech Methods. 2005;15(2):121–124. [DOI] [PubMed] [Google Scholar]
- 28.Gigante M, Santangelo L, Diella S, Caridi G, Argentiero L, D’'Alessandro MM, Martino M, Stea ED, Ardissino G, Carbone V, Pepe S, Scrutinio D, Maringhini S, Ghiggeri GM, Grandaliano G, Giordano M, Gesualdo L. Mutational spectrum of CYP24A1 gene in a cohort of italian patients with idiopathic infantile hypercalcemia. Nephron. 2016;133(3):193–204. [DOI] [PubMed] [Google Scholar]
- 29.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9(4):310–322. [DOI] [PubMed] [Google Scholar]