Abstract
It is generally accepted that natural human embryo mortality during pregnancy is high – losses of 70% and higher from fertilisation to birth are frequently claimed. The first external sign of pregnancy occurs two weeks after fertilisation with a missed menstrual period. Establishing the fate of embryos before this is challenging, and hampered by a lack of data on the efficiency of fertilisation under natural conditions. Four distinct sources are cited to justify quantitative claims regarding embryo loss: (i) a hypothesis published by Roberts & Lowe in The Lancet is widely cited but has no quantitative value; (ii) life table analyses give consistent assessments of clinical pregnancy loss, but cannot illuminate losses at earlier stages of development; (iii) studies that measure human chorionic gonadotrophin (hCG) reveal losses in the second week of development and beyond, but not before; and (iv) the classic studies of Hertig and Rock offer the only direct insight into the fate of human embryos from fertilisation under natural conditions. Re-examination of Hertig’s data demonstrates that his estimates for fertilisation rate and early embryo loss are highly imprecise and casts doubt on the validity of his numerical analysis. A recent re-analysis of hCG study data suggests that approximately 40-60% of embryos may be lost between fertilisation and birth, although this will vary substantially between individual women. In conclusion, it is clear that some published estimates of natural embryo mortality are exaggerated. Although available data do not provide a precise estimate, natural human embryo mortality is lower than is often claimed.
Keywords: early pregnancy loss, occult pregnancy, embryo mortality, human chorionic gonadotrophin, Hertig, pre-implantation embryo loss
Introduction
It is widely accepted that under natural circumstances, human embryo mortality is high, particularly immediately after fertilisation. Quantitative estimates of embryo loss are found in diverse media including television documentaries (“You made it through the first round” presented by Michael Mosley: video at http://www.bbc.co.uk/timelines/z84tsg8; transcript at http://a.files.bbci.co.uk/bam/live/content/z3b87hv/transcript: accessed on 22 nd October, 2016), online educational videos (“Bill Nye: Can We Stop Telling Women What to Do With Their Bodies?” presented by Bill Nye, the Science Guy: video at https://www.youtube.com/watch?v=4IPrw0NYkMg: accessed on 22 nd October, 2016), online museum exhibits (“Who Am I? What happens in week 1?” presented by The Science Museum; available at http://www.sciencemuseum.org.uk/WhoAmI/FindOutMore/Yourbody/Wheredidyoucomefrom/Howdoyougrowinthewomb/Whathappensinweek1: accessed on 22 nd October, 2016), news reports (“Scientists get ‘gene-editing’ go-ahead” by James Gallagher: article at http://www.bbc.co.uk/news/health-35459054: accessed on 22 nd October, 2016), as well as academic philosophical articles 1 and legal judgements 2. Among reputable scientific publications, including medical and reproductive biology text books, scientific reviews and primary research articles, reported mortality estimates include: 30–70% before and during implantation 3; >50% 4, 73% 5 and 80% 6 before the 6 th week; 75% before the 8 th week 7; 70% in the first trimester 8; 40–50% in the first 20 weeks 9; and 49% 10, >50% 11, 12, 53% 13, 54% 14, 60% 15, >60% 16, 63% 17, 18, 70% 19– 23, 50–75% 24, 76% 5, 25, 78% 26, 80–85% 27, >85% 28, and 90% 29 total loss from fertilisation to term. The variance in these estimates is striking. 90% intrauterine mortality implies a maximal live birth fecundability of 10%, and only then if all other stages of the reproductive process are 100% efficient. Observed human fecundability is low compared to other animals 13, but at approximately 20–30% 4, 30 it is still higher than implied by such a high embryo mortality rate.
Early human embryo mortality is of interest not only to reproductive biologists and fertility doctors, but also to ethicists 31, theologians 32 and lawyers 2. Nevertheless, becoming pregnant and having children is of primary and personal importance to many women and their families. As with all biological processes, nothing works perfectly all the time 33, and failure to conceive and pregnancy loss are common problems. However, inconsistent estimates of early pregnancy loss are not reassuring, nor do they provide a sound basis for either a quantitative understanding of natural human reproductive biology or an unbiased appraisal of artificial reproductive technologies. The divergent and excessive values noted above therefore invite scrutiny of the evidence that supports them. In this article, I identify and re-evaluate published data that contribute to claims regarding natural human embryo mortality.
A quantitative framework for embryo mortality
A quantitative framework has been proposed to facilitate the calculation and comparison of embryo mortalities from fecundability and pregnancy loss data 34. The model comprises conditional probabilities ( π) of the following biological processes: (1) reproductive behaviours resulting in sperm-ovum-co-localisation per cycle = π SOC; (2) successful fertilisation given sperm-ovum-co-localisation = π FERT; (3) implantation of a fertilised ovum as indicated by increased levels of human chorionic gonadotrophin (hCG) = π HCG; (4) progression of an implanted embryo to a clinically recognised pregnancy = π CLIN; (5) survival of a clinical pregnancy to live birth = π LB.
Fecundability is the probability of reproductive success per cycle, but may take different values depending on the definition of success. The following four fecundabilities broadly follow Leridon 30:
-
1.
Total (all fertilisations): FEC TOT = π SOC × π FERT
-
2.
Detectable (implantation): FEC HCG = π SOC × π FERT × π HCG
-
3.
Apparent (clinical): FEC CLIN = π SOC × π FERT × π HCG × π CLIN
-
4.
Effective (live birth): FEC LB = π SOC × π FERT × π HCG × π CLIN × π LB
Hence, the probability that a fertilised egg will perish prior to implantation is [1 - π HCG], and prior to clinical recognition is [1 - ( π HCG × π CLIN)]. In theory, embryonic mortality may be estimated at different stages; however, in practice, this depends on available data. Clinical and live birth fecundabilities are most easily quantified and most frequently reported. Total and detectable fecundabilities are less frequently reported, although of direct relevance.
What the data say
Publications containing data relevant to early human embryo mortality were identified primarily by tracing citations found in articles, reviews and textbooks. Systematic online searches did not capture all of these studies. Some are particularly old, many were not conducted to address the specific question, and others are in books or publications that are not adequately indexed. If not entirely complete, nevertheless the data presented form a substantial proportion of relevant, available scientific information on natural early human embryo mortality.
Studies that contribute analysis and data relevant to the quantification of natural human embryo mortality fall into the following four categories and will be considered in turn.
-
1.
A speculative hypothesis published in The Lancet.
-
2.
Life tables of intra-uterine mortality.
-
3.
Studies of early pregnancy by biochemical detection of hCG.
-
4.
Anatomical studies of Dr Arthur Hertig and Dr John Rock.
1. Where have all the conceptions gone?
In 1975, a short hypothesis published in The Lancet entitled “ Where Have All The Conceptions Gone?” concluded that 78% of all conceptions were lost before birth 26. It has been widely cited by both scientists 4, 17, 19, 20, 35 and non-scientists 36, 37 alike. Conceptions among married women aged 20–29 in England and Wales in 1971 were estimated and compared to infants born in the same period. In this analysis ( Table 1) there are reliable values, e.g., census data, and simple arithmetical calculations. However, speculative values are necessary to perform the calculations. Three are biological: (1) fertilisation rate following unprotected coitus during the fertile period was estimated as 50% and supported by reference to Hertig 38 (although his estimate was 84% 33); (2) the length of a menstrual cycle (28 days); and (3) the duration of the fertile period (2 days). These latter values are plausible, but also variable. No justification is provided for three behavioural variables: (1) coital frequency estimated at twice per week; (2) proportion of unprotected coital acts estimated at 25%; and (3) either a random or regular distribution of coital acts during menstrual cycles such that 1/14 of all coital acts fall within a fertile period.
Table 1. Numerical estimates of conceptions and their loss in married women aged 20–29 in England and Wales in 1971.
The table replicates the values and calculations of Roberts & Lowe 26 with more explanatory detail. In addition, it illustrates how introducing variance into speculative estimates influences the final calculated value of embryo loss. *Data type indicates whether the numerical value is reliable (e.g., derived from census data), the result of a simple arithmetical calculation, or speculative (shown in italics). §Values are the 2.5 th and 97.5 th percentile boundaries, assuming a normal distribution for the variables centred on Roberts & Lowe’s values with a coefficient of variation of 20%. †Speculative values were adjusted either up or down by 25% compared to Roberts & Lowe’s values. Values for ‘Length of menstrual cycle’ were adjusted by 10%. ‡The median values of the 2.5 th and 97.5 th percentile boundaries from 1,000 simulations, each containing 10,000 separate estimates for embryo loss. The derivation of these values is described in the text. Briefly, each separate estimate of embryo loss was calculated using variable speculative values that were obtained by random sampling from a normal distribution with a mean equal to the Roberts & Lowe value and a coefficient of variation of 20%. The median value of the mean percentage loss was 73.3% and of the median was 76.5%. ¥The most frequent duration of a menstrual cycle is 28 days but there is substantial variability and the mean length is generally 30–31 days 30.
| Description of data | Data type* | Roberts &
Lowe values |
Low estimate
values † |
High estimate
values † |
95% data range
(CV = 20%) § |
|---|---|---|---|---|---|
| Married women aged 20–29
in 1971 |
Reliable value | 2,437,000 | 2,437,000 | 2,437,000 | - |
|
Frequency of coitus per
married woman per week |
Speculative
value |
2 | 1.5 | 2.5 | [1.2, 2.8] |
| Weeks per year | Reliable value | 52 | 52 | 52 | - |
| Acts of coitus among
married women per year |
Calculation | 253,448,000 | 190,086,000 | 316,810,000 | - |
|
Percentage of acts of coitus
that are unprotected |
Speculative
value |
25% | 19% | 31% | [15%, 35%] |
| Acts of unprotected coitus
per year |
Calculation | 63,362,000 | 35,641,125 | 99,003,125 | - |
|
Length of menstrual cycle
(days) |
Speculative
value |
28 | 31 ¥ | 25 | [17, 39] |
|
Length of fertile period in
each cycle (days) |
Speculative
value |
2 | 1.5 | 2.5 | [1.2, 2.8] |
| Acts of unprotected coitus
during fertile period per year |
Calculation | 4,525,857 | 1,735,769 | 9,821,739 | |
| Probability of fertilisation |
Speculative
value |
50% | 38% | 63% | [30%, 70%] |
| Total fertilised ova per year | Calculation | 2,262,929 | 650,913 | 6,138,587 | - |
| Number of infants born (live
and still) in 1971 |
Reliable value | 505,000 | 505,000 | 505,000 | - |
| Total number of lost embryos
in 1971 |
Calculation | 1,757,929 | 145,913 | 5,633,587 | - |
|
Percentage of embryos
lost before live birth |
Calculation | 78% | 22% | 92% | [37%, 90%] ‡ |
The validity of Roberts & Lowe’s conclusion depends largely on the accuracy and precision of these speculative values. The following two simple analyses illustrate the sensitivity of their conclusion on the speculative values.
