ABSTRACT
Patients with refractory or recurrent B-lineage hematologic malignancies have less than 50% of chance of cure despite intensive therapy and innovative approaches are needed. We hypothesize that gene modification of haematopoietic stem cells (HSC) with an anti-CD19 chimeric antigen receptor (CAR) will produce a multi-lineage, persistent immunotherapy against B-lineage malignancies that can be controlled by the HSVsr39TK suicide gene. High-titer third-generation self-inactivating lentiviral constructs were developed to deliver a second-generation CD19-specific CAR and the herpes simplex virus thymidine kinase HSVsr39TK to provide a suicide gene to allow ablation of gene-modified cells if necessary. Human HSC were transduced with such lentiviral vectors and evaluated for function of both CAR and HSVsr39TK. Satisfactory transduction efficiency was achieved; the addition of the suicide gene did not impair CAR expression or antigen-specific cytotoxicity, and determined marked cytotoxicity to ganciclovir. NSG mice transplanted with gene-modified human HSC showed CAR expression not significantly different between transduced cells with or without HSVsr39TK, and expression of anti-CD19 CAR conferred anti-tumor survival advantage. Treatment with ganciclovir led to significant ablation of gene-modified cells in mouse tissues. Haematopoietic stem cell transplantation is frequently part of the standard of care for patients with relapsed and refractory B cell malignancies; following HSC collection, a portion of the cells could be modified to express the CD19-specific CAR and give rise to a persistent, multi-cell lineage, HLA-independent immunotherapy, enhancing the graft-versus-malignancy activity.
KEYWORDS: CAR, cancer immunotherapy, gene therapy, HSC, suicide gene
Introduction
Chimeric antigen receptors (CAR) are generated by fusing the antigen binding domain of a monoclonal antibody to intracellular signaling domains capable to activate immune cells, leading to engineered specificity to direct cellular cytotoxicity against malignant cells bearing the target for the antigen recognition domain of the CAR.1-4 CD19 represents an ideal target for B cell malignancies as it is present in the majority of B cell malignancies and is absent from haematopoietic stem cells (HSC). Although it is expressed on non-malignant B cells, the clinical experience with rituximab therapy has demonstrated the feasibility of survival without B lymphocytes by routine intravenous gamma-globulin replacement. Given the poor outcome demonstrated in patients with relapsed or refractory B cell malignancies, there is a clinical need for novel therapeutics directed toward this patient population.5,6
Although the majority of the published work with CAR has focused on CAR modified T cells,7-12 the expression and effective antigen-specific cytotoxicity of CAR has also been shown in natural killer (NK) cells and myeloid cells.13-17 Our group and others have published effective transgene expression and antigen targeting by gene modification of HSC with CAR18,19 or transgenic T cell receptor (TCR).20-24 Such approaches lead to continuous in vivo lymphopoiesis and proliferation of gene-modified T cells, potentially leading to long-term persistence of antigen-specific immunity. CAR modification of HSC increases the immune effector cells by its expression and directed antigen specificity in multiple lineages (T cells, NK cells and myeloid cells).
To increase the safety of the modification of HSC, a suicide gene can be inserted into the gene transfer vector to eradicate the modified cells in the setting of toxicity.19,23,25 The most extensively used suicide gene is the herpes simplex virus thymidine kinase (HSV-TK), which phosphorylates the prodrugs acyclovir or ganciclovir (GCV). The safety and efficacy of the HSV-TK suicide gene has been demonstrated in the setting of donor lymphocyte infusions, where administration of acyclovir terminated graft vs. host disease.26-28 The hyper-active sr39 mutant of HSV-TK (HSVsr39TK) has been used due to significantly increased sensitivity to acyclovir and GCV.29,30
Here we report the pre-clinical evaluation of gene modification of human HSC with lentiviral vectors co-delivering CD19-specific CAR and HSVsr39TK for immunotherapy of B lineage hematological malignancies.
Results
Promoter comparison in gene modification of Jurkat cells and primary human T cells
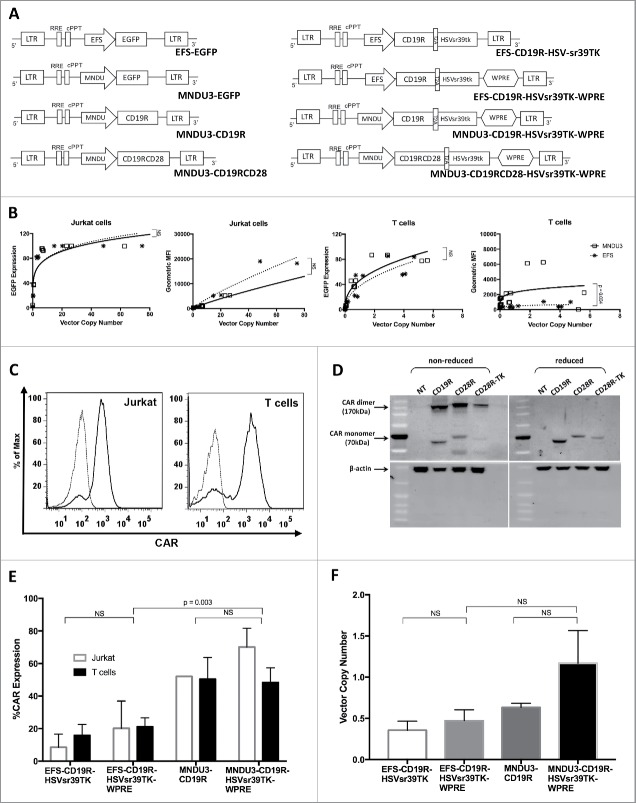
Transgene expression relies upon the construct promoter with variable efficacy depending on the transduced cell. We have initially evaluated 2 different promoters for the lentiviral vector constructs: the human EFS (human elongating factor-1 short)31 and the retrovirus-derived MNDU3.32 High-titer lentiviral vectors were produced carrying enhanced green fluorescent protein (EGFP) under either the EFS or MNDU3 promoters, and used for gene modification of Jurkat cells and primary human T cells (Fig. 1A).
Figure 1.
Lentiviral vectors and transduction of Jurkat and primary human T cells. (A) Schematics of the different lentiviral vector constructs. (B) VCN (in number of viral copies/cell) and geometric MFI of Jurkat and T cells transduced with lentiviral vectors delivering EGFP. (C) Representative flow cytometry histograms of CAR+ Jurkat and T cells, unstained and stained with anti-human IgG Fc gamma F(ab’)2 (D) Western Blot analysis of CAR in Jurkat cells transduced with different constructs delivering CAR and HSVsr39TK. (E) CAR expression (in % of total cells) of Jurkat and T cells transduced with lentiviral vectors delivering CAR and HSVsr39TK; (F) VCN of T cells transduced with lentiviral vectors delivering CAR and HSVsr39TK. Values represent arithmetic means of results from multiple experiments and error bars represent mean + SEM. EGFP: enhanced green fluorescent protein. MFI: mean fluorescence intensity. NS: not statistically significant. SEM: standard error of mean. VCN: vector copy number.
