Abstract
Cardiogenic shock (CS) remains a major cause of in-hospital mortality in the setting of acute myocardial infarction. CS begins as a hemodynamic problem with impaired cardiac output leading to reduced systemic perfusion, increased residual volume within the left and right ventricles, and increased cardiac filling pressures. A critical step towards the development of future algorithms is a clear understanding of the treatment objectives for CS. In this review, we introduce the “door to support” time as an emerging target of therapy to improve outcomes associated with CS, define four key treatment objectives in the management of CS, discuss the importance of early hemodynamic assessment and appropriate selection of acute mechanical circulatory support (AMCS) devices for CS, and introduce a classification scheme that identifies subtypes of CS based on cardiac filling pressures.
Keywords: ventricular unloading, acute mechanical circulatory support, cardiogenic shock, hemodynamics, percutaneous ventricular assist device
The “door to support” time in cardiogenic shock
Cardiogenic shock (CS) remains a major cause of in-hospital mortality in the setting of acute myocardial infarction (AMI). Several recent reports identified an increase in the prevalence of CS among patients with AMI from 6–7% to 10–12% 1, 2. Despite early revascularization, an estimated one in three patients will die during their hospitalization for AMI-CS and one in five patients will die within the first year after discharge for AMI 3, 4. More sobering is the fact that over 30% of AMI-CS survivors develop recurrent heart failure (HF) within the first year after discharge 5. The natural history of HF is a progressive decline in ventricular function as compensatory remodeling ultimately fails and patients present with recurrent episodes of acutely decompensated HF and ultimately CS owing to advanced HF (CS-HF). A recent analysis of the Interagency for Mechanical Circulatory Support (INTERMACS) registry identified that 52.5% of patients with advanced HF referred for surgical left ventricular (LV) assist device (LVAD) placement present with CS-HF defined as INTERMACS levels 1 or 2 HF 6. By 2030, 8 million people in the United States alone will be diagnosed with HF 7. Collectively, these data identify CS as a persistent clinical problem and further suggest that the distribution of CS patients may be shifting from CS-AMI to CS-HF over the next decade.
Irrespective of the injurious mechanism, CS begins as a hemodynamic problem with impaired cardiac output leading to reduced systemic perfusion, increased residual volume within both ventricles, and increased cardiac filling pressures. If these hemodynamic derangements persist, reduced tissue perfusion and elevated filling pressures lead to multi-organ ischemia, increased lactate accumulation, hepatic and venous congestion, and worsening multi-organ function 8. At this stage, CS has transitioned from a potentially reversible hemodynamic problem to a more complex “hemo-metabolic” problem that may not respond to treatment of the underlying cause or hemodynamic support alone ( Figure 1). For this reason, early identification of CS and application of hemodynamic support in CS may improve clinical outcomes. Rapid triage and treatment algorithms for CS require a similar approach currently employed for ST-segment elevation myocardial infarction (STEMI), whereby early diagnosis, emergent network activation, and short “door to balloon” (DTB) coronary reperfusion times have substantially reduced in-hospital mortality associated with STEMI. For CS, a similar quality metric that reflects the time between onset of CS and initiation of acute mechanical circulatory support (AMCS) should be developed as the “door to support” (DTS) time. Several recent reports support the concept of a DTS time and have observed improved survival with early initiation of AMCS before percutaneous coronary revascularization or before the initiation of inotropes and vasopressors in the setting of AMI-CS 9– 11. Future studies quantifying the optimal DTS time in CS are required.
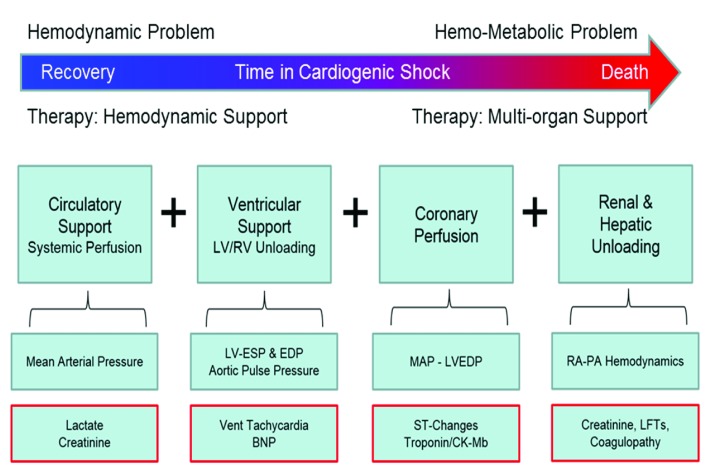
Figure 1. The hemodynamic support equation.