-
1.
When four of the speculative values are reduced by 25% (e.g., coital frequency reduced to 1.5/week) and cycle length increased by 10% (from 28 days to 31 days 30), the estimate for embryo loss drops to 22%. The opposite operation (e.g., coital frequency increased to 2.5/week) results in an estimate of 92% ( Table 1). Embryo loss of 22% is barely sufficient to account for observed clinical losses, and 92% indicates a maximum FEC LB of 8%. Neither scenario is biologically plausible.
-
2.
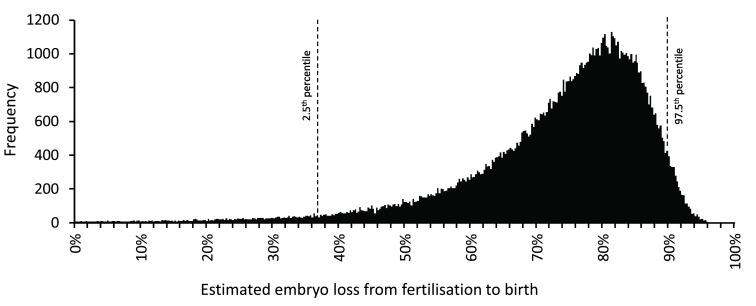
A non-zero variance was applied to each speculative value reflecting their uncertain nature. Using the random number generator in Microsoft ® Excel (Office 2010) simulated values were obtained by random sampling from normal distributions with means equal to Roberts & Lowe’s speculative values with coefficients of variation equal to 20%. For simplicity, it was assumed that there was no covariance between the different speculative values. Table 1 shows the expected range within which 95% of these simulated values fall (e.g., coital frequency is 1.2–2.8/week). For each simulated record, a new estimate of embryo loss was calculated and from 10,000 of these, the mean, median and 2.5 th and 97.5 th percentiles of embryo loss were determined. This was repeated 1,000 times: the mean value of the simulated means was 73.3% and of the simulated medians was 76.5%. The mean values of the 2.5 th and 97.5 th percentile boundaries for embryo loss were 37% and 90% ( Table 1). The same simulation was also performed using NONMEM 7.3.0 ® (Icon PLC, Dublin, Eire) and generated 100,000 data records. The outcome of this is shown in Figure 1. The code and simulated data values are in Dataset 1.
Figure 1. Distribution of embryo loss estimates from fertilisation to birth derived using a modified version of the model of Roberts & Lowe 26.
Embryo loss values were calculated using alternative speculative values (see text and Table 1) obtained by randomly sampling from normal distributions with mean values equal to the Roberts & Lowe’s values with a coefficient of variation of 20%. 100,000 simulated embryo loss values were obtained. Frequencies within a bin size of 0.25% are shown. The 2.5 th and 97.5 th percentiles are indicated. The simulation was performed using NONMEM 7.3.0® (Icon PLC, Dublin, Eire). Simulated values are in Dataset 1.
See README.docx for a description of the file.
Copyright: © 2016 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The sole purpose of these simple sensitivity analyses is to illustrate that modest adjustments to Roberts & Lowe’s original speculative values can result in any biologically plausible estimate for embryo loss. The output from the calculation is therefore substantially dependent on the subjectively selected input. Such an analysis has no practical quantitative value.
Other sources of bias in their model include the failure to account for intentionally terminated pregnancies and the reduced fecundability of already pregnant women and nursing mothers. Despite this, it was described as “ persuasive” 39 and it has been claimed that “ it is still difficult to better the original calculations of Roberts and Lowe (1975)” 19. By contrast, others have noted that “ their calculations can be criticized” 4 and are “ tenuous” 40. Considering its quantitative limitations, it has been cited surprisingly often 8, 20, 41.
2. Life tables of intrauterine mortality
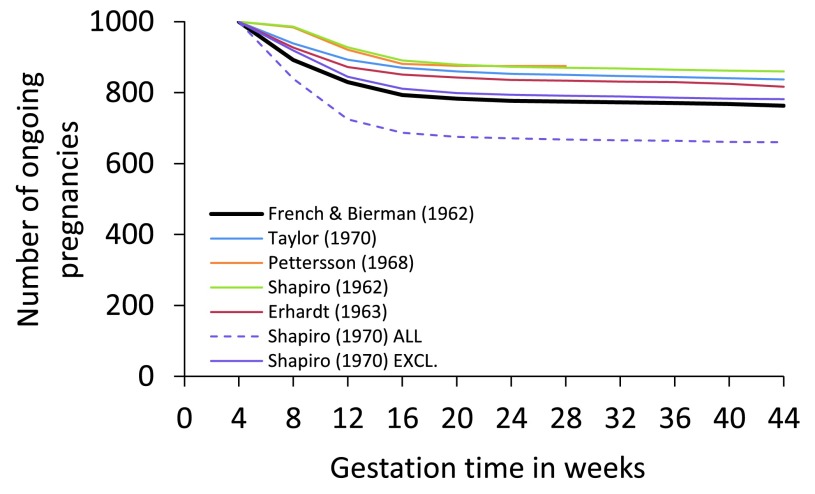
Constructing a life table of intrauterine mortality is challenging since embryonic death may occur even before the presence of an embryo is recognised. Nevertheless, in 1977 Henri Leridon published a complete life table of intrauterine mortality 18. Leridon highlighted the consequences of inappropriate analysis and the quantitative biases produced by alternative numerical methods. Overall, he discussed sixteen studies, and provided detailed commentary on six 42– 47. These data are summarised in Figure 2 and suggest that 12–24% embryos alive at 4 weeks’ gestation (i.e., approx. 2 weeks’ post-fertilisation) will perish before birth.
Figure 2. Graphical representation of the fate of 1,000 pregnancies in progress at 4 weeks’ gestation (2 weeks’ post-fertilisation).
The figure is generated using values in Table 4.3 of Leridon 18 and are derived from six different studies (see text). The Kauai Pregnancy Study data 42 are shown in thick black. Data from Shapiro (1970) 46 were analysed either with all pregnancies included (ALL) or with those pregnancies excluded that aborted within one week of study entry (EXCL.). The greater loss observed with ALL may be due to a correlation between study entry and abortion risk. Based on these data, the risk of losing a pregnancy ongoing at 4 weeks’ gestation ranges from 12.5% to 23.7% (excluding Shapiro (1970) ALL). Values are in Dataset 2.
See README.docx for a description of the file.
Copyright: © 2016 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Leridon described the Kauai Pregnancy Study 42 in particular detail. In this study, an attempt was made to identify every pregnancy on Kauai from 1953–56. Women were encouraged to enrol as soon as they missed a period. Early pregnancy loss may therefore have been overestimated, since not all amenorrhoea is caused by conception, although other studies that relied upon medically-identified pregnancies probably underestimated early pregnancy loss by not capturing all cases 48. Whatever the truth, it is clear that, among the studies reviewed by Leridon, the Kauai Pregnancy Study revealed the highest levels of pregnancy loss ( Figure 2).
All recorded pregnancies in the Kauai study were categorised by date of enrolment in four week intervals, beginning with 4–7 weeks’ gestation. This time-staggered approach enabled risk of miscarriage to be associated with stage of gestation. However, despite considerable efforts, only 19% of the 3,197 recorded Kauai pregnancies were enrolled between 4–7 weeks’ gestation, thereby reducing the precision of pregnancy loss estimates for this earliest of time intervals. Although pregnancies were grouped in four week periods, Leridon suggested that early mortality may change week by week, resulting in underestimation of pregnancy loss. He re-allocated the 592 study entries and 32 pregnancy losses for weeks 4–7 ( Table 2) generating an overall probability of pregnancy loss during this period of 15.0%, higher than 10.8% originally reported 42. Leridon’s own description of this interpolation as “ risky” can be illustrated by adjusting his re-allocation 18. Transferring just two of the pregnancy losses out of or into the first week results in estimates of the 4–7 week pregnancy loss of 10.9% and 19.1% respectively ( Table 2). The validity of adjusting Leridon’s re-allocation may be questioned. However, pregnancy loss in week 4–5 of the Kauai Study would manifest as a menstrual period delayed by up to one week. This is far from being a robust pregnancy diagnosis and in different study 46, exclusion of pregnancy losses reported within one week of study entry resulted in substantially different loss probabilities ( Figure 2) suggesting a confounding correlation between entry and loss 18. Nevertheless, the re-allocation does reinforce a concern highlighted by Leridon, namely the uncertainty that affects the first probability. Clearly, these estimates of early loss should be treated with caution.
Table 2. A speculative numerical re-allocation of entries and pregnancy losses during weeks 4–7 in the Kauai Pregnancy Study (KPS) 42.
Minor differences in the re-allocation of the earliest pregnancy losses have a substantial effect on the overall measure of pregnancy loss for that period. (Adapted from Table 4.2 in Leridon 18.)
| Time period
of gestation |
New entries into study
in each time period |
Actual pregnancy losses
in each time period |
% pregnancy loss in
each time period |
Surviving pregnancies
in each time period |
||||
|---|---|---|---|---|---|---|---|---|
| Leridon’s re-
allocation |
KPS | Leridon’s re-
allocation & [variants] |
KPS | Leridon’s re-
allocation & [variants] |
KPS | Leridon’s re-
allocation & [variants] |
KPS | |
| 4–5 | 80 | 592 | 2 [0, 4] | 32 | 5.0 [0.0, 10.0] | 10.8 | 100 [100, 100] | 100 |
| 5–6 | 120 | 6 [6, 6] | 4.3 [4.3, 4.4] | 95 [100, 90] | ||||
| 6–7 | 180 | 10 [11, 9] | 3.5 [3.9, 3.2] | 91 [96, 86] | ||||
| 7–8 | 212 | 14 [15, 13] | 3.0 [3.2, 2.8] | 88 [92, 83] | ||||
| 4–8 | 85 [89, 81] | 89.2 | ||||||
| % loss | 15.0 [10.9, 19.1] | 10.8 | ||||||
A more fundamental problem is that these data offer no insight into the fate of embryos prior to the earliest possible point of clinical pregnancy detection. Leridon completed his life table with values from Hertig’s analysis 33. He concluded that among 100 ova exposed to the risk of fertilisation, 16 are not fertilised, 15 die in week one (before implantation), and 27 die in week two (before the menstrual period). After two weeks his life table follows the Kauai probabilities closely ending with 31 live births. Leridon’s table therefore indicates an embryo mortality of 50% (42/84) within the first two weeks after fertilisation and a total mortality of 63% (53/84) from fertilisation to birth.
Leridon’s account of intrauterine mortality has been widely cited. However, its accuracy depends entirely on the quality and interpretation of the data from Hertig 33 and French & Bierman 42. French & Bierman’s approach probably resulted in an overestimate of total pregnancy loss and is certainly imprecise in its estimate of embryo loss in the four weeks following the first missed menstrual period. The reliability of Hertig’s estimates of embryo loss in the two weeks following fertilisation is considered below.