In Jurkat cells, comparing similar transduction concentrations of high-titer vectors (vector MNDU3-EGFP at 2.6×1010 TU/mL and EFS-EGFP at 7.7 × 1010 TU/mL), the transduction efficiency measured by flow cytometry for EGFP and vector copy numbers (VCN) of the MNDU3 promoter and the EFS promoter were similar, reaching a plateau above 15 copies/cell (Fig. 1B 1st panel). As expected, geometric mean fluorescence index (MFI) increased with higher copy number for both vector constructs, with non-significant difference between the mean MFI achieved by both vector constructs (Fig. 1B, 2nd panel).
In primary human T cells, the MNDU3 promoter construct was also found to have similar transduction efficiency when compared with the EFS promoter, at lower final copy numbers, reaching a plateau around 5 copies/cell (Fig. 1B, 3rd panel). A difference between the promoters in primary T cells was noted when analyzing EGFP MFI per integrated vector copy number/cell (Fig. 1B, 4th panel). The EFS vector reached a lower plateau on MFI despite increasing copy numbers, while the MNDU3 consistently achieved about 2 to 3-fold higher MFI (p = 0.004). This suggests that in primary cells the MNDU3 promoter has an increased advantage by promoting higher expression at comparable, or even lower, integrated vector copies/cell (Fig. 1B and Fig. S1).
Lentiviral co-delivery of CAR and HSVsr39TK (Figures 1A–F)
To compare the MNDU3 and EFS promoters on the expression of CAR, lentiviral constructs (Fig. 1A) were then produced for transduction of Jurkat cell line and primary T cells with the first-generation CD19-specific CAR (CD19R) and HSVsr39RK, for evaluation of VCN by qPCR, CAR expression by flow cytometry, and transgene function by antigen-specific cytotoxicity and GCV killing. Small-scale production of such constructs generated the vectors MNDU3-CD19R (mean titer 4.9 × 106 TU/mL), MNDU3-CD19R-HSVsr39TK-WPRE (mean titer 1.7 × 106 TU/mL), EFS-CD19R-HSVsr39TK (mean titer 1.3 × 106 TU/mL) and EFS-CD19R-HSVsr39TK-WPRE (mean titer 1.2 × 106 TU/mL) (Fig. 1A). CAR expression through transduction with the different vectors was confirmed by flow cytometry (Fig. 1C) and Western Blot (Fig. 1D). The CAR expression by flow cytometry demonstrated higher expression and MFI with the MNDU3 promoter compared with the EFS promoter (p = 0.003) in primary T cells (Fig. 1E) similar to our findings with lentiviral transductions delivering EGFP (Fig. 1B); the EFS promoter without woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) had the lowest mean CAR expression. As expected from the CAR expression, the mean VCN was highest with the MNDU3 promoter compared with the EFS in T cells (Fig. 1F), although not reaching statistical significance (p = 0.15).
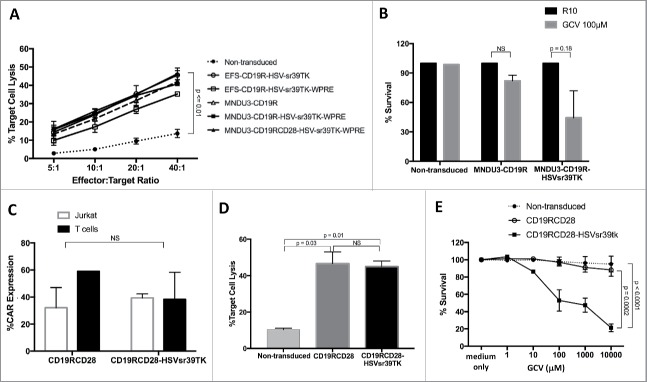
The cytotoxicity of the transduced T cells against CD19-expressing Raji cells was then used to evaluate the antigen-specific cytotoxicity determined by CAR, comparing the different vector constructs. The results showed similar target cell lysis with the MNDU3-CD19R, MNDU3-CD19R-HSVsr39TK-WPRE, EFS-CD19R-HSVsr39TK and EFS-CD19R-HSVsr39TK-WPRE constructs, all 4 had significantly greater target cell killing than the non-transduced cells (p < 0.03 for all 4 comparisons). In addition, the percentage of Raji cells killed increased significantly faster as the effect to target ratio increased in the 4 constructs compared with the non-transduced cells (p≤0.02 for the comparison of slopes) (Fig. 2A). The presence of GCV decreased the survival of the cells containing the HSVsr39TK vector compared with the non-transduced and control vector MNDU3-CD19R (Fig. 2B). Based upon these findings, the MNDU3-CD19R-HSVsr39TK-WPRE vector backbone was chosen for further studies with the addition of the stimulatory molecule CD28 to generate a second-generation CAR.
Figure 2.
Transgene function in gene-modified Jurkat and primary human T cells. (A) Antigen-specific cytotoxicity of CAR-modified T cells against CD19+ Raji cell line, expressed in % of target cell lysis. (B) Cytotoxicity of gene-modified Jurkat cells cultured with GCV (100 μM), expressed in % of surviving cells. (C) CAR expression in Jurkat and T cells modified with second-generation lentiviral vector CAR constructs. (D) Anti-CD19 cytotoxicity of T cells modified with second-generation lentiviral vector CAR constructs. (E) Cytotoxicity of gene-modified T cells incubated with different GCV concentrations. Values represent arithmetic means of results from multiple experiments and error bars represent mean + SEM. GCV: ganciclovir. NS: not statistically significant. SEM: standard error of mean.
Addition of the CD28 costimulatory molecule
Given importance of a second signal in addition the CD3 ζ, for T cell proliferation and cytotoxicity, the costimulatory molecule CD28 was added proximal to the CD3 ζ to create the second-generation CAR, CD19RCD28.33,34 A lentiviral vector delivering CD19RCD28 was used as a control for comparison to the vector co-delivering CD19RCD28 and HSVsr39TK (Fig. 1A, last vector diagram). Human T cells were used to evaluate both vectors for transduction efficiency, cytotoxicity and GCV killing against non-transduced T cells as controls. The mean CAR expression by flow cytometry was 59.1% for MNDU3-CD19RCD28 and 38.3% for MNDU3-CD19RCD28-HSVsr39TK-WPRE (p = 0.26) (Fig. 2C). The cytotoxicity against CD19-positive Raji cells was also similar between MNDU3-CD19RCD28- and MNDU3-CD19RCD28-HSVsr39TK-WPRE-transduced T cells, with less than 10% target cell lysis by the non-transduced cells (p≤0.03) (Fig. 2D). As demonstrated with the first-generation construct, the MNDU3-CD19RCD28-HSVsr39TK-WPRE determined significantly decreased survival after exposure to GCV compared with the other transduced and non-transduced controls, based on comparison of the difference in slopes (p≤0.0002) (Fig. 2E). These results led to further assessment of this vector for gene modification of human HSC.
