Version Changes
Revised. Amendments from Version 1
Version 2 has a new figure and glossary to assist readers and help them to follow the timings of key reproductive events. Sections of text in the Introduction and Discussion have been re-ordered. In text dates have been provided to aid with understanding of the chronology of studies. A brief commentary on a study into implantation by Brosens et al. (2014) has been included. Methods for the Roberts & Lowe simulation study, the calculation of bootstrap confidence intervals, and the calculation of estimated loss from implantation to birth have been clarified. Comments have been included to note value in Roberts & Lowe’s analysis, to affirm the significance of Leridon’s critique, and to better contextualise the subjects from Hertig’s study. Some additional studies have been referenced.
Abstract
How many human embryos die between fertilisation and birth under natural conditions? It is widely accepted that natural human embryo mortality is high, particularly during the first weeks after fertilisation, with total prenatal losses of 70% and higher frequently claimed. However, the first external sign of pregnancy occurs two weeks after fertilisation with a missed menstrual period, and establishing the fate of embryos before this is challenging. Calculations are additionally hampered by a lack of data on the efficiency of fertilisation under natural conditions. Four distinct sources are used to justify quantitative claims regarding embryo loss: (i) a hypothesis published by Roberts & Lowe in The Lancet is widely cited but has no practical quantitative value; (ii) life table analyses give consistent assessments of clinical pregnancy loss, but cannot illuminate losses at earlier stages of development; (iii) studies that measure human chorionic gonadotrophin (hCG) reveal losses in the second week of development and beyond, but not before; and (iv) the classic studies of Hertig and Rock offer the only direct insight into the fate of human embryos from fertilisation under natural conditions. Re-examination of Hertig’s data demonstrates that his estimates for fertilisation rate and early embryo loss are highly imprecise and casts doubt on the validity of his numerical analysis. A recent re-analysis of hCG study data concluded that approximately 40-60% of embryos may be lost between fertilisation and birth, although this will vary substantially between individual women. In conclusion, natural human embryo mortality is lower than often claimed and widely accepted. Estimates for total prenatal mortality of 70% or higher are exaggerated and not supported by the available data.
Keywords: early pregnancy loss, occult pregnancy, embryo mortality, human chorionic gonadotrophin, Hertig, pre-implantation embryo loss
Introduction
Early human embryo mortality is a matter of considerable interest not only to reproductive biologists and fertility doctors, but also to philosophers 1, 2, theologians 3 and lawyers 4. Most especially, becoming pregnant and having children is of overwhelming and personal importance to many women and their families. As with all biological processes, nothing works perfectly all the time 5, and failure to conceive and pregnancy loss are common problems. However, among reputable scientific publications, including medical and reproductive biology text books, scientific reviews and primary research articles, reported mortality estimates are surprisingly varied and include: 30–70% 6, >50% 7 and 75% 8 before and during implantation; >50% 9, 73% 10 and 80% 11 before the 6 th week; 75% before the 8 th week 12; 70% in the first trimester 13; 40–50% in the first 20 weeks 14; and 46% 7, 49% 15, 50% 16– 18, >50% 19, 20, 53% 21, 54% 22, 60% 23, >60% 24, 63% 25, 26, 70% 27– 31, 50–75% 32, 76% 10, 33, 78% 34, 80–85% 35, >85% 36, and 90% 37 total loss from fertilisation to term. The variance in these estimates is striking and the scale of some implausible. 90% intrauterine mortality implies a maximal live birth fecundability of 10%, and only then if all other stages of the reproductive process are 100% efficient. Observed human fecundability is low compared to other animals 21, but at approximately 20–30% 9, 38 it is still higher than implied by such a high embryo mortality rate. Such inconsistent estimates of pregnancy loss are not reassuring, nor do they provide a sound basis for either a quantitative understanding of natural human reproductive biology or an unbiased appraisal of artificial reproductive technologies. These divergent and excessive values therefore invite scrutiny of the evidence that supports them. In this article, I identify and re-evaluate published data that contribute to claims regarding natural human embryo mortality. Using the available data, I attempt to answer the question: “ How many human embryos die between fertilisation and birth under natural conditions?”
A quantitative framework for embryo mortality
A quantitative framework has recently been proposed to facilitate the calculation and comparison of embryo mortalities from fecundability and pregnancy loss data 39. Briefly, the model comprises conditional probabilities ( π) of the following biological processes: (1) reproductive behaviours resulting in sperm-ovum-co-localisation per cycle = π SOC; (2) successful fertilisation given sperm-ovum-co-localisation = π FERT; (3) implantation of a fertilised ovum as indicated by increased levels of human chorionic gonadotrophin (hCG) = π HCG; (4) progression of an implanted embryo to a clinically recognised pregnancy = π CLIN; (5) survival of a clinical pregnancy to live birth = π LB .
Fecundability ( FEC) is the probability of reproductive success per cycle, but may take different values depending on the definition of success. The following four fecundabilities broadly follow Leridon (1977) 38:
1. Total (all fertilisations): FEC TOT = π SOC × π FERT
2. Detectable (implantation): FEC HCG = π SOC × π FERT × π HCG
3. Apparent (clinical): FEC CLIN = π SOC × π FERT × π HCG × π CLIN
4. Effective (live birth): FEC LB = π SOC × π FERT × π HCG × π CLIN × π LB
Hence, the probability that a fertilised egg will perish prior to implantation is [1 - π HCG], and prior to clinical recognition is [1 - ( π HCG × π CLIN)]. In theory, embryonic mortality may be estimated at different stages; however, in practice, this depends on available data. Clinical and live birth fecundabilities are most easily quantified and most frequently reported. Total and detectable fecundabilities are less frequently reported, although of direct relevance.
To aid understanding of the reproductive processes described in this article definitions of key terms have been provided in Box 1, and Figure 1 illustrates the timelines for key biological events associated with fecund and non-fecund cycles.
BOX 1: Glossary of Key Reproductive Terms.
-
1.
Ovum: A female gamete, also known as an egg or oocyte. Ova ( pl) are produced by the ovaries of the woman.
-
2.
Spermatozoon: A male gamete. Sperm (or spermatozoa, pl) are produced in the testes of the man.
-
3.
Ovulation: The release of an ovum from the ovary. In humans, ovulation usually involves the release of a single egg in each menstrual cycle.
-
4.
Fallopian tube: A narrow tubular extension of the uterus, which opens out next to the ovary. It is also called the oviduct. Following ovulation, the ovum passes into the opening of the fallopian tube and travels towards the uterus.
-
5.
Coitus: An act of sexual intercourse between a man and woman, usually resulting in the deposition of sperm within the reproductive tract of the woman.
-
6.
Menstrual cycle: An interval of approximately 28 days, which commences with the onset of menstruation. Ovulation occurs mid-way though a menstrual cycle, approximately 14 days before the onset of the next cycle.
-
7.
Amenorrhoea: The absence of menstruation. A missed menstrual period is often the first observable sign that pregnancy has commenced, although there are many other causes.
-
8.
Fertile period: The time in a woman’s menstrual cycle during which coitus may result in pregnancy. This period probably varies considerably between women. Coitus up to 6 days prior to and 1 day after ovulation may result in pregnancy although the most fertile days are the day of ovulation and the 2 days beforehand 40.
-
9.
Fertilization: The fusion of a spermatozoon and an ovum, which usually takes place in the fallopian tube up to 24 hours after ovulation.
-
10.
Conception: A biologically imprecise term meaning either ‘the coming into existence of a new human being’ or ‘the beginning of a pregnancy’. It is often used synonymously with fertilisation but may also refer to implantation.
-
11.
Embryo: A newly fertilised ovum until the eighth week of development.
-
12.
Zygote: The newly fertilised ovum: a one-cell embryo.
-
13.
Blastocyst: An embryo approximately 5–6 days after fertilisation.
-
14.
Implantation: The biological process that begins when a blastocyst attaches to the lining of the uterus approximately 6–7 days after fertilisation. The embryo subsequently becomes embedded within the uterine lining.
-
15.
Human chorionic gonadotrophin (hCG): A protein produced by the embryo. It signals to the mother that an embryo is present and prevents menstruation and the loss of the embryo. Elevated levels of hCG can be detected in the serum or urine of a woman from around the time of implantation.
-
16.
Fecundability: A measure of reproductive potential. It is the probability of becoming pregnant in a single menstrual cycle. Fecundity is often used to mean the probability of achieving a live birth in a single cycle. A fecund cycle is one in which fertilisation occurs.
-
17.
Pregnancy: The condition of a woman harbouring an embryo, fetus or unborn child. When pregnancy begins is a matter of some confusion 7 ( Figure 1). Pregnancy may be considered to commence with fertilisation and lasts approximately 38 weeks. Clinicians often time the onset of pregnancy from day 1 of the last menstrual cycle, 2 weeks before fertilisation, and refer to subsequent time as a period of gestation. On this account, pregnancy or gestation lasts approximately 40 weeks. Some scientists and legal judgements define pregnancy as beginning with implantation, one week after fertilisation. This definition is of particular utility in the context of IVF treatment where evidence of implantation is the earliest sign that a transferred embryo has developed normally and that fertility treatment has, up to that point, been successful. For some women, the start of a pregnancy may be noted with the first missed menstrual period, approximately 2 weeks after fertilisation, or a positive pregnancy test.
-
18.
Miscarriage: The premature termination of a pregnancy leading to loss of a developing embryo or fetus. Embryo loss may occur before a woman knows she is pregnant. Miscarriage late in pregnancy is often called abortion, with a cut-off of approximately 20 weeks gestation used to distinguish between miscarriage and abortion.
-
19.
Early Pregnancy Loss: This usually refers to the loss of an embryo very early in pregnancy, even before a clinical diagnosis is made, when a woman would not be aware of the pregnancy. Such losses are also called occult, because they are hidden, or biochemical, because they can only be identified by detecting hCG. Pregnancy loss shortly after a clinical diagnosis may also be described as early.
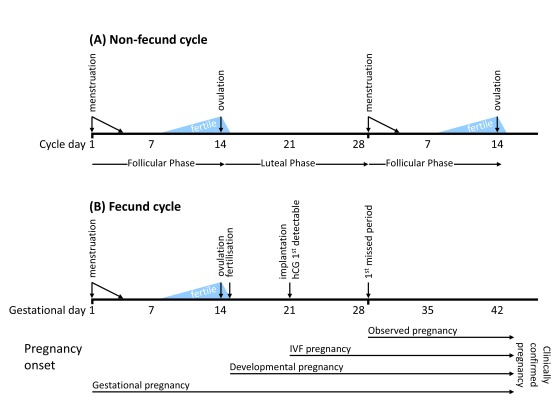
Figure 1. Schematic representation of timelines and key events in ( A) non-fecund and ( B) fecund menstrual cycles.
Menstrual cycle lengths vary considerably and most fall within a range of 20 to 40 days 45. A typical menstrual cycle is usually represented as lasting for 28 days, as shown here. Differences in cycle length are mostly due to variations in the duration of the follicular phase, the time from the onset of menstruation to ovulation. The time from ovulation to the onset of the next cycle, the luteal phase, is more consistently 14 days. Therefore, in the typical 28 day cycle, ovulation occurs midway at around 14 days. The fertile period (shown in light blue) is the time during which coitus may result in a pregnancy. The probability of pregnancy is highest when coitus occurs in the two days leading up to ovulation 40. In a normal fecund cycle, fertilisation occurs within hours of ovulation in the fallopian tube, after which point an embryo is present and development begins. Embryonic development may fail at any stage from fertilisation through to birth. 6–7 days after fertilisation, the embryo begins to implant in the uterine wall at which stage human chorionic gonadotrophin (hCG) produced by the embryo becomes detectable in urine or serum samples. The onset and duration of pregnancy may be defined in various ways: gestational pregnancy (typically used in clinical practice) is timed from the first day of the last menstrual period; developmental pregnancy begins with fertilisation; in an IVF treatment cycle, although an embryo is present in the uterus immediately following embryo transfer, pregnancy is not considered to be established until there is evidence of implantation, usually provided by elevated hCG levels. The earliest point at which a woman could observe that she is pregnant is approximately 14 days after ovulation/fertilisation with the first missed period. The stage at which pregnancies are clinically confirmed depends on study design and clinical practice, and may be at gestational day 28 (i.e., first missed menstrual period, Zinaman (1996) 46, French & Bierman (1962) 47), gestational day 42 (Wang (2003) 48), or following a positive pregnancy test (Wilcox (1988) 49) or satisfactory ultrasound scan.
What the data say
Publications containing data relevant to early human embryo mortality were identified primarily by manually tracing citations found in articles, reviews and textbooks. A PubMed search ("early pregnancy loss" [All Fields]) identified some, but not all relevant studies. Certain studies were not conducted to address the specific question, and others are in books or publications that are not adequately indexed. If not entirely complete, nevertheless the data presented form a substantial proportion of relevant, available scientific data on natural early human embryo mortality.