3. Biochemical detection of pregnancy using hCG
Quantification of pregnancy loss requires pregnancy diagnosis. The earliest outward sign of pregnancy is a missed menstrual period, approximately 2 weeks after fertilisation, although amenorrhoea in women of reproductive age is not exclusively associated with fertilisation 49, 50. Several potentially diagnostic pregnancy-associated proteins have been identified 51 of which only one, Early Pregnancy Factor (EPF) 52, has been claimed to be produced by embryos within one day of fertilisation. However, there is doubt about the utility of EPF for diagnosing early pregnancy 53 and little has been published on it in the past five years.
Modern pregnancy tests detect human chorionic gonadotrophin (hCG), a highly glycosylated 37 kDa protein hormone produced by embryonic trophoblast cells 54. Mid-cycle elevation of hCG is associated with embryo implantation 19, 20, 55. Early assays for the detection of hCG were probably confounded by antibody cross-reactivity with luteinizing hormone 56 but modern tests are more specific and a positive result is a reliable indicator of early pregnancy. Highly sensitive assays have revealed low levels of hCG in non-pregnant women and healthy men 57; hence, quantitative criteria are required to distinguish between non-pregnant women and those harbouring early embryos 55.
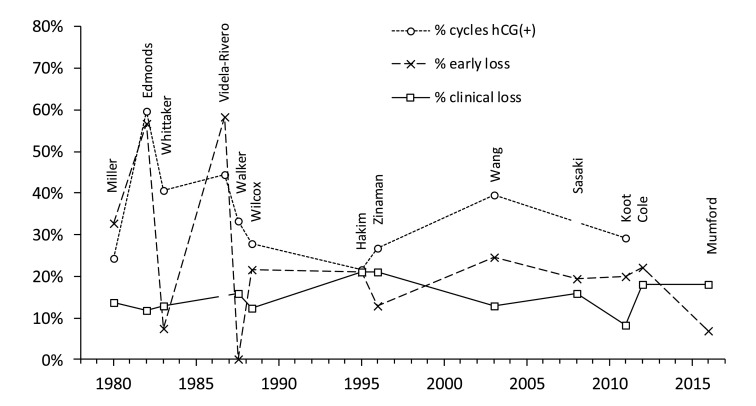
Figure 3 and Table 3 summarise findings from thirteen studies that used hCG to identify so-called early, occult or biochemical pregnancy loss, i.e., pregnancy loss between the initiation of implantation and clinical recognition 58– 70. Notwithstanding design and subject differences, estimates for clinical pregnancy loss, ranging from 8.3% - 21.2% ( Figure 3), are similar to previous estimates ( Figure 2). Estimates for early/occult loss ranged from 0% to 58.3% in studies 58– 62 prior to Wilcox in 1988 63. This high variance was probably due to reduced specificity and sensitivity of the hCG assays and sub-optimal study design 48, 51, 71– 74. Studies from 1988 63 onwards have produced more consistent data indicating early/occult loss of approximately 20% ( Figure 3). In the three largest studies 63, 66, 70 pregnancies were clinically recognised only if they lasted ≥6 weeks after the onset of the last menstrual period 66, 75. Hence, early pregnancy losses in these studies included those lost up to approximately two weeks after a missed menstrual period: this may influence comparison of study results 34, 73. An overview of the thirteen studies suggests that overall pregnancy loss from first detection of hCG through to live birth is approximately one third ( Table 3). This is consistent with another recent study which found that 98 out of 301 (32.6%) singleton pregnancies diagnosed by an early positive hCG test and followed-up to either birth or miscarriage were lost 76.
Figure 3. Summary of findings from thirteen studies that used hCG detection to diagnose early pregnancy.
Data are arranged by publication date and the first author of the study is shown. Three datasets are shown: (i) the percentage of at risk reproductive cycles that were hCG positive; (ii) the percentage of hCG positive cycles that did not manifest as clinical pregnancies = early pregnancy loss; and (iii) the percentage of clinical pregnancies lost prior to 12 or 28 weeks or live birth (definitions vary between studies). A clinical pregnancy may be manifest by a missed period although criteria vary between studies. Videla-Rivero et al. 61, Sasaki et al. 67, Cole 69 and Mumford et al. 70 do not report sufficient data to calculate all three values. Values are in Dataset 3.
Table 3. Summary data from thirteen studies using hCG detection to diagnose pregnancy and identify early pregnancy loss.
Raw FEC HCG is the ratio of hCG pregnancies detected and the number of cycles monitored in each study. Where available, mean (SD) ages of the participating women are taken directly from the published study. In some cases mean and SD (indicated by *) or SD (indicated by †) were estimated based on published demographic characteristics. §These data relate to the whole study cohort (n=124) which included known sub-fertile women, and not just to the 74 apparently fertile women. ‡Mean value from Wilcox et al. (2001) 78. ¶Some studies only provide data up to late pregnancy (e.g., up to 28 weeks) rather than to term. ND = no data. ¤Wilcox subsequently reported an additional hCG pregnancy which had not been detected and reported in the 1988 paper, making a total of 199 hCG pregnancies and 44 pre-clinical losses in the study group 75. #Mumford reported data from aspirin- and placebo-treated subjects who had at least one prior miscarriage. Summary data from both treatment groups are included as there was no effect of aspirin 70.
| First
author |
Year | Number
of women |
Age mean
(SD) [range] |
Number
of cycles |
hCG
pregnancies detected |
Raw
FEC HCG |
Clinical
pregnancies detected |
% survival
from hCG to clinical detection |
% loss
from hCG detection to live birth ¶ |
|---|---|---|---|---|---|---|---|---|---|
| Miller | 1980 | 197 | 27 (4)* | 623 | 152 | 24.4% | 102 | 67.1% | 42.4% |
| Edmonds | 1982 | 82 | 27 (4)* | 198 | 118 | 59.6% | 51 | 43.2% | 61.9% |
| Whittaker | 1983 | 91 | 30 (3.7) † | 226 | 92 | 40.7% | 85 | 92.4% | 19.6% |
| Videla-Rivero | 1987 | 27 | ND | 27 | 12 | 44.4% | 5 | 41.7% | ND |
| Walker | 1988 | 38 | 27.4 [22–38] | 75 | 25 | 33.3% | 25 | 100% | 16.0% |
| Wilcox | 1988 | 221 | 30 ‡ (4)* | 707 | 198 ¤ | 28.0% | 155 | 78.3% | 31.3% |
| Hakim | 1995 | 74 | 31 (3)* § | 305 | 66 | 21.6% | 52 | 78.8% | 37.9% |
| Zinaman | 1996 | 200 | 30.6 (3.3) | 432 | 116 | 26.9% | 101 | 87.1% | 31.3% |
| Wang | 2003 | 518 | 24.9 (1.7) | 1,561 | 618 | 39.6% | 466 | 75.4% | 35.7% |
| Sasaki | 2008 | 110 | [21–36] | ND | 62 | ND | 50 | 80.6% | 32.3% |
| Koot | 2011 | 46 | 28.7 (3.3) | 103 | 30 | 29.1% | 24 | 80.0% | 26.7% |
| Cole | 2012 | 168 | 28.8 (4.4) | ND | 127 | ND | 99 | 78.0% | 36.2% |
| Mumford | 2016 | 1088 # | 28.7 (4.8) | ND | 785 | ND | 730 | 93.0% | 23.9% |
See README.docx for a description of the file.
Copyright: © 2016 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The much cited Wilcox study 63 is the earliest of several large well-designed studies that made use of a specific and sensitive hCG assay and led to numerous further publications 75, 77– 83. Two other studies (Zinaman 65 and Wang 66) were similar in purpose, design and execution. These studies provide some of the best available data to calculate pregnancy loss between implantation and birth 34. In each study, women intending to become pregnant and with no known fertility problems were recruited and hCG levels monitored cycle by cycle in daily urine samples until they became pregnant. Most women were followed through to late pregnancy or birth. Although these studies provide evidence regarding the outcome of both clinical and hCG pregnancies, determining the fate of embryos prior to implantation is more difficult. To relate the study results to pre-implantation embryo loss, it is necessary to determine fecundability. In each study FEC CLIN declined in successive cycles as the proportion of sub-fertile women increased. Hence, reported FEC HCG values of 30% 65 and 40% 66, and FEC CLIN values of 25% 63 and 30% 66 are biased underestimates of the fecundability of normal fertile women. A recent re-analysis of these data provides statistical evidence for discrete fertile and sub-fertile sub-cohorts within the study populations 34. The proportions of sub-fertile women (mean [95% CI]) were estimated as 28.1% [20.6, 36.9] (Wilcox); 22.8% [12.9, 37.2] (Zinaman); and 6.0% [2.8, 12.3] (Wang). For normally fertile women, FEC HCG was, respectively: 43.2% [35.6, 51.1]; 38.1% [32.7, 43.7]; and 46.2% [42.8, 49.6]. FEC CLIN was: 33.9% [29.4, 38.6]; 33.3% [27.6, 39.6]; and 34.9% [33.0, 36.8]. There was no apparent difference in π CLIN between fertile and sub-fertile sub-cohorts, which was estimated as: 78.3% [69.2, 85.3]; 87.5% [76.0, 93.9]; and 75.4% [71.5, 79.0] 34.
Why do a proportion of menstrual cycles in women attempting to conceive fail to show any increase in hCG? Since FEC HCG = π SOC × π FERT × π HCG, there can be various causes for this failure including mistimed coitus, anovulation, failure of fertilisation or pre-implantation embryo death. Although FEC HCG puts limits on the extent of pre-implantation embryo loss, uncertainty in the estimates of π SOC, π FERT and π HCG translates into uncertainty in estimates of pre-implantation embryo mortality. In the Wang study, for normally fertile women, FEC HCG = 46.2%; hence, the absolute maximum value for pre-implantation embryo loss must be 53.8%, although only if π SOC = π FERT = 1, conditions both extreme and unlikely 34. Studies of the relationship between coital frequency and conception indicate that fecundability is greater with daily compared to alternate day intercourse 34, 84, 85. Hence, when coital frequency is less than once per day a proportion of reproductive failure will be due to mistimed coitus, i.e., π SOC < 1. In the Wilcox study, coitus occurred on only 40% of the six pre-ovulatory days 34, 79, and in the Zinaman study participants were advised that alternate day intercourse was optimal 65. Based on the difference in fecundability between daily and alternate day intercourse as modelled by Schwartz 85, a value of π SOC = 0.80 was used to calculate pre-implantation embryo mortality 34. However, this is a speculative estimate, and in reality the value may be higher, or lower.