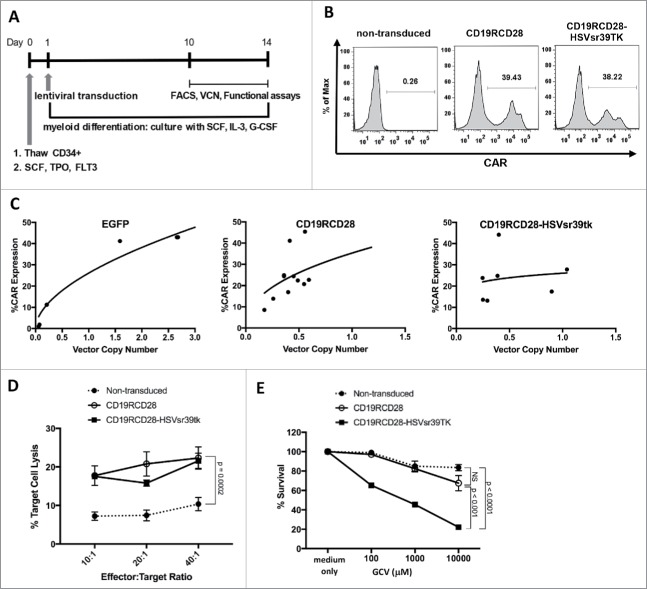
Modification of human HSC with the lentiviral vector for co-delivery of CD19RCD28 and HSVsr39TK
To evaluate the transduction efficiency and function of the suicide gene-containing vector in vitro, human umbilical cord blood CD34 cells were transduced with either the concentrated MNDU3-CD19RCD28-HSVsr39TK-WPRE vector (mean titer 1.49 × 109 TU/mL) or concentrated MNDU3-CD19RCD28 vector control (mean titer 1.97 × 109 TU/mL), or non-transduced cells, and cultured under myeloid differentiation conditions.18 Fig. 3A outlines the modification of HSC, myeloid differentiation and in vitro studies. Flow cytometry on day +11 following transduction revealed effective CAR expression of both vectors with 29.3% mean CAR expression from the MNDU3-CD19RCD28 vector and 27.2% mean CAR expression from the MNDU3-CD19RCD28-HSVsr39TK-WPRE vector (Fig. 3B and 3C). VCN was similar between the 2 vectors (Fig. 3C). Anti-CD19 CAR successfully directed myeloid cells cytotoxicity against CD19+ Raji cells, similarly to T cells, as opposed to minimal target cell cytotoxicity by non-transduced myeloid cells derived from non-gene modified HSC (p = 0.002) (Fig. 3D). GCV effectively eradicated the HSVsr39TK-modified cells with minimal impact on the non-transduced or MNDU3-CD19RCD28-transduced cells, determined by comparison in slopes (p < 0.0001) (Fig. 3E).
Figure 3.
Transduction of human HSC with second-generation CAR constructs. (A) Timeline of thawing, transduction and functional assays of human CD34+ HSC. (B) Representative flow cytometry histograms of gene-modified HSC stained with anti-human IgG Fc gamma F(ab’).2 (C) VCN and CAR expression of HSC (in % of total cells). (D) Antigen-specific cytotoxicity of CAR-modified myeloid cells differentiated from HSC against CD19+ Raji cell line. (E) Cytotoxicity of gene-modified HSC incubated with different GCV concentrations. Values represent arithmetic means of results from multiple experiments and error bars represent mean + SEM. NS: not statistically significant. SEM, standard error of mean. FLT3: FMS-related tyrosine kinase 3. GCV: ganciclovir. G-CSF: granulocyte colony-stimulating factor. IL-3: interleukin 3. SCF: stem cell factor; TPO: thrombopoietin.
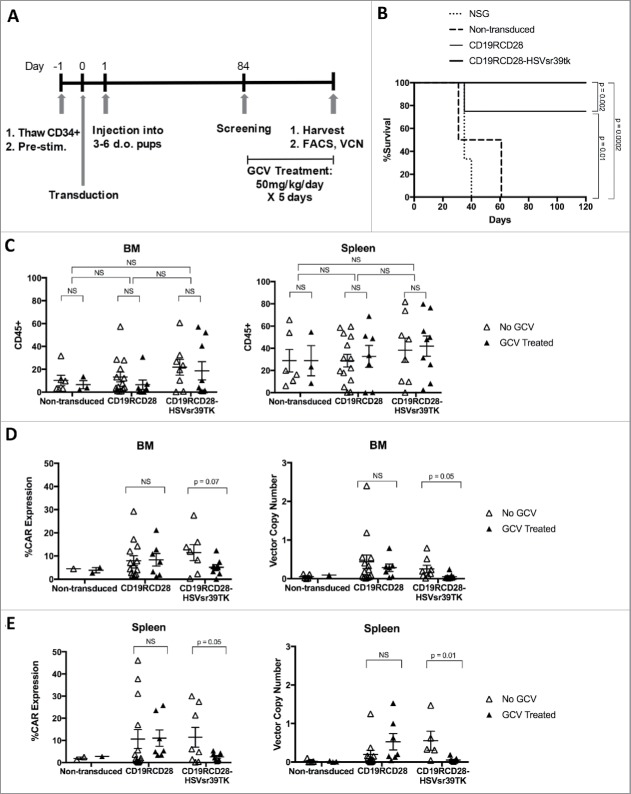
In vivo engraftment and ablation of gene-modified HSC in humanized NSG mice
In vivo assessment of the engraftment, proliferation, and differentiation of modified human HSC cells were performed following the intrahepatic injection of non-transduced, CD19RCD28-, and CD19RCD28-HSVsr39TK-transduced human HSC into sublethally irradiated NOD scid gamma (NSG) pups (Fig. 4A outlines in vivo experiments). There was no significant difference of engraftment of human cells between the non-transduced, CD19RCD28-, or CD19RCD28-HSVsr39TK-transduced arms, as defined by flow cytometry detection of human CD45-positive cells (Fig. 4C). CAR expression was also detected in the mice engrafted with transduced HSC, and absent in the mice engrafted with non-transduced cells (Fig. 4D and 4E).
Figure 4.
In vivo experiments with humanized NSG. (A) Timeline of thawing, transduction, pup injection and in vivo studies. (B) Kaplan-Meier curves of NSG injected with CD19+ Raji lymphoblast-like cells. (C) Detection by flow cytometry of human cells (huCD45+) in bone marrows (BM) and spleens (S) of humanized NSG. (D-E) Detection by flow cytometry and qPCR of gene-modified human cells in bone marrows (D) and spleens (E) of humanized NSG. Values represent arithmetic means of results from mice from multiple experiments and error bars represent mean + SEM. GCV: ganciclovir. NS: not statistically significant. SEM, standard error of mean.
