Abstract
Vaccine information of varying quality is available through many different sources. We describe the creation, release and utilization of ReadyVax, a new mobile smartphone app providing access to trustworthy, evidence-based vaccine information for a target audience of healthcare providers, pharmacists, and patients (including parents of children). We describe the information content and technical development of ReadyVax. Between the hard launch of the app on February 12, 2015 and October 8, 2016, the app has been downloaded by 5,142 unique users, with 6,841 total app sessions initiated, comprising a total of 15,491 screen views (2.3 screens/session on average). ReadyVax has been downloaded by users in 102 different countries; most users (52%) are from the United States. We are continuing outreach efforts to increase app use, and planning for development of an Android-compatible version of ReadyVax, to increase the available market for the app.
Keywords: app, smartphone, vaccination, vaccine
Introduction
Given recent experiences with pandemic influenza,1,2 seasonal influenza epidemics,3 and pertussis outbreaks,4,5 there is an ever-present need to relay important vaccine-related information to populations particularly vulnerable to the effects of infectious diseases. Depending on the nature of the threat and populations at risk, messaging may need to take different forms.6,7 Routine vaccine promotion activities are typically educational in nature, informing the target population about vaccines, vaccine-preventable diseases, and populations indicated for vaccination. However, changing recommendations in response to new evidence necessitates widespread and targeted dissemination of new information. For example, in the context of pertussis and influenza outbreaks, messaging to inform pregnant women of the importance of routine tetanus, diphtheria, and acellular pertussis (Tdap) and seasonal influenza vaccination during every pregnancy takes on heightened importance.
Smartphone ownership is high in the United States – 64% among adults in the general population8 and 80% among physicians.9 Similarly, healthcare information seeking is common. In 2012, more than half (52%) of smartphone users reported accessing health information on their phones, with 19% having a health-related app on their mobile device.10 Additionally, many physicians report using smartphone during medical rounds.11-13 Having a readily available reference on a smartphone or other mobile device without having to consult a book or website on a computer may add efficiency to the clinical encounter with regard to providing vaccine information to patients. With an ever-increasing number of preventive services recommended and less time available to spend on patient-level counseling,14 methods such as mobile app usage for checking key references may offer advantages to providing timely and appropriate preventive care.
Information needs related to vaccines and vaccine-preventable diseases are varied. Our previous research has highlighted the information needs and preferred delivery mechanisms for vaccine-related information.15-18 These findings, including provision of information before clinical encounters and providing access to information of increasing levels of detail to be used by the user to meet their needs (e.g. per the Elaboration Likelihood Model19,20), were used to develop a multi-level intervention to improve vaccine uptake among pregnant women that included a tablet-based educational model.21 The use of mobile technologies for vaccine information provision during clinical encounters is a logical step toward improving vaccination recommendations.
Developing a mobile smartphone application (“app”) for use by traditional vaccine providers, pharmacists, and patients or parents of pediatric patients to readily obtain vaccine-related information is a valuable public health systems tool. In addition to providing access to standard information about vaccines, this tool can be used in the event of an emergency to empower public health authorities to convey messages to target populations as quickly as possible.
To assess the use of a smartphone app to provide access to information about vaccines and the diseases they prevent, we created ReadyVax. ReadyVax is a new mobile smartphone app designed to provide access to trustworthy, evidence-based vaccine information. We have made ReadyVax available for free download in the Apple iTunes app store with regard to routine vaccination recommendations, to determine the potential for this app to be used. Here we describe the creation, release and to-date utilization of ReadyVax.
App development and evaluation
Information content was structured around four primary categories: Vaccines, Diseases, Answers to Common Questions, and Resources. For vaccines, information was obtained from relevant CDC publications, including the Morbidity and Mortality Weekly Report-based publication of the Advisory Committee on Immunization Practice (ACIP) recommendations22 for each vaccine as well as the book “Epidemiology and Prevention of Vaccine-Preventable Diseases,” also known as the CDC “Pink Book.”23 Disease specific information and answers to common questions were synthesized from MMWR publications of ACIP recommendations,22 the “Pink Book,”23 and peer-reviewed published manuscripts as needed. The list of web-based resources was collated from the project team's knowledge of available organizations and information repositories.