Studies that contribute analysis and data relevant to the quantification of natural human embryo mortality fall into the following four categories and will be considered in turn.
1. A speculative hypothesis published in The Lancet.
2. Life tables of intra-uterine mortality.
3. Studies of early pregnancy by biochemical detection of hCG.
4. Anatomical studies of Dr Arthur Hertig and Dr John Rock.
1. Where have all the conceptions gone?
In 1975, a short hypothesis published in The Lancet entitled “ Where Have All The Conceptions Gone?” concluded that 78% of all conceptions were lost before birth 34. It has been widely cited by both scientists 9, 25, 27, 28, 41 and non-scientists 42, 43 alike. Conceptions among married women aged 20–29 in England and Wales in 1971 were estimated and compared to infants born in the same period. In this analysis ( Table 1) there are reliable values, e.g., census data, and simple arithmetical calculations. However, speculative values are necessary to perform the calculations. Three are biological: (1) fertilisation rate following unprotected coitus during the fertile period was estimated as 50% and supported by reference to Hertig 44 (although his estimate was 84% 5); (2) the length of a menstrual cycle (28 days); and (3) the duration of the fertile period (2 days). These latter values are plausible, but also variable ( Figure 1). No justification is provided for three behavioural variables: (1) coital frequency estimated at twice per week; (2) proportion of unprotected coital acts estimated at 25%; and (3) either a random or regular distribution of coital acts during menstrual cycles such that 1/14 of all coital acts fall within a fertile period.
The validity of Roberts & Lowe’s conclusion depends largely on the accuracy and precision of these speculative values. The following two simple analyses illustrate the sensitivity of their conclusion on the speculative values.
1. When four of the speculative values are reduced by 25% (e.g., coital frequency reduced to 1.5/week) and cycle length increased by 10% (from 28 days to 31 days 38), the estimate for embryo loss drops to 22%. The opposite operation (e.g., coital frequency increased to 2.5/week) results in an estimate of 92% ( Table 1). Embryo loss of 22% is barely sufficient to account for observed clinical losses, and 92% indicates a maximum FEC LB of 8%. Neither scenario is biologically plausible.
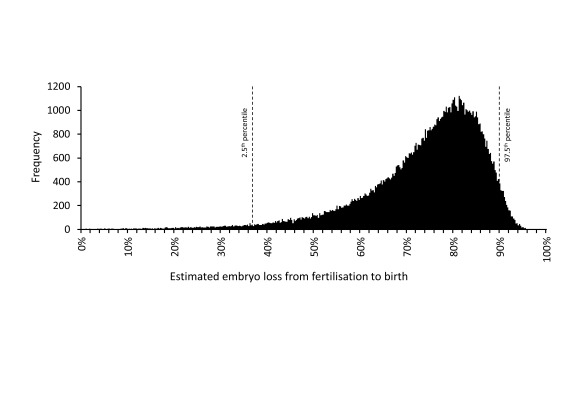
2. A non-zero variance was applied to each speculative value reflecting their uncertain nature. Using the random number generator in Microsoft ® Excel (Office 2010) simulated values were obtained by random sampling from normal distributions with means equal to Roberts & Lowe’s speculative values with coefficients of variation equal to 20%. For simplicity, it was assumed that there was no covariance between the different speculative values. Table 1 shows the expected range within which 95% of these simulated values fall (e.g., coital frequency is 1.2–2.8/week). For each simulated record, a new estimate of embryo loss was calculated, and from 10,000 of these, the mean, median and 2.5 th and 97.5 th percentiles of embryo loss were determined. This step was repeated 1,000 times: the mean value of the simulated means was 73.3% and of the simulated medians was 76.5%. The mean values of the 2.5 th and 97.5 th percentile boundaries for embryo loss were 37% and 90% ( Table 1). Separately, the same simulation was performed using NONMEM 7.3.0 ® (Icon PLC, Dublin, Eire) to generate 100,000 data records which are represented in Figure 2. The code and simulated data values are in Dataset 1.
Figure 2. Distribution of embryo loss estimates from fertilisation to birth derived using a modified version of the model of Roberts & Lowe 34.
Embryo loss values were calculated using alternative speculative values (see text and Table 1) obtained by randomly sampling from normal distributions with means equal to the Roberts & Lowe values and a coefficient of variation of 20%. 100,000 simulated embryo loss values were obtained. Frequencies within a bin size of 0.25% are shown. The 2.5 th and 97.5 th percentiles are indicated. The simulation was performed using NONMEM 7.3.0® (Icon PLC, Dublin, Eire). Simulated values are in Dataset 1.
Table 1. Numerical estimates of conceptions and their loss in married women aged 20–29 in England and Wales in 1971.
The table replicates the values and calculations of Roberts & Lowe 34 with more explanatory detail. In addition, it illustrates how introducing variance into speculative estimates influences the final calculated value of embryo loss. *Data type indicates whether the numerical value is reliable (e.g., derived from census data), the result of a simple arithmetical calculation, or speculative (shown in italics). §Values are the 2.5 th and 97.5 th percentile boundaries, assuming a normal distribution for the variables centred on Roberts & Lowe’s values with a coefficient of variation of 20%. †Speculative values were adjusted either up or down by 25% compared to Roberts & Lowe’s values. Values for ‘Length of menstrual cycle’ were adjusted by 10%. ‡The mean values of the 2.5 th and 97.5 th percentile boundaries from 1,000 simulations, each containing 10,000 separate estimates for embryo loss. The derivation of these values is described in the text. Briefly, each separate estimate of embryo loss was calculated using variable speculative values that were obtained by random sampling from a normal distribution with a mean equal to the Roberts & Lowe value and a coefficient of variation of 20%. The mean value of the mean percentage loss was 73.3% and of the median was 76.5%. ¥The most frequent duration of a menstrual cycle is 28 days but there is substantial variability and the mean length is generally 30–31 days 38.
| Description of data | Data type* | Roberts &
Lowe values |
Low estimate
values † |
High estimate
values † |
95% data range
(CV = 20%) § |
|---|---|---|---|---|---|
| Married women aged 20–29
in 1971 |
Reliable value | 2,437,000 | 2,437,000 | 2,437,000 | - |
|
Frequency of coitus per
married woman per week |
Speculative
value |
2 | 1.5 | 2.5 | [1.2, 2.8] |
| Weeks per year | Reliable value | 52 | 52 | 52 | - |
| Acts of coitus among
married women per year |
Calculation | 253,448,000 | 190,086,000 | 316,810,000 | - |
|
Percentage of acts of coitus
that are unprotected |
Speculative
value |
25% | 19% | 31% | [15%, 35%] |
| Acts of unprotected coitus
per year |
Calculation | 63,362,000 | 35,641,125 | 99,003,125 | - |
|
Length of menstrual cycle
(days) |
Speculative
value |
28 | 31 ¥ | 25 | [17, 39] |
|
Length of fertile period in
each cycle (days) |
Speculative
value |
2 | 1.5 | 2.5 | [1.2, 2.8] |
| Acts of unprotected coitus
during fertile period per year |
Calculation | 4,525,857 | 1,735,769 | 9,821,739 | |
| Probability of fertilisation |
Speculative
value |
50% | 38% | 63% | [30%, 70%] |
| Total fertilised ova per year | Calculation | 2,262,929 | 650,913 | 6,138,587 | - |
| Number of infants born (live
and still) in 1971 |
Reliable value | 505,000 | 505,000 | 505,000 | - |
| Total number of lost embryos
in 1971 |
Calculation | 1,757,929 | 145,913 | 5,633,587 | - |
|
Percentage of embryos
lost before live birth |
Calculation | 78% | 22% | 92% | [37%, 90%] ‡ |
See README.docx for a description of the file.
Copyright: © 2017 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The sole purpose of these simple sensitivity analyses is to illustrate that modest adjustments to Roberts & Lowe’s original speculative values can result in any biologically plausible estimate for embryo loss. Whilst their analysis is useful for highlighting factors that influence observed fecundity, the output from the calculation remains substantially dependent on the subjectively selected input. Consequently, their analysis has no practical quantitative value.
Other sources of bias in their model include the failure to account for intentionally terminated pregnancies and the reduced fecundability of already pregnant women and nursing mothers. Despite this, it was described as “ persuasive” 50 and it has been claimed that “ it is still difficult to better the original calculations of Roberts and Lowe (1975)” 27. By contrast, others have noted that “ their calculations can be criticized” 9 and are “ tenuous” 51. Considering its quantitative limitations, it has been cited surprisingly often 13, 28, 52, 53.
2. Life tables of intrauterine mortality
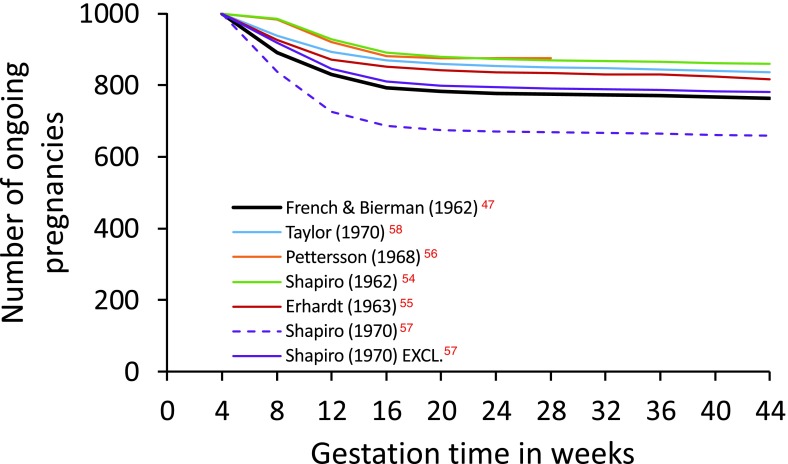
Constructing a life table of intrauterine mortality is challenging since embryonic death may occur even before the presence of an embryo is recognised. Nevertheless, in 1977, the distinguished demographer Henri Leridon published an impressive critique and analysis of pregnancy loss data, and a complete life table of intrauterine mortality 26. Leridon highlighted the consequences of inappropriate analysis and the quantitative biases produced by alternative numerical methods. Overall, he discussed sixteen studies, and provided detailed commentary on six published between 1962 and 1970 47, 54– 58. These data are summarised in Figure 3 and suggest that 12–24% embryos alive at 4 weeks’ gestation (i.e., approx. 2 weeks’ post-fertilisation, see Figure 1) will perish before birth.
Figure 3. Graphical representation of the fate of 1,000 pregnancies in progress at 4 weeks’ gestation (2 weeks’ post-fertilisation).
The figure is generated using values in Table 4.3 of Leridon (1977) 26 and are derived from six different studies (see text). The Kauai Pregnancy Study data published by French & Bierman (1962) 47 are shown in thick black. Data from Shapiro (1970) 57 were analysed either with all pregnancies included (ALL) or with those pregnancies excluded that aborted within one week of study entry (EXCL.). The greater loss observed with ALL may be due to a correlation between study entry and abortion risk. Based on these data, the risk of losing a pregnancy ongoing at 4 weeks’ gestation ranges from 12.5% to 23.7% (excluding Shapiro (1970) ALL). Values are in Dataset 2.
See README.docx for a description of the file.
Copyright: © 2017 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Leridon described the Kauai Pregnancy Study published by French & Bierman (1962) 47 in particular detail. In this study, an attempt was made to identify every pregnancy on Kauai from 1953–56. Women were encouraged to enrol as soon as they missed a period. Pregnancy loss may therefore have been overestimated, since not all amenorrhoea is caused by conception, although other studies that relied upon medically-identified pregnancies probably underestimated pregnancy loss by not capturing all cases 16. Whatever the truth, it is clear that, among the studies reviewed by Leridon, the Kauai Pregnancy Study revealed the highest levels of pregnancy loss ( Figure 3).