The Hemodynamic Support Equation encompasses the four major management objectives for patients with cardiogenic shock, which include: circulatory support, ventricular unloading, myocardial perfusion, and decongestive strategies. BNP, brain natriuretic peptide; CK-MB, creatinine kinase and its MB isozyme; EDP, end-diastolic pressure; ESP, end-systolic pressure; LFT, liver function test; LV, left ventricle; MAP, mean arterial pressure; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
The hemodynamic support equation
A critical step towards the development of future algorithms is a clear understanding of the treatment objectives for CS. These four primary objectives are summarized in the “hemodynamic support equation” and include 1) circulatory support, 2) ventricular unloading, 3) myocardial perfusion, and 4) decongestion ( Figure 1). Adequate circulatory support is defined by an increase in mean arterial pressure and enhanced microvascular organ perfusion. Ventricular unloading is defined as a reduction in myocardial work and wall stress, which is best achieved by reducing native ventricular pressure and volume 12. Myocardial perfusion is defined as increased epicardial and microvascular coronary blood flow and is often associated with successful circulatory and ventricular support. Decongestion refers to a reduction in total body volume and elevated venous filling pressures, which is commonly associated with worsening renal function, hepatic failure, bowel edema, and subsequent sepsis. To solve the hemodynamic support equation, all four objectives must be achieved in a timely manner.
Pharmacologic approaches fail to solve the hemodynamic support equation. Often drug therapy will solve one part of the equation but at the cost of another. For example, early use of vasopressors such as norepinephrine in CS may increase mean arterial pressure but not microvascular organ perfusion. Furthermore, increased mean arterial pressure will increase LV afterload, thereby increasing myocardial work and wall stress, which promotes myocardial ischemia, impairs cardiac function, and increases cardiac filling pressures. Similarly, inotropic therapy in CS may increase mean arterial pressure but directly increases myocardial work, thereby potentially worsening myocardial ischemia. For these reasons, CS refractory to one or more vasopressors or inotropes is associated with increased in-hospital mortality.
Solving the hemodynamic support equation with acute mechanical circulatory support devices
In the contemporary era, the hemodynamic support equation can be readily addressed with early and appropriate use of AMCS devices, which can be broadly categorized by their mechanism of action as pulsatile or rotary flow pumps ( Figure 2).
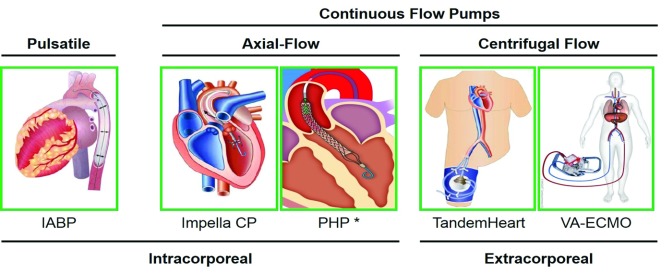
Figure 2. Left ventricular acute mechanical circulatory support devices.
Contemporary acute mechanical circulatory support devices for left ventricular support are illustrated and categorized by mode of action (pulsatile or continuous-flow pumps), type of rotary flow pump (axial- or centrifugal flow), and pump location (intracorporeal or extracorporeal). IABP, intra-aortic balloon counter-pulsation pump; PHP, percutaneous heart pump; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
The intra-aortic balloon counter-pulsation pump (IABP) is a catheter-mounted balloon that augments pulsatile blood flow by inflating during diastole, thereby increasing diastolic pressure in the aortic root and enhancing coronary blood flow, while also displacing blood volume in the descending aorta. During systole, rapid deflation of the intra-aortic balloon generates a pressure sink, which reduces LV afterload and increases LV cardiac output 13. The magnitude of hemodynamic support generated by an IABP is directly related to LV cardiac output. Recent studies confirm that the more dysfunctional the LV, the less effective an IABP becomes 14– 16. In 2012, the IABP-SHOCK II study reported no benefit with IABP therapy in patients with AMI-CS. No large, randomized studies have evaluated the utility of IABP therapy in HF-CS 17.