Specifications for app functionality were defined by the project team, and are presented in Table 1. Technical development of the app was conducted by AnyPresence, LLC (Reston VA), which specializes in mobile backend as a service (MBaaS) app development and optimization. The project team developed a variety of data flow models to address user accessibility to the app content. Proposed data flow models were presented to the technical developer and optimized through iterative discussions between the project team and developer. Following finalization of data flow, the vendor developed wireframes to guide app development. Routine technical and vaccine-related discussions occurred between the project team and the technical developer to refine the wireframes and ultimately the app.
Table 1.
ReadyVax app technical specifications.
| Native app that can be downloaded and installed directly on smartphone or tablet |
| Information content will be available even with no network connection. |
| Updated information will be downloaded automatically when app is used on a network connection |
| Information will be browsable and searchable |
| Information content can be tailored to specific user groups |
| User type specifications: Healthcare provider, Pharmacist, Patient/Parent |
| Information content can be updated through a web-based dashboard interface |
| Messages can be pushed out to app users, with Alert Notifications appearing on the users' device |
| Links to multimedia (e.g., YouTube videos) can be embedded |
The project team received the initial version of the ReadyVax app for pilot testing on November 12, 2014, and it was initially housed for download on the Emory University Information Technology internal app store. Members of the project team and other colleagues were invited to download the ReadyVax app and conduct user testing. Feedback from this user testing was provided to the technical vendor to make the requested updates. The production version of the app was received by the project team in early January 2015 and submitted to the Apple iTunes app store. ReadyVax was available for public download on January 23, 2015. Following project team-led downloading and use of the app to ensure that the publicly available version worked properly, we began promotion of the app (i.e. our “hard launch”) to public health partners on February 12, 2015. ReadyVax is available through the Apple iTunes store, at https://itunes.apple.com/us/app/readyvax/id957851259?mt=8.
We worked with public health partners in Georgia, Oregon, Washington and New York State to publicize the availability of the ReadyVax app. Additionally, press releases were provided by the Emory University Media Relations office to media outlets, and project team members worked with vaccine advocacy groups to promote the availability of the app on their websites and through their email newsletters.
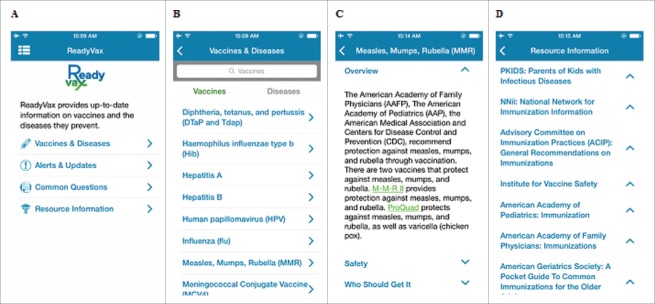
The project team monitored app acquisition and usage through Google Analytics. We routinely monitored the number of unique acquisitions of the app, total user sessions initiated, engagement time, and number of screen views within the app. For ReadyVax a screen view consists of viewing information in one area. There are ten relevant screens available in ReadyVax: Home (general navigation home screen; see Fig. 1, upper left panel), VaccineDiseases (general home screen from which users can select to view either vaccines or diseases; see Fig. 1, upper right panel), Vaccine (information on individual vaccines; see Fig. 1, lower left panel), Disease (information on individual diseases), Questions (frequently asked questions), Resources (list of external vaccine information resources; see Fig. 1, lower right panel), Alerts (messages provided through push message notifications), Role (screen to set user role), About (general information about the app), and Menu (general menu to view User Role and About information). Within a given screen, multiple pieces of information can be accessed. For example, one screen view of Questions could consist of the user viewing multiple questions and their answers. Likewise, one screen view of Vaccine could involve viewing information on more than one vaccine.
Figure 1.
Representative screenshots of ReadyVax: A. Home screen; B. Vaccines tab of the Vaccines & Diseases screen; C. Representative vaccine entry; D. Resource Information list.
The ReadyVax app project was approved as Exempt research by the Emory University Institutional Review Board.
Results
Representative screenshots of the ReadyVax app are shown in Figure 1.
Between the ReadyVax “hard launch” on February 12, 2015 and October 8, 2016, the app has been downloaded by 5,142 unique users, with 6,841 total app sessions initiated (average 1.3 sessions/user), comprising a total of 15,491 screen views (average 2.3 screens/session). On average, a session lasted approximately 55 seconds. There is a subset of users (N = 427 (8.3%), as of October 8, 2016) who are repeat users of ReadyVax. These users are responsible for 1,648 unique app sessions (average of 3.9 sessions per user), with an average of 5.6 screen views per session and an average session duration of one minute 53 seconds.