All recorded pregnancies in the Kauai study were categorised by date of enrolment in four week intervals, beginning with 4–7 weeks’ gestation. This time-staggered approach enabled risk of miscarriage to be associated with stage of gestation. However, despite considerable efforts, only 19% of the 3,197 recorded Kauai pregnancies were enrolled between 4–7 weeks’ gestation, thereby reducing the precision of pregnancy loss estimates for this earliest of time intervals. Although pregnancies were grouped in four week periods, Leridon suggested that early mortality may change week by week, resulting in underestimation of pregnancy loss. He re-allocated the 592 study entries and 32 pregnancy losses for weeks 4–7 ( Table 2) generating an overall probability of pregnancy loss during this period of 15.0%, higher than 10.8% originally reported 47. Leridon’s own description of this interpolation as “ risky” can be illustrated by adjusting his re-allocation 26. Transferring just two of the pregnancy losses out of or into the first week results in estimates of the 4–7 week pregnancy loss of 10.9% and 19.1% respectively ( Table 2). The validity of adjusting Leridon’s re-allocation may be questioned. However, pregnancy loss in week 4–5 of the Kauai Study would manifest as a menstrual period delayed by up to one week. This is far from being a robust pregnancy diagnosis and in a different study 57, exclusion of pregnancy losses reported within one week of study entry resulted in substantially different loss probabilities ( Figure 3) suggesting a confounding correlation between entry and loss 26. Nevertheless, the re-allocation does reinforce a concern highlighted by Leridon, namely the uncertainty that affects the first probability. Clearly, these estimates of early loss should be treated with caution.
Table 2. A speculative numerical re-allocation of entries and pregnancy losses during weeks 4–7 in the Kauai Pregnancy Study (KPS) 47.
Minor differences in the re-allocation of the earliest pregnancy losses have a substantial effect on the overall measure of pregnancy loss for that period. (Adapted from Table 4.2 in Leridon 26.)
| Time period
of gestation |
New entries into study
in each time period |
Actual pregnancy losses
in each time period |
% pregnancy loss in
each time period |
Surviving pregnancies
in each time period |
||||
|---|---|---|---|---|---|---|---|---|
| Leridon’s re-
allocation |
KPS | Leridon’s re-
allocation & [variants] |
KPS | Leridon’s re-
allocation & [variants] |
KPS | Leridon’s re-
allocation & [variants] |
KPS | |
| 4–5 | 80 | 592 | 2 [0, 4] | 32 | 5.0 [0.0, 10.0] | 10.8 | 100 [100, 100] | 100 |
| 5–6 | 120 | 6 [6, 6] | 4.3 [4.3, 4.4] | 95 [100, 90] | ||||
| 6–7 | 180 | 10 [11, 9] | 3.5 [3.9, 3.2] | 91 [96, 86] | ||||
| 7–8 | 212 | 14 [15, 13] | 3.0 [3.2, 2.8] | 88 [92, 83] | ||||
| 4–8 | 85 [89, 81] | 89.2 | ||||||
| % loss | 15.0 [10.9, 19.1] | 10.8 | ||||||
A more fundamental problem is that these data offer no insight into the fate of embryos prior to the earliest possible point of clinical pregnancy detection. Leridon completed his life table with values from Hertig’s 1967 analysis 5. He concluded that among 100 ova exposed to the risk of fertilisation, 16 are not fertilised, 15 die in week one (between fertilisation and implantation), and 27 die in week two (between implantation and the time of the first missed period). After two weeks his life table follows the Kauai probabilities closely ending with 31 live births. Leridon’s table therefore indicates an embryo mortality of 50% (42/84) within the first two weeks after fertilisation and a total mortality of 63% (53/84) from fertilisation to birth.
Leridon’s account of intrauterine mortality has been widely cited. However, its accuracy depends entirely on the quality and interpretation of the data from Hertig 5 and French & Bierman 47. French & Bierman’s approach probably resulted in an overestimate of total pregnancy loss and is certainly imprecise in its estimate of embryo loss in the four weeks following the first missed menstrual period. The reliability of Hertig’s estimates of embryo loss in the two weeks following fertilisation is considered below.
3. Biochemical detection of pregnancy using hCG
Quantification of pregnancy loss requires pregnancy diagnosis. The earliest outward sign of pregnancy is a missed menstrual period, approximately 2 weeks after fertilisation, although amenorrhoea in women of reproductive age is not exclusively associated with fertilisation 59, 60. Several potentially diagnostic pregnancy-associated proteins have been identified 61 of which only one, Early Pregnancy Factor (EPF) 62, has been claimed to be produced by embryos within one day of fertilisation. However, there is doubt about the utility of EPF for diagnosing early pregnancy 63 and little has been published on it in the past five years.
Modern pregnancy tests detect human chorionic gonadotrophin (hCG), a highly glycosylated 37 kDa protein hormone produced by embryonic trophoblast cells 64. Elevation of hCG around 6–7 days after ovulation is associated with embryo implantation 27, 28, 65 ( Figure 1). Early assays for the detection of hCG were probably confounded by antibody cross-reactivity with luteinizing hormone 66 but modern tests are more specific and a positive result is a reliable indicator of early pregnancy. Highly sensitive assays have revealed low levels of hCG in non-pregnant women and healthy men 67; hence, quantitative criteria and appropriate design are required to distinguish between non-pregnant women and those harbouring early embryos 65, 68, 69.
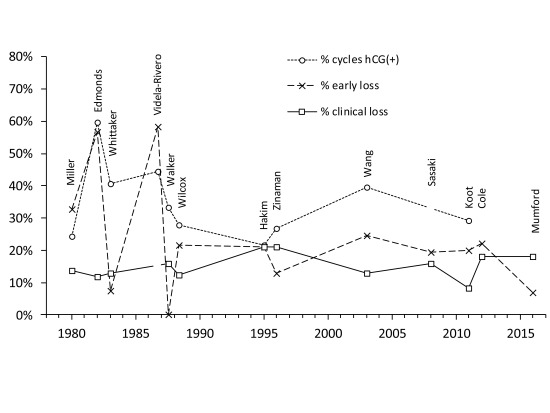
Figure 4 and Table 3 summarise findings from thirteen studies that used hCG to identify so-called early, occult or biochemical pregnancy loss, i.e., pregnancy loss between the initiation of implantation and clinical recognition 46, 48, 49, 70– 79. (Ellish et al. (1996) 69 is not included since the hCG assay was positive for only 72.5% of clinical pregnancies. By contrast, among the thirteen studies in Table 3 only one clinically-recognised pregnancy was reported undetected by hCG testing 70. Nevertheless, their estimates of early pregnancy loss (17.4%) and clinical loss (13.7%) are comparable to these other studies.) Each study measured urinary hCG levels except two, which measured hCG in serum 72, 73. Notwithstanding design and subject differences, estimates for clinical pregnancy loss, ranging from 8.3% – 21.2% ( Figure 4), are similar to previous estimates ( Figure 3). Estimates for early/occult pregnancy loss ranged from 0% to 58.3% in studies 70– 74 prior to Wilcox (1988) 49. This high variance was probably due to reduced specificity and sensitivity of the hCG assays and sub-optimal study design 16, 61, 80– 83. Studies from Wilcox (1988) 49 onwards have produced more consistent data indicating early/occult pregnancy loss of approximately 20% ( Figure 4). In the largest studies 48, 79 pregnancies were clinically recognised only if they lasted ≥6 weeks after the onset of the last menstrual period; hence, early pregnancy losses in these studies included those lost up to approximately two weeks after a missed menstrual period. Definition of clinical pregnancies can influence comparison of study results 39, 82. For example, Wilcox originally reported 43 (later 44 85) pre-clinical and 19 clinical losses from 198 detected pregnancies 49, giving 21.7% early/occult and 12.3% clinical loss rates. In later reports, 4 cases were re-allocated resulting in 48 early and 15 clinical losses (i.e., 24.1% early/occult and 9.9% clinical loss rates) 40, 86– 88. Variable definitions can generate confusion, although in this case the overall picture is not greatly affected. Based on the eight most recent studies, beginning with Wilcox (1988), pregnancy loss from first detection of hCG through to live birth is approximately one third ( Table 3). This is consistent with another recent study which found that 98 out of 301 (32.6%) singleton pregnancies diagnosed by an early positive hCG test and followed-up to either birth or miscarriage were lost 89.
Figure 4. Summary of findings from thirteen studies that used hCG detection to diagnose early pregnancy.
Data are arranged by publication date and the first author of the study is shown. Three datasets are shown: (i) the percentage of at risk menstrual cycles that were hCG positive; (ii) the percentage of hCG positive cycles that did not manifest as clinical pregnancies = early pregnancy loss; and (iii) the percentage of clinical pregnancies lost prior to 12 or 28 weeks or live birth (definitions vary between studies). A clinical pregnancy may be manifest by a missed period although criteria vary between studies. Videla-Rivero et al. 73, Sasaki et al. 76, Cole 78 and Mumford et al. 79 do not report sufficient data to calculate all three values. Values are in Dataset 3.
Table 3. Summary data from thirteen studies using hCG detection to diagnose pregnancy and identify early pregnancy loss.
Raw FEC HCG is the ratio of hCG pregnancies detected and the number of cycles monitored in each study. Where available, mean (SD) ages of the participating women are taken directly from the published study. In some cases mean and SD (indicated by *) or SD (indicated by †) were estimated based on published demographic characteristics. §These data relate to the whole study cohort (n=124) which included known sub-fertile women, and not just to the 74 apparently fertile women. ‡Mean value from Wilcox et al. (2001) 84. ¶Some studies only provide data up to late pregnancy (e.g., up to 28 weeks) rather than to term. ND = no data. ¤Wilcox subsequently reported an additional hCG pregnancy which had not been detected and reported in the 1988 paper, making a total of 199 hCG pregnancies and 44 pre-clinical losses in the study group 85. #Mumford reported data from aspirin- and placebo-treated subjects who had at least one prior miscarriage. Summary data from both treatment groups are included as there was no effect of aspirin 79.
| First
author |
Year | Number
of women |
Age mean
(SD) [range] |
Number
of cycles |
hCG
pregnancies detected |
Raw
FEC HCG |
Clinical
pregnancies detected |
% survival
from hCG to clinical detection |
% loss
from hCG detection to live birth ¶ |
|---|---|---|---|---|---|---|---|---|---|
| Miller | 1980 | 197 | 27 (4)* | 623 | 152 | 24.4% | 102 | 67.1% | 42.4% |
| Edmonds | 1982 | 82 | 27 (4)* | 198 | 118 | 59.6% | 51 | 43.2% | 61.9% |
| Whittaker | 1983 | 91 | 30 (3.7) † | 226 | 92 | 40.7% | 85 | 92.4% | 19.6% |
| Videla-
Rivero |
1987 | 27 | ND | 27 | 12 | 44.4% | 5 | 41.7% | ND |
| Walker | 1988 | 38 | 27.4 [22–38] | 75 | 25 | 33.3% | 25 | 100% | 16.0% |
| Wilcox | 1988 | 221 | 30 ‡ (4)* | 707 | 198 ¤ | 28.0% | 155 | 78.3% | 31.3% |
| Hakim | 1995 | 74 | 31 (3)* § | 305 | 66 | 21.6% | 52 | 78.8% | 37.9% |
| Zinaman | 1996 | 200 | 30.6 (3.3) | 432 | 116 | 26.9% | 101 | 87.1% | 31.3% |
| Wang | 2003 | 518 | 24.9 (1.7) | 1,561 | 618 | 39.6% | 466 | 75.4% | 35.7% |
| Sasaki | 2008 | 110 | [21–36] | ND | 62 | ND | 50 | 80.6% | 32.3% |
| Koot | 2011 | 46 | 28.7 (3.3) | 103 | 30 | 29.1% | 24 | 80.0% | 26.7% |
| Cole | 2012 | 168 | 28.8 (4.4) | ND | 127 | ND | 99 | 78.0% | 36.2% |
| Mumford | 2016 | 1088 # | 28.7 (4.8) | ND | 785 | ND | 730 | 93.0% | 23.9% |
See README.docx for a description of the file.
Copyright: © 2017 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The much cited Wilcox (1988) study 49 is the earliest of several large well-designed studies that made use of a specific and sensitive hCG assay and led to numerous further publications 40, 84– 87, 90– 92. Two other studies (Zinaman (1996) 46 and Wang (2003) 48) were similar in purpose, design and execution. These studies provide some of the best available data to calculate pregnancy loss between implantation and birth 39. In each study, women intending to become pregnant and with no known fertility problems were recruited and hCG levels monitored cycle by cycle in daily urine samples until they became pregnant. Most women were followed through to late pregnancy or birth. Although these studies provide evidence regarding the outcome of both clinical and hCG pregnancies, determining the fate of embryos prior to implantation is more difficult. To relate the study results to pre-implantation embryo loss, it is necessary to determine fecundability. In each study FEC CLIN declined in successive cycles as the proportion of sub-fertile women increased. Hence, reported raw FEC HCG values of 30% 46 and 40% 48, and FEC CLIN values of 25% 49 and 30% 48 are biased underestimates of the fecundability of normal fertile women. A recent re-analysis of these data provides statistical evidence for discrete fertile and sub-fertile sub-cohorts within the study populations 39. The proportions of sub-fertile women (mean [95% CI]) were estimated as 28.1% [20.6, 36.9] (Wilcox); 22.8% [12.9, 37.2] (Zinaman); and 6.0% [2.8, 12.3] (Wang). For normally fertile women, FEC HCG was, respectively: 43.2% [35.6, 51.1]; 38.1% [32.7, 43.7]; and 46.2% [42.8, 49.6]. FEC CLIN was: 33.9% [29.4, 38.6]; 33.3% [27.6, 39.6]; and 34.9% [33.0, 36.8]. There was no apparent difference in π CLIN between fertile and sub-fertile sub-cohorts, which was estimated as: 78.3% [69.2, 85.3]; 87.5% [76.0, 93.9]; and 75.4% [71.5, 79.0] 39.