A further critical missing piece of the equation is knowledge of the efficiencies of fertilisation and implantation under normal, natural, propitious circumstances. Assuming that either of these processes may be up to 90% efficient, and based on data from the three hCG studies 63, 65, 66, a plausible range for pre-implantation embryo loss in normally fertile women is 10–40% and for loss from fertilisation to birth, 40–60% 34. Even with these wide ranges of mathematically possible outcomes, it is clear that estimates for total embryonic loss of 90% 29, 85% 28, 83% 31, 80–85% 6, 27, 78% 26, 76% 5, 25 and 70% 19– 23 are excessive.
A previous review concluded that “ at least 73% of natural single conceptions have no real chance of surviving 6 weeks of gestation” 5, 86. Live birth fecundability was estimated as “ not over 15%”, substantially lower than Leridon’s 31%. Despite this discrepancy, Boklage’s conclusions were derived from a review of data including several hCG studies 55, 58– 61, 63 and Leridon’s analysis 18. He derived a model describing the survival probability of human embryos comprising the sum of two exponential functions:
in which t is the time in days post-fertilization. This is the source of the 73% in the conclusion.
There are, however, serious problems with this analysis. Firstly, data presented as embryo survival probabilities at different times post-fertilization 55, 58, 59, 61, 63 are fecundabilities, i.e., successes per cycle, not per fertilised embryo. Secondly, for reasons that are unclear, data from Whittaker 60 and Leridon 18 were excluded from the modelling analysis and the data from an earlier Wilcox report 55 were included twice since this preliminary data had been incorporated into the later report 63. Thirdly, the modelled data were normalised to a survival probability of 0.287 at 21 days post-fertilization. This value was derived from data published by Barrett & Marshall on the relationship between coital frequency and conception 84. Barrett & Marshall had concluded that coitus during a single day alone, 2 days before ovulation resulted in a conception probability of 0.30. Boklage’s value of 0.287 is his calculated equivalent. However, conception in this study was “ identified by the absence of menstruation, after ovulation” 84. Hence, 0.30 (and similarly, 0.287) is a clinical fecundability and not a measure of embryo survival. Furthermore, 0.30 is a non-maximal fecundability, since it was an estimate based on coitus on a single day (2 days before ovulation) within the cycle. Barrett & Marshall clearly report that as coital frequency increased so did the fecundability, up to a maximum of 0.68 associated with daily coitus 84.
Boklage’s analysis can only make biological sense if it is assumed that every cycle in the Barrett & Marshall study resulted in fertilisation. Under these circumstances, failure to detect conception in 71.3% (1 – 0.287) of cycles would be due entirely to embryo mortality. However, this is highly implausible and explicitly contradicted by the higher estimate of fecundability reported 84. Boklage’s implicit assumption also contradicts his further conclusion that “ only 60–70% of all oocytes are successfully fertilized given optimum timing of natural insemination” 5. The vertical normalisation of the hCG study data to a value of 0.287 at 21 days is the principal determinant of the parameters that define the two exponential model. Any change in this value would commensurately alter the balance between the two implied sub-populations of embryos. Since it is evident that the value of 0.287 is neither an embryo survival rate nor even a maximal fecundability, it follows that quantitative conclusions from this analysis in relation to the survival of naturally conceived human embryos are of doubtful validity.
However, Boklage is right about two things: firstly, the difficulty of calculating pre-clinical losses, because “ In the place of the necessary numbers for the first few weeks of pregnancy we find editorially acceptable estimates which, while perhaps not far wrong, are difficult to defend with any precision”, and secondly, that the source of some of the only directly relevant data (even though he excluded it from his modelling analysis), namely, “ Hertig’s sample is, and will probably remain, unique”.
4. The anatomical studies of Dr Arthur Hertig
At the start of the 1930s, no-one had ever seen a newly fertilised human embryo. It was barely 60 years since Oscar Hertwig had first observed fertilisation in sea urchins 87, and just 40 years before the birth of the first test tube baby 88, 89. In Boston, Dr Arthur Hertig and Dr John Rock’s search to find early human embryos generated an irreplaceable collection which has left an indelible mark on our understanding of human embryology.
Hertig and Rock recruited 210 married women of proven fertility who presented for gynaecological surgery 38. (In most of their publications, the number is given as 210 33, 90, 91 although 211 subjects are mentioned elsewhere 38.) Of these, 107 were considered optimal for finding an embryo because they apparently: (i) demonstrated ovulation; (ii) had at least one recorded coital date within 24 hours before or after the estimated time of ovulation; (iii) lacked pathologic conditions that would interfere with conception. Hertig examined the excised uteri and fallopian tubes, and over fifteen years found 34 human embryos aged up to 17 days 33, 38, 90– 97. Of these, 24 were normal and 10 abnormal 33, 90. (There is some confusion over this: in three publications 38, 91, 97, 21 embryos are described as normal and 13 as abnormal. It appears that the three alternatively described embryos (C-8299; C-8000; C-8290) were originally defined as abnormal based on their position or depth of implantation 38.) Table 4 provides information about the 34 embryos found in these 107 women. Although the study was primarily intended to find and describe early human embryos, Hertig subsequently used the data to derive estimates of reproductive efficiency including early embryo wastage 33, 90.
Table 4. Summary of the characteristics of Hertig’s 34 embryos (values are taken from Figure 4 in Hertig et al. (1959)).
The embryos were collected from 107 out of 210 women. *In Hertig’s figure, day 28 of the ovulatory cycle is identified with day 1 of the next cycle and is the day of the presumed missed period in cases where pregnancy had commenced. The 36 cases that provide the evidential foundation for his numerical analysis are shown in bold.
| Day of
cycle |
Biological description/
stage |
Approx. age
of embryos (days) |
Number
of cases |
Embryos
found |
Normal
embryos |
Abnormal
embryos |
Detection
rate (%) |
|---|---|---|---|---|---|---|---|
| 14 | Ovulation ± fertilisation | 0 | 0 | 0 | 0 | 0 | |
| 16–17 | Embryo suspended in
fallopian tube |
2–3 | 9 | 1 | 1 | 0 | 11.1% |
| 18–19 | Embryo suspended in
uterus |
4–5 | 15 | 7 | 3 | 4 | 46.7% |
| 20–24 | Implantation | 6–10 | 47 | 5 | 5 | 0 | 10.6% |
| 25–3 |
First missed period on
day 28/1* |
11–16 | 36 | 21 | 15 | 6 | 58.3% |
| Total | 107 | 34 | 24 | 10 | 31.8% |
Hertig’s analysis 33, 90 relies heavily on the 15 normal and 6 abnormal implanted embryos found in 36 women from cycle day 25 onwards. He assumed the 6 abnormal embryos would perish around the time of the first period concluding that fertility (% pregnant) at this stage = 42% (15/36). Of the 8 pre-implantation embryos identified (7 in the uterus and 1 in the fallopian tubes), 4 were abnormal. Hertig assumed the 4 normal embryos would implant successfully but that some of the abnormal ones would not, such that the proportion of normal embryos would increase from 50% (4/8) before implantation to 71% (15/21) after implantation as observed. Hence, among the 36 post-cycle day 25 cases, in addition to the 15 normal embryos, there must have been 15 abnormal pre-implantation embryos of which 60% (9/15) failed to implant and were not observed, and 40% (6/15) did implant and were observed, although these 6 would have perished shortly afterwards. This left 6/36 eggs that must have been unfertilised. The ratio of ‘unfertilised’ : ‘fertilised abnormal’ : ‘fertilised normal’ was therefore 6:15:15, matching the 16% infertility (no fertilisation), 42% sterility (post-fertilisation death) and 42% fertility (reproductive success) reported in Figure 9 of Hertig’s article, “ The Overall Problem in Man” 33. This is the source of Hertig’s 84% fertilisation rate and 50% embryo loss before and during implantation, and is reproduced in Leridon’s life table 18 as 84/100 eggs surviving at time zero (ovulation and fertilisation) and 42 surviving to 2 weeks (time of first missed period).
Hertig provides almost the entire body of evidence used to quantify natural human embryo loss in the first week post-fertilisation. Most claims regarding early human embryo mortality find their source here. Before considering how reliable the figures are, it is worth repeating Hertig’s own caveat, namely, the lack of data on the efficiency of natural fertilisation 33. All estimates of embryo mortality from fertilisation onwards are subject to commensurate inaccuracy in the absence of reliable fertilisation probabilities (i.e., π FERT), which are “ surprisingly difficult to estimate” 13.
There are several problems with Hertig’s analysis. As noted by others, the observations are cross-sectional, but the inferences are longitudinal 48. Hertig detected 21 embryos from 36 cases (58.3%) from cycle day 25 onwards. If this detection rate were representative, then on average, prior to day 25, the detection rate should either be the same or higher; however, they are all lower, and substantially so ( Table 4). Hertig suggested that this was due to the technical difficulty of finding newly fertilised embryos. However, the detection rate for cycle days 18–19 was good (46.7%) and embryos one or two days younger would not have been much smaller, at which stage the detection rate was poor (11.1%). An alternative explanation for this discrepancy might simply be random variation. Furthermore, from cycle day 25 onwards, embryos would probably have produced hCG and therefore FEC HCG would have been at least 58%. This is approximately double the equivalent values observed in more recent and robust hCG studies ( Table 3) further suggesting that this subset of the data is not representative.
Despite having proven fertility, these women presented with gynaecological problems, suggesting sub-optimal reproductive function. Furthermore, Hertig’s reproductively ‘optimal’ coital pattern does not include 2 days pre-ovulation and does include one day post-ovulation, conditions which are known not to maximise fertilisation 34, 79, 84, 85, 98. Hence, detection rates before cycle day 25 may be more representative than those after. Given the numerical discrepancies, they cannot both be.
Hertig does not provide error estimates with his conclusions. In order to estimate the precision of his derived proportions, a bootstrap analysis was performed as follows: Hertig’s 107 optimal cases were categorised according to stage of cycle (Category 1 = cycle days 16–19 (n=24); Category 2 = cycle days 20–24 (n=47); Category 3 = cycle days ≥25 (n=36)), and presence and type of embryos (Category 0 = no embryo (n=73); Category 1 = normal embryo (n=24); Category 3 = abnormal embryo (n=10)). Five hundred pseudo-datasets each containing 107 cases were generated using a balanced random re-sampling method using Microsoft Excel ®. The original and pseudo datasets are in Dataset 4.
See README.docx for a description of the files.