ReadyVax has been downloaded by users in 107 countries (Fig. 2); of the 4,106 unique users for whom we have location information 2,183 (52%) were from the United States; US users accounted for 3,550 of the 5,770 sessions (62%) and 13,089 of the 15,471 screen views (85%) for which we have location information. Of the 453 users who have repeatedly accessed ReadyVax for whom we have location information, 383 (85%) were from the United States; US-based users accounted for 1,367 of 1,645 sessions (84%) and 7,773 of 9,208 screen views (84%) by repeat users. There were a total of 37 countries from which returning users originated.
Figure 2.
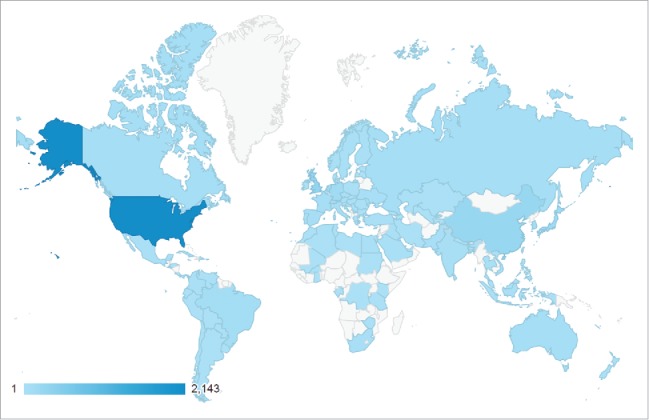
World map showing countries from which ReadyVax has been accessed, February 12, 2015 to August 4, 2016.
View counts and engagement of individual screens are summarized in Table 2. The most common content-focused screen accessed was Vaccine, which details information about specific immunizations (2,590 views overall, with 1,519 views among the 427 returning users).
Table 2.
User engagement with specific ReadyVax screens, February 12, 2015 to October 8, 2016.
| All Users (N = 5,142) |
Returning users (N = 427) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screen views (N) | Screen views per session | Average time on screen (mm:ss) | % exiting app | Screen views (N) | Screen views per session | Average time on screen (mm:ss) | % exiting app | ||
| Content screens | |||||||||
| VaccinesDiseases | 3,676 | 0.54 | 0:12 | 6.1 | 2,119 | 1.29 | 0:12 | 7.2 | |
| Vaccine | 2,590 | 0.38 | 0:55 | 30.5 | 1,519 | 0.92 | 1:03 | 34.2 | |
| Disease | 451 | 0.07 | 0:23 | 9.3 | 282 | 0.17 | 0:25 | 9.2 | |
| Alerts | 1,277 | 0.19 | 0:25 | 35.1 | 841 | 0.51 | 0:22 | 41.7 | |
| Questions | 519 | 0.08 | 0:56 | 31.0 | 274 | 0.17 | 0:52 | 32.1 | |
| Resources | 314 | 0.05 | 0:21 | 32.5 | 177 | 0.11 | 0:25 | 36.2 | |
| Administrative screens | |||||||||
| Home | 4,878 | 0.71 | 0:16 | 9.4 | 2,906 | 1.76 | 0:15 | 9.3 | |
| Menu | 1,281 | 0.19 | 0:08 | 2.4 | 778 | 0.47 | 0:10 | 2.7 | |
| Role | 369 | 0.05 | 0:14 | 5.1 | 225 | 0.14 | 0:18 | 6.7 | |
| About | 136 | 0.02 | 0:51 | 19.9 | 95 | 0.06 | 0:42 | 21.1 | |
| Total | 15,491 | 2.26 | N/A | N/A | 9,216 | 5.59 | N/A | N/A | |
The most common vaccine-related screen events (i.e., data elements accessed on a particular screen) were for diphtheria, tetanus, and pertussis vaccine (18% of all vaccine data screen events); general vaccine information (17% of all vaccine data screen events); measles, mumps, rubella vaccine (11% of all vaccine data screen events); and pneumococcal vaccines (11% of all vaccine data screen events) (Table 3).
Table 3.