Why do a proportion of menstrual cycles in women attempting to conceive fail to show any increase in hCG? Since FEC HCG = π SOC × π FERT × π HCG , there can be various causes for this failure including mistimed coitus, anovulation, failure of fertilisation or pre-implantation embryo death. Although FEC HCG puts limits on the extent of pre-implantation embryo loss, uncertainty in the estimates of π SOC, π FERT and π HCG translates into uncertainty in estimates of pre-implantation embryo mortality. In the Wang study, for normally fertile women, FEC HCG = 46.2%; hence, the absolute maximum value for pre-implantation embryo loss must be 53.8%, although only if π SOC = π FERT = 1, conditions both extreme and unlikely 39. Studies of the relationship between coital frequency and conception indicate that fecundability is greater with daily compared to alternate day intercourse 39, 93, 94. Hence, when coital frequency is less than once per day a proportion of reproductive failure will be due to mistimed coitus, i.e., π SOC < 1. In the Wilcox study, coitus occurred on only 40% of the six pre-ovulatory days 39, 40, and in the Zinaman study participants were advised that alternate day intercourse was optimal 46. Based on the difference in fecundability between daily and alternate day intercourse as modelled by Schwartz 94, a value of π SOC = 0.80 was used to calculate pre-implantation embryo mortality 39. However, this is a speculative estimate, and in reality the value may be higher, or lower.
A further critical missing piece of the equation is knowledge of the efficiencies of fertilisation and implantation under normal, natural, propitious circumstances. Assuming that either of these processes may be up to 90% efficient, and based on data from the three hCG studies 46, 48, 49, a plausible range for pre-implantation embryo loss in normally fertile women is 10–40% and for loss from fertilisation to birth, 40–60% 39. Even with these wide ranges of mathematically possible outcomes, it is clear that estimates for total embryonic loss of 90% 37, 85% 36, 83% 2, 80–85% 11, 35, 78% 34, 76% 10, 33 and 70% 27– 31 are excessive.
In 1990, Charles Boklage concluded that “ at least 73% of natural single conceptions have no real chance of surviving 6 weeks of gestation” 10, 95. Live birth fecundability was estimated as “ not over 15%”, substantially lower than Leridon’s 31%. Despite this discrepancy, Boklage’s conclusions were derived from a review of data including several hCG studies 49, 65, 70– 73 and Leridon’s analysis 26. He derived a model describing the survival probability of human embryos comprising the sum of two exponential functions:
P t(pregnancy survival) = 0.73 e -0.155 t + 0.27 e -0.00042 t
in which t is the time in days post-fertilization. This is the source of the 73% in the conclusion.
There are, however, serious problems with this analysis. Firstly, data presented as embryo survival probabilities at different times post-fertilization 49, 65, 70, 71, 73 are fecundabilities, i.e., successes per cycle, not per fertilised embryo. Secondly, for reasons that are unclear, data from Whittaker 72 and Leridon 26 were excluded from the modelling analysis and the data from an earlier Wilcox report 65 were included twice since this preliminary data had been incorporated into the later report 49. Thirdly, the modelled data were normalised to a survival probability of 0.287 at 21 days post-fertilization. This value was derived from data published by Barrett & Marshall (1969) on the relationship between coital frequency and conception 93. Barrett & Marshall had concluded that coitus during a single day alone, 2 days before ovulation, resulted in a conception probability of 0.30. Boklage’s value of 0.287 is his calculated equivalent. However, conception in this study was “ identified by the absence of menstruation, after ovulation” 93. Hence, 0.30 (and similarly, 0.287) is a clinical fecundability and not a measure of embryo survival. Furthermore, 0.30 is a non-maximal fecundability, since it was an estimate based on coitus on a single day (2 days before ovulation) within the cycle. Barrett & Marshall clearly report that as coital frequency increased so did the fecundability, up to a maximum of 0.68 associated with daily coitus 93.
Boklage’s analysis can only make biological sense if it is assumed that every cycle in the Barrett & Marshall study resulted in fertilisation. Under these circumstances, failure to detect conception in 71.3% (1 – 0.287) of cycles would be due entirely to embryo mortality. However, this is highly implausible and explicitly contradicted by the higher estimate of fecundability reported 93. Boklage’s implicit assumption also contradicts his further conclusion that “ only 60–70% of all oocytes are successfully fertilized given optimum timing of natural insemination” 10. The vertical normalisation of the hCG study data to a value of 0.287 at 21 days is the principal determinant of the parameters that define the two exponential model. Any change in this value would commensurately alter the balance between the two implied sub-populations of embryos. Since it is evident that the value of 0.287 is neither an embryo survival rate nor even a maximal fecundability, it follows that quantitative conclusions from this analysis in relation to the survival of naturally conceived human embryos are of doubtful validity.
However, Boklage was right about two things. Firstly, the difficulty of calculating pre-clinical losses: as he put it, “ In the place of the necessary numbers for the first few weeks of pregnancy we find editorially acceptable estimates which, while perhaps not far wrong, are difficult to defend with any precision”. Secondly, the source of some of the only directly relevant data (even though he excluded it from his modelling analysis), namely, “ Hertig’s sample is, and will probably remain, unique”.
4. The anatomical studies of Dr Arthur Hertig
At the start of the 1930s, no-one had ever seen a newly fertilised human embryo. It was barely 60 years since Oscar Hertwig had first observed fertilisation in sea urchins 96, and just 40 years before the birth in 1978 of Louise Brown, the first test tube baby 97, 98. In Boston, Dr Arthur Hertig and Dr John Rock’s search for early human embryos generated an irreplaceable collection, and set an influential benchmark for the scale of early human embryo mortality.
The so-called “Boston Egg Hunt” began in 1938 99. Hertig and Rock recruited 210 married women of proven fertility who presented for gynaecological surgery 44. (In most of their publications, the number is given as 210 5, 100, 101 although 211 subjects are mentioned elsewhere 44.) Of these, 107 were considered optimal for finding an embryo because they apparently: (i) demonstrated ovulation; (ii) had at least one recorded coital date within 24 hours before or after the estimated time of ovulation; (iii) lacked pathologic conditions that would interfere with conception. Hertig examined the excised uteri and fallopian tubes, and over fifteen years found 34 human embryos aged up to 17 days 5, 44, 100– 107. Of these, 24 were normal and 10 abnormal 5, 100. (There is some confusion over this: in three publications 44, 101, 107, 21 embryos are described as normal and 13 as abnormal. It appears that the three alternatively described embryos (C-8299; C-8000; C-8290) were originally defined as abnormal based on their position or depth of implantation 44.) Table 4 provides information about the 34 embryos found in these 107 women. Although the study was primarily intended to find and describe early human embryos, Hertig subsequently used the data to derive estimates of reproductive efficiency including early embryo wastage 5, 100.
Table 4. Summary of the characteristics of Hertig’s 34 embryos (values are taken from Figure 4 in Hertig et al. (1959)) 100.
The embryos were collected from 107 out of 210 women. *In Hertig’s figure, day 28 of the ovulatory cycle is identified with day 1 of the next cycle and is the day of the presumed missed period in cases where pregnancy had commenced. The 36 cases that provide the evidential foundation for his numerical analysis are shown in bold.
| Day of
cycle |
Biological description/
stage |
Approx. age
of embryos (days) |
Number
of cases |
Embryos
found |
Normal
embryos |
Abnormal
embryos |
Detection
rate (%) |
|---|---|---|---|---|---|---|---|
| 14 | Ovulation ± fertilisation | 0 | 0 | 0 | 0 | 0 | |
| 16–17 | Embryo suspended in
fallopian tube |
2–3 | 9 | 1 | 1 | 0 | 11.1% |
| 18–19 | Embryo suspended in
uterus |
4–5 | 15 | 7 | 3 | 4 | 46.7% |
| 20–24 | Implantation | 6–10 | 47 | 5 | 5 | 0 | 10.6% |
| 25–3 |
First missed period on
day 28/1* |
11–16 | 36 | 21 | 15 | 6 | 58.3% |
| Total | 107 | 34 | 24 | 10 | 31.8% |
Hertig’s analysis 5, 100 relies heavily on the 15 normal and 6 abnormal implanted embryos found in 36 women from cycle day 25 onwards. He assumed the 6 abnormal embryos would perish around the time of the first period concluding that fertility (% pregnant) at this stage = 42% (15/36). Of the 8 pre-implantation embryos identified (7 in the uterus and 1 in the fallopian tubes), 4 were abnormal. Hertig assumed the 4 normal embryos would implant successfully but that some of the abnormal ones would not, such that the proportion of normal embryos would increase from 50% (4/8) before implantation to 71% (15/21) after implantation as observed. Hence, among the 36 post-cycle day 25 cases, in addition to the 15 normal embryos, there must have been 15 abnormal pre-implantation embryos of which 60% (9/15) failed to implant and were not observed, and 40% (6/15) did implant and were observed, although these 6 would have perished shortly afterwards. This left 6/36 eggs that must have been unfertilised. The ratio of ‘unfertilised’: ‘fertilised abnormal’: ‘fertilised normal’ was therefore 6:15:15, matching the 16% infertility (no fertilisation), 42% sterility (post-fertilisation death) and 42% fertility (reproductive success) reported in Figure 9 of Hertig’s 1967 article, “ The Overall Problem in Man” 5. This is the source of Hertig’s 84% fertilisation rate and 50% embryo loss before and during implantation, and is reproduced in Leridon’s life table 26 as 84/100 eggs surviving at time zero (ovulation and fertilisation) and 42 surviving to 2 weeks (time of first missed period).
Hertig provides almost the entire body of evidence used to quantify natural human embryo loss in the first week post-fertilisation. Most claims regarding early human embryo mortality find their source here. Before considering how reliable the figures are, it is worth repeating Hertig’s own caveat, namely, the lack of data on the efficiency of natural fertilisation 5. All estimates of embryo mortality from fertilisation onwards are subject to commensurate inaccuracy in the absence of reliable fertilisation probabilities (i.e., π FERT), which are “ surprisingly difficult to estimate” 21.
There are several problems with Hertig’s analysis. As noted by others, the observations are cross-sectional, but the inferences are longitudinal 108. Hertig detected 21 embryos from 36 cases (58.3%) from cycle day 25 onwards. If this detection rate were representative, then on average, prior to day 25, the detection rate should either be the same or higher; however, they are all lower, and substantially so ( Table 4). Hertig suggested that this was due to the technical difficulty of finding newly fertilised embryos. However, the detection rate for cycle days 18–19 was good (46.7%) and embryos one or two days younger would not have been much smaller, at which stage the detection rate was poor (11.1%). An alternative explanation for this discrepancy might simply be random variation. Furthermore, from cycle day 25 onwards, embryos would probably have produced hCG and therefore FEC HCG would have been at least 58%. This is approximately double the equivalent values observed in more recent and robust hCG studies ( Table 3) further suggesting that this subset of the data is not representative.
Despite having proven fertility, these women presented for gynaecological surgery which, according to Hertig, was “medically essential” 99. This suggests that the women may have had sub-optimal reproductive function, although the effect of this on the quantitative outcome of the study is difficult to gauge. Furthermore, Hertig’s reproductively ‘optimal’ coital pattern does not include 2 days pre-ovulation and does include one day post-ovulation, conditions which are known not to maximise fertilisation 39, 40, 93, 94, 109. Hence, detection rates before cycle day 25 may be more representative than those after. Given the numerical discrepancies, they cannot both be.
Hertig does not provide error estimates with his conclusions. In order to estimate the precision of his derived proportions, a bootstrap analysis was performed as follows: Hertig’s 107 optimal cases were categorised according to stage of cycle (Category 1 = cycle days 16–19 (n=24); Category 2 = cycle days 20–24 (n=47); Category 3 = cycle days ≥25 (n=36)), and presence and type of embryos (Category 0 = no embryo (n=73); Category 1 = normal embryo (n=24); Category 3 = abnormal embryo (n=10)). Five hundred pseudo-datasets each containing 107 cases were generated using a balanced random re-sampling method using Microsoft Excel ®. The original and pseudo datasets are in Dataset 4.
See README.docx for a description of the files.