In contrast to counter-pulsation balloons, rotary-flow pumps generate rotational kinetic energy, which increases blood flow. Rotary flow pumps can be further categorized based on the type of motor as axial-flow or centrifugal-flow systems 18. Axial-flow AMCS pumps are placed across the aortic valve and displace blood from the LV into the ascending aorta. The net result of these trans-valvular axial pumps is a reduction in LV pressure and volume with a concomitant increase in mean aortic root pressure. As a result, systemic perfusion is increased, LV wall stress is reduced, and the trans-myocardial perfusion gradient (aortic diastolic pressure – LV diastolic pressure) is increased. Furthermore, several prior studies have shown that under ischemic conditions, coronary blood flow is increased after activation of a trans-valvular axial-flow pump 19, 20. Trans-valvular axial-flow pumps directly solve three of the four major objectives in the hemodynamic support equation by increasing mean arterial pressure, reducing LV pressure and volume, and increasing coronary blood flow. Contemporary trans-valvular axial-flow pumps include the Impella (Abiomed Inc, Danvers, MA) or the HeartMate percutaneous heart pump (PHP) (Abbott Inc, Chicago IL) 21, 22. The PHP device is currently under investigation in the United States as part of the SHIELD II trial. The Impella devices are the only AMCS pumps approved by the US Food and Drug Administration for use in CS.
Centrifugal-flow pumps include the TandemHeart device (TandemLife, Pittsburgh, PA) and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) 22. The TandemHeart and VA-ECMO systems draw blood from the left or right atrium, respectively, into an extracorporeal pump that displaces the blood into the femoral artery, thereby pressurizing the arterial tree and increasing mean arterial pressure. Since VA-ECMO displaces venous blood into the arterial system, an oxygenator is placed in the circuit prior to the return of blood to the femoral artery. The distinct location of the inflow cannula has a profound impact on the hemodynamic effects of these two systems 23. Since VA-ECMO drains blood from a large venous reservoir, at typical flow rates of 4 to 6 liters/minute, VA-ECMO does not significantly reduce LV volume. As a result, VA-ECMO increases LV pressure, wall stress, and myocardial work and fails to solve the hemodynamic support equation. In contrast to VA-ECMO, by displacing blood from the left atrium, the TandemHeart device effectively reduces LV preload, thereby reducing LV volume, wall stress, and workload, while increasing systemic mean arterial pressure and myocardial perfusion 24. The TandemHeart system is able to solve the same three objectives of the hemodynamic support equation as do trans-valvular axial-flow pumps; however, a major technical limitation of the TandemHeart device is the need for a puncture across the interatrial septum to deliver the 21 French cannula that drains the left atrium.
In summary, the trans-valvular axial-flow pumps and the TandemHeart left atrial-to-femoral artery centrifugal-flow pump successfully achieve three of the four major objectives of the hemodynamic support equation: circulatory support, ventricular unloading, and enhanced coronary perfusion.
Right ventricular acute mechanical circulatory support devices
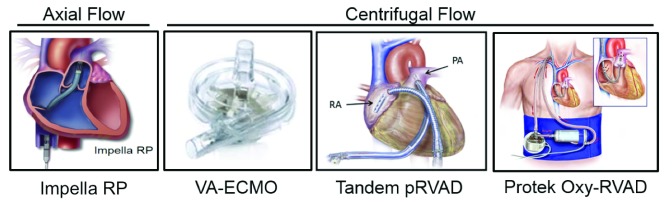
Over the past 5 years, the introduction of right ventricular (RV) non-surgical AMCS devices has advanced our ability to support patients with CS and either isolated RV failure or biventricular (BiV) failure. Options for RV-AMCS include the Impella RP, the TandemHeart RVAD, and VA-ECMO ( Figure 3). The Impella RP and TandemHeart RVAD function by displacing blood from the right atrium (RA) to the pulmonary artery, whereas VA-ECMO drains the RA and displaces blood into the arterial system. The use of RV-AMCS devices has increased awareness of RV dysfunction in the setting of AMI, CS, and HF and after LVAD surgery. While clinical reports support the hemodynamic effects of RV-AMCS 25– 27, no guidelines regarding their use have been developed to date.