Vaccine- and disease-specific screen events captured within the Vaccine, Disease, or VaccineDisease screens, ReadyVax, February 12, 2015 to October 8, 2016.
| Vaccine data screens |
Disease data screens |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All users (N = 350 events) |
Returning users (N = 203 events) |
All users (N = 132 events) |
Returning users (N = 92 events) |
||||||
| Vaccine name | N | % | N | % | Disease name | N | % | N | % |
| Diphtheria, tetanus, and pertussis (DTaP and Tdap) | 64 | 18.3% | 36 | 17.7% | Diphtheria, tetanus, and pertussis (DTaP and Tdap) | 24 | 18.2% | 23 | 25.0% |
| Haemophilus influenzae type b (Hib) | 8 | 2.3% | 4 | 2.0% | Haemophilus influenzae type b (Hib) | 9 | 6.8% | 7 | 7.6% |
| Hepatitis A | 18 | 5.1% | 13 | 6.4% | Hepatitis A | 18 | 13.6% | 11 | 12.0% |
| Hepatitis B | 3 | 0.9% | 1 | 0.5% | Hepatitis B | 7 | 5.3% | 6 | 6.5% |
| Human papillomavirus (HPV) | 17 | 4.9% | 11 | 5.4% | Human papillomavirus (HPV) | 13 | 9.8% | 10 | 10.9% |
| Influenza (flu) | 35 | 10.0% | 25 | 12.3% | Influenza (flu) | 9 | 6.8% | 5 | 5.4% |
| Measles, Mumps, Rubella | 39 | 11.1% | 13 | 6.4% | Measles | 6 | 4.5% | 2 | 2.2% |
| (MMR) | Mumps | 3 | 2.3% | 2 | 2.2% | ||||
| Rubella | 6 | 4.5% | 5 | 5.4% | |||||
| Meningococcal (Meningitis) Vaccines* | 33 | 9.4% | 24 | 11.8% | Meningococcal disease | 16 | 12.1% | 10 | 10.9% |
| Meningococcal Conjugate Vaccine (MCV4) | 10 | 2.9% | 8 | 3.9% | |||||
| Pneumococcal vaccines | 38 | 10.9% | 18 | 8.9% | Pneumococcal disease | 7 | 5.3% | 4 | 4.3% |
| Polio | 6 | 1.7% | 4 | 2.0% | Polio | 2 | 1.5% | 1 | 1.1% |
| Rotavirus | 7 | 2.0% | 2 | 1.0% | Rotavirus | 4 | 3.0% | 2 | 2.2% |
| Varicella (chickenpox) | 4 | 1.1% | 4 | 2.0% | Varicella | 1 | 0.8% | 1 | 1.1% |
| Zoster (shingles) | 9 | 2.6% | 5 | 2.5% | Zoster (shingles) | 7 | 5.3% | 3 | 3.3% |
| General vaccine information | 59 | 16.9% | 35 | 17.2% | |||||
Includes general information on meningitis vaccines, including information on the Serogroup B vaccine.
Discussion and next steps
With over 5,100 app downloads in one year for a mobile app focused on a very specific medical and public health topic area that is available for only one major smartphone operating system, we believe that ReadyVax is capable of establishing itself as a primary source of vaccine information. ReadyVax provides a comprehensive suite of information on the vaccines and the diseases they prevent. While there are other mobile apps related to vaccines, most exist solely as a way to either record immunization history or view immunization schedules (e.g., CDC Vaccine Schedules; My Immunizations). We are aware of only one other comprehensive vaccine information app – Vaccines on the Go, published by Children's Hospital of Philadelphia. Because of funding constraints during the initial development of ReadyVax, we were only able to provide an Apple iOS-compatible version. As of March 2015, Android smartphones accounted for 52% of the smartphone market share in the use, followed by Apple iOS (43%),24 so expansion to the Android platform could easily double the availability of the app.
With slightly less than 10% of ReadyVax users repeatedly accessing the app, we recognize the need to query ReadyVax users regarding their reasons for use or non-use of the app. However, this does not appear to be a situation unique to ReadyVax. A recent Nielsen consumer survey found that even though app downloads are common – the average smartphone user has 42 apps downloaded – most go unused, as more than half of smartphone users report using only between one and four of the apps on their device daily.25 In light of these Nielsen findings, ReadyVax is not unique as an app that may be downloaded but not routinely accessed. However, given the degree of engagement observed among our repeat ReadyVax users, there is evidence that value is seen in this type of app.
We are currently planning for expansion of ReadyVax to the Android platform, as well as making an HTML5 compatible version. By increasing the market reach of ReadyVax, and continuing development and refinement of app content and messaging, we anticipate increasing the ability of healthcare providers and the public to be better informed about vaccines. Through this type of routine usage, we believe that ReadyVax can prove itself as a trusted source of vaccine information. Additionally, while we have used the push notification function of the app in limited capacity (e.g., to highlight newly recommended vaccines), routine utilization of this messaging function can have the potential to reach users rapidly in the event of a public health emergency. This is a focus area of the ReadyVax project team moving forward. As part of enhanced dissemination efforts, we are planning more in-depth data collection on users' perceptions of the app and reasons for and against repeat use. Included in these data collection methods, we plan to use the push notification function within ReadyVax to alert users to an online survey, with a direct link to the survey in the notification message. These evaluations will assess perceptions of content, layout, user interface, and perceived utility of the ReadyVax app. As part of the development of the new version of ReadyVax, we are also designing outreach efforts to healthcare providers for pre-release testing and feedback on usability and design, in addition to content. Additionally, we will conduct pre-release usability assessments with parents. As part of both of these outreach efforts, we will evaluate user's perspectives on presentation or functionality that will increase repeat use.
In a short period of time, we have developed and disseminated a new mobile smartphone app to provide easy access to evidence-based information about vaccines and vaccination. Initial uptake and use has shown that this type of app can be accepted by smartphone users. We plan to continue updating and monitoring the use of ReadyVax to improve the app, to allow us to serve the vaccine information needs of healthcare providers and the general public. This includes routine information needs using the standard content in ReadyVax as well as rapidly updated information through the enhanced use of push notifications.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
Portions of this manuscript have been presented in poster form at the 2015 ID Week meeting, San Diego CA, October 2015.
Funding
This project was supported by Centers for Disease Control and Prevention (CDC) grant #5P01TP000300 for the Emory University Preparedness and Emergency Response Research Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Contributions
RAB, EAW, and SBO conceived the project. RAB was the technical lead for the project team, working with the app developer on all technical requirements, and drafted the manuscript. PMF and DAS oversaw the development of information content and reviewed initial versions of the app during development. SBO supervised and oversaw the overall project. All authors have read and contributed to the drafting of the manuscript and approved the final version.
References
- [1].Association of State and Territorial Health Officials Addressing Communication Challenges During an Infectious Disease Emergency Response: State Experiences from the H1N1 Pandemic. 2014; We describe the creation, release and utilization of ReadyVax, a new mobile smartphone app designed to provide access to trustworthy, evidence-based vaccine information Accessed December18, 2015. [Google Scholar]
- [2].Institute of Medicine (US) Forum on Medical and Public Health Preparedness for Catastrophic Events The 2009 H1N1 influenza vaccination campaign: Summary of a workshop series. Washington DC: National Academies Press; 2010 [PubMed] [Google Scholar]
- [3].Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Petrie JG, McLean HQ, Belongia EA, et al.. Early estimates of seasonal influenza vaccine effectiveness-United States, January 2015. MMWR Morb Mortal Week Rep 2015; 64(1):10-5; PMID:25590680 [PMC free article] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention Pertussis Outbreak Trends. 2015; http://www.cdc.gov/pertussis/outbreaks/trends.html. Accessed December18, 2015. [Google Scholar]