Copyright: © 2017 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Hertig’s numerical calculations, as detailed above, were repeated for each pseudo-dataset thereby generating 500 estimates for each parameter, from which were derived median values and [95% CIs] using the percentile method 110: fertility = 42% [26%, 59%]; sterility = 42% [5%, 182%]; infertility = 16% [-127%, 61%]; pre-implantation embryo survival probability = 69% [27%, 128%]; post-implantation to week two survival probability = 71% [50%, 91%]; detection rate for cycle day 25 onwards = 58% [41%, 74%]. Median values matched estimates calculated from the original dataset. Bootstrap 95% CIs for the day 25 detection rate (58%) matched those calculated using the “exact” method of Clopper & Pearson 111, [41%, 74%], which are a little wider than those calculated using the “more exact” method of Agresti & Coull 112, [42%, 73%]. (These analyses were performed using an online GraphPad ® calculator accessed on 18 th April 2017: http://www.graphpad.com/quickcalcs/ConfInterval1.cfm.) The congruence between these confidence intervals and the point estimates provides some reassurance that that the bootstrap procedure worked effectively. Estimates of parameters other than the day 25 detection rate (58%) are derived from more complex proportional relationships, and are therefore less precise. Table 5 reproduces a life table in the style of Leridon 26 and includes probabilities for each reproductive step with confidence intervals. These intervals (and some noted above) are impossibly wide highlighting further problems with Hertig’s analysis.
Table 5. Life Table of egg survival and probabilities during the first two weeks of development derived solely from Hertig’s data.
The table is modelled on Leridon’s life table 26 and includes his values for survivors and data from Hertig 5. Probabilities are also shown for each stage of the early development process. Medians and 95% confidence intervals derived from a bootstrap analysis of Hertig’s data indicate the precision in the estimates for fertilisation and embryo loss in the first two weeks. *Although Leridon’s values are based on Hertig, they do not fully match. Leridon reports losses of 15 and 27 in the first and second weeks respectively. However, Hertig’s 60% loss of abnormal pre-implantation embryos implies 25 (0.6 × 42) losses in the first week leaving 58, and 16 (58 × (6/21)) losses in the second week, leaving 42. ¥A value of π SOC = 0.90 was used to avoid the calculation of probabilities greater than 1.
| Week after Ovulation | Biological Description | Survivors (Leridon 26) | Survivors (Hertig 5) | Bootstrap Median
[95% CIs] |
|---|---|---|---|---|
| Number of Cycles | 100 | 100 | 100 [100, 100] | |
| 0 | Fertilised Eggs | 84 | 83 | 84 [39, 227] |
| 1 | Implanted Embryos | 69* | 58 | 58 [41, 74] |
| 2 | Missed First Period | 42 | 42 | 42 [26, 59] |
| Probabilities | Biological Description | Probabilities | Probabilities |
Bootstrap Median
[95% CIs] |
| π SOC × π FERT | Fertilisation per cycle | 0.84 | 0.83 | 0.84 [0.39, 2.27] |
| π FERT (when π SOC = 0.90 ¥) | Fertilisation per ideal insemination | 0.93 | 0.93 | 0.93 [0.43, 2.52] |
| π HCG | Fertilised egg implanting | 0.82* | 0.70 | 0.69 [0.27, 1.28] |
| π CLIN | Implanted egg to clinical recognition | 0.61* | 0.71 | 0.71 [0.50, 0.91] |
| π HCG × π CLIN | Fertilised egg to clinical recognition | 0.50 | 0.50 | 0.50 [0.20, 0.88] |
Hertig’s analysis omits 47 cases from cycle days 20–24, comprising 44% of his data. It is clear why he cannot use it, since all five embryos were normal and, given his mathematical and biological assumptions, five normal implanting embryos could not become 29% (6/21) abnormal post-implantation. Others have also noted that these “missing data are sufficient to engender an entirely different result” 16. Furthermore, the data that define the 50% proportion of abnormal pre-implantation embryos (i.e., 4/8) are so few that any numerical variation will make a substantial difference to derived proportions. If he had observed 3/8 abnormal embryos, his estimate of pre-implantation loss would have been 13% rather than 30%: for 5/8 it would have been 48%, with a fertilisation rate of 111%, which is clearly impossible. It seems therefore, that Hertig designed his analysis based on a post-hoc examination and selective use of the data. His own caveat about the lack of relevant and necessary data should be taken at least as seriously as his conclusions.
Hertig and Rock’s contribution to human embryology is undeniable and their quantitative conclusions have profoundly influenced our impression of the extent of early human embryo mortality. Regrettably, their estimates have a cripplingly low precision, which undermines their biological credibility or utility. In conclusion, Hertig’s data and flawed analysis cannot be regarded as a reliable quantitative foundation upon which to evaluate and understand natural human reproduction.
Discussion
Answering the question “ How many fertilised human embryos die before or during implantation under natural conditions?” is difficult. Relevant, credible data are in short supply. Among regularly cited publications, the Lancet hypothesis 34 is entirely speculative and in the view of the current author should cease to be used as an authoritative source. Clinical pregnancy studies are only useful for quantifying clinical pregnancy loss and contribute nothing to estimates of embryo mortality in the first two weeks’ post-fertilisation. Even Hertig’s unique dataset is inadequate to draw quantitative conclusions and oft-repeated values should be treated with scepticism. The hCG studies from 1988 onwards provide the best data for estimating embryo mortality although a lack of information on fertilisation success rates 5, 16, 21, 23, 113 prevents satisfactory completion of the calculations. A recent re-analysis of these data proposed plausible limits for reproductively normal women indicating that approximately 10–40% of embryos perish before implantation and 40–60% do so between fertilisation and birth 39. However, these ranges are wide, particularly for pre-implantation mortality, reflecting the lack of appropriate data. Is there any possibility of narrowing down the numbers?
In the 1980s, two separate groups collected embryos from women following carefully timed artificial insemination as part of fertility treatment. Insemination around the time of ovulation in women of proven fertility was followed 5 days later by uterine lavage to recover ova 114– 117. These data appear to hold promise for determining fertilisation efficiency and some authors have made quantitative inferences about embryo mortality from them 24, 27, 28. However, such inferences are complicated by numerous confounding factors. For example, in one series 116, from 88 uterine lavages following artificial insemination by donor (AID), 4 unfertilised eggs, 6 fragmented eggs, and 27 embryos from 2 cell to blastocyst stage were retrieved. In the 51 cycles in which no egg or embryo was retrieved, there was one retained pregnancy suggesting that the lavage and ova retrieval efficiency was reasonably high, albeit not perfect. These data therefore suggest that FEC TOT was low (≈31/88 = 35%) although a proportion of fertilised eggs may have completely degenerated within the first 5 days. Assuming π SOC was high (given the targeted insemination), this suggests that π FERT ≈ 50%. In the context of the recent analysis 39, this implies that π HCG is high and that levels of embryo mortality are therefore towards the lower end of the 10–40% and 40–60% ranges. However, the clinical pregnancy rate following transfer of the embryos was only 40%. This is equivalent to π HCG × π CLIN. If π CLIN ≈ 75%, as suggested by the hCG studies ( Table 3), this would mean that π HCG ≈ 50%. This would imply that π FERT is high, fertilised egg degeneration is high, occurs before day 5 and was therefore unobserved, and hence levels of embryo mortality tend towards the upper end of the 10–40% and 40–60% ranges.
It is possible that the lavage/transfer procedure reduced implantation and early developmental efficiency thereby reducing π HCG × π CLIN. A comparison of AID pregnancy rates may provide some insight as suggested by the authors 116. The clinical pregnancy rate in their pharmacologically unstimulated cohort was 12.5% (11/88) which is lower than an equivalent 18.9% observed for fresh semen AID 118, and also the live birth rate (which also incorporates clinical pregnancy losses) of 14.7% reported by the HFEA for AID in 2012 in unstimulated women aged 18–34 119. These different success rates suggest that the lavage/transfer procedure did adversely affect implantation and early gestation with clear implications for quantitative extrapolation. Furthermore, the women who were embryo recipients were receiving fertility treatment and their overall fertility may have been lower than expected in a normal healthy cohort. In summary, it seems that there are too many unresolved variables in these data to narrow down estimates of fertilization ( π FERT) or implantation ( π HCG) rates.
With high fecundability, the range of possible embryo mortality rates falls. Red deer hinds have pregnancy rates of >85% following natural mating 120: establishing numerical limits for embryo mortality under these efficient reproductive circumstances is more straightforward. By contrast, humans lack the instinct to mate predominantly during fertile periods thereby reducing observed reproductive efficiency substantially. In studies of early pregnancy loss, owing to sub-optimal coital frequency and cohorts including sub-fertile couples, natural fecundability was almost certainly not maximised 39. Combining data on coital frequency and hCG elevation may help to address this. In 1995, applying the Schwartz model 94 to his 1988 hCG data 49, Wilcox calculated a FEC HCG value of 36% for high coital frequencies (>4 days with intercourse in 6 pre-ovulatory days) 40. However, the Schwartz model assumed that cycle viability was evenly distributed among couples, a condition which the authors recognised was not true and is contradicted by a subsequent analysis which suggests that approximately a quarter of the Wilcox cohort was sub-fertile 39. If possible, focussing analytical attention on normally fertile women with the highest coital frequencies may help to narrow the range of plausible embryo mortality.
In this review of natural early embryo mortality no use has been made of data from in vitro fertilisation (IVF) and associated laboratory studies. Sub-optimal conditions for embryo culture mean that it was 121, 122 and probably still is 123 doubtful that reliable values can be extrapolated from laboratory in vitro to natural in vivo circumstances 28. Importantly, the reproductive stages are also altered. In IVF, π SOC = 1 and for transferred embryos π FERT = 1. Furthermore, transferred embryos are selected based on quality criteria, however inexact those may be 123, 124. IVF program manipulations may reduce π HCG compared to natural circumstances 6 and implantation failure remains a substantial issue for IVF 125, 126. Although for IVF cycles, the reported live birth rate per cycle has gone up (from 14% in 1991 to 25.4% in 2012 119), comparison of IVF success rates and natural live birth fecundability values involves too many undefined variables to shed numerical light on early natural embryo development and mortality.
In vitro fertilisation per se may provide some insight into values of π FERT, since π SOC = 1, and successful fertilisation can be observed. In seven studies of natural cycle IVF, fertilisation was successful in 70.9% (443/625) of attempts 127– 133. If this represented natural, in vivo fertilisation, based on the recent analysis 39, it implies that π HCG ≈ 0.75, focusing estimates for pre-implantation embryo loss on 25%, and for total loss on 50%. However, high frequencies of chromosomal aberrations caused by the in vitro handling of human oocytes 134 can render any comparison of natural and assisted reproduction open to criticism 9.
In calculating summary values of embryo mortality, it is important to note that human fertility is as numerically heterogeneous as it could possibly be. Some couples are infertile and some are highly fertile. Excessive attention to averages and neglect of variances fosters a misleading appreciation of reality. The hCG studies clearly had both fertile and sub-fertile participants: use of overall values underestimated fecundability for the fertile majority 39. Furthermore, apparently ‘optimal’ conditions for conception may not maximise human biological fecundability. Other biological factors also contribute to reproductive heterogeneity in humans; however, even after controlling for age-related decline, fecundability remains highly variable 119, 135. For intercourse occurring 2 days prior to ovulation, average fecundabilities resembled those previously published 88, but for couples at the 5 th and 95 th percentiles, fecundabilities were 5% and 83%. 83% fecundability implies a very low embryo mortality rate. In conclusion, apparent low fecundability in humans need not necessarily be caused by embryo mortality, but also defects of ovulation, mistimed coitus, or fertilisation failure 39. Where fecundability is low, any or all of these factors may contribute.
Embryo mortality and pregnancy loss are not only a matter of academic scientific interest, and diverse quantitative estimates can also be found in popular media. For example, 70% loss in the first six days is claimed by Michael Mosley in “You made it through the first round” ( http://www.bbc.co.uk/timelines/z84tsg8; transcript at http://a.files.bbci.co.uk/bam/live/content/z3b87hv/transcript: accessed on 20 th April, 2017). By contrast a 25% pre-implantation loss is reported by the Science Museum’s online exhibit, “Who Am I?” ( http://www.sciencemuseum.org.uk/WhoAmI/FindOutMore/Yourbody/Wheredidyoucomefrom/Howdoyougrowinthewomb/Whathappensinweek1: accessed on 20 th April, 2017). News reports, often associated with ethical controversies, also feature estimates of embryo loss. On 1 st February 2016, James Gallagher reported that only 13/100 fertilised eggs develop beyond 3 months ( http://www.bbc.co.uk/news/health-35459054: accessed on 20 th April, 2017) and on 4 th May 2016, Sarah Knapton reported in the online Daily Telegraph that “two thirds of pregnancies fail because the embryo does not implant properly” ( http://www.telegraph.co.uk/science/2016/05/04/human-embryos-kept-alive-in-lab-for-unprecedented-13-days-so-sci/: accessed on 20 th April, 2017). In an ethical advocacy video, Bill Nye (“The Science Guy”) begins by claiming that “Many, many, many, many more hundreds of eggs are fertilized than become humans” ( https://www.youtube.com/watch?v=4IPrw0NYkMg: accessed on 20 th April, 2017). Additionally, academic philosophical articles 1 and legal judgements 4 have considered the significance of the scale of embryo loss. Given the breadth of societal interest in this facet of human reproductive biology, it is vital that scientists report plausible and defensible estimates for natural embryo mortality. Above all, it is obvious that women wishing to have children deserve reliable and unbiased estimates of reproductive success and pregnancy failure.