Mice engrafted with cells from the 3 different arms were challenged with subcutaneous injection of CD19-positive Raji cells to evaluate anti-tumor response (Fig. 4B). NSG mice with no humanization had to be killed up to 40 d post-injection, while mice engrafted with non-transduced human HSC developed tumors and were killed up to 60 d post-challenge. Mice engrafted with CAR-transduced HSC presented anti-tumor protection, and did not develop tumors on follow-up to 120 d (Fig. 4B).
At 16-20 weeks post-transplant, mice in each cohort (CD19RCD28, CD19RCD28-HSVsr39TK, and non-transduced) were treated with either intraperitoneal GCV 50 mg/kg daily or PBS for 5 consecutive days. Ten days following GCV treatment, all mice were harvested and cells from bone marrow, spleen, and peripheral blood were evaluated by flow cytometry for detection of human CD45+ cells and CAR expression, and by qPCR for integrated vector copy number analysis (Fig. 4C–E).
When evaluating for CAR expression by flow cytometry, human cells from bone marrow (BM) and spleen (S) of CD19RCD28-HSVsr39TK transplanted mice were 11.47 +/− 3.47 (BM) and 11.43 +/− 4.47 (S) if treated with PBS, vs. 5.15 +/− 1.14 (BM) and 2.54 +/− 0.61 (S), if treated with GCV (p = 0.07 for BM, p = 0.05 for S) (Fig. 4D). While the percentages of human CD45+ cells did not change with the drug treatments (Fig. 4C), the mice engrafted with HSC transduced with the CD19RCD28-HSVsr39TK vector demonstrated a decrease in CAR expression in the GCV-treated group compared with GCV-untreated group (Fig. 4D and 4E).
When analyzing the harvested cells transduction by VCN, mice transplanted with non-transduced and CD19RCD28 HSC treated with PBS had mean VCN of 0.05 +/− 0.03 and 0.43 +/− 0.17 (BM), and 0.02 +/− 0.02 and 0.197 +/− 0.11 (S). After treatment with GCV, mice transplanted with non-transduced and CD19RCD28 HSC had mean VCN of 0.09 +/− 0 and 0.28 +/− 0.096 (BM), and 0.013 +/− 0.009 and 0.53 +/− 0.21 (S) which were not statistically different (Fig. 4D and 4E). Bone marrows from mice transplanted with the CD19RCD28-HSVsr39TK-transduced HSC had mean VCN of 0.25 +/− 0.09 when treated with PBS and 0.05 +/− 0.025 when treated with GCV (p = 0.05) (Fig. 4D). Spleens from mice transplanted with the CD19RCD28-HSVsr39TK vector had mean VCN of 0.554 +/− 0.247 when treated with PBS and 0.05 +/− 0.21 when treated with GCV (p = 0.01) (Fig. 4E). These PCR results corroborate the flow cytometry findings of ablation of gene-modified HSC carrying HSVsr39TK.
Discussion
Our group has previously published that CAR-modified HSC can engraft and lead to CAR-expressing immune cells in the NSG humanized mouse model.,18,23 In this report we demonstrate that human HSC can be effectively modified with a lentiviral vector to co-express CAR and HSVsr39TK, and successfully engraft in a humanized mouse model, generating human cells that have engineered specificity and are amenable to ablation through activation of the suicide gene (Fig. 4C–E).
To our knowledge this is the first report showing engraftment of gene-modified HSC expressing HSVsr39TK and CAR. The effective use of the HSVsr39TK suicide gene for ablation of HSC is consistent with previously published data in mice2230 and Rhesus monkeys,35 further demonstrating the ability of the HSVsr39TK to function as a suicide gene in HSC. The use of HSVsr39TK in this work was chosen due to the extensive clinical experience available from multiple clinical trials.26,36 Multiple cited disadvantages of the clinical application of HSV TK are cell cycle dependence, relatively slow mechanism of action, the avoidance of use of a clinically used antiviral drug, and the risk of immunogenicity to a foreign molecule.19 However, the use of this suicide gene in the HSCT context represents the optimal situation for the application, as it decreases immunogenicity and favors induction of tolerance.17,27,36
The construct used in this study used a second-generation CAR with the CD28 costimulatory domain, which has been shown in previous studies to effectively activate T cells, NK cells and myeloid cells.18,37-39 Preclinical studies have shown mixed results regarding different costimulatory domains, with CD137 (4–1BB)8,9 presenting the most successful responses in clinical trials using gene-modified T cells. Further work is necessary to determine the preferred costimulatory domain for modification of HSC. It is important to highlight that the CAR construct used directed antigen-specific cytotoxicity in T cells (Fig. 2A and 2D) and myeloid cells (Fig 3D), as we have demonstrated in previous publications.18,19,23
Upon evaluation of the vector response to ganciclovir treatment, no difference was seen in the CAR expression or vector copy number of the mice with CD19RCD28 transduced HSC (Fig. 4C). In those mice engrafted with HSC transduced with CD19RCD28-HSVsr39TK there was a significant difference in both CAR expression and vector copy number when comparing treatment with ganciclovir or PBS (Fig. 4D and 4E). CAR expression was significantly decreased in the spleen after ganciclovir treatment as well as vector copy number in both the spleen and the bone marrow (Fig. 4D and 4E). Based on these results, ablation of HSC transduced with the suicide gene HSVsr39TK is possible with ganciclovir treatment. In our experimental findings it is important to note that the transplant of gene-modified HSC was performed one day after lentiviral transduction and without any selection before injection into mice. The transgenes are not expected to be expressed until days later after transduction, when the cells would have started to differentiate if kept in culture, losing pluripotency and proliferation potential. Optimization of lentiviral transduction may still be attempted, keeping vector integration to a maximum of 1–3 copies/cell, and carefully evaluating proliferation of gene-modified cells, genotoxicity and potential development of malignant transformation.
Lymphodepletion preceding infusion of CAR-modified T cells enhance the anti-cancer immune targeting in animal models and clinical trials.11,40,41 The experience from gene transfer protocols for ADA-SCID has shown that a conditioning regimen is necessary for sufficient engraftment of the gene-modified cells.42,43 The treatment of relapsed and refractory B cell malignancies lends itself well to immunotherapy approaches as haematopoietic stem cell transplant is frequently part of the standard of care for these patients. Following progenitor cell collection, a portion of these cells could be modified to express the CD19-specific CAR and give rise to a persistent, multi-cell lineage, HLA-independent immunotherapy, enhancing the graft-vs.-malignancy activity. Based on current clinical trials of gene therapy,23,43 gene modification of a partial portion of HSC would be sufficient to give rise to a large number of antigen-specific immune cells, and ablation of such gene-modified cells would not cause myeloablation. The generation of NK and myeloid cells bearing CAR would allow earlier directed anti-tumor activity until post-transplant thymopoiesis can take place, enhancing the graft-vs.-cancer activity.