- [5].Wolf ER, Opel D, DeHart MP, Warren J, Rowhani-Rahbar A. Impact of a pertussis epidemic on infant vaccination in Washington state. Pediatrics 2014; 134(3):456-64; PMID:25136046; http://dx.doi.org/ 10.1542/peds.2013-3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Frew PM, Owens LE, Saint-Victor DS, Benedict S, Zhang S, Omer SB. Factors associated with maternal influenza immunization decision-making. Evidence of immunization history and message framing effects. Hum Vaccin Immunother 2014; 10(9):2576-83; PMID:25483468; http://dx.doi.org/ 10.4161/hv.32248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marsh HA, Malik F, Shapiro E, Omer SB, Frew PM. Message framing strategies to increase influenza immunization uptake among pregnant African American women. Matern Child Health J 2014; 18(7):1639-47; PMID:24337776; http://dx.doi.org/ 10.1007/s10995-013-1404-9 [DOI] [PubMed] [Google Scholar]
- [8].Pew Research Center U.S. smartphone use in 2015. 2015; http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed April8, 2016. [Google Scholar]
- [9].Dolan B. In-Depth: mobile adoption among US physicians. 2014; http://mobihealthnews.com/32232/in-depth-mobile-adoption-among-us-physicians. Accessed April8, 2016. [Google Scholar]
- [10].Pew Research Center Mobile Health 2012. 2012; http://www.pewinternet.org/2012/11/08/mobile-health-2012/. Accessed December21, 2015. [Google Scholar]
- [11].Franko OI, Tirrell TF. Smartphone app use among medical providers in ACGME training programs. J Med Syst 2012; 36(5):3135-9; PMID:22052129; http://dx.doi.org/ 10.1007/s10916-011-9798-7 [DOI] [PubMed] [Google Scholar]
- [12].Katz-Sidlow RJ, Ludwig A, Miller S, Sidlow R. Smartphone use during inpatient attending rounds: prevalence, patterns and potential for distraction. J Hosp Med 2012; 7(8):595-9; PMID:22744793; http://dx.doi.org/ 10.1002/jhm.1950 [DOI] [PubMed] [Google Scholar]
- [13].Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. J Med Internet Res 2012; 14(5):e128; PMID:23017375; http://dx.doi.org/ 10.2196/jmir.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health 2003; 93(4):635-41; PMID:12660210; http://dx.doi.org/ 10.2105/AJPH.93.4.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chamberlain AT, Seib K, Wells K, Hannan C, Orenstein WA, Whitney EA, Hinman AR, Berkelman RL, Omer SB. Perspectives of immunization program managers on 2009–10 H1N1 vaccination in the United States: a national survey. Biosecur Bioterror 2012; 10(1):142-50; PMID:22360580; http://dx.doi.org/ 10.1089/bsp.2011.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moriarty LF, Omer SB, Seib K, Chamberlain A, Wells K, Whitney E, Berkelman R, Bednarczyk RA. Changes in immunization program managers' perceptions of programs' functional capabilities during and after vaccine shortages and pH1N1. Public Health Rep 2014; 129 Suppl 4:42-8; PMID:25355974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seib K, Gleason C, Richards JL, Chamberlain A, Andrews T, Watson L, Whitney E, Hinman AR, Omer SB. Partners in immunization: 2010 survey examining differences among H1N1 vaccine providers in Washington state. Public Health Rep 2013; 128(3):198-211; PMID:23633735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Link-Gelles R, Chamberlain AT, Schulkin J, Ault K, Whitney E, Seib K, Omer SB. Missed opportunities: a national survey of obstetricians about attitudes on maternal and infant immunization. Matern Child Health J 2012; 16(9):1743-7; PMID:22198260; http://dx.doi.org/ 10.1007/s10995-011-0936-0 [DOI] [PubMed] [Google Scholar]
- [19].Petty R, Cacioppo J. Communication and Persuasion: Central and peripheral routes to attitude change. New York: Springer-Verlag; 1986 [Google Scholar]
- [20].Petty RE, Cacioppio JT. Central and peripheral routes to persuasion: Application to advertising In: Percy L, Woodside A, eds. Advertising and Consumer Psychology. Lexington, MA: Lexington Books; 1983:3-23. [Google Scholar]
- [21].Chamberlain AT, Seib K, Ault KA, Rosenberg ES, Frew PM, Cortés M, Whitney EA, Berkelman RL, Orenstein WA, Omer SB. Improving influenza and Tdap vaccination during pregnancy: A cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine 2015; 33(30):3571-9; PMID:26044495; http://dx.doi.org/ 10.1016/j.vaccine.2015.05.048 [DOI] [PubMed] [Google Scholar]
- [22].Centers for Disease Control and Prevention ACIP Vaccine Recommendations. 2015; http://www.cdc.gov/vaccines/hcp/acip-recs/. Accessed December18, 2015. [Google Scholar]
- [23].Hamborsky J, Kroger A, Wolfe S, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. Washington DC: Public Health Foundation; 2015. Centers for Disease Control and Prevention, ed. [Google Scholar]
- [24].comScore Inc comScore Reports March 2015 US smartphone subscriber market share. 2015; https://www.comscore.com/Insights/Market-Rankings/comScore-Reports-March-2015-US-Smartphone-Subscriber-Market-Share Accessed December18, 2015. [Google Scholar]
- [25].The Nielsen Company. Tech-or-treat: Consumers are sweet on mobile apps. 2014; http://www.nielsen.com/us/en/insights/news/2014/tech-or-treat-consumers-are-sweet-on-mobile-apps.html. Accessed December21, 2015. [Google Scholar]