Pregnancy loss and embryo mortality under natural conditions are real and substantial. However, estimates of 90% 37, 85% 36, 80% 11, 35, 78% 34, 76% 10, 33 and 70% 27– 31 total loss are excessive and not supported by available data. Estimates for clinical pregnancy loss are approximately 10–20%. For women of reproductive age, losses between implantation and clinical recognition are approximately 10–25%. Loss from implantation to birth is approximately one third 39, 46, 48, 49.
Natural pre-implantation embryo loss remains quantitatively undefined. In the absence of knowledge of π SOC and π FERT it is almost impossible to estimate precisely. Hertig’s estimate is 30%; however, mathematically and biologically implausible confidence intervals [-28%, 73%] betray the quantitative weaknesses in his data and analysis. The best available data for quantifying early pregnancy loss are from studies monitoring daily hCG levels in women attempting to conceive. A recent re-analysis 39 of data from three studies 46, 48, 49 concluded that, in normal healthy women, 10–40% is a plausible range for pre-implantation embryo loss and overall pregnancy loss from fertilisation to birth is approximately 40–60%. This latter range is consistent with Kline's estimate of 50% 16, and similar to, although a little narrower than the 25–70% suggested by Professor Robert Edwards 136.
In the absence of suitable data to quantify pre-implantation loss, many published articles and reviews merely restate previously published values 11, 28, 29. It has been suggested that “ claimed research findings may often be simply accurate measures of the prevailing bias” 137. Widely held views on early embryo mortality may reflect an entrenched and biased view of the biology. For example, the Macklon “Black Box” review 28 has been cited 212 times (Web of Science TM citations on 12 th April 2017) with many articles explicitly referencing its 30% survival/70% failure values 13, 29, 125, 138– 146. Macklon’s quantitative summary in his “Pregnancy Loss Iceberg” (30% implantation failure; 30% early pregnancy loss; 10% clinical miscarriage; 30% live births) is a direct, unedited reproduction of estimates published over 10 years previously 27. 30% pre-implantation loss fairly represents Hertig’s conclusions although, as has been shown, this estimate is highly imprecise. However, Macklon misrepresents the best data which he reviews 46, 49. Wilcox reports early pregnancy loss (i.e., [1 - π CLIN]) of 21.7% whereas Macklon’s iceberg implies that 43% (30/70) of implanting embryos fail before clinical recognition. The iceberg’s clinical loss rate of 25% (10/40) is also higher than relevant data indicate ( Figure 3 & Figure 4). Total loss of implanting (hCG+) embryos (i.e., [1 - ( π CLIN × π LB]) is 57% (40/70) according to the iceberg. By contrast, Wilcox 49 and Zinaman 46, both included in Macklon’s review, report that only 31% of hCG positive pregnancies fail.
Early pregnancy loss of 10–25% is not trivial. Despite difficulties associated with extrapolating in vitro observations to in vivo circumstances, implantation is clearly a crucial biological milestone in embryonic development and pregnancy. Recent studies suggest that biological mediators from embryos may regulate endometrial receptivity resulting in selective implantation of fitter embryos 18, 147, 148. Interestingly, supernatant from developmentally impaired IVF embryos deemed unsuitable for transfer provoked a different response in endometrial cells compared to supernatant from developmentally competent embryos that produced an ongoing pregnancy after transfer 18. However, it would be of interest to compare these responses to that provoked by supernatant from developmentally competent embryos that did not successfully implant and produce an ongoing pregnancy. Arrested or developmentally impaired embryos may lose cellular integrity and release mediators that disrupt endometrial receptivity; however, the intrinsic developmental competence of such embryos must also determine the success of implantation. Implantation failure is a substantial issue for IVF 125, and distinguishing between obviously impaired and competent embryos is not the principal challenge, but rather distinguishing from among apparently competent embryos those that will successfully implant and those that will not. Striking evidence suggests that only 9% of IVF embryos have a normal karyotype in all their cells 145, 149 raising the possibility that the rate of aneuploidy in IVF embryos is artefactually high, or that a degree of mosaic aneuploidy in human embryos is not necessarily developmentally deleterious, or indeed both. Implantation failure of a normal embryo may well be an uncommon event 51, but defining what is normal is a considerable scientific and conceptual challenge.
If Macklon’s 28 (and Chard’s 27) 70% estimate for embryo loss is excessive, as the data suggest, this casts doubt on claims 125, 143 that the frequency of embryonic abnormalities observed in vitro is representative of the natural in vivo situation. In turn, this implies that many of the chromosomal abnormalities observed in in vitro human embryos may be, to a greater extent than currently recognised 125, an artefact of the clinical and experimental interventions of assisted reproductive technologies.
This is not the first time that attention has been drawn to unsatisfactory estimates of early embryo loss. Faced with some of the same data, others have noted that “ a claim of ‘no significant difference’ might easily be sustained against any interpretation proffered” 16 and that estimates are “ difficult to defend with any precision” 10. Conclusions have been based on “ poor estimates of fertilization failure rate and the mortality at 2 weeks after fertilisation” 23 and drawn “ from unusual or biased samples” 150. Nevertheless, although precision may be elusive, exaggeration can be avoided. It is hoped that this critical re-evaluation of the data describing early human embryo mortality will serve as a robust foundation upon which to make informed biological, ethical, legal and personal judgements.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
F1000Research: Dataset 1. Figure 2 data, 10.5256/f1000research.8937.d140569 151
F1000Research: Dataset 2. Figure 3 data, 10.5256/f1000research.8937.d140570 152
F1000Research: Dataset 3. Figure 4 data, 10.5256/f1000research.8937.d140571 153
F1000Research: Dataset 4. Pseudo-datasets of Hertig’s study, obtained via a bootstrap procedure, 10.5256/f1000research.8937.d140572 154
Acknowledgements
Thanks are due to Professor David Paton, Dr Paul Schofield and Dr Amanda Sferruzzi-Perri for reviewing and providing helpful comments during the writing of this article.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 1 approved
References
- 1. Ord T: The scourge: moral implications of natural embryo loss. Am J Bioeth. 2008;8(7):12–9. 10.1080/15265160802248146 [DOI] [PubMed] [Google Scholar]
- 2. Harris J: Stem cells, sex, and procreation. Camb Q Healthc Ethics. 2003;12(4):353–71. 10.1017/S096318010312405X [DOI] [PubMed] [Google Scholar]
- 3. Rahner K: The Problem of Genetic Manipulation. Theological Investigations, Vol IX Translation by Graham Harrison, London: Darton, Longman & Todd;1972;225–6. [Google Scholar]
- 4. R (on the application of Smeaton) v Secretary of State for Health. [2002] EWHC 610 (Admin), [2002] All ER (D) 115 (Apr),2002. Reference Source [Google Scholar]
- 5. Hertig AT: The Overall Problem in Man. In: Benirschke K, editor. Comparative Aspects of Reproductive Failure An International Conference at Dartmouth Medical School. Berlin: Springer Verlag;1967;11–41. 10.1007/978-3-642-48949-5_2 [DOI] [Google Scholar]
- 6. Kennedy TG: Physiology of implantation. In Vitro Fert Ass Rep. 1997;729–35. Reference Source [Google Scholar]
- 7. Jones RE, Lopez KH: Chapter 10: Pregnancy. Human Reproductive Biology.4th ed. Amsterdam: Elsevier;2014;175–204. 10.1016/B978-0-12-382184-3.00010-6 [DOI] [Google Scholar]
- 8. Sharkey AM, Macklon NS: The science of implantation emerges blinking into the light. Reprod Biomed Online. 2013;27(5):453–60. 10.1016/j.rbmo.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 9. Benagiano G, Farris M, Grudzinskas G: Fate of fertilized human oocytes. Reprod Biomed Online. 2010;21(6):732–41. 10.1016/j.rbmo.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 10. Boklage CE: Survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35(2):75, 79–80, 81–94. [PubMed] [Google Scholar]
- 11. Vitzthum VJ, Spielvogel H, Thornburg J, et al. : A prospective study of early pregnancy loss in humans. Fertil Steril. 2006;86(2):373–9. 10.1016/j.fertnstert.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 12. Bainbridge DR: Making Babies. A Visitor Within. London: Phoenix;2001;101–62at 59ff. Reference Source [Google Scholar]
- 13. Ramos-Medina R, García-Segovia Á, León JA, et al. : New decision-tree model for defining the risk of reproductive failure. Am J Reprod Immunol. 2013;70(1):59–68. 10.1111/aji.12098 [DOI] [PubMed] [Google Scholar]
- 14. Norwitz ER, Schust DJ, Fisher SJ: Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–8. 10.1056/NEJMra000763 [DOI] [PubMed] [Google Scholar]
- 15. James WH: The incidence of spontaneous abortion. Popul Stud (Camb). 1970;24(2):241–5. 10.1080/00324728.1970.10406127 [DOI] [PubMed] [Google Scholar]
- 16. Kline J, Stein Z, Susser M: Conception and Reproductive Loss: Probabilities. Conception to Birth. Epidemiology of Prenatal Development.New York: OUP;1989;43–68. [Google Scholar]
- 17. Rai R, Regan L: Recurrent miscarriage. Lancet. 2006;368(9535):601–11. 10.1016/S0140-6736(06)69204-0 [DOI] [PubMed] [Google Scholar]
- 18. Brosens JJ, Salker MS, Teklenberg G, et al. : Uterine selection of human embryos at implantation. Sci Reports. 2014;4: 3894. 10.1038/srep03894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silver RM, Branch DW: Sporadic and recurrent pregnancy loss. In: Reece EA, Hobbins JC, editors. Clinical Obstetrics: The Fetus and Mother.3rd ed: Blackwell Publishing;2007;143–60. 10.1002/9780470753323.ch9 [DOI] [Google Scholar]
- 20. Nishimura H: Fate of human fertilized eggs during prenatal life: present status of knowledge. Okajimas Folia Anat Jpn. 1970;46(6):297–305. 10.2535/ofaj1936.46.6_297 [DOI] [PubMed] [Google Scholar]
- 21. Short RV: When a conception fails to become a pregnancy. Ciba Found Symp. 1978; (64):377–94. 10.1002/9780470720479.ch16 [DOI] [PubMed] [Google Scholar]
- 22. Opitz JM: The Farber lecture. Prenatal and perinatal death: the future of developmental pathology. Pediatr Pathol. 1987;7(4):363–94. 10.3109/15513818709161402 [DOI] [PubMed] [Google Scholar]
- 23. Biggers JD: Risks of In Vitro Fertilization and Embryo Transfer in Humans. In: Crosignani PG, Rubin BL, editors. In Vitro Fertilization and Embryo Transfer.London: Academic Press;1983;393–410. Reference Source [Google Scholar]
- 24. Johnson MH: Chapter 15: Fetal Challenges. Essential Reproduction 7th ed. Oxford: Wiley-Blackwell;2013;258–69. Reference Source [Google Scholar]
- 25. Biggers JD: In vitro fertilization and embryo transfer in human beings. N Engl J Med. 1981;304(6):336–42. 10.1056/NEJM198102053040607 [DOI] [PubMed] [Google Scholar]
- 26. Leridon H: Intrauterine Mortality. Human Fertility: The Basic Components Chicago: The University of Chicago Press;1977;48–81. Reference Source [Google Scholar]
- 27. Chard T: Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. 1991;5(1):179–89. 10.1016/S0950-3552(05)80077-X [DOI] [PubMed] [Google Scholar]
- 28. Macklon NS, Geraedts JP, Fauser BC: Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–43. 10.1093/humupd/8.4.333 [DOI] [PubMed] [Google Scholar]
- 29. Ford HB, Schust DJ: Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 30. McCoy RC, Demko Z, Ryan A, et al. : Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Science. 2015;348(6231):235–8. 10.1126/science.aaa3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loke YW, King A: Human Implantation: Cell Biology and Immunology. Cambridge: Cambridge University Press;1995. Reference Source [Google Scholar]
- 32. American College of Obstetricians and Gynecologists: Early pregnancy loss. Int J Gynaecol Obstet. 1995;51(3):278–85. 10.1016/0020-7292(95)80036-0 [DOI] [PubMed] [Google Scholar]
- 33. Drife JO: What proportion of pregnancies are spontaneously aborted? Brit Med J. 1983;286(6361):294. [Google Scholar]
- 34. Roberts CJ, Lowe CR: Where have all the conceptions gone? Lancet. 1975;305:498–9. 10.1016/S0140-6736(75)92837-8 [DOI] [Google Scholar]
- 35. Johnson MH, Everitt BJ: Chapter 15: Fertility. Essential Reproduction 5th ed. Oxford: Wiley-Blackwell;2000;251–74. Reference Source [Google Scholar]
- 36. Braude PR, Johnson MH: The Embryo in Contemporary Medical Science. In: Dunstan GR, editor. The Human Embryo: Aristotle and the Arabic and European Traditions Exeter: University of Exeter Press;1990;208–21. Reference Source [Google Scholar]
- 37. Opitz JM: Human Development - The Long and the Short of it. In: Furton EJ, Mitchell LA, editors. What is Man, O Lord? The Human Person in a Biotech Age; Eighteenth Workshop for Bishops Boston, MA: The National Catholic Bioethics Center;2002;131–53. [Google Scholar]
- 38. Leridon H: Fecundability. Human Fertility: The Basic Components Chicago: The University of Chicago Press;1977;22–47. Reference Source [Google Scholar]
- 39. Jarvis GE: Estimating limits for natural human embryo mortality [version 1; referees: 2 approved]. F1000Res. 2016;5:2083 10.12688/f1000research.9479.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilcox AJ, Weinberg CR, Baird DD: Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–21. 10.1056/NEJM199512073332301 [DOI] [PubMed] [Google Scholar]
- 41. Bulletti C, Flamigni C, Giacomucci E: Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum Reprod Update. 1996;2(2):118–36. 10.1093/humupd/2.2.118 [DOI] [PubMed] [Google Scholar]
- 42. Devolder K, Harris J: The ambiguity of the embryo: Ethical inconsistency in the human embryonic stem cell debate. Metaphilosophy. 2007;38(2–3):153–69. 10.1111/j.1467-9973.2007.00480.x [DOI] [Google Scholar]
- 43. Green RM: The Human Embryo Research Debates: Bioethics in the Vortex of Controversy. Oxford: Oxford University Press;2001. Reference Source [Google Scholar]
- 44. Hertig AT, Rock J, Adams EC: A description of 34 human ova within the first 17 days of development. Am J Anat. 1956;98(3):435–93. 10.1002/aja.1000980306 [DOI] [PubMed] [Google Scholar]
- 45. Treloar AE, Boynton RE, Behn BG, et al. : Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 46. Zinaman MJ, Clegg ED, Brown CC, et al. : Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503–9. 10.1016/S0015-0282(16)58144-8 [DOI] [PubMed] [Google Scholar]
- 47. French FE, Bierman JM: Probabilities of fetal mortality. Public Health Rep. 1962;77(10):835–47. [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Chen C, Wang L, et al. : Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577–84. 10.1016/S0015-0282(02)04694-0 [DOI] [PubMed] [Google Scholar]
- 49. Wilcox AJ, Weinberg CR, O'Connor JF, et al. : Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. 10.1056/NEJM198807283190401 [DOI] [PubMed] [Google Scholar]
- 50. Rushton DI, Scott JS, Jenkins DM, et al. : Letter: Where have all the conceptions gone? Lancet. 1975;1(7907):636–7. 10.1016/S0140-6736(75)91920-0 [DOI] [PubMed] [Google Scholar]
- 51. Cooke ID: Failure of implantation and its relevance to subfertility. J Reprod Fertil Suppl. 1988;36:155–9. [PubMed] [Google Scholar]
- 52. Catalano RA, Saxton KB, Bruckner TA, et al. : Hormonal evidence supports the theory of selection in utero. Am J Hum Biol. 2012;24(4):526–32. 10.1002/ajhb.22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orzack SH, Zuckerman JE: Referee Report For: Early embryo mortality in natural human reproduction: What the data say [version 1; referees: 1 approved, 2 approved with reservations]. F1000Res. 2016;5:2765 10.5256/f1000research.9616.r22945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shapiro S, Jones EW, Densen PM: A life table of pregnancy terminations and correlates of fetal loss. Milbank Mem Fund Q. 1962;40(1):7–45. 10.2307/3348609 [DOI] [PubMed] [Google Scholar]
- 55. Erhardt CL: Pregnancy Losses in New York City, 1960. Am J Public Health Nations Health. 1963;53(9):1337–52. 10.2105/AJPH.53.9.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pettersson F: Epidemiology of Early Pregnancy Wastage. Stockholm: Svenska Bokförlaget;1968. Reference Source [Google Scholar]
- 57. Shapiro S, Levine HS, Abramowicz M: Factors associated with early and late fetal loss. Adv Planned Parenthood. 1970;6:45–63. [Google Scholar]
- 58. Taylor WF: The Probability of Fetal Death. In: Fraser FC, McCusick VA, editors. Congenital Malformations Amsterdam: Excerpta Medica;1970;307–20. [Google Scholar]
- 59. Master-Hunter T, Heiman DL: Amenorrhea: evaluation and treatment. Am Fam Physician. 2006;73(8):1374–82. [PubMed] [Google Scholar]
- 60. Committee on Practice Bulletins—Gynecology: Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206. 10.1097/AOG.0b013e318262e320 [DOI] [PubMed] [Google Scholar]
- 61. Grudzinskas JG, Nysenbaum AM: Failure of human pregnancy after implantation. Ann N Y Acad Sci. 1985;442:38–44. 10.1111/j.1749-6632.1985.tb37503.x [DOI] [PubMed] [Google Scholar]
- 62. Morton H, Rolfe B, Clunie GJ: An early pregnancy factor detected in human serum by the rosette inhibition test. Lancet. 1977;1(8008):394–7. 10.1016/S0140-6736(77)92605-8 [DOI] [PubMed] [Google Scholar]
- 63. Chard T, Grudzinskas JG: Early pregnancy factor. Biol Res Pregnancy Perinatol. 1987;8(2 2D Half):53–6. [PubMed] [Google Scholar]
- 64. Cole LA: hCG, the wonder of today's science. Reprod Biol Endocrinol. 2012;10:24. 10.1186/1477-7827-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilcox AJ, Weinberg CR, Wehmann RE, et al. : Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44(3):366–74. [PubMed] [Google Scholar]
- 66. Regan L: A prospective study of spontaneous abortion. In: Beard RW, Sharp F, editors. Early Pregnancy Loss: Mechanisms and Treatment Springer-Verlag;1988;23–37. 10.1007/978-1-4471-1658-5_4 [DOI] [Google Scholar]
- 67. Odell WD, Griffin J: Pulsatile secretion of human chorionic gonadotropin in normal adults. N Engl J Med. 1987;317(27):1688–91. 10.1056/NEJM198712313172702 [DOI] [PubMed] [Google Scholar]
- 68. Cho SI, Goldman MB, Ryan LM, et al. : Reliability of serial urine HCG as a biomarker to detect early pregnancy loss. Hum Reprod. 2002;17(4):1060–6. 10.1093/humrep/17.4.1060 [DOI] [PubMed] [Google Scholar]
- 69. Ellish NJ, Saboda K, O’Connor J, et al. : A prospective study of early pregnancy loss. Hum Reprod. 1996;11(2):406–12. 10.1093/HUMREP/11.2.406 [DOI] [PubMed] [Google Scholar]
- 70. Miller JF, Williamson E, Glue J, et al. : Fetal loss after implantation. A prospective study. Lancet. 1980;2(8194):554–6. 10.1016/S0140-6736(80)91991-1 [DOI] [PubMed] [Google Scholar]
- 71. Edmonds DK, Lindsay KS, Miller JF, et al. : Early embryonic mortality in women. Fertil Steril. 1982;38(4):447–53. 10.1016/S0015-0282(16)46579-9 [DOI] [PubMed] [Google Scholar]
- 72. Whittaker PG, Taylor A, Lind T: Unsuspected pregnancy loss in healthy women. Lancet. 1983;1(8334):1126–7. 10.1016/S0140-6736(83)92865-9 [DOI] [PubMed] [Google Scholar]
- 73. Videla-Rivero L, Etchepareborda JJ, Kesseru E: Early chorionic activity in women bearing inert IUD, copper IUD and levonorgestrel-releasing IUD. Contraception. 1987;36(2):217–26. 10.1016/0010-7824(87)90017-5 [DOI] [PubMed] [Google Scholar]
- 74. Walker EM, Lewis M, Cooper W, et al. : Occult biochemical pregnancy: fact or fiction? Br J Obstet Gynaecol. 1988;95(7):659–63. 10.1111/j.1471-0528.1988.tb06526.x [DOI] [PubMed] [Google Scholar]
- 75. Hakim RB, Gray RH, Zacur H: Infertility and early pregnancy loss. Am J Obstet Gynecol. 1995;172(5):1510–7. 10.1016/0002-9378(95)90489-1 [DOI] [PubMed] [Google Scholar]
- 76. Sasaki Y, Ladner DG, Cole LA: Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. 2008;89(6):1781–6. 10.1016/j.fertnstert.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 77. Koot YE, Boomsma CM, Eijkemans MJ, et al. : Recurrent pre-clinical pregnancy loss is unlikely to be a 'cause' of unexplained infertility. Hum Reprod. 2011;26(10):2636–41. 10.1093/humrep/der217 [DOI] [PubMed] [Google Scholar]
- 78. Cole LA: Hyperglycosylated hCG and pregnancy failures. J Reprod Immunol. 2012;93(2):119–22. 10.1016/j.jri.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 79. Mumford SL, Silver RM, Sjaarda LA, et al. : Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod. 2016;31(3):657–65. 10.1093/humrep/dev329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wilcox AJ, Baird DD, Weinberg CR, et al. : The use of biochemical assays in epidemiologic studies of reproduction. Environ Health Perspect. 1987;75:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brattebø G: Occult biochemical pregnancy: fact or fiction? Br J Obstet Gynaecol. 1989;96(2):252–4. 10.1111/j.1471-0528.1989.tb01676.x [DOI] [PubMed] [Google Scholar]
- 82. Walker EM, Lewis M, Howie PW: Authors' reply. Br J Obstet Gynaecol. 1989;96(2):253–4. 10.1111/j.1471-0528.1989.tb01677.x [DOI] [Google Scholar]
- 83. Wilcox AJ, Weinberg CR, Baird DD: Subclinical embryonic loss. Fertil Steril. 1989;51(5):907–8. 10.1016/S0015-0282(16)60691-X [DOI] [PubMed] [Google Scholar]
- 84. Wilcox AJ, Dunson DB, Weinberg CR, et al. : Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63(4):211–5. 10.1016/S0010-7824(01)00191-3 [DOI] [PubMed] [Google Scholar]
- 85. Wilcox AJ, Weinberg CR, Baird DD: Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–5. [DOI] [PubMed] [Google Scholar]
- 86. Wilcox AJ, Baird DD, Weinberg CR: Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–9. 10.1056/NEJM199906103402304 [DOI] [PubMed] [Google Scholar]
- 87. Weinberg CR, Moledor E, Baird DD, et al. : Is there a seasonal pattern in risk of early pregnancy loss? Epidemiology. 1994;5(5):484–9. [PubMed] [Google Scholar]
- 88. Wilcox AJ, Weinberg CR, Baird DD: Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13(2):394–7. 10.1093/humrep/13.2.394 [DOI] [PubMed] [Google Scholar]
- 89. Sapra KJ, Buck Louis GM, Sundaram R, et al. : Signs and symptoms associated with early pregnancy loss: findings from a population-based preconception cohort. Hum Reprod. 2016;31(4):887–96. 10.1093/humrep/dew010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wilcox AJ, Baird DD, Dunson D, et al. : Natural limits of pregnancy testing in relation to the expected menstrual period. JAMA. 2001;286(14):1759–61. 10.1001/jama.286.14.1759 [DOI] [PubMed] [Google Scholar]
- 91. Weinberg CR, Gladen BC, Wilcox AJ: Models relating the timing of intercourse to the probability of conception and the sex of the baby. Biometrics. 1994;50(2):358–67. 10.2307/2533379 [DOI] [PubMed] [Google Scholar]
- 92. Weinberg CR, Hertz-Picciotto I, Baird DD, et al. : Efficiency and bias in studies of early pregnancy loss. Epidemiology. 1992;3(1):17–22. 10.1097/00001648-199201000-00005 [DOI] [PubMed] [Google Scholar]