Copyright: © 2016 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Hertig’s numerical calculations, as detailed above, were repeated for each pseudo-dataset thereby generating 500 estimates for each parameter, from which median values and [95% CIs] were derived: fertility = 42% [26%, 59%]; sterility = 42% [5%, 182%]; infertility = 16% [-127%, 61%]; pre-implantation embryo survival probability = 69% [27%, 128%]; post-implantation to week two survival probability = 71% [50%, 91%]; detection rate for cycle day 25 onwards = 58% [41%, 74%]. Median values matched estimates calculated from the original dataset. Bootstrap 95% CIs for the day 25 detection rate (58%) matched those calculated using the “exact” method of Clopper & Pearson 99, [41%, 74%], which are a little wider than those calculated using the “more exact” method of Agresti & Coull 100, [42%, 73%]. (These analyses was performed using an online GraphPad ® calculator accessed on 21 st October 2016: http://www.graphpad.com/quickcalcs/ConfInterval1.cfm.) The congruence between these confidence intervals and the point estimates provides some reassurance that that the bootstrap procedure worked effectively. Estimates of parameters other than the day 25 detection rate (58%) are derived from more complex proportional relationships, and are therefore less precise. Table 5 reproduces a life table in the style of Leridon 18 and includes probabilities for each reproductive step with confidence intervals. These intervals (and some noted above) are impossibly wide highlighting further problems with Hertig’s analysis.
Table 5. Life Table of egg survival and probabilities during the first two weeks of development derived solely from Hertig’s data.
The table is modelled on Leridon’s life table 18 and includes his values for survivors and data from Hertig 33. Probabilities are also shown for each stage of the early development process. Medians and 95% confidence intervals derived from a bootstrap analysis of Hertig’s data indicate the precision in the estimates for fertilisation and embryo loss in the first two weeks. *Although Leridon’s values are based on Hertig, they do not fully match. Leridon reports losses of 15 and 27 in the first and second weeks respectively. However, Hertig’s 60% loss of abnormal pre-implantation embryos implies 25 (0.6 7#215; 42) losses in the first week leaving 58, and 16 (58 7#215; (6/21)) losses in the second week, leaving 42. ¥A value of π SOC = 0.90 was used to avoid the calculation of probabilities greater than 1.
| Week after Ovulation | Biological Description | Survivors (Leridon 18) | Survivors (Hertig 33) | Bootstrap Median
[95% CIs] |
|---|---|---|---|---|
| Number of Cycles | 100 | 100 | 100 [100, 100] | |
| 0 | Fertilised Eggs | 84 | 83 | 84 [39, 227] |
| 1 | Implanted Embryos | 69* | 58 | 58 [41, 74] |
| 2 | Missed First Period | 42 | 42 | 42 [26, 59] |
| Probabilities | Biological Description | Probabilities | Probabilities |
Bootstrap Median
[95% CIs] |
| π SOC × π FERT | Fertilisation per cycle | 0.84 | 0.83 | 0.84 [0.39, 2.27] |
| π FERT (when π SOC = 0.90 ¥) | Fertilisation per ideal insemination | 0.93 | 0.93 | 0.93 [0.43, 2.52] |
| π HCG | Fertilised egg implanting | 0.82* | 0.70 | 0.69 [0.27, 1.28] |
| π CLIN | Implanted egg to clinical
recognition |
0.61* | 0.71 | 0.71 [0.50, 0.91] |
| π HCG × π CLIN | Fertilised egg to clinical
recognition |
0.50 | 0.50 | 0.50 [0.20, 0.88] |
Hertig’s analysis omits 47 cases from cycle days 20–24, comprising 44% of his data. It is clear why he cannot use it, since all five embryos were normal and, given his mathematical and biological assumptions, five normal implanting embryos could not become 29% (6/21) abnormal post-implantation. Furthermore, the data that define the 50% proportion of abnormal pre-implantation embryos (i.e., 4/8) are so few that any numerical variation will make a substantial difference to derived proportions. If he had observed 3/8 abnormal embryos, his estimate of pre-implantation loss would have been 13% rather than 30%: for 5/8 it would have been 48%, with a fertilisation rate of 111%, which is clearly impossible. It seems therefore, that Hertig designed his analysis based on a post-hoc examination and selective use of the data. His own caveat about the lack of relevant and necessary data should be taken at least as seriously as his conclusions.
Hertig and Rock’s contribution to human embryology is undeniable. However, their quantitative conclusions regarding early embryo mortality have a low precision that undermines their biological credibility or utility. Such estimates cannot be regarded as a reliable foundation upon which to evaluate and understand natural human reproduction.
Discussion
Answering the question “ How many fertilised human embryos die before or during implantation under natural conditions?” is difficult. Relevant, credible data are in short supply. Among regularly cited publications, the Lancet hypothesis 26 is entirely speculative and in the view of the current author should cease to be used as an authoritative source. Clinical pregnancy studies are only useful for quantifying clinical pregnancy loss and contribute nothing to estimates of embryo mortality in the first two weeks’ post-fertilisation. Even Hertig’s unique dataset is inadequate to draw quantitative conclusions and oft-repeated values should be treated with scepticism. The hCG studies from 1988 onwards provide the best data for estimating embryo mortality although a lack of information on fertilisation rates 13, 15, 33, 48, 101 prevents satisfactory completion of the calculations. A recent re-analysis of these data has proposed plausible limits for reproductively normal women indicating that approximately 10–40% of embryos perish before implantation and 40–60% do so between fertilisation and birth 34. However, these ranges are wide, particularly for pre-implantation mortality, reflecting the lack of appropriate data. Is there any possibility of narrowing down the numbers?
Two separate groups have previously collected embryos from women following carefully timed artificial insemination as part of fertility treatment. Insemination around the time of ovulation in women of proven fertility was followed 5 days later by uterine lavage to recover ova 102– 105. These data appear to hold promise for determining fertilisation efficiency and some authors have made quantitative inferences about embryo mortality from them 16, 19, 20. However, such inferences are complicated by numerous confounding factors. For example, in one series 104, from 88 uterine lavages following artificial insemination by donor (AID), 4 unfertilised eggs, 6 fragmented eggs and 27 embryos from 2 cell to blastocyst stage were retrieved. In the 51 cycles in which no egg or embryo was retrieved, there was one retained pregnancy suggesting that the lavage and ova retrieval efficiency was reasonably high, albeit not perfect. These data therefore suggest that FEC TOT was low (≈31/88 = 35%) although a proportion of fertilised eggs may have completely degenerated within the first 5 days. Assuming π SOC was high (given the targeted insemination), this suggests that π FERT ≈ 50%. In the context of the recent analysis 34, this implies that π HCG is high and that levels of embryo mortality are therefore towards the lower end of the 10–40% and 40–60% ranges. However, the clinical pregnancy rate following transfer of the embryos was only 40%. This is equivalent to π HCG × π CLIN. If π CLIN ≈ 75%, as suggested by the hCG studies, this would mean that π HCG ≈ 50%. This would imply that π FERT is high, fertilised egg degeneration is high, occurs before day 5 and was therefore unobserved, and hence levels of embryo mortality tend towards the upper end of the 10–40% and 40–60% ranges.
It is possible that the lavage/transfer procedure reduced implantation and early developmental efficiency thereby reducing π HCG × π CLIN. A comparison of AID pregnancy rates may provide some insight as suggested by the authors 104. The clinical pregnancy rate in their pharmacologically unstimulated cohort was 12.5% (11/88) which is lower than an equivalent 18.9% observed for fresh semen AID 106, and also the live birth rate (which also incorporates clinical pregnancy losses) of 14.7% reported by the HFEA for AID in 2012 in unstimulated women aged 18–34 107. These different success rates suggest that the lavage/transfer procedure did adversely affect implantation and early gestation with clear implications for quantitative extrapolation. Furthermore, the women who were embryo recipients were receiving fertility treatment and their overall fertility may have been lower than expected in a normal healthy cohort. In summary, it seems that there are too many unresolved variables in these data to narrow down estimates of fertilization ( π FERT) or implantation ( π HCG) rates.
With high fecundability, the range of possible embryo mortality rates falls. Red deer hinds have pregnancy rates of >85% following natural mating 108: establishing numerical limits for embryo mortality under these efficient reproductive circumstances is more straightforward. By contrast, humans lack the instinct to mate predominantly during fertile periods thereby reducing observed reproductive efficiency substantially. In studies of early pregnancy loss, owing to sub-optimal coital frequency and cohorts including sub-fertile couples, natural fecundability was almost certainly not maximised 34. Combining data on coital frequency and hCG elevation may help to address this. In a later analysis, applying the Schwartz model 85 to hCG data, Wilcox calculated a FEC HCG value of 36% for high coital frequencies (>4 days with intercourse in 6 pre-ovulatory days) 79. However, the model assumed that cycle viability was evenly distributed among couples, a condition which the authors recognised was not true and is contradicted by a subsequent analysis which suggests that approximately a quarter of the Wilcox cohort was sub-fertile 34. If possible, focussing analytical attention on normally fertile women with the highest coital frequencies may help to further narrow the range of plausible embryo mortality.
In this review of natural early embryo mortality no use has been made of data from in vitro fertilisation (IVF) and associated laboratory studies. Sub-optimal conditions for embryo culture mean that it was 109, 110 and probably still is 111 doubtful that reliable values can be extrapolated from laboratory in vitro to natural in vivo circumstances 20. Importantly, the reproductive stages are also altered. In IVF, π SOC = 1 and for transferred embryos π FERT = 1. Furthermore, transferred embryos are selected based on quality criteria, however inexact those may be 111, 112. IVF program manipulations may reduce π HCG compared to natural circumstances 3 and implantation failure remains a substantial issue for IVF 113, 114. Although for IVF cycles, the reported live birth rate per cycle has gone up (from 14% in 1991 to 25.4% in 2012 34), comparison of IVF success rates and natural live birth fecundability values involves too many undefined variables to shed numerical light on early natural embryo development and mortality.
In vitro fertilisation per se may provide some insight into values of π FERT, since π SOC = 1, and successful fertilisation can be observed. In seven studies of natural cycle IVF, fertilisation was successful in 70.9% (443/625) of attempts 115– 121. If this represented natural, in vivo fertilisation, based on the recent analysis 34, it implies that π HCG ≈ 0.75, focusing estimates for pre-implantation embryo loss on 25%, and for total loss on 50%. However, high frequencies of chromosomal aberrations caused by the in vitro handling of human oocytes 122 can render any comparison of natural and assisted reproduction open to criticism 4.
In calculating summary values of embryo mortality, it is important to note that human fertility is as numerically heterogeneous as it could possibly be. Some couples are infertile and some are highly fertile. Excessive attention to averages and neglect of variances fosters a misleading appreciation of reality. The hCG studies clearly had both fertile and sub-fertile participants: use of overall values underestimated fecundability for the fertile majority 34. Furthermore, apparently ‘optimal’ conditions for conception may not maximise human biological fecundability. Other biological factors also contribute to reproductive heterogeneity in humans; however, even after controlling for age-related decline, fecundability remains highly variable 107, 123. For intercourse occurring 2 days prior to ovulation, average fecundabilities resembled those previously published 124, but for couples at the 5 th and 95 th percentiles, fecundabilities were 5% and 83%. 83% fecundability implies a very low embryo mortality rate. In conclusion, apparent low fecundability in humans need not necessarily be caused by embryo mortality, but also defects of ovulation, mistimed coitus, or fertilisation failure 34. Where fecundability is low, any or all of these factors may contribute.