Figure 3. Right ventricular acute mechanical circulatory support devices.
Contemporary acute mechanical circulatory support devices for right ventricular support are illustrated and categorized by type of rotary flow pump (axial- or centrifugal-flow). pRVAD, percutaneous right ventricular assist device; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Decongestion in cardiogenic shock: an important target of therapy
A critical barrier to successful clinical outcomes in advanced HF and CS is persistent systemic volume overload or congestion. Recent studies have identified that elevated right heart filling pressures are directly related to worsening renal function and further that elevated BiV filling pressures are associated with increased short-term mortality 28, 29. In CS, adequate circulating volume is necessary to maintain cardiac output; however, excess circulating volume may be detrimental to multi-organ function. As described above, AMCS devices can effectively address parts of the hemodynamic support equation. However, in isolation, AMCS devices alone cannot address the fourth objective, namely, decongestion. Decongestive approaches such as concomitant diuretic therapy or renal replacement therapy should be considered early in CS for patients with elevated BiV filling pressures refractory to diuretics and AMCS device support.
Hemodynamic profiles in cardiogenic shock
The contemporary definition of CS must evolve beyond metrics associated with the early stages of hemo-metabolic shock such as hypotension and evidence of low perfusion, including cold and clammy extremities and end-organ dysfunction 19, 30. At this stage, CS is becoming irreversible. Emerging evidence supports the use of pulmonary artery catheters (PACs) to identify CS before metabolic failure ensues and to define the hemodynamic condition of patients in advanced HF and CS 31. PAC guidance must be strongly considered in patients with suspected CS to confirm the presence of CS (low cardiac output), define the congestive profile in CS (cardiac filing pressures), and to evaluate the patient’s response to therapeutic interventions.
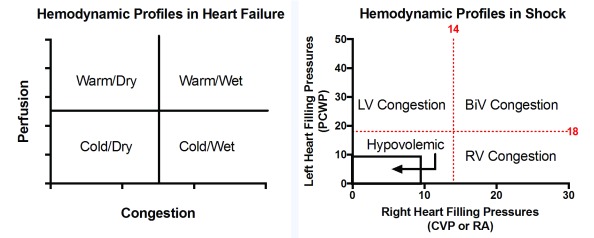
Early acquisition of hemodynamic data also helps to define CS as univentricular or BiV. Beginning in the early 1980s, several studies identified the importance of right and left heart filling pressures in AMI, CS, and advanced HF 32– 34. The relationship between RA and pulmonary capillary wedge pressure (RA:PCWP ratio) has been used to identify RV failure in AMI and is associated with prognosis in advanced HF. Analogous to the 2×2 evaluation of patients with advanced HF as being “warm or cold and dry or wet” 35, the RA:PCWP ratio allows us to classify CS based on congestive state into four hemodynamic profiles: hypovolemic, LV-, RV-, or BiV-dominant congestion 36. For patients with CS failing to improve despite the initiation of one vasopressor or inotrope, each of these four categories may require a different therapeutic approach ( Figure 4). The hypovolemic-CS patient may require volume resuscitation. The LV-CS or RV-CS patients may require specific approaches to modulate univentricular preload or afterload or treatment with a left- or right-sided AMCS device, respectively. The BiV-CS patient may require more aggressive decongestive therapy along with LV or BiV AMCS therapy. Future studies are required to determine whether defining CS based on hemodynamic profile alters management strategies and leads to improved clinical outcomes. Now is the time for a series of prospective, randomized trials or prospective registries confirming the clinical utility of hemodynamic assessment and AMCS device therapy in CS. One recently launched prospective registry is the Detroit Shock Initiative, which involves early application of the Impella trans-valvular axial-flow pump in the setting of AMI-CS 37.
Figure 4. Congestive profiles in cardiogenic shock.
Clinical assessment of hemodynamic conditions in decompensated heart failure is traditionally categorized into four groups based on systemic perfusion and congestive status using a two-by-two table. We now propose a similar two-by-two construct to define hemodynamic profiles in cardiogenic shock based on congestive state using measures of left and right heart filling pressures. Cardiogenic shock is categorized as having LV-, RV-, or BiV-dominant congestion or hypovolemia. Treatment approaches may be tailored to each of these four categories. BiV, biventricular; CVP, central venous pressure; LV, left ventricular; PCWP, pulmonary capillary wedge pressure; RA, right atrial; RV, right ventricular.
In conclusion, as our options to stabilize and rescue patients from the slippery slope of hemodynamic to hemo-metabolic CS grow, we must develop new guidelines that involve 1) early hemodynamic assessment of CS, 2) early use of AMCS devices for refractory CS, 3) identification of the optimal DTS time, 4) appropriate AMCS device selection based on the clinical scenario, and 5) early use of decongestive therapies to reduce the propensity for worsening metabolic failure despite adequate circulatory support.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Daniel Steinberg, Medical University of South Carolina, Charleston, SC, USA
Nader Moazami, Kaufman Center for Heart Failure, Heart and Vascular Institute, Cleveland Clinic, Ohio, Ohio, USA
Daniel Burkhoff, Cardiovascular Research Foundation, New York, New York, NY, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Menees DS, Peterson ED, Wang Y, et al. : Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369(10):901–9. 10.1056/NEJMoa1208200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. McNamara RL, Kennedy KF, Cohen DJ, et al. : Predicting In-Hospital Mortality in Patients With Acute Myocardial Infarction. J Am Coll Cardiol. 2016;68(6):626–35. 10.1016/j.jacc.2016.05.049 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Wayangankar SA, Bangalore S, McCoy LA, et al. : Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc Interv. 2016;9(4):341–51. 10.1016/j.jcin.2015.10.039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Shah RU, de Lemos JA, Wang TY, et al. : Post-Hospital Outcomes of Patients With Acute Myocardial Infarction With Cardiogenic Shock: Findings From the NCDR. J Am Coll Cardiol. 2016;67(7):739–47. 10.1016/j.jacc.2015.11.048 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Ezekowitz JA, Kaul P, Bakal JA, et al. : Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53(1):13–20. 10.1016/j.jacc.2008.08.067 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Kirklin JK, Naftel DC, Pagani FD, et al. : Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. 10.1016/j.healun.2015.10.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Heidenreich PA, Albert NM, Allen LA, et al. : Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19. 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Reynolds HR, Hochman JS: Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–97. 10.1161/CIRCULATIONAHA.106.613596 [DOI] [PubMed] [Google Scholar]
- 9. Basir MB, Schreiber TL, Grines CL, et al. : Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017;119(6):845–51. 10.1016/j.amjcard.2016.11.037 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Meraj PM, Doshi R, Schreiber T, et al. : Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol. 2017. 10.1111/joic.12377 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. O'Neill WW, Schreiber T, Wohns DH, et al. : The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(1):1–11. 10.1111/joic.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Burkhoff D, Naidu SS: The science behind percutaneous hemodynamic support: a review and comparison of support strategies. Catheter Cardiovasc Interv. 2012;80(5):816–29. 10.1002/ccd.24421 [DOI] [PubMed] [Google Scholar]
- 13. van Nunen LX, Noc M, Kapur NK, et al. : Usefulness of Intra-aortic Balloon Pump Counterpulsation. Am J Cardiol. 2016;117(3):469–76. 10.1016/j.amjcard.2015.10.063 [DOI] [PubMed] [Google Scholar]
- 14. Sintek MA, Gdowski M, Lindman BR, et al. : Intra-Aortic Balloon Counterpulsation in Patients With Chronic Heart Failure and Cardiogenic Shock: Clinical Response and Predictors of Stabilization. J Card Fail. 2015;21(11):868–76. 10.1016/j.cardfail.2015.06.383 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Patel MR, Smalling RW, Thiele H, et al. : Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–37. 10.1001/jama.2011.1280 [DOI] [PubMed] [Google Scholar]
- 16. Kapur NK, Paruchuri V, Majithia A, et al. : Hemodynamic effects of standard versus larger-capacity intraaortic balloon counterpulsation pumps. J Invasive Cardiol. 2015;27(4):182–8. [PubMed] [Google Scholar]
- 17. Thiele H, Schuler G, Neumann FJ, et al. : Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock: design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163(6):938–45. 10.1016/j.ahj.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 18. Moazami N, Fukamachi K, Kobayashi M, et al. : Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant. 2013;32(1):1–11. 10.1016/j.healun.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 19. Remmelink M, Sjauw KD, Henriques JP, et al. : Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70(4):532–7. 10.1002/ccd.21160 [DOI] [PubMed] [Google Scholar]
- 20. Merhige ME, Smalling RW, Cassidy D, et al. : Effect of the hemopump left ventricular assist device on regional myocardial perfusion and function. Reduction of ischemia during coronary occlusion. Circulation. 1989;80(5 pt 2):III158–66. [PubMed] [Google Scholar]
- 21. Rihal CS, Naidu SS, Givertz MM, et al. : 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. 10.1016/j.jacc.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 22. Kapur NK, Esposito M: Hemodynamic support with percutaneous devices in patients with heart failure. Heart Fail Clin. 2015;11(2):215–30. 10.1016/j.hfc.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 23. Esposito ML, Shah N, Dow S, et al. : Distinct Effects of Left or Right Atrial Cannulation on Left Ventricular Hemodynamics in a Swine Model of Acute Myocardial Injury. ASAIO J. 2016;62(6):671–6. 10.1097/MAT.0000000000000416 [DOI] [PubMed] [Google Scholar]
- 24. Burkhoff D, Sayer G, Doshi D, et al. : Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 2015;66(23):2663–74. 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Kapur NK, Paruchuri V, Korabathina R, et al. : Effects of a percutaneous mechanical circulatory support device for medically refractory right ventricular failure. J Heart Lung Transplant. 2011;30(12):1360–7. 10.1016/j.healun.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 26. Kapur NK, Paruchuri V, Jagannathan A, et al. : Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013;1(2):127–34. 10.1016/j.jchf.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 27. Anderson MB, Goldstein J, Milano C, et al. : Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34(12):1549–60. 10.1016/j.healun.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 28. Mullens W, Abrahams Z, Francis GS, et al. : Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96. 10.1016/j.jacc.2008.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper LB, Mentz RJ, Stevens SR, et al. : Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J Card Fail. 2016;22(3):182–9. 10.1016/j.cardfail.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Hochman JS, Sleeper LA, Webb JG, et al. : Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625–34. 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 31. O'Connor CM, Starling RC, Hernandez AF, et al. : Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. 10.1056/NEJMoa1100171 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Lopez-Sendon J, Coma-Canella I, Gamallo C: Sensitivity and specificity of hemodynamic criteria in the diagnosis of acute right ventricular infarction. Circulation. 1981;64(3):515–25. 10.1161/01.CIR.64.3.515 [DOI] [PubMed] [Google Scholar]
- 33. Drazner MH, Hellkamp AS, Leier CV, et al. : Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1(3):170–7. 10.1161/CIRCHEARTFAILURE.108.769778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kormos RL, Teuteberg JJ, Pagani FD, et al. : Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139(5):1316–24. 10.1016/j.jtcvs.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 35. Nohria A, Lewis E, Stevenson LW: Medical management of advanced heart failure. JAMA. 2002;287(5):628–40. 10.1001/jama.287.5.628 [DOI] [PubMed] [Google Scholar]
- 36. Kapur NK, Esposito ML: Door to Unload: A New Paradigm for the Management of Cardiogenic Shock. Curr Cardiovasc Risk Rep. 2016;10:41 10.1007/s12170-016-0524-3 [DOI] [Google Scholar]
- 37. Meyer Z: Detroit Hospitals See Hope For Heart Attacks With New Pump. Detroit Free Press,2017; Accessed March 8, 2017. Reference Source [Google Scholar]