- 93. Barrett JC, Marshall J: The risk of conception on different days of the menstrual cycle. Popul Stud (Camb). 1969;23(3):455–61. 10.1080/00324728.1969.10405297 [DOI] [PubMed] [Google Scholar]
- 94. Schwartz D, Macdonald PD, Heuchel V: Fecundability, coital frequency and the viability of Ova. Popul Stud (Camb). 1980;34(2):397–400. 10.1080/00324728.1980.10410398 [DOI] [PubMed] [Google Scholar]
- 95. Boklage CE: The frequency and and survival probability of natural twin conceptions. In: Keith LG, Papiernik E, Keith DM, Lukie B, editors. Multiple Pregnancy: Epidemiology, Gestation and Perinatal Outcome New York: Parthenon Publishing Group;1995;41–50. Reference Source [Google Scholar]
- 96. Hertwig O: Beiträge zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies (Contributions to the knowledge of the formation, fertilization and division of the animal egg). Morphol Jahrb. 1876; 1:347–434. [Google Scholar]
- 97. Steptoe PC, Edwards RG: Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. 10.1016/S0140-6736(78)92957-4 [DOI] [PubMed] [Google Scholar]
- 98. Clift D, Schuh M: Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14(9):549–62. 10.1038/nrm3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McLaughlin L: Chapter 4: The Human Egg Hunt. The Pill, John Rock, and the Church: The Biography of a Revolution.Boston: Little, Brown & Co.;1982;58–71. [Google Scholar]
- 100. Hertig AT, Rock J, Adams EC, et al. : Thirty-four fertilized human ova, good, bad and indifferent, recovered from 210 women of known fertility; a study of biologic wastage in early human pregnancy. Pediatrics. 1959;23(1 Part 2):202–11. [PubMed] [Google Scholar]
- 101. Hertig AT: A fifteen-year search for first-stage human ova. JAMA. 1989;261(3):434–5. 10.1001/jama.1989.03420030108040 [DOI] [PubMed] [Google Scholar]
- 102. Rock J, Hertig AT: Some aspects of early human development. Am J Obstet Gynecol. 1942;44(6):973–83. 10.1016/S0002-9378(42)90806-8 [DOI] [Google Scholar]
- 103. Hertig AT, Rock J: On a human blastula recovered from the uterine cavity 4 days after ovulation. Anat Rec. 1946;94:469. [PubMed] [Google Scholar]
- 104. Hertig AT, Rock J: A series of potentially abortive ova recovered from fertile women prior to the first missed menstrual period. Am J Obstet Gynecol. 1949;58(5):968–93, illust. 10.1016/0002-9378(49)90203-3 [DOI] [PubMed] [Google Scholar]
- 105. Hertig AT, Rock J: Two human ova of the pre-villous stage, having a developmental age of about 8 and 9 days respectively. Contrib Embryol. 1949;33(213–221):169–86. [PubMed] [Google Scholar]
- 106. Hertig AT, Adams EC, McKay DG, et al. : A thirteen-day human ovum studied histochemically. Am J Obstet Gynecol. 1958;76(5):1025–40; discussion 40–3. 10.1016/0002-9378(58)90185-6 [DOI] [PubMed] [Google Scholar]
- 107. Hertig AT, Rock J: Searching for early fertilized human ova. Gynecol Invest. 1973;4(3):121–39. 10.1159/000301716 [DOI] [PubMed] [Google Scholar]
- 108. Kline J, Stein Z, Susser M: Preface. Conception to Birth. Epidemiology of Prenatal Development.New York: OUP;1989;v–i. [Google Scholar]
- 109. Barrett JC: Fecundability and coital frequency. Popul Stud (Camb). 1971;25(2):309–13. 10.1080/00324728.1971.10405806 [DOI] [PubMed] [Google Scholar]
- 110. Efron B: Chapter 10: Nonparametric confidence intervals. The Jackknife, the Bootstrap and Other Resampling Plans Philadelphia: SIAM;1982;75–90. 10.1137/1.9781611970319.ch10 [DOI] [Google Scholar]
- 111. Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13. 10.2307/2331986 [DOI] [Google Scholar]
- 112. Agresti A, Coull BA: Approximate is better than "exact" for interval estimation of binomial proportions. Am Stat. 1998;52(2):119–26. 10.1080/00031305.1998.10480550 [DOI] [Google Scholar]
- 113. Edwards RG: The Cleaving Embryo and the Blastocyst. In: Conception in the Human Female.London: Academic Press;1980;668–766at 47ff. [Google Scholar]
- 114. Buster JE, Bustillo M, Rodi IA, et al. : Biologic and morphologic development of donated human ova recovered by nonsurgical uterine lavage. Am J Obstet Gynecol. 1985;153(2):211–7. 10.1016/0002-9378(85)90116-4 [DOI] [PubMed] [Google Scholar]
- 115. Formigli L, Formigli G, Roccio C: Donation of fertilized uterine ova to infertile women. Fertil Steril. 1987;47(1):162–5. 10.1016/S0015-0282(16)49953-X [DOI] [PubMed] [Google Scholar]
- 116. Formigli L, Roccio C, Belotti G, et al. : Non-surgical flushing of the uterus for pre-embryo recovery: possible clinical applications. Hum Reprod. 1990;5(3):329–35. 10.1093/oxfordjournals.humrep.a137099 [DOI] [PubMed] [Google Scholar]
- 117. Sauer MV, Bustillo M, Rodi IA, et al. : In-vivo blastocyst production and ovum yield among fertile women. Hum Reprod. 1987;2(8):701–3. 10.1093/oxfordjournals.humrep.a136617 [DOI] [PubMed] [Google Scholar]
- 118. Richter MA, Haning RV, Jr, Shapiro SS: Artificial donor insemination: fresh versus frozen semen; the patient as her own control. Fertil Steril. 1984;41(2):277–80. 10.1016/S0015-0282(16)47604-1 [DOI] [PubMed] [Google Scholar]
- 119. HFEA: Fertility Treatment in 2013 - trends and figures. Human Fertilisation & Embryology Authority. 2013. Reference Source [Google Scholar]
- 120. Asher GW: Reproductive cycles of deer. Anim Reprod Sci. 2011;124(3–4):170–5. 10.1016/j.anireprosci.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 121. Bolton VN, Braude PR: Development of the human preimplantation embryo in vitro. Curr Top Dev Biol. 1987;23:93–114. 10.1016/S0070-2153(08)60621-3 [DOI] [PubMed] [Google Scholar]
- 122. Jones HW, Jr, Oehninger S, Bocca S, et al. : Reproductive efficiency of human oocytes fertilized in vitro. Facts Views Vis Obgyn. 2010;2(3):169–71. [PMC free article] [PubMed] [Google Scholar]
- 123. Bolton VN, Leary C, Harbottle S, et al. : How should we choose the 'best' embryo? A commentary on behalf of the British Fertility Society and the Association of Clinical Embryologists. Hum Fertil (Camb). 2015;18(3):156–64. 10.3109/14647273.2015.1072646 [DOI] [PubMed] [Google Scholar]
- 124. Machtinger R, Racowsky C: Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26(3):210–21. 10.1016/j.rbmo.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 125. Niakan KK, Han J, Pedersen RA, et al. : Human pre-implantation embryo development. Development. 2012;139(5):829–41. 10.1242/dev.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Koot YE, Teklenburg G, Salker MS, et al. : Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822(12):1943–50. 10.1016/j.bbadis.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 127. Daya S, Gunby J, Hughes EG, et al. : Natural cycles for in-vitro fertilization: cost-effectiveness analysis and factors influencing outcome. Hum Reprod. 1995;10(7):1719–24. 10.1093/oxfordjournals.humrep.a136162 [DOI] [PubMed] [Google Scholar]
- 128. Zayed F, Lenton EA, Cooke ID: Natural cycle in-vitro fertilization in couples with unexplained infertility: impact of various factors on outcome. Hum Reprod. 1997;12(11):2402–7. 10.1093/humrep/12.11.2402 [DOI] [PubMed] [Google Scholar]
- 129. Bassil S, Godin PA, Donnez J: Outcome of in-vitro fertilization through natural cycles in poor responders. Hum Reprod. 1999;14(5):1262–5. 10.1093/humrep/14.5.1262 [DOI] [PubMed] [Google Scholar]
- 130. Roesner S, Pflaumer U, Germeyer A, et al. : Natural cycle IVF: evaluation of 463 cycles and summary of the current literature. Arch Gynecol Obstet. 2014;289(6):1347–54. 10.1007/s00404-013-3123-2 [DOI] [PubMed] [Google Scholar]
- 131. Omland AK, Fedorcsák P, Storeng R, et al. : Natural cycle IVF in unexplained, endometriosis-associated and tubal factor infertility. Hum Reprod. 2001;16(12):2587–92. 10.1093/humrep/16.12.2587 [DOI] [PubMed] [Google Scholar]
- 132. Janssens RM, Lambalk CB, Vermeiden JP, et al. : In-vitro fertilization in a spontaneous cycle: easy, cheap and realistic. Hum Reprod. 2000;15(2):314–8. 10.1093/humrep/15.2.314 [DOI] [PubMed] [Google Scholar]
- 133. Fahy UM, Cahill DJ, Wardle PG, et al. : In-vitro fertilization in completely natural cycles. Hum Reprod. 1995;10(3):572–5. [DOI] [PubMed] [Google Scholar]
- 134. Braude PR, Johnson MH, Pickering SJ, et al. : Mechanisms of Early Embryonic Loss In Vivo and In Vitro . In: Chapman. M, Grudzinskas G, Chard T, editors. The Embryo: Normal and Abnormal Development and Growth London: Springer-Verlag;1991;1–10. 10.1007/978-1-4471-1802-2_1 [DOI] [Google Scholar]
- 135. Dunson DB, Colombo B, Baird DD: Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–403. 10.1093/humrep/17.5.1399 [DOI] [PubMed] [Google Scholar]
- 136. Edwards RG: Sexuality and Coitus. In: Conception in the Human Female London: Academic Press;1980;525–72at 60ff. [Google Scholar]
- 137. Ioannidis JP: Why most published research findings are false. PLoS Med. 2005;2(8):e124. 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McCoy RC, Demko ZP, Ryan A, et al. : Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genet. 2015;11(10):e1005601. 10.1371/journal.pgen.1005601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Harris J: Germline Modification and the Burden of Human Existence. Camb Q Healthc Ethics. 2016;25(1):6–18. 10.1017/S0963180115000237 [DOI] [PubMed] [Google Scholar]
- 140. Saravelos SH, Regan L: Early pregnancy failure after assisted reproductive technology. Pregnancy after Assisted Reproductive Technology. 2012;51–65. 10.1017/CBO9780511902604.005 [DOI] [Google Scholar]
- 141. Jones DG, Towns CR: Navigating the quagmire: the regulation of human embryonic stem cell research. Hum Reprod. 2006;21(5):1113–6. 10.1093/humrep/dei461 [DOI] [PubMed] [Google Scholar]
- 142. Dupont C, Froenicke L, Lyons LA, et al. : Chromosomal instability in rhesus macaque preimplantation embryos. Fertil Steril. 2009;91(4):1230–7. 10.1016/j.fertnstert.2008.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Daughtry BL, Chavez SL: Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences. Cell Tissue Res. 2016;363(1):201–25. 10.1007/s00441-015-2305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shorten PR, Peterson AJ, O'Connell AR, et al. : A mathematical model of pregnancy recognition in mammals. J Theor Biol. 2010;266(1):62–9. 10.1016/j.jtbi.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 145. Vanneste E, Voet T, Le Caignec C, et al. : Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15(5):577–83. 10.1038/nm.1924 [DOI] [PubMed] [Google Scholar]
- 146. Teklenberg G, Salker M, Heijnen C, et al. : The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol Hum Repro. 2010;16(12):886–95. 10.1093/molehr/gaq079 [DOI] [PubMed] [Google Scholar]
- 147. Teklenberg G, Salker M, Molokhia M, et al. : Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5(4):e10258. 10.1371/journal.pone.0010258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Macklon N, Brosens JJ: The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91(4):98. 10.1095/biolreprod.114.122846 [DOI] [PubMed] [Google Scholar]
- 149. Ledbetter DH: Chaos in the embryo. Nat Med. 2009;15(5):490-1. 10.1038/nm0509-490 [DOI] [PubMed] [Google Scholar]
- 150. Potts M, Diggory P, Peel J: Spontaneous Abortion. Abortion Cambridge: Cambridge University Press;1977;45–64. Reference Source [Google Scholar]
- 151. Jarvis G: Dataset 1 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jarvis G: Dataset 2 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Jarvis G: Dataset 3 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jarvis G: Dataset 4 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]