Pregnancy loss and embryo mortality under natural conditions are real and substantial. However, estimates of 90% 29, 85% 28, 80% 6, 27, 78% 26, 76% 5, 25 and 70% 19– 23 loss are excessive and not supported by available data. Estimates for clinical pregnancy loss are approximately 10–20%. For women of reproductive age, losses between implantation and clinical recognition are approximately 10–25%. Loss from implantation to birth is approximately one third 34, 63, 65, 66.
Natural pre-implantation embryo loss remains quantitatively undefined. In the absence of knowledge of π SOC and π FERT it is almost impossible to estimate precisely. Hertig’s estimate is 30%; however, mathematically and biologically implausible confidence intervals [-28%, 73%] betray the quantitative weaknesses in his data and analysis. The best available data are from studies monitoring daily hCG levels in women attempting to conceive 63, 65, 66. Based on analyses of these data, in normal healthy women, 10–40% is a plausible range for pre-implantation embryo loss and overall pregnancy loss from fertilisation to birth is approximately 40–60% 34. This latter range is similar to, although a little narrower than the 25–70% suggested by Professor Robert Edwards 125.
In the absence of suitable data to quantify pre-implantation loss, many published articles and reviews merely restate previously published values 6, 20, 21. It has been suggested that “ for many current scientific fields, claimed research findings may often be simply accurate measures of the prevailing bias” 126. Widely held views on early embryo mortality may reflect an entrenched and biased view of the biology. For example, the Macklon “Black Box” review 20 has been cited over 200 times (Web of Knowledge citations on 10 th October 2016) with many articles explicitly referencing its 30% survival/70% failure value 8, 21, 113, 127– 133. Macklon’s quantitative summary in his “Pregnancy Loss Iceberg” (30% implantation failure; 30% early pregnancy loss; 10% clinical miscarriage; 30% live births) is a direct, unedited reproduction of estimates published over 10 years previously 19. 30% pre-implantation loss fairly represents Hertig’s conclusions although, as has been shown, this estimate is highly imprecise. However, Macklon misrepresents the best data which he reviews 63, 65. Wilcox reports early pregnancy loss (i.e., [1 - π CLIN]) of 21.7% whereas Macklon’s iceberg implies that 43% (30/70) of implanting embryos fail before clinical recognition. The iceberg’s clinical loss rate of 25% (10/40) is also higher than relevant data indicate ( Figure 2 & Figure 3). Total loss of implanting (hCG+) embryos (i.e., [1 - ( π CLIN × π LB]) is 57% (40/70) according to the iceberg. By contrast, Wilcox 63 and Zinaman 65, both included in Macklon’s review, both report that only 31% of hCG positive pregnancies fail.
If Macklon’s (and Chard’s 19) estimates are excessive as the data suggest, this casts doubt on claims 113, 132 that the frequency of embryonic abnormalities observed in vitro is representative of the natural in vivo situation. In turn, this implies that many of the chromosomal abnormalities observed in in vitro human embryos are, to a greater extent than currently recognised 113, an artefact of the clinical and experimental context of assisted reproduction technologies.
In attempting to quantify pre-implantation embryo mortality it is easy to appreciate why “ a claim of ‘no significant difference’ might easily be sustained against any interpretation proffered” 48, and why estimates are “ difficult to defend with any precision” 5. In conclusion, “ poor estimates of fertilization failure rate and the mortality at 2 weeks after fertilisation” 15 drawn “ from unusual or biased samples” 134 indicate that the “black box” of early pregnancy loss 20 is not as wide open as has been thought.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2016 Jarvis GE
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
F1000Research: Dataset 1. Figure 1 data, 10.5256/f1000research.8937.d140569 135
F1000Research: Dataset 2. Figure 2 data, 10.5256/f1000research.8937.d140570 136
F1000Research: Dataset 3. Figure 3 data, 10.5256/f1000research.8937.d140571 137
F1000Research: Dataset 4. Pseudo-datasets of Hertig’s study, obtained via a bootstrap procedure, 10.5256/f1000research.8937.d140572 138
Acknowledgements
Thanks are due to Professor David Paton, Dr Paul Schofield and Dr Amanda Sferruzzi-Perri for reviewing and providing helpful comments during the writing of this article.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 1 approved
References
- 1. Ord T: The scourge: moral implications of natural embryo loss. Am J Bioeth. 2008;8(7):12–9. 10.1080/15265160802248146 [DOI] [PubMed] [Google Scholar]
- 2. R (on the application of Smeaton) v Secretary of State for Health. [2002] EWHC 610 (Admin), [2002] All ER (D) 115 (Apr),2002. Reference Source [Google Scholar]
- 3. Kennedy TG: Physiology of implantation. In Vitro Fert Ass Rep. 1997;729–35. Reference Source [Google Scholar]
- 4. Benagiano G, Farris M, Grudzinskas G: Fate of fertilized human oocytes. Reprod Biomed Online. 2010;21(6):732–41. 10.1016/j.rbmo.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 5. Boklage CE: Survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35(2):75, 79–80, 81–94. [PubMed] [Google Scholar]
- 6. Vitzthum VJ, Spielvogel H, Thornburg J, et al. : A prospective study of early pregnancy loss in humans. Fertil Steril. 2006;86(2):373–9. 10.1016/j.fertnstert.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 7. Bainbridge DR: Making Babies. A Visitor Within. London: Phoenix;2001;101–62at 59ff. Reference Source [Google Scholar]
- 8. Ramos-Medina R, García-Segovia Á, León JA, et al. : New decision-tree model for defining the risk of reproductive failure. Am J Reprod Immunol. 2013;70(1):59–68. 10.1111/aji.12098 [DOI] [PubMed] [Google Scholar]
- 9. Norwitz ER, Schust DJ, Fisher SJ: Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–8. 10.1056/NEJMra000763 [DOI] [PubMed] [Google Scholar]
- 10. James WH: The incidence of spontaneous abortion. Popul Stud (Camb). 1970;24(2):241–5. 10.1080/00324728.1970.10406127 [DOI] [PubMed] [Google Scholar]
- 11. Silver RM, Branch DW: Sporadic and recurrent pregnancy loss. In: Reece EA, Hobbins JC, editors. Clinical Obstetrics: The Fetus and Mother.3rd ed: Blackwell Publishing;2007;143–60. 10.1002/9780470753323.ch9 [DOI] [Google Scholar]
- 12. Nishimura H: Fate of human fertilized eggs during prenatal life: present status of knowledge. Okajimas Folia Anat Jpn. 1970;46(6):297–305. 10.2535/ofaj1936.46.6_297 [DOI] [PubMed] [Google Scholar]
- 13. Short RV: When a conception fails to become a pregnancy. Ciba Found Symp. 1978; (64):377–94. [DOI] [PubMed] [Google Scholar]
- 14. Opitz JM: The Farber lecture. Prenatal and perinatal death: the future of developmental pathology. Pediatr Pathol. 1987;7(4):363–94. 10.3109/15513818709161402 [DOI] [PubMed] [Google Scholar]
- 15. Biggers JD: Risks of In Vitro Fertilization and Embryo Transfer in Humans. In: Crosignani PG, Rubin BL, editors. In Vitro Fertilization and Embryo Transfer.London: Academic Press;1983;393–410. Reference Source [Google Scholar]
- 16. Johnson MH: Chapter 15: Fetal Challenges. Essential Reproduction 7th ed. Oxford: Wiley-Blackwell;2013;258–69. Reference Source [Google Scholar]
- 17. Biggers JD: In vitro fertilization and embryo transfer in human beings. N Engl J Med. 1981;304(6):336–42. 10.1056/NEJM198102053040607 [DOI] [PubMed] [Google Scholar]
- 18. Leridon H: Intrauterine Mortality. Human Fertility: The Basic Components Chicago: The University of Chicago Press;1977;48–81. Reference Source [Google Scholar]
- 19. Chard T: Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. 1991;5(1):179–89. 10.1016/S0950-3552(05)80077-X [DOI] [PubMed] [Google Scholar]
- 20. Macklon NS, Geraedts JP, Fauser BC: Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–43. 10.1093/humupd/8.4.333 [DOI] [PubMed] [Google Scholar]
- 21. Ford HB, Schust DJ: Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 22. McCoy RC, Demko Z, Ryan A, et al. : Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Science. 2015;348(6231):235–8. 10.1126/science.aaa3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loke YW, King A: Human Implantation: Cell Biology and Immunology. Cambridge: Cambridge University Press;1995. Reference Source [Google Scholar]
- 24. American College of Obstetricians and Gynecologists: Technical Bulletin No. 212: Early pregnancy loss. Int J Gynaecol Obstet. 1995;51(3):278–85. 10.1016/0020-7292(95)80036-0 [DOI] [PubMed] [Google Scholar]
- 25. Drife JO: What proportion of pregnancies are spontaneously aborted? Brit Med J. 1983;286(6361):294. [Google Scholar]
- 26. Roberts CJ, Lowe CR: Where have all the conceptions gone? Lancet. 1975;305:498–9. 10.1016/S0140-6736(75)92837-8 [DOI] [Google Scholar]
- 27. Johnson MH, Everitt BJ: Chapter 15: Fertility. Essential Reproduction 5th ed. Oxford: Wiley-Blackwell;2000;251–74. Reference Source [Google Scholar]
- 28. Braude PR, Johnson MH: The Embryo in Contemporary Medical Science. In: Dunstan GR, editor. The Human Embryo: Aristotle and the Arabic and European Traditions Exeter: University of Exeter Press;1990;208–21. Reference Source [Google Scholar]
- 29. Opitz JM: Human Development - The Long and the Short of it. In: Furton EJ, Mitchell LA, editors. What is Man, O Lord? The Human Person in a Biotech Age; Eighteenth Workshop for Bishops Boston, MA: The National Catholic Bioethics Center;2002;131–53. [Google Scholar]
- 30. Leridon H: Fecundability. Human Fertility: The Basic Components Chicago: The University of Chicago Press;1977;22–47. Reference Source [Google Scholar]