The experimental results here reported give proof of principle for CAR-modified HSC regulated by suicide gene, and further studies are needed to enable full clinical translation of this approach. Different ablation approaches, such as inducible caspase 9 or co-delivery of inert cell surface markers (truncated CD20, truncated EGFR) may be more efficient on ablating gene-modified cells and should be evaluated.11,19 The feasibility of co-delivery of a suicide system to inactivate gene-modified HSC ensures increased safety for clinical translation of this approach, as a potential “off-switch” in case of undesired side effects or complications.
Materials and methods
Lentiviral vector constructs
The third-generation self-inactivating lentiviral vectors used the pCCL−c backbone44 and contained the Human Elongation Factor α short (EFS)31 or MND LTR U3 (MNDU3)32 promoter (Fig. 1A). The single chain variable fragment (scFv) targeting CD19 was connected to the intracellular domain of the human CD3ζ T cell intracellular domain through a “stalk” hinge region from the human IgG1Fc protein.45 The hypersensitive HSVsr39TK suicide gene sequence was cloned into the plasmid.22,30 The plasmids containing the EFS promoter were prepared with and without the mutated woodchuck hepatitis virus posttranscriptional regulatory element (WPRE).46,47 The costimulatory domain CD28 was cloned into the plasmid, creating a second-generation CAR.18,37
Lentiviral vector production
Lentiviral supernatant was created through transfection of 293T cells with gag/pol plasmid, VSV-G envelope plasmid, and the lentiviral plasmids of the constructs shown in Fig. 1A. High-titer vectors were produced by tangential flow filtration.48 The first-generation anti-CD19 CAR, MNDU3-CD19R, was used a control. The titer determination of the vectors was performed through transduction of HT-29 cells with 3 independent dilutions of 10−1 vector. The cells were harvested after 72 hours of incubation, and the DNA was extracted using the Qiagen® DNeasy kit (Qiagen 69504). The titer was determined through qPCR of the DNA extraction product.48 Each vector construct was produced at least in 3 different batches to determine mean titer and evaluate transduction efficiency.
Human cell lines and primary human cells
The Raji lymphoblast-like cell line was used as a CD19-expressing cell line for target in cytotoxicity assays and in vivo tumor challenges (ATCC CCL86). Peripheral blood from anonymous donors was used to isolate peripheral blood mononuclear cells (PBMC) through Ficoll-Paque Plus (GE Healthcare Life Sciences 17–1440-02) density gradient separation. Dynabeads® Human T-Activator CD3/CD28 beads (ThermoFisher Scientific 11132D) were used to activate T lymphocytes through incubation for 72 hours. After 72 hours, the T cells were harvested and beads removed through a magnetic column system. Once the beads were removed, 5 × 105 cells were transduced in R10 at 4 × 107 TU/mL. These cells were kept in culture in RPMI plus 10% FBS (R10) with rhuIL-2 (R&D Systems 202-IL-500) and reactivated with the anti-CD3/CD28 beads every 7–10 d.
Umbilical cord blood units were obtained through anonymous collection from the delivery rooms at UCLA. Isolation of human CD34+ cells were obtained through immunomagnetic beads (MACS CD34 MicroBead Separation Kit, Miltenyi 130–046-702) and stored at −170°C. After thawing, these cells were prestimulated for 14 hours in X-Vivo15 medium (Lonza 04–744Q) with human stem cell factor (SCF), Flt-3 ligand, and thrombopoietin (R&D Systems 255-SC-200, 308-FK and 288-TP). 1 × 106 cells in 1 mL with wells coated with recombinant human fibronectin fragment RetroNectin (Clontech T100B) were transduced at a vector concentration of 5 × 107 TU/mL for 24 hours.18 Myeloid differentiation conditions were created by culture for 12–15 d in Iscove's Modified Dulbecco's medium (IMDM) (Corning 10–016-CM) with 10% FBS, plus SCF and IL-3.18
Flow cytometry
All flow cytometry acquisitions were made using a Fortessa cytometer (BD Biosciences), and analyses performed using BD FACS Diva Software 6.1 (BD Biosciences). The presence of the CAR was detected through flow cytometry using a Fluorescein isothiocyanate (FITC)-labeled goat anti-human IgG Fc gamma F(ab’)2 (Jackson ImmunoResearch Laboratories 109–096-008) which binds to the IgG1 Fcγ hinge region of the CAR construct.18,37 Cell surface markers were assessed by staining with fluorescent-labeled murine monoclonal antibodies for 20 min in the dark at 4°C, followed by washing in PBS with 2.5% FBS and fixation using BD stabilizing fixative (BD Biosciences 338036) as described previously.18 All experiments with determinations of geometric MFI (Fig. 1B and Fig. S1) were performed using the same protocol, fluorochrome voltages and cytometer.
Vector copy number assessment
The Qiagen DNeasy Blood and Tissue kit (Qiagen 69504) was used to extract DNA from samples. The DNA was quantified using the Sigma-Aldrich DNA Quantification Fluorescence Assay kit (Sigma-Aldrich DNAQF-1KT) The HIV-1 ψ region of the vector provirus was detected using Taqman primers and probes and compared with a standard curve generated from genomic DNA obtained from a cell line with an established lentiviral vector copy number.18 The quantitative real-time-PCR (qPCR) was performed using the ABI 7700 Sequence Detector (Applied Biosystems).
Western blot
For immunoblot analysis of CAR expression, Jurkat cells transduced with the lentiviral vectors were lysed with Denaturing Cell Extraction Buffer (ThermoFisher Scientific FNN0091) in presence of protease inhibitor (Roche Diagnostics 04693116001), and protein lysates were quantified using BCA assay (Pierce BCA Protein Assay kit (ThermoFisher Scientific 23227). The appropriate protein samples were collected and placed at 95°C for 10 mins and subjected to SDS-PAGE and immunoblotting. The immunoreactive protein bands of CAR were visualized with a peroxidase-conjugated goat anti-human IgG Fcγ fragment specific (Jackson ImmunoResearch 109–03–008) and actin bands were visualized with a primary murine monoclonal anti-β-actin antibody (Sigma Aldrich A1978) followed by a peroxidase-conjugated secondary anti-mouse IgG (Fc specific) antibody (Sigma Aldrich A0168), using Pierce ECL Plus western blotting substrate (ThermoFisher Scientific 32134) and a Typhoon™ FLA 900 biomolecular imager (GE Healthcare Life Sciences). Reduced protein samples were treated with NuPAGE sample reducing agent 0.7X (ThermoFisher Scientific NP0004).
Cytotoxicity assays
The cytotoxicity analysis was performed using a non-radiation based assay flow cytometry Live/Dead Cell Mediated Cytotoxicity Kit (ThermoFisher Scientific L7010) 5 × 105 target cells per well in a “U” bottom 96-well plate. The target cells used were Raji and K562 cell lines. These cells were stained with 3,3′ diethyloxacarbocyanine iodide (DiOC) and placed in R10 media containing propidium iodide (PI). Cells positive for both DiOC and PI were considered non-viable. The cytotoxicity was assessed at the effector to target ratios of 5:1, 10:1, 20:1, and 40:1 using a Fortessa cytometer (BD Biosciences).18
Ganciclovir cell death assays
CAR transduced Jurkat cells or primary human cells were plated at 1 × 105 cells in 1 mL in a 48-well tissue culture treated plate in RPMI with 10% FBS and 1% penicillin-streptomycin. Non-transduced cells and cells transduced with a CAR that does not contain the suicide gene were used as controls. The cells were incubated with R10 or ganciclovir at 1 µM, 10 µM, 100 µM, 1,000 µM or 10,000 µM for 96 hours at 37°C.30,49,50 The cells were harvested and stained with a FITC anti-IgG FC. DAPI was added and the cells were assessed for survival using flow cytometry.
In vivo studies
NOD/SCID/γ chain null mice (NSG) (Jackson Laboratory 005557) were kept according to the protocol approved by the UCLA Office of Animal Research Oversight. All animals were handled in laminar flow hoods and housed in microinsulator cages in a pathogen-free colony in a biocontainment vivarium facility. At 3–7 d of life the NSG pups were irradiated with 150cGy of sublethal total body irradiation from a 137Ce source with attenuator. One day following irradiation, the pups received 3 × 105 cells/pup of gene-modified or non-modified CD34-positive cells isolated from human umbilical cord blood via intrahepatic injection, one day after vector transduction, with cells injected without any selection. After transplant, mice were allowed to engraft over 12 weeks.18 At 12 weeks post-transplantation, the pups were screened for the presence of human CD45-positive cells and CAR modified cells in the peripheral blood through retro-orbital venous blood sampling by flow cytometry. Once engraftment was determined, a cohort of mice from each arm were treated with intraperitoneal GCV 50 mg/kg daily for 5 d.30 The mice were harvested 10–15 d following ganciclovir treatment and evaluated for the presence of CAR-modified cells in the bone marrow and spleen, by flow cytometry and qPCR.22 Separate humanized mice with the different transduction arms were challenged with subcutaneous injection of 1 million Raji cells (CD19-positive human lymphoblast-like cell line) to evaluate anti-tumor response. Mice were killed if tumors developed a diameter greater than 1.5 cm, interfered with bodily functions, or if the tumors showed ulceration.
Statistical analyses
Summary statistics (means, standard deviations and standard errors) were computed for each group. The Student's t-test was used to compare the means of outcome measures between groups. We used linear regression models to compare the effect of concentration (ganciclovir) or effector to target ratio vs. the percentage of surviving cells. In these models we used interaction effects to test for differences in the relationship between concentration (or effector ratio) and survival between the different cell types. P values ≤0.05 were used for assigning statistical significance. Analyses of experimental data were performed using GraphPad Prism 7 and SAS (ver 9.14).
Supplementary Material
Abbreviations
- CAR
chimeric antigen receptors
- CD19R
first-generation CD19-specific CAR
- DiOC
diethyloxacarbocyanine iodide
- EFS
elongation factor α short
- EGFP
enhanced green fluorescent protein
- FBS
fetal bovine serum
- G-CSF
granulocyte colony-stimulating factor
- GCV
ganciclovir
- HSC
haematopoietic stem cells
- HSV-TK
herpes simplex virus-thymidine kinase
- IL
interleukin
- MFI
mean fluorescence intensity
- NK
natural killer
- NS
not statistically significant
- NSG
NOD scid gamma null mouse strain
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PI
propidium iodide
- qPCR
quantitative real-time-PCR
- SCF
stem cell factor
- scFv
single chain variable fragment
- SEM
standard error of mean
- TCR
T cell receptor
- TU
transducing unit
- VCN
vector copy number
- WPRE
woodchuck hepatitis virus posttranscriptional regulatory element
Disclosure of potential conflicts of interest
SL is a consultant for Bristol-Myers Squibb. TT is currently employee of Genentech, hired after having completed her participation in this project.
Acknowledgments
All the authors acknowledge the training and technical support of the Flow Cytometry Core of the UCLA Broad Stem Cell Research Center (BSCRC), where all flow cytometry experiments were performed. We also acknowledge the help on data acquisition and analysis from Perseus Patel, Therese Dinoso and Nezia Rahman.
Funding
Support was provided by the UCLA Department of Pediatrics (K-12 UCLA Child Health Research Center Development Award (CHRCDA)), Gwynne Hazen Cherry Memorial Fund, Pediatric Cancer Research Foundation, Lights Camera Cure, Miranda D. Beck Pediatric Cancer Research Foundation, UCLA Cancer Research Coordinating Committee award ID CRN-15–380486, Hyundai Hope on Wheels, UCLA Children's Discovery and Innovation Institute, UCLA Jonsson Comprehensive Cancer Center, UCLA/CFAR Virology Core Lab, UCLA Clinical and Translational Science Institute Grant UL1TR000124, St. Baldrick's Foundation and American Society of Hematology.
References
- [1].Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen M, Cooper LJ, June CH, Rosenberg S, Sadelain M, Heslop HE. CARs on track in the clinic. Mol Ther [Internet] 2011. [cited 2013June26]; 19:432-8. Available from: http://www.nature.com/doifinder/10.1038/mt.2011.1; PMID:21358705; http://dx.doi.org/ 10.1038/mt.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dotti G, Savoldo B, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: “Are we nearly there yet?” Hum Gene Ther [Internet] 2009. [cited 2013June25]; 20:1229-39. Available from: http://www.liebertonline.com/doi/abs/10.1089/hum.2009.142; PMID:19702437; http://dx.doi.org/ 10.1089/hum.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev [Internet] 2014; 257:107-26. Available from: http://doi.wiley.com/10.1111/imr.12131; PMID:24329793; http://dx.doi.org/ 10.1111/imr.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jensen MC, Riddell SR. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev [Internet] 2014; 257:127-44. Available from: http://doi.wiley.com/10.1111/imr.12139; PMID:24329794; http://dx.doi.org/ 10.1111/imr.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N, et al.. Induction therapy for adults with acute lymphoblastic leukemia : results of more than 1500 patients from the international ALL trial : MRC UKALL XII / ECOG E2993. Blood [Internet] 2005; 106:3760-7. Available from: http://www.bloodjournal.org/content/106/12/3760?sso-checked = true; PMID:16105981; http://dx.doi.org/ 10.1182/blood-2005-04-1623 [DOI] [PubMed] [Google Scholar]
- [6].Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, et al.. Salvage regimens with autologous transplantation for relapsed large B-Cell Lymphoma in the Rituximab Era. J Clin Oncol [Internet] 2010. [cited 2015July15]; 28:4184-90. Available from: http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2010.28.1618; PMID:20660832; http://dx.doi.org/ 10.1200/JCO.2010.28.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol [Internet] 2013. [cited 2013June4]; 10:267-76. Available from: http://www.nature.com/doifinder/10.1038/nrclinonc.2013.46; PMID:23546520; http://dx.doi.org/ 10.1038/nrclinonc.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med [Internet] 2011. [cited 2013June25]; 365:725-33. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1103849; PMID:21830940; http://dx.doi.org/ 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al.. Chimeric antigen receptor T cells for sustained remissions in Leukemia. N Engl J Med [Internet] 2014; 371:1507-17. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1407222; PMID:25317870; http://dx.doi.org/ 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells : CARs take the front seat for hematologic malignancies. Blood [Internet] 2015; 123:2625-36. Available from: http://www.bloodjournal.org/content/123/17/2625?sso-checked=true; http://dx.doi.org/ 10.1182/blood-2013-11-492231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corrigan-Curay J, Kiem H-P, Baltimore D, O'Reilly M, Brentjens RJ, Cooper L, Forman S, Gottschalk S, Greenberg P, Junghans R, et al.. T-Cell immunotherapy: looking forward. Mol Ther [Internet] 2014; 22:1564-74. Available from: http://www.nature.com/doifinder/10.1038/mt.2014.148; PMID:25186558; http://dx.doi.org/ 10.1038/mt.2014.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davila ML, Bouhassira DCG, Park JH, Curran KJ, Smith EL, Pegram HJ, Brentjens R. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int J Hematol [Internet] 2014; 99:361-71. Available from: http://link.springer.com/10.1007/s12185-013-1479-5; PMID:24311149; http://dx.doi.org/ 10.1007/s12185-013-1479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tran AC, Zhang D, Byrn R, Roberts MR. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol [Internet] 1995. [cited 2013June18]; 155:1000-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7608531; PMID:7608531 [PubMed] [Google Scholar]
- [14].Hege KM, Cooke KS, Finer MH, Zsebo KM, Roberts MR. Systemic T cell-independent tumor immunity after transplantation of universal receptor-modified bone marrow into SCID mice. J Exp Med [Internet] 1996. [cited 2013June18]; 184:2261-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8976181; PMID:8976181; http://dx.doi.org/ 10.1084/jem.184.6.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schirrmann T, Pecher G. Specific targeting of CD33+ leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res [Internet] 2005. [cited 2015July15]; 29:301-6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0145212604002474; PMID:15661266; http://dx.doi.org/ 10.1016/j.leukres.2004.07.005 [DOI] [PubMed] [Google Scholar]
- [16].Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, Wolfraim LA, Fujisaki H, Campana D, Chopas N, et al.. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther [Internet] 2010. [cited 2010July19]; 17:147-54. Available from: http://www.nature.com/doifinder/10.1038/cgt.2009.61; PMID:19745843; http://dx.doi.org/ 10.1038/cgt.2009.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol [Internet] 2015; 6:1-7. Available from: http://journal.frontiersin.org/Article/10.3389/fphar.2015.00021/abstract; PMID:25805991; http://dx.doi.org/ 10.3389/fphar.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Oliveira SN, Ryan C, Giannoni F, Hardee CL, Tremcinska I, Katebian B, Wherley J, Sahaghian A, Tu A, Grogan T, et al.. Modification of hematopoietic stem/progenitor cells with CD19-specific chimeric antigen receptors as a novel approach for cancer immunotherapy. Hum Gene Ther [Internet] 2013; 24:824-39. Available from: http://online.liebertpub.com/doi/abs/10.1089/hum.2012.202; PMID:23978226; http://dx.doi.org/ 10.1089/hum.2012.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larson S, De Oliveira SN. Gene-modified hematopoietic stem cells for cancer immunotherapy. Hum Vaccin Immunother [Internet] 2014. [cited 2014May26]; 10:982-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24398603; PMID:24398603; http://dx.doi.org/ 10.4161/hv.27637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kitchen SG, Bennett M, Galić Z, Kim J, Xu Q, Young A, Lieberman A, Joseph A, Goldstein H, Ng H, et al.. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS One [Internet] 2009. [cited 2013May23]; 4:e8208. Available from: http://dx.plos.org/10.1371/journal.pone.0008208; PMID:19997617; http://dx.doi.org/ 10.1371/journal.pone.0008208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giannoni F, Hardee CL, Wherley J, Gschweng E, Senadheera S, Kaufman ML, Chan R, Bahner I, Gersuk V, Wang X, et al.. Allelic exclusion and peripheral reconstitution by TCR transgenic T cells arising from transduced human hematopoietic stem/progenitor cells. Mol Ther [Internet] 2013. [cited 2013May22]; 21:1044-54. Available from: http://www.nature.com/doifinder/10.1038/mt.2013.8; PMID:23380815; http://dx.doi.org/ 10.1038/mt.2013.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gschweng EH, McCracken MN, Kaufman ML, Ho M, Hollis RP, Wang X, Saini N, Koya RC, Chodon T, Ribas A, et al.. HSV-sr39TK positron emission tomography and suicide gene elimination of human hematopoietic stem cells and their progeny in humanized mice. Cancer Res [Internet] 2014. [cited 2015February19]; 74:5173-83. Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-14-0376; PMID:25038231; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gschweng E, De Oliveira S, Kohn DB. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev [Internet] 2014. [cited 2013December18]; 257:237-49. Available from: http://doi.wiley.com/10.1111/imr.12128; PMID:24329801; http://dx.doi.org/ 10.1111/imr.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Starck L, Popp K, Pircher H, Uckert W. Immunotherapy with TCR-redirected T cells: Comparison of TCR-transduced and TCR-engineered hematopoietic stem cell-derived T Cells. J Immunol [Internet] 2014; 192:206-13. Available from: http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.1202591; PMID:24293634; http://dx.doi.org/ 10.4049/jimmunol.1202591 [DOI] [PubMed] [Google Scholar]
- [25].Najjar AM, Manuri PR, Olivares S, Flores L, Mi T, Huls H, Shpall EJ, Champlin RE, Turkman N, Paolillo V, et al.. Imaging of sleeping beauty-modified CD19-specific T cells expressing HSV1-Thymidine Kinase by positron emission tomography. Mol Imaging Biol [Internet] 2016; Epub ahead of print. Available from: http://link.springer.com/10.1007/s11307-016-0971-8; PMID:27246312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, et al.. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science [Internet] 1997. [cited 2013July9]; 276:1719-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9180086; PMID:9180086; http://dx.doi.org/ 10.1126/science.276.5319.1719 [DOI] [PubMed] [Google Scholar]
- [27].Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, Bonini C, Bordignon C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood [Internet] 2007. [cited 2013July9]; 109:4708-15. Available from: http://www.bloodjournal.org/cgi/doi/10.1182/blood-2006-04-015230; PMID:17327417; http://dx.doi.org/ 10.1182/blood-2006-04-015230 [DOI] [PubMed] [Google Scholar]
- [28].Bonini C, Bondanza A, Perna SK, Kaneko S, Traversari C, Ciceri F, Bordignon C. The suicide gene therapy challenge: How to improve a successful gene therapy approach. Mol Ther [Internet] 2007; 15:1248-52. Available from: http://www.nature.com/doifinder/10.1038/sj.mt.6300190; PMID:17505474; http://dx.doi.org/ 10.1038/sj.mt.6300190 [DOI] [PubMed] [Google Scholar]
- [29].Kokoris MS, Black ME. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci [Internet] 2009; 11:2267-72. Available from: http://doi.wiley.com/10.1110/ps.2460102; http://dx.doi.org/ 10.1110/ps.2460102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blumenthal M, Skelton D, Pepper KA, Jahn T, Methangkool E, Kohn DB. Effective suicide gene therapy for Leukemia in a model of insertional oncogenesis in mice. Mol Ther [Internet] 2007; 15:183-92. Available from: http://www.nature.com/doifinder/10.1038/sj.mt.6300015; PMID:17164790; http://dx.doi.org/ 10.1038/sj.mt.6300015 [DOI] [PubMed] [Google Scholar]
- [31].Thornhill SI, Schambach A, Howe SJ, Ulaganathan M, Grassman E, Williams D, Schiedlmeier B, Sebire NJ, Gaspar HB, Kinnon C, et al.. Self-inactivating Gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol Ther [Internet] 2008; 16:590-8. Available from: http://www.nature.com/doifinder/10.1038/sj.mt.6300393; PMID:18180772; http://dx.doi.org/ 10.1038/sj.mt.6300393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Challita PM, Skelton D, El-Khoueiry A, Yu XJ, Weinberg K, Kohn DB. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J Virol [Internet] 1995; 69:748-55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7815539; PMID:7815539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature [Internet] 1992; 356:607-9. Available from: http://www.nature.com/doifinder/10.1038/356607a0; PMID:1313950; http://dx.doi.org/ 10.1038/356607a0 [DOI] [PubMed] [Google Scholar]
- [34].Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nat Biotechnol [Internet] 2002; 20:70-5. Available from: http://www.nature.com/doifinder/10.1038/nbt0102-70; PMID:11753365; http://dx.doi.org/ 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- [35].Barese CN, Krouse AE, Metzger ME, King CA, Traversari C, Marini FC, Donahue RE, Dunbar CE. Thymidine kinase suicide gene-mediated ganciclovir ablation of autologous gene-modified rhesus hematopoiesis. Mol Ther [Internet] 2012; 20:1932-43. Available from: http://www.nature.com/doifinder/10.1038/mt.2012.166; PMID:22910293; http://dx.doi.org/ 10.1038/mt.2012.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lupo-Stanghellini MT, Provasi E, Bondanza A, Ciceri F, Bordignon C, Bonini C. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther [Internet] 2010. [cited 2013June26]; 21:241-50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20121594; PMID:20121594; http://dx.doi.org/ 10.1089/hum.2010.014 [DOI] [PubMed] [Google Scholar]
- [37].Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJN. CD28 Costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res [Internet] 2006; 66:10995-1004. Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-06-0160; PMID:17108138; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0160 [DOI] [PubMed] [Google Scholar]
- [38].Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al.. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest [Internet] 2011. [cited 2013June25]; 121:1822-6. Available from: http://www.jci.org/articles/view/46110; PMID:21540550; http://dx.doi.org/ 10.1172/JCI46110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, Victor A, Meisen WH, Kim S, Grandi P, et al.. CAR-Engineered NK cells targeting Wild-Type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep [Internet] 2015; 5:11483. Available from: http://www.nature.com/articles/srep11483; PMID:26155832; http://dx.doi.org/ 10.1038/srep11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred Tumor-specific T Cells. J Immunother [Internet] 2010; 33:1-7. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00002371-201001000-00001; PMID:19952961; http://dx.doi.org/ 10.1097/CJI.0b013e3181b88ffc [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev [Internet] 2014; 257:56-71. Available from: http://doi.wiley.com/10.1111/imr.12132; PMID:24329789; http://dx.doi.org/ 10.1111/imr.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Aiuti A. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science (80) [Internet] 2002; 296:2410-3. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1070104; http://dx.doi.org/ 10.1126/science.1070104 [DOI] [PubMed] [Google Scholar]
- [43].Kohn DB, Pai S-Y, Sadelain M. Gene therapy through autologous transplantation of gene-modified hematopoietic stem cells. Biol Blood Marrow Transplant [Internet] 2013. [cited 2013June21]; 19:S64-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1083879112003990; PMID:23032601; http://dx.doi.org/ 10.1016/j.bbmt.2012.09.021 [DOI] [PubMed] [Google Scholar]
- [44].Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol [Internet] 1998; 72:8463-71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9765382; PMID:9765382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cooper LJN. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood [Internet] 2003. [cited 2010July3]; 101:1637-44. Available from: http://www.bloodjournal.org/cgi/doi/10.1182/blood-2002-07-1989; PMID:12393484; http://dx.doi.org/ 10.1182/blood-2002-07-1989 [DOI] [PubMed] [Google Scholar]
- [46].Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, Kohn DB, Malik P. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther [Internet] 2007; 14:1298-304. Available from: http://www.nature.com/doifinder/10.1038/sj.gt.3302979; PMID:17597793; http://dx.doi.org/ 10.1038/sj.gt.3302979 [DOI] [PubMed] [Google Scholar]
- [47].Zanta-Boussif MA, Charrier S, Brice-Ouzet A, Martin S, Opolon P, Thrasher AJ, Hope TJ, Galy A. Validation of a mutated PRE sequence allowing high and sustained transgene expression while abrogating WHV-X protein synthesis: application to the gene therapy of WAS. Gene Ther [Internet] 2009; 16:605-19. Available from: http://www.nature.com/doifinder/10.1038/gt.2009.3; PMID:19262615; http://dx.doi.org/ 10.1038/gt.2009.3 [DOI] [PubMed] [Google Scholar]
- [48].Cooper AR, Patel S, Senadheera S, Plath K, Kohn DB, Hollis RP. Highly efficient large-scale lentiviral vector concentration by tandem tangential flow filtration. J Virol Methods [Internet] 2011; 177:1-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0166093411002837; PMID:21784103; http://dx.doi.org/ 10.1016/j.jviromet.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zu B, Shi Y, Xu M, You G, Huang Z, Gao M, Feng W. ARE/SUZ12 dual specifically-regulated adenoviral TK/GCV system for CML blast crisis cells. J Exp Clin Cancer Res [Internet] 2015; 34:56. Available from: http://jeccr.biomedcentral.com/articles/10.1186/s13046-015-0139-4; PMID:26017281; http://dx.doi.org/ 10.1186/s13046-015-0139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dong XY, Wang WQ, Zhao Y, Li XD, Fang ZG, Lin DJ, Xiao RZ, Huang R-W, Pan GJ, Liu JJ. Antibody-directed double suicide gene therapy targeting of MUC1- positive leukemia cells in vitro and in vivo. Curr Gene Ther [Internet] 2013; 13:346-57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24060312; PMID:24060312; http://dx.doi.org/ 10.2174/15665232113136660029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.