- 31. Harris J: Stem cells, sex, and procreation. Camb Q Healthc Ethics. 2003;12(4):353–71. 10.1017/S096318010312405X [DOI] [PubMed] [Google Scholar]
- 32. Rahner K: Theological Investigations, Vol IX. London: DLT;1972. [Google Scholar]
- 33. Hertig AT: The Overall Problem in Man. In: Benirschke K, editor. Comparative Aspects of Reproductive Failure An International Conference at Dartmouth Medical School. Berlin: Springer Verlag;1967. 10.1007/978-3-642-48949-5_2 [DOI] [Google Scholar]
- 34. Jarvis GE: Estimating limits for natural human embryo mortality [version 1; referees: 2 approved]. F1000Res. 2016;5:2083 10.12688/f1000research.9479.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bulletti C, Flamigni C, Giacomucci E: Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum Reprod Update. 1996;2(2):118–36. 10.1093/humupd/2.2.118 [DOI] [PubMed] [Google Scholar]
- 36. Devolder K, Harris J: The ambiguity of the embryo: Ethical inconsistency in the human embryonic stem cell debate. Metaphilosophy. 2007;38(2–3):153–69. 10.1111/j.1467-9973.2007.00480.x [DOI] [Google Scholar]
- 37. Green RM: The Human Embryo Research Debates: Bioethics in the Vortex of Controversy. Oxford: Oxford University Press;2001. Reference Source [Google Scholar]
- 38. Hertig AT, Rock J, Adams EC: A description of 34 human ova within the first 17 days of development. Am J Anat. 1956;98(3):435–93. 10.1002/aja.1000980306 [DOI] [PubMed] [Google Scholar]
- 39. Letter: Where have all the conceptions gone? Lancet. 1975;1(7907):636–7. 10.1016/S0140-6736(75)91920-0 [DOI] [PubMed] [Google Scholar]
- 40. Cooke ID: Failure of implantation and its relevance to subfertility. J Reprod Fertil Suppl. 1988;36:155–9. [PubMed] [Google Scholar]
- 41. Catalano RA, Saxton KB, Bruckner TA, et al. : Hormonal evidence supports the theory of selection in utero. Am J Hum Biol. 2012;24(4):526–32. 10.1002/ajhb.22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. French FE, Bierman JM: Probabilities of fetal mortality. Public Health Rep. 1962;77(10):835–47. [PMC free article] [PubMed] [Google Scholar]
- 43. Shapiro S, Jones EW, Densen PM: A life table of pregnancy terminations and correlates of fetal loss. Milbank Mem Fund Q. 1962;40(1):7–45. [PubMed] [Google Scholar]
- 44. Erhardt CL: Pregnancy Losses in New York City, 1960. Am J Public Health Nations Health. 1963;53(9):1337–52. 10.2105/AJPH.53.9.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pettersson F: Epidemiology of Early Pregnancy Wastage. Stockholm: Svenska Bokförlaget;1968. Reference Source [Google Scholar]
- 46. Shapiro S, Levine HS, Abramowicz M: Factors associated with early and late fetal loss. Adv Planned Parenthood. 1970;6:45–63. [Google Scholar]
- 47. Taylor WF: The Probability of Fetal Death. In: Fraser FC, McCusick VA, editors. Congenital Malformations Amsterdam: Excerpta Medica;1970;307–20. [Google Scholar]
- 48. Kline J, Stein Z, Susser M: Conception and Reproductive Loss: Probabilities. Conception to Birth. Epidemiology of Prenatal Development.New York: OUP;1989;43–68. [Google Scholar]
- 49. Master-Hunter T, Heiman DL: Amenorrhea: evaluation and treatment. Am Fam Physician. 2006;73(8):1374–82. [PubMed] [Google Scholar]
- 50. Committee on Practice Bulletins—Gynecology: Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206. 10.1097/AOG.0b013e318262e320 [DOI] [PubMed] [Google Scholar]
- 51. Grudzinskas JG, Nysenbaum AM: Failure of human pregnancy after implantation. Ann N Y Acad Sci. 1985;442:38–44. 10.1111/j.1749-6632.1985.tb37503.x [DOI] [PubMed] [Google Scholar]
- 52. Morton H, Rolfe B, Clunie GJ: An early pregnancy factor detected in human serum by the rosette inhibition test. Lancet. 1977;1(8008):394–7. 10.1016/S0140-6736(77)92605-8 [DOI] [PubMed] [Google Scholar]
- 53. Chard T, Grudzinskas JG: Early pregnancy factor. Biol Res Pregnancy Perinatol. 1987;8(2 2D Half):53–6. [PubMed] [Google Scholar]
- 54. Cole LA: hCG, the wonder of today's science. Reprod Biol Endocrinol. 2012;10:24. 10.1186/1477-7827-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilcox AJ, Weinberg CR, Wehmann RE, et al. : Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44(3):366–74. [PubMed] [Google Scholar]
- 56. Regan L: A prospective study of spontaneous abortion. In: Beard RW, Sharp F, editors. Early Pregnancy Loss: Mechanisms and Treatment Springer-Verlag;1988;23–37. 10.1007/978-1-4471-1658-5_4 [DOI] [Google Scholar]
- 57. Odell WD, Griffin J: Pulsatile secretion of human chorionic gonadotropin in normal adults. N Engl J Med. 1987;317(27):1688–91. 10.1056/NEJM198712313172702 [DOI] [PubMed] [Google Scholar]
- 58. Miller JF, Williamson E, Glue J, et al. : Fetal loss after implantation. A prospective study. Lancet. 1980;2(8194):554–6. 10.1016/S0140-6736(80)91991-1 [DOI] [PubMed] [Google Scholar]
- 59. Edmonds DK, Lindsay KS, Miller JF, et al. : Early embryonic mortality in women. Fertil Steril. 1982;38(4):447–53. 10.1016/S0015-0282(16)46579-9 [DOI] [PubMed] [Google Scholar]
- 60. Whittaker PG, Taylor A, Lind T: Unsuspected pregnancy loss in healthy women. Lancet. 1983;1(8334):1126–7. 10.1016/S0140-6736(83)92865-9 [DOI] [PubMed] [Google Scholar]
- 61. Videla-Rivero L, Etchepareborda JJ, Kesseru E: Early chorionic activity in women bearing inert IUD, copper IUD and levonorgestrel-releasing IUD. Contraception. 1987;36(2):217–26. 10.1016/0010-7824(87)90017-5 [DOI] [PubMed] [Google Scholar]
- 62. Walker EM, Lewis M, Cooper W, et al. : Occult biochemical pregnancy: fact or fiction? Br J Obstet Gynaecol. 1988;95(7):659–63. 10.1111/j.1471-0528.1988.tb06526.x [DOI] [PubMed] [Google Scholar]
- 63. Wilcox AJ, Weinberg CR, O'Connor JF, et al. : Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. 10.1056/NEJM198807283190401 [DOI] [PubMed] [Google Scholar]
- 64. Hakim RB, Gray RH, Zacur H: Infertility and early pregnancy loss. Am J Obstet Gynecol. 1995;172(5):1510–7. 10.1016/0002-9378(95)90489-1 [DOI] [PubMed] [Google Scholar]
- 65. Zinaman MJ, Clegg ED, Brown CC, et al. : Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503–9. 10.1016/S0015-0282(16)58144-8 [DOI] [PubMed] [Google Scholar]
- 66. Wang X, Chen C, Wang L, et al. : Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577–84. 10.1016/S0015-0282(02)04694-0 [DOI] [PubMed] [Google Scholar]
- 67. Sasaki Y, Ladner DG, Cole LA: Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. 2008;89(6):1781–6. 10.1016/j.fertnstert.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 68. Koot YE, Boomsma CM, Eijkemans MJ, et al. : Recurrent pre-clinical pregnancy loss is unlikely to be a 'cause' of unexplained infertility. Hum Reprod. 2011;26(10):2636–41. 10.1093/humrep/der217 [DOI] [PubMed] [Google Scholar]
- 69. Cole LA: Hyperglycosylated hCG and pregnancy failures. J Reprod Immunol. 2012;93(2):119–22. 10.1016/j.jri.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 70. Mumford SL, Silver RM, Sjaarda LA, et al. : Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod. 2016;31(3):657–65. 10.1093/humrep/dev329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilcox AJ, Baird DD, Weinberg CR, et al. : The use of biochemical assays in epidemiologic studies of reproduction. Environ Health Perspect. 1987;75:29–35. 10.1289/ehp.877529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brattebø G: Occult biochemical pregnancy: fact or fiction? Br J Obstet Gynaecol. 1989;96(2):252–4. 10.1111/j.1471-0528.1989.tb01676.x [DOI] [PubMed] [Google Scholar]
- 73. Walker EM, Lewis M, Howie PW: Authors' reply. Br J Obstet Gynaecol. 1989;96(2):253–4. 10.1111/j.1471-0528.1989.tb01677.x [DOI] [Google Scholar]
- 74. Wilcox AJ, Weinberg CR, Baird DD: Subclinical embryonic loss. Fertil Steril. 1989;51(5):907–8. 10.1016/S0015-0282(16)60691-X [DOI] [PubMed] [Google Scholar]
- 75. Wilcox AJ, Weinberg CR, Baird DD: Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–5. [DOI] [PubMed] [Google Scholar]
- 76. Sapra KJ, Buck Louis GM, Sundaram R, et al. : Signs and symptoms associated with early pregnancy loss: findings from a population-based preconception cohort. Hum Reprod. 2016;31(4):887–96. 10.1093/humrep/dew010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wilcox AJ, Baird DD, Weinberg CR: Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–9. 10.1056/NEJM199906103402304 [DOI] [PubMed] [Google Scholar]
- 78. Wilcox AJ, Dunson DB, Weinberg CR, et al. : Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63(4):211–5. 10.1016/S0010-7824(01)00191-3 [DOI] [PubMed] [Google Scholar]
- 79. Wilcox AJ, Weinberg CR, Baird DD: Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333(23):1517–21. 10.1056/NEJM199512073332301 [DOI] [PubMed] [Google Scholar]
- 80. Wilcox AJ, Baird DD, Dunson D, et al. : Natural limits of pregnancy testing in relation to the expected menstrual period. JAMA. 2001;286(14):1759–61. 10.1001/jama.286.14.1759 [DOI] [PubMed] [Google Scholar]
- 81. Weinberg CR, Gladen BC, Wilcox AJ: Models relating the timing of intercourse to the probability of conception and the sex of the baby. Biometrics. 1994;50(2):358–67. 10.2307/2533379 [DOI] [PubMed] [Google Scholar]
- 82. Weinberg CR, Moledor E, Baird DD, et al. : Is there a seasonal pattern in risk of early pregnancy loss? Epidemiology. 1994;5(5):484–9. [PubMed] [Google Scholar]
- 83. Weinberg CR, Hertz-Picciotto I, Baird DD, et al. : Efficiency and bias in studies of early pregnancy loss. Epidemiology. 1992;3(1):17–22. 10.1097/00001648-199201000-00005 [DOI] [PubMed] [Google Scholar]
- 84. Barrett JC, Marshall J: The risk of conception on different days of the menstrual cycle. Popul Stud (Camb). 1969;23(3):455–61. 10.1080/00324728.1969.10405297 [DOI] [PubMed] [Google Scholar]
- 85. Schwartz D, Macdonald PD, Heuchel V: Fecundability, coital frequency and the viability of Ova. Popul Stud (Camb). 1980;34(2):397–400. 10.1080/00324728.1980.10410398 [DOI] [PubMed] [Google Scholar]
- 86. Boklage CE: The frequency and and survival probability of natural twin conceptions. In: Keith LG, Papiernik E, Keith DM, Lukie B, editors. Multiple Pregnancy: Epidemiology, Gestation and Perinatal Outcome New York: Parthenon Publishing Group;1995;41–50. Reference Source [Google Scholar]
- 87. Hertwig O: Beiträge zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies (Contributions to the knowledge of the formation, fertilization and division of the animal egg). Morphol Jahrb. 1876; 1:347–434. [Google Scholar]
- 88. Steptoe PC, Edwards RG: Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. 10.1016/S0140-6736(78)92957-4 [DOI] [PubMed] [Google Scholar]
- 89. Clift D, Schuh M: Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14(9):549–62. 10.1038/nrm3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hertig AT, Rock J, Adams EC, et al. : Thirty-four fertilized human ova, good, bad and indifferent, recovered from 210 women of known fertility; a study of biologic wastage in early human pregnancy. Pediatrics. 1959;23(1 Part 2):202–11. [PubMed] [Google Scholar]
- 91. Hertig AT: A fifteen-year search for first-stage human ova. JAMA. 1989;261(3):434–5. 10.1001/jama.1989.03420030108040 [DOI] [PubMed] [Google Scholar]
- 92. Rock J, Hertig AT: Some aspects of early human development. Am J Obstet Gynecol. 1942;44(6):973–83. 10.1016/S0002-9378(42)90806-8 [DOI] [Google Scholar]
- 93. Hertig AT, Rock J: On a human blastula recovered from the uterine cavity 4 days after ovulation. Anat Rec. 1946;94:469. [PubMed] [Google Scholar]
- 94. Hertig AT, Rock J: A series of potentially abortive ova recovered from fertile women prior to the first missed menstrual period. Am J Obstet Gynecol. 1949;58(5):968–93, illust. 10.1016/0002-9378(49)90203-3 [DOI] [PubMed] [Google Scholar]
- 95. Hertig AT, Rock J: Two human ova of the pre-villous stage, having a developmental age of about 8 and 9 days respectively. Contrib Embryol. 1949;33(213–221):169–86. [PubMed] [Google Scholar]
- 96. Hertig AT, Adams EC, McKay DG, et al. : A thirteen-day human ovum studied histochemically. Am J Obstet Gynecol. 1958;76(5):1025–40; discussion 40-3. 10.1016/0002-9378(58)90185-6 [DOI] [PubMed] [Google Scholar]
- 97. Hertig AT, Rock J: Searching for early fertilized human ova. Gynecol Invest. 1973;4(3):121–39. 10.1159/000301716 [DOI] [PubMed] [Google Scholar]
- 98. Barrett JC: Fecundability and coital frequency. Popul Stud (Camb). 1971;25(2):309–13. 10.1080/00324728.1971.10405806 [DOI] [PubMed] [Google Scholar]
- 99. Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13. 10.2307/2331986 [DOI] [Google Scholar]
- 100. Agresti A, Coull BA: Approximate is better than "exact" for interval estimation of binomial proportions. Am Stat. 1998;52(2):119–26. 10.1080/00031305.1998.10480550 [DOI] [Google Scholar]
- 101. Edwards RG: The Cleaving Embryo and the Blastocyst. In: Conception in the Human Female.London: Academic Press;1980;668–766at 47ff. [Google Scholar]
- 102. Buster JE, Bustillo M, Rodi IA, et al. : Biologic and morphologic development of donated human ova recovered by nonsurgical uterine lavage. Am J Obstet Gynecol. 1985;153(2):211–7. 10.1016/0002-9378(85)90116-4 [DOI] [PubMed] [Google Scholar]
- 103. Formigli L, Formigli G, Roccio C: Donation of fertilized uterine ova to infertile women. Fertil Steril. 1987;47(1):162–5. 10.1016/S0015-0282(16)49953-X [DOI] [PubMed] [Google Scholar]
- 104. Formigli L, Roccio C, Belotti G, et al. : Non-surgical flushing of the uterus for pre-embryo recovery: possible clinical applications. Hum Reprod. 1990;5(3):329–35. [DOI] [PubMed] [Google Scholar]
- 105. Sauer MV, Bustillo M, Rodi IA, et al. : In-vivo blastocyst production and ovum yield among fertile women. Hum Reprod. 1987;2(8):701–3. [DOI] [PubMed] [Google Scholar]
- 106. Richter MA, Haning RV, Jr, Shapiro SS: Artificial donor insemination: fresh versus frozen semen; the patient as her own control. Fertil Steril. 1984;41(2):277–80. 10.1016/S0015-0282(16)47604-1 [DOI] [PubMed] [Google Scholar]
- 107. HFEA: Fertility Treatment in 2013 - trends and figures. Human Fertilisation & Embryology Authority. 2013. Reference Source [Google Scholar]
- 108. Asher GW: Reproductive cycles of deer. Anim Reprod Sci. 2011;124(3–4):170–5. 10.1016/j.anireprosci.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 109. Bolton VN, Braude PR: Development of the human preimplantation embryo in vitro. Curr Top Dev Biol. 1987;23:93–114. [DOI] [PubMed] [Google Scholar]
- 110. Jones HW, Jr, Oehninger S, Bocca S, et al. : Reproductive efficiency of human oocytes fertilized in vitro. Facts Views Vis Obgyn. 2010;2(3):169–71. [PMC free article] [PubMed] [Google Scholar]
- 111. Bolton VN, Leary C, Harbottle S, et al. : How should we choose the 'best' embryo? A commentary on behalf of the British Fertility Society and the Association of Clinical Embryologists. Hum Fertil (Camb). 2015;18(3):156–64. 10.3109/14647273.2015.1072646 [DOI] [PubMed] [Google Scholar]
- 112. Machtinger R, Racowsky C: Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26(3):210–21. 10.1016/j.rbmo.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 113. Niakan KK, Han J, Pedersen RA, et al. : Human pre-implantation embryo development. Development. 2012;139(5):829–41. 10.1242/dev.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Koot YE, Teklenburg G, Salker MS, et al. : Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822(12):1943–50. 10.1016/j.bbadis.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 115. Daya S, Gunby J, Hughes EG, et al. : Natural cycles for in-vitro fertilization: cost-effectiveness analysis and factors influencing outcome. Hum Reprod. 1995;10(7):1719–24. [DOI] [PubMed] [Google Scholar]
- 116. Zayed F, Lenton EA, Cooke ID: Natural cycle in-vitro fertilization in couples with unexplained infertility: impact of various factors on outcome. Hum Reprod. 1997;12(11):2402–7. 10.1093/humrep/12.11.2402 [DOI] [PubMed] [Google Scholar]
- 117. Bassil S, Godin PA, Donnez J: Outcome of in-vitro fertilization through natural cycles in poor responders. Hum Reprod. 1999;14(5):1262–5. 10.1093/humrep/14.5.1262 [DOI] [PubMed] [Google Scholar]
- 118. Roesner S, Pflaumer U, Germeyer A, et al. : Natural cycle IVF: evaluation of 463 cycles and summary of the current literature. Arch Gynecol Obstet. 2014;289(6):1347–54. 10.1007/s00404-013-3123-2 [DOI] [PubMed] [Google Scholar]
- 119. Omland AK, Fedorcsák P, Storeng R, et al. : Natural cycle IVF in unexplained, endometriosis-associated and tubal factor infertility. Hum Reprod. 2001;16(12):2587–92. 10.1093/humrep/16.12.2587 [DOI] [PubMed] [Google Scholar]
- 120. Janssens RM, Lambalk CB, Vermeiden JP, et al. : In-vitro fertilization in a spontaneous cycle: easy, cheap and realistic. Hum Reprod. 2000;15(2):314–8. 10.1093/humrep/15.2.314 [DOI] [PubMed] [Google Scholar]
- 121. Fahy UM, Cahill DJ, Wardle PG, et al. : In-vitro fertilization in completely natural cycles. Hum Reprod. 1995;10(3):572–5. [DOI] [PubMed] [Google Scholar]
- 122. Braude PR, Johnson MH, Pickering SJ, et al. : Mechanisms of Early Embryonic Loss In Vivo and In Vitro . In: Chapman. M, Grudzinskas G, Chard T, editors. The Embryo: Normal and Abnormal Development and Growth London: Springer-Verlag;1991;1–10. 10.1007/978-1-4471-1802-2_1 [DOI] [Google Scholar]
- 123. Dunson DB, Colombo B, Baird DD: Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–403. 10.1093/humrep/17.5.1399 [DOI] [PubMed] [Google Scholar]
- 124. Wilcox AJ, Weinberg CR, Baird DD: Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13(2):394–7. 10.1093/humrep/13.2.394 [DOI] [PubMed] [Google Scholar]
- 125. Edwards RG: Sexuality and Coitus. In: Conception in the Human Female London: Academic Press;1980;525–72at 60ff. [Google Scholar]
- 126. Ioannidis JP: Why most published research findings are false. PLoS Med. 2005;2(8):e124. 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McCoy RC, Demko ZP, Ryan A, et al. : Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genet. 2015;11(10):e1005601. 10.1371/journal.pgen.1005601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Harris J: Germline Modification and the Burden of Human Existence. Camb Q Healthc Ethics. 2016;25(1):6–18. 10.1017/S0963180115000237 [DOI] [PubMed] [Google Scholar]
- 129. Saravelos SH, Regan L: Early pregnancy failure after assisted reproductive technology. Pregnancy after Assisted Reproductive Technology. 2012;51–65. [Google Scholar]
- 130. Jones DG, Towns CR: Navigating the quagmire: the regulation of human embryonic stem cell research. Hum Reprod. 2006;21(5):1113–6. 10.1093/humrep/dei461 [DOI] [PubMed] [Google Scholar]
- 131. Dupont C, Froenicke L, Lyons LA, et al. : Chromosomal instability in rhesus macaque preimplantation embryos. Fertil Steril. 2009;91(4):1230–7. 10.1016/j.fertnstert.2008.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Daughtry BL, Chavez SL: Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences. Cell Tissue Res. 2016;363(1):201–25. 10.1007/s00441-015-2305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shorten PR, Peterson AJ, O'Connell AR, et al. : A mathematical model of pregnancy recognition in mammals. J Theor Biol. 2010;266(1):62–9. 10.1016/j.jtbi.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 134. Potts M, Diggory P, Peel J: Spontaneous Abortion. Abortion Cambridge: Cambridge University Press;1977;45–64. Reference Source [Google Scholar]
- 135. Jarvis G: Dataset 1 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]
- 136. Jarvis G: Dataset 2 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]
- 137. Jarvis G: Dataset 3 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]
- 138. Jarvis G: Dataset 4 in: Early embryo mortality in natural human reproduction: What the data say. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed]