ABSTRACT
Two rotavirus vaccines are currently available in Japan. We estimated the incremental cost-effectiveness ratio (ICER) of routine infant rotavirus immunisation program without defining which vaccine to be evaluated, which reflects the current deliberation at the Health Science Council in charge of Immunisation and Vaccine established by the Ministry of Health, Labor and Welfare of Japan. Three ICERs were estimated, one from payers' perspective and 2 from societal perspective depending on the scenarios to uptake vaccines. The health statuses following the birth cohort were as follows: not infected by rotavirus, asymptomatic infection, outpatients after infection, hospitalised after infection, developing encephalitis/encephalopathy followed by recovery, sequelae, and death. Costs of per course of vaccination was ¥30,000 (US$283; US$1 = ¥106). The model runs for 60 months with one month cycle. From payers' perspective, estimated ICERs were ¥6,877,000 (US$64,877) per QALY. From societal perspective, immunisation program turns out to be cost-saving for 75% simultaneous vaccination scenario, while it is at ¥337,000 (US$3,179) per QALY gained with vaccine alone scenario. The probability of rotavirus immunisation program to be under ¥5,000,000 (US$47,170) per QALY was at 19.8%, 40.7%, and 75.6% when costs per course of vaccination were set at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189), respectively. Rotavirus immunisation program has a potential to be cost-effective from payers' perspective and even cost-saving from societal perspective in Japan, however, caution should be taken with regard to the interpretation of the results as cost-effectiveness is critically dependent on vaccination costs.
KEYWORDS: cost-effectiveness, economic evaluation, gastroenteritis, rotavirus vaccine, routine vaccination, quality adjusted life year
Introduction
Approximately 453,000 rotavirus gastroenteritis (RVGE)-associated annual child deaths occur worldwide.1 In developed countries, though mortality due to RVGE is low, there is still a high level of morbidity which imposes a large health and economic burden on the healthcare system.2-6 Also, rotavirus can cause severe complications such as encephalopathy/encephalitis, which lead to sequelae or death.7-10 RVGE has no antiviral therapy and its treatment is limited to symptomatic measures.1 Two rotavirus vaccines (RVs), monovalent vaccine (RV1) and pentavalent vaccine (RV5), are currently available for the immunisation of infants under 6 months. These 2 vaccine products differ in composition and schedule of administration. RV5 contains 5 reassortant rotaviruses developed from human and bovine parent rotavirus strains with 3 doses given in the series, while RV1 contains an attenuated human rotavirus strain having 2 doses given in the series.1 Both live attenuated RVs given orally have shown similar high efficacy and good safety.11-14 Recently, studies, which compared RV1 and RV5 with each other, suggested that there is no statistical difference in vaccine performance between RV1 and RV5 in real-world settings.15,16 As of April 1, 2015, 77 countries have introduced RVs in their national immunisation programmes, among them 18 are World Bank-classified high-income countries, such as Australia, Austria, Belgium, Finland, United Kingdom, and United States.17
In Japan, about 80,000 children under 5 y.o. are hospitalised every year due to RVGE, while mortality due to it is rare.6,18 No data is available on rotavirus-related mortality, however, there were 19 deaths recorded due to infectious gastroenteritis (ICD10, A09) for children under 5 y.o. in 2015.19 A study conducted between January 2008 and December 2009 in eight regional core hospitals in an urban area of Japan estimated that 4.8% of all hospitalisation in children aged under 6 are due to rotavirus.6 Both RV1 and RV5 are currently available for voluntary vaccination, to which a limited number of municipalities give subsidy on their own accord, in Japan. The introduction of RVs into the routine vaccination schedule has become one of the current topics in health policy and has raised the need to evaluate its value for money. We were able to identify 2 relevant cost-effectiveness studies which compared the routine rotavirus immunisation program to no immunisation program in Japan.20,21 A study, which assumed that there is no difference in both vaccination costs and effectiveness between the 2 vaccines, reported that immunisation program is almost cost-effective (¥9.8 million or US$ 92,453 per quality adjusted life year (QALY) gained; 1US$ = ¥106, average of 2014) from the health care perspective and highly cost-effective (¥0.9 million or US$ 8,491 per QALY gained) from the societal perspective.20 On the other hand, a manufacturer-sponsored study identified that universal vaccination with RV5 may be cost-effective from both payers' and societal perspectives (¥8 million or US$75,472 and ¥4 million or US$37,736 per QALY gained, respectively).21 In order to further augment the current knowledge for immunisation program implementation, we compared rotavirus immunisation program with no immunisation program without defining which vaccine (RV1 or RV5) will be subsidised, which reflects the current deliberation at the Health Science Council in charge of Immunisation and Vaccine established by the Ministry of Health, Labour and Welfare (MHLW) of Japan.22 The introduction of RVs into the national immunisation programmes, without defining which vaccine (RV1 or RV5) will be subsidised, has been under discussion based on a mandatory fact sheet presented by National Institute of Infectious Diseases stating that the effectiveness of both vaccines in this context is similar.23 Other reasons for not defining which vaccine to be evaluated are: (1) the information from websites of municipalities where subsidies were given to their inhabitant vaccinees to uptake RV1/RV5 shows that costs per course of the 2 are around the same, whereas costs per dose of RV1 is higher than that of RV5. Even though there is a difference with regard to the number of doses per course between RV1 and RV5, 2 doses and 3 doses, respectively, this difference on vaccine cost is eventually offset by the doctor's fee for medical advice and technical fee for administering the vaccine; around ¥ 3,500 or US$33 per shot, (2) Japan Pediatric Society offers no vaccine preference between the 2, which implies that when the municipality implements the rotavirus immunisation program, RV1 and RV5 are considered to be equal, (3) 2 relevant cost-effectiveness studies which compared the routine rotavirus immunisation program to no immunisation program in Japan were identified, one is a manufacturer-sponsored study which evaluated cost-effectiveness of RV5 immunisation program,21 the other is an academic-based study, which assumed that there is no difference in both vaccination costs and effectiveness between the 2 vaccines,20 and (4) since there is no difference in the effectiveness and costs of both vaccines, our study evaluated mainly the efficiency of the immunisation program, and not that of the products. We took into account asymptomatic infected cases and rotavirus related-encephalitis/encephalopathy as a novelty of this study. We also updated some epidemiological data to have a more comprehensive picture of the impact of vaccination, which we hope can contribute to the ongoing discussion of the implementation of routine rotavirus vaccination program in Japan.
Results
Results of base-case analyses
Table 1 shows the results of base-case analyses. When comparing immunisation program with no immunisation program, estimated average incremental effects per child were at 0.00174 QALYs. Immunisation program reduces both disease treatment costs and care-giver's productivity loss due to disease treatment. However, when the care-giver's productivity loss was not included, the reduced disease treatment costs alone cannot offset the vaccination costs, which means that the program turned out to be “gained more, but cost more.” Estimated incremental cost-effectiveness ratios (ICERs) were ¥6,877,000 (US$64,877) per QALY gained. When care-giver's productivity loss was included, they were at ¥337,000 (US$3,179) per QALY gained with vaccine alone scenario, while it turned out to be cost-saving with 75% simultaneous vaccination scenario.
Table 1.
Results of base-case analysis (cost per child, effectiveness per child and ICER).
| Vaccination cost (¥) | Treatment cost (¥) | Productivity lost to accompany vaccination (¥) | Productivity lost to accompany treatment (¥) | Effectiveness (QALY) | ICER* (¥/QALY) | |
|---|---|---|---|---|---|---|
| Payers' perspective | ||||||
| No immunisation programme | 0 | 20,055 | — | — | 4.71242 | — |
| Immunisation programme | 21,000 | 11,048 | — | — | 4.71416 | 6,877,000 |
| Societal perspective | ||||||
| No immunisation programme | 0 | 20,055 | 0 | 70,536 | 4.71242 | — |
| Immunisation programme: Vaccinated alone | 21,000 | 11,048 | 11,777 | 47,353 | 4.71416 | 337,000 |
| Immunisation programme: 75% simultaneous vaccination | 2,000 | 11,048 | 2,944 | 47,353 | 4.71416 | (4,728,294) cost less, gained more |
All ICERs were rounded to the nearest thousand.
Results of one-way sensitivity analyses and probabilistic analyses
From payers' perspective, the variables which were found to increase/decrease the ICER more than ¥1,000,000 (US$9,434) by one-way sensitivity analyses are as follows: (1) costs of per course of vaccination (lower/upper limit: ± 50% of base-case), (2) utility weight of outpatient episode for children age ≥18 months (lower limit: −20% of base-case, upper limit: 1), (3) utility weight of outpatient episode for children age <18 months (lower limit: −20% of base-case, upper limit: 1), (4) treatment costs per hospitalisation episode (lower/upper limit: ± 50% of base-case), (5) treatment costs per outpatient episode (lower/upper limit: ± 50% of base-case), (6) treatment costs per encephalitis episode (lower/upper limit: ± 50% of base-case), and (7) VE on decreasing GP visit (lower limit: −20% of base-case, upper limit: 100%) (Fig. 1). ICER increases from ¥6,877,000 per QALY to ¥7,510,000 per QALY, if VE decreased by 10% every year from the base-case (Table 2). The upper limit of costs per vaccination course to gain one QALY under /5,000,000 (US$47,170) was ¥25,000 (US$236) (Fig. 2).
Figure 1.
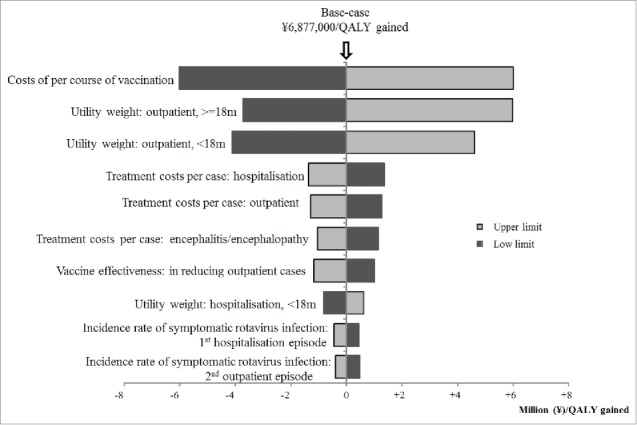
Result of one-way sensitivity analyses.
Table 2.
Results of sensitivity analyses on VE from payers' perspective: decreased VE by 10% every year from the base-case.
| Vaccination cost (¥) | Treatment cost (¥) | Productivity lost to accompany vaccination (¥) | Productivity lost to accompany treatment (¥) | Effectiveness (QALY) | ICER* (¥/QALY) | |
|---|---|---|---|---|---|---|
| No immunisation programme | 0 | 20,055 | — | — | 4.71242 | — |
| Immunisation programme | 21,000 | 11,483 | — | — | 4.71407 | 7,510,000 |
All ICERs were rounded to the nearest thousand.
Figure 2.
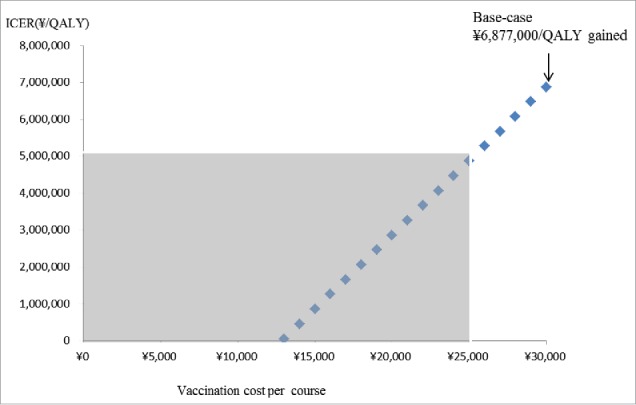
The effect on ICERs by changing vaccination costs per course. Grey area shows ICER less than ∞ 5,000,00 per QALY gained.
Figure 3 and Figure 4 show the results of probabilistic analyses when the costs of per course of vaccination were at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189), respectively. Each dot on Fig 3 represents the incremental cost and effect obtained from one simulation following the random draw of model parameters from distribution. The dots dispersed in the horizontal direction reflected the distribution being used for the variables. The 3 cost-effectiveness acceptability curves (CEACs) show that in immunisation program vs. no immunisation program, among 1000 ICERs produced by Monte Carlo simulations, the probabilities that ICER is under ¥5,000,000 (US$47,170) per QALY gained was at 19.8%, 40.7%, and 75.6% when the costs of per course of vaccination were at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189), respectively. The probabilities that immunisation program is cost-saving, i.e., gained more cost less, were at 7.7%, 3.5%, and 9.6% when the costs of per course of vaccination were at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189), respectively (Fig. 4).
Figure 3.
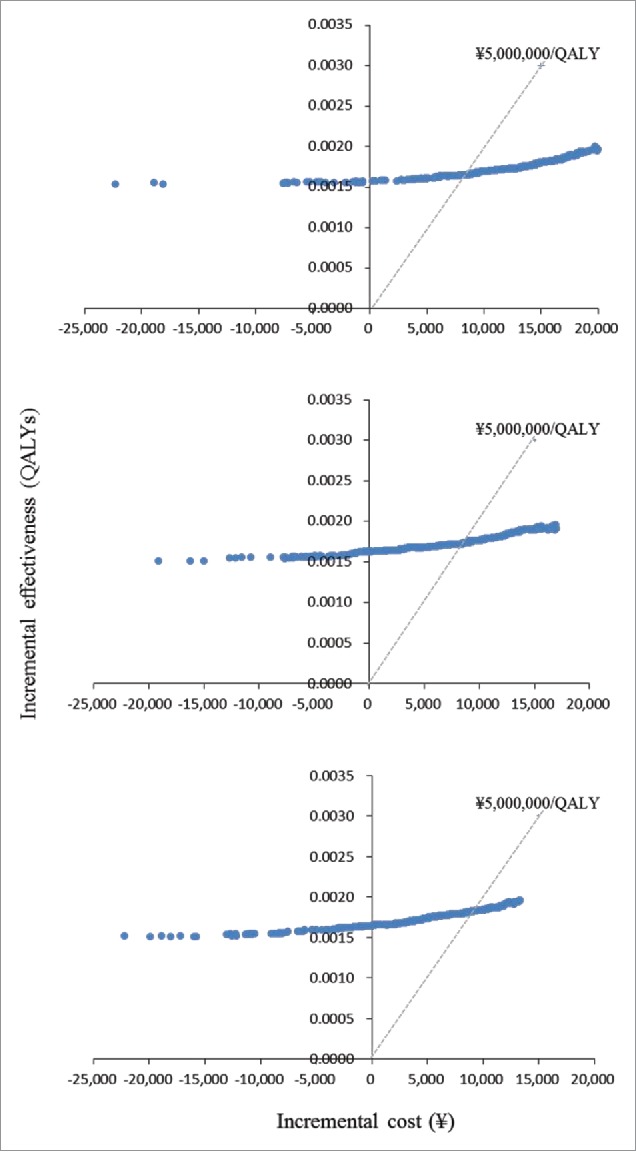
Results of Probabilistic analyses: Scatterplot of incremental cost and incremental effectiveness per person of immunisation programme vs. no immunisation programme. (a) Vaccination costs per course ∞ 30.000. (b) Vaccination costs per course = ∞ 25.000. (3) Vaccination costs per course = ∞ 20,000.
Figure 4.
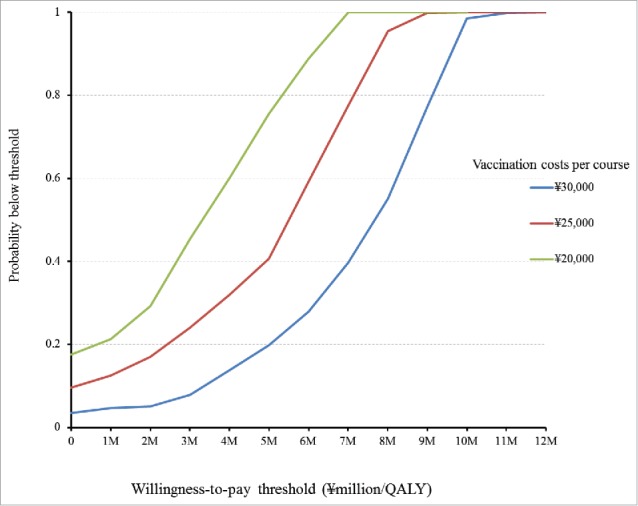
Cost-effictiveness acceptability curves (CEACs) of immunisation programme vs no. immunisation programme.
Discussion
We estimated the cost-effectiveness of rotavirus immunisation program in Japan without defining which vaccine (RV1 or RV5) to be evaluated, which reflects the current deliberation at the Health Science Council in charge of Immunisation and Vaccine established by the MHLW of Japan. ICERs were calculated from 2 perspectives (payers' and societal). Furthermore, in estimating ICER from societal perspective, we created the vaccinated alone and 75% simultaneous vaccination for the RV uptake scenarios.
From payers' perspective, our base-case analyses, set with the costs per vaccination course at ¥30,000 (US$283), show that ICER of immunisation program were ¥6,877,000 (US$64,877). From societal perspective, these were at ¥337,000 (US$3,179) per QALY gained for vaccinated alone scenario; cost saving for 75% simultaneous vaccination scenario. From the payers' perspective, ICER was less than the “favorable” level (US$ 10,000∼100,000 per QALY) set by Committee to Study Priority for Vaccine Development in the United States24 or WHO's cost-effective criterion for intervention, i.e., less than 3 times of GDP per capita ( ¥11,000,000 or US$103,774 in Japan),25 while it was slightly higher than a willingness-to-pay threshold suggested for healthcare intervention, i.e., ¥5,000,000 (US$47,170) per QALY gained in Japan.26
Sensitivity analyses on costs per vaccination course indicated that from the payers' perspective, the upper limit of the cost to gain one QALY under /5,000,000 (US$47,170) was ¥25,000 (US$236). The probabilistic sensitivity analyses showed that the probabilities of immunisation program to be cost-saving were at 7.7%, 3.5%, and 9.6% when costs per course of vaccination were set at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189), respectively; to be under ¥5,000,000 (US$47,170) per QALY were at 19.8%, 40.7%, 75.6%, respectively. From societal perspective, immunisation program turned out to be highly cost-effective or even cost-saving if 75% vaccinees uptake RVs simultaneously with other vaccines on the list. In Japan, a study showed that PCV, an on the list of routine immunisation, was found to have ¥7,400,000 (US$69,811) per QALY gained.27 If ICERs of rotavirus vaccination estimated in our study were to be compared with that of PCV's, ICERs of rotavirus immunisation program can still be considered as acceptable.
Our results are robust based on the results from our one-way sensitivity analyses with only eight variables found to increase/decrease the ICER more than ¥1,000,000 (US$9,434). Among the eight, three are from the treatment costs. In Japan, treatment costs are based on national fee schedule, therefore, are not likely to decrease/increase as large as 50%.
Over the last few years, several studies evaluated the cost-effectiveness of routine infant rotavirus vaccination around the world and reported varying results even studies from the same country;20,21,28-35 among them, 2 are from Japan.20,21 The significant difference between our model and those of the previous studies is the incorporation of rotavirus-related encephalitis/encephalopathy, and sequelae in the model, which were not present in the previous ones. These might be considerably influential factors, which have contributed to favorable ICERs compared with those of the 2 previous studies.
As pointed out by many studies, several key issues in assessing rotavirus vaccination efficiency need to be further discussed, such as: (1) the extent of VE waning, has varying impacts because of the substantial difference in the assumptions. In our study, we conducted sensitivity analysis on VE which assumed to decrease by 10% every year, and found that results did not change significantly which is consistent with that of Giammanco et al.'s.36 (2) The proportion of hospitalised patients who developed encephalitis/encephalopathy is adapted from overseas, and the disease definition (CNS diagnosis) is broader than encephalitis/encephalopathy. The adaption of the data definitely brought some uncertainty to our results, however, our one-way sensitivity analysis revealed that the impact of this variable is small. (3) The inclusion of QALY losses of care-givers. Given that the consensus was not obtained, we remained conservative and did not include it in the model. If it were to be included, ICER for societal perspective will definitely turn out to be more favorable. (4) The inclusion of herd protection benefit within and outside the vaccinated cohort. Due to data insufficiency related to both transmission model of rotavirus and shift in circulating rotavirus type in Japan, we deferred its incorporation to avoid presenting a too optimistic result. (5) Utility weights used to estimate QALY were based on studies performed in UK,37 which has been used in several economic studies related to rotavirus vaccination.38-40 In addition to these limitations, proportion of asymptomatic infection cases to symptomatic infection cases and relative risks of subsequent rotavirus infection were adapted from studies carried out in other countries since no similar study has been done in Japan. There should be differences in vaccine strains, in ethnicity as well as in healthcare system between those countries and Japan.
Regardless of these limitations, we consider our model to be robust, which included the potential impact of asymptomatic infected cases and rotavirus related-encephalitis/encephalopathy. Likewise, we were also able to determine the ICERs of different VE scenarios together with the results of probabilistic analyses. We believe that the model and the results provide a more comprehensive picture of the impact of vaccination and will contribute to the ongoing discussion in the implementation of routine rotavirus immunisation program in Japan.
Conclusion
From our analyses, we found that rotavirus vaccination has a potential to be cost-effective from payers' perspective and even cost-saving from societal perspective; however, caution should be taken with regard to interpreting cost-effectiveness since it is critically dependent on vaccination costs per course. In this study, ICERs estimated from both payers' and societal perspectives are more favorable than those observed in the 2 previous studies from Japan.
Method
We conducted a cost-effectiveness analysis with Markov modeling to evaluate the efficiency of rotavirus immunisation program for the birth cohort in Japan (1.001 million in 2014). ICERs of immunisation program compared with no immunisation program were calculated to determine the resource use efficiency. ICER is defined as difference in cost between immunisation program and no immunisation program, divided by the difference in their effectiveness (QALYs). QALYs were estimated by assigning transition probabilities and utility weights from literature to the Markov model. We estimated ICERs from both payers' (not including care-giver's productivity cost) and societal perspectives (including care-giver's productivity cost).
In defining the immunisation programmes and constructing the model, we conducted a literature survey to find out the best available evidence. Studies pertaining to epidemiology and prognosis of rotavirus-relevant disease in Japan's setting were accessed from Medline database, Igaku Chuo Zasshi database, MHLW Grant System, and annual statistic reports published by the government. Igaku Chuo Zasshi (Japana Centra Revuo Medicina) is a Japanese medical bibliographic database which contains over 10 million citations originating from Japan. Due to insufficient evidences from Japan, overseas' reports from Medline, The Cochrane Database of Systematic Reviews, Health Technology Assessment database, and The NHS Economic Evaluation Database regarding vaccine effectiveness and utility weight to estimate QALY were used instead.
Program and model
We compared rotavirus immunisation program with no immunisation program without defining which vaccines to be evaluated, which reflects the current deliberation at the Health Science Council in charge of Immunisation and Vaccine established by MHLW of Japan.22
We assumed that the vaccine uptake rate was 72% for the vaccination program, which was the same as with the third-dose vaccine uptake rate of Haemophilus influenzae type b (Hib) vaccine and 13-valent pneumococcal conjugate vaccine (PCV-13) in 2013. From societal perspective, we set 2 scenarios to uptake vaccine as follows: (1) 100% vaccinees uptake RV alone (vaccinated alone), and (2) 75% vaccinees simultaneously uptaking RV with other vaccines listed on the national immunisation schedule (75% simultaneous vaccination). The 75% simultaneous vaccination was based on the uptake of Hib and PCV-13 vaccine.41 Even though some municipalities have provided subsidies for their residents, most of the municipalities are yet to provide a program; therefore, we set the vaccine coverage of no immunisation program at 0%. In total, 3 ICERs were estimated with one coming from payers' perspective and 2 from societal perspective (vaccinated alone scenario and 75% simultaneous vaccination scenario).
Figure 5 shows the Markov model of various health status followed by the birth cohort under consideration. A Markov cycle for each stage was set at 1 month. Time horizon was 60 months. In each cycle the cohort was exposed to the risk of a first to third episode of rotavirus infection which could either be symptomatic or asymptomatic. An asymptomatic episode, though may not incur any treatment costs directly, still has an impact on ICER by reducing the symptomatic episodes. Usually in Japan, an RVGE episode for children will all lead to either an outpatient visit or hospitalisation. Non-access of medical care was not considered in the model, because in Japan, the 20% co-payment of medical services is mostly borne by the municipality, which encourages parents to bring their children to the medical facilities to access the service. Death directly from RVGE is rare and the proportion is unknown, therefore we omitted this status from our model.6,42 However, since rotavirus is the third most common pathogen of acute encephalopathy in Japan, which caused 4% of all the acute-encephalopathy,9 we incorporated encephalitis/encephalopathy followed by recovery, sequelae, and death into the model, instead. Repeated rotavirus infections have been shown to induce progressive natural immunity that protects against subsequent infections.43 In our model, children will have up to 3 symptomatic or asymptomatic episodes with the second and subsequent RVGE infections being less severe and the third symptomatic infection never leading to encephalitis/encephalopathy. After the third infection all subsequent infections will be asymptomatic.43 Adverse effects of vaccination were not incorporated based on reports from large clinical trials and from post-marketing surveys.44-46 Herd effects were not considered in the mode as its incorporation might pose some bias to the result. Though several studies do provide some evidence for the existence of such an effect, further evidence is required before definite interpretations can be made.
Figure 5.
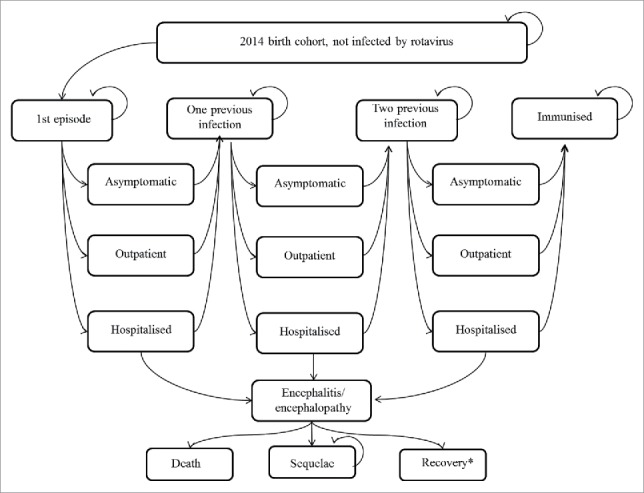
Markov Model.* After recovery, individuals may proceed to the following health status: “One previous infection“, “Two previous infection” or “Immunised”.
Annual incidence rates and case fatality rates
We used the age-specific annual incidence rates of RVGE outpatients without vaccination from a meta-analysis of 4 studies from geographically diverse locations in Japan.47 Hospitalisation incidence rates were taken from a 10-year retrospective, hospital-based study.48 Causal relationship of encephalitis/encephalopathy with rotavirus infection has been putative since 1980s49 and has recently been observed in studies from Japan and from United States.9,10,49,50 In Japan, a study supported by the MHLW conducted a nationwide survey on epidemiology of acute encephalopathy from 2007–2010 reported that rotavirus was the third most common pathogen of acute encephalopathy.9 The other study also supported by MHLW, conducted a nationwide survey on rotavirus–associated encephalopathy and sudden unexpected death between 2009 and 2011.10 The same study reported that among rotavirus-associated encephalopathy cases, 25.9% cases had neurological sequelae and 12.1% cases demonstrated fatal outcome. As to the proportion of rotavirus-related hospitalised patients who developed encephalitis/encephalopathy, since there was no straightforward report on it in Japan, we adapted data from Lynch et al.'s study, whereby among rotavirus hospitalisation cases 2.5% (36/1452) developed encephalitis/encephalopathy.49 The original purpose of Lynch et al.'s study was to compare the hospitalisations and frequency of associated CNS diagnoses between children with rotavirus diarrhea and with bacterial diarrhea in children <5 y by using data from National Hospital Discharge Survey. Since the definition of CNS diagnosis (unspecified convulsions, infantile spasms, generalized non-convulsive epilepsy, generalized convulsive epilepsy, unspecified viral encephalitis, acute encephalitis, and unspecified viral meningitis) is broader than encephalitis/encephalopathy, we conducted one-way sensitivity analysis to explore the uncertainty derived by this variable. From this proportion, 25.9% of the patients had neurological sequelae, and 12.1% had fatal outcomes.10 Deaths of causes other than the above diseases were taken from the vital statistics. By setting the first episode as the reference point, we assumed that children with one or 2 previous episodes will have lower risk of subsequent rotavirus infection (0.62 and 0.4 multiplied with the first episode risk, respectively), than children who had no previous infection.41 Ratio of asymptomatic infection cases to symptomatic infection cases were estimated from a study by Velazquez et al.43 In Table 2 of Velazquez et al.'s study, they reported cases of any infection (including symptomatic infection, asymptomatic infection, and infections for which the symptom status was unknown) and cases of asymptomatic infection of the first (164, 71), second (102, 47), and third infections (40, 17), respectively. Using these figures we estimated ratios of asymptomatic infection cases to symptomatic infection cases at 0.763 [71÷(164–71)], 0.855 [47÷(102–47)], and 0.739 [17÷(40–17)] for the first, second, and third infections, respectively. There are 2 studies which estimated the health-related quality of life lost to RVGE or utility scores of RVGE in children.37,51 In Brisson et al.'s study, caregivers evaluated health-related quality of life of their children suffering from RVGE and found out that QALY decreased by 0.0022 without differentiating between disease severity and age.51 On the other hand, Martin et al.'s study showed that GPs/pediatrician were able to estimate the utility weight in children with RVGE according to disease severity and age.37 In our base-case analysis, we adopted the data set from GP estimated by Martin et al., because it fits our model better, in terms of health states after RVGE (primary care only and hospitalization) and the length of Markov cycle (1 month) taking into account the utility weight of hospitalised patients to be lower than patients who only need primary care. Utility weight for neurological sequelae was set at 0.57.27 All these data are shown in Table 3.
Table 3.
Model inputs.
| Variables |
Base-case |
Ranges and distributions used in probabilistic sensitivity analysis (PSA)a |
Reference |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidences of symptomatic rotavirus infection; per 100 persons | Lower | Upper | Distribution | |||||||
| Annually |
Monthly |
Monthly |
[47,48] | |||||||
| Age (months) |
Outpatient |
Hospitalised |
Outpatient |
Hospitalised |
Outpatientb |
Hospitalisedc |
Outpatientb |
Hospitalisedd |
Uniform |
|
| 0–2 m | 3.13 | 0.27 | 0.265 | 0.023 | 0.253 | 0.022 | 0.278 | 0.024 | Uniform | |
| 3–5 m | 3.13 | 1.32 | 0.265 | 0.111 | 0.253 | 0.106 | 0.278 | 0.116 | Uniform | |
| 6–11 m | 15.13 | 2.27 | 1.358 | 0.191 | 1.327 | 0.187 | 1.389 | 0.195 | Uniform | |
| 12–17 m | 27.07 | 2.32 | 2.596 | 0.195 | 2.562 | 0.192 | 2.630 | 0.198 | Uniform | |
| 18–23 m | 27.07 | 1.59 | 2.596 | 0.133 | 2.562 | 0.131 | 2.630 | 0.135 | Uniform | |
| 24–29 m | 10.98 | 0.57 | 0.965 | 0.048 | 0.953 | 0.047 | 0.978 | 0.049 | Uniform | |
| 30–35 m | 10.98 | 0.32 | 0.965 | 0.027 | 0.953 | 0.027 | 0.978 | 0.027 | Uniform | |
| 36–41 m | 10.98 | 0.30 | 0.965 | 0.025 | 0.953 | 0.025 | 0.978 | 0.025 | Uniform | |
| 42–47 m | 10.98 | 0.27 | 0.965 | 0.023 | 0.953 | 0.023 | 0.978 | 0.023 | Uniform | |
| 48–53 m | 4.67 | 0.11 | 0.398 | 0.009 | 0.390 | 0.009 | 0.406 | 0.009 | Uniform | |
| 54–59 m |
4.67 |
0.25 |
0.398 |
0.021 |
0.390 |
0.021 |
0.406 |
0.021 |
Uniform |
|
| |
|
|
|
Base-case |
Lower limit |
|
Upper limit |
SD |
|
|
| Proportions of asymptomatic infection cases to symptomatic infection cases | ||||||||||
| First infection | 76.3% | 61.0% | 91.6% | Uniform | [43] | |||||
| Second infection | 85.5% | 68.4% | 100% | Uniform | ||||||
| Third infection | 73.9% | 59.1% | 88.7% | Uniform | ||||||
| Relative risks of subsequent rotavirus infection | ||||||||||
| No previous infection | 1 | |||||||||
| With one previous infection | 0.620 | 0.496 | 0.744 | Uniform | ||||||
| With two previous infections | 0.400 | 0.320 | 0.480 | Uniform | [43] | |||||
| Proportion of hospitalised patients developed encephalitis/encephalopathy | 2.5% | 2.0% | 3.0% | Uniform | [49] | |||||
| Proportion of encephalitis/encephalopathy cases resulted in neurological sequelae | 25.9% | 20.7% | 31.1% | Uniform | [10] | |||||
| Proportion of encephalitis/encephalopathy cases resulted in death | 12.1% | 9.7% | 14.5% | Uniform | [10] | |||||
| Vaccine effectiveness | [12, 52-54] | |||||||||
| In reducing outpatient cases (<12 m/> = 12 m) | 85%/82% | 68.0%/65.6% | 100%/98.4% | Uniform | ||||||
| In reducing hospitalisation cases | 90% | 72.0% | 100% | Uniform | ||||||
| Utility weights | ||||||||||
| No rotavirus infection | 1 | |||||||||
| Outpatient (≦18 m/>18 m) | 0.781/0.688 | 0.263/0.345e | Normal | [37] | ||||||
| Hospitalisation (≦18 m/>18 m) | 0.425/0.200 | 0.243/0.386e | Normal | [37] | ||||||
| Neurological sequelae | 0.570 | 0.523 | 0.617 | Uniform | [27] | |||||
| Death | 0 | |||||||||
| Costs | ||||||||||
| Costs of per course of vaccination | ¥30000 | assumed | ||||||||
| Outpatient treatment costs per case | ¥15,000 | α = 1, β = cost/cost2 | Gamma | [55] | ||||||
| Hospitalisation treatment costs per case | ¥221,000 | α = 1, β = cost/cost2 | Gamma | [6] | ||||||
| Encephalitis/encephalopathy | ¥852,642 | α = 1, β = cost/cost2 | Gamma | [56] | ||||||
| Neurological sequelae (Long-term treatment cost per year) | ¥420,464 | α = 1, β = cost/cost2 | Gamma | [56] | ||||||
| Variables related to care-giver's productivity loss | ||||||||||
| Proportion of uptaking rotavirus simultaneously with other vaccine list on routine vaccination schedule | 75% | [41] | ||||||||
| Care-giver to accompany a child for one uptake of vaccine (vaccinated alone scenario) | 4h | assumed | ||||||||
| Mean duration of illness of an outpatient case | 7 days | [6, 58] | ||||||||
| Mean duration of hospitalisation days without encephalitis | 5 days | [6, 58] | ||||||||
| Mean duration of hospitalisation days with encephalitis | 22.7 days | [59] | ||||||||
| Average hourly wage of Japanese women labourers | ¥1402 | [57] | ||||||||
| Discount rate | 3% | 5% | [60] | |||||||
PSA was performed from payers’ perspective, therefore, variables related to productivity loss by care-giver were not included.
95% CI reported by Yokoo et al.47
Base-case value of hospitalised × (lower value of outpatient÷base-case value of outpatient).
Base-case value of hospitalised × (upper value of outpatient ÷base-case value of outpatient).
SD reported by Martin et al.37
Due to data insufficiency, variables without footnote were assumed to have a uniform distribution corresponding to the range tested in one way sensitivity analyses, i.e., ±20% of base-case.
Vaccine effectiveness
Similar estimates of vaccine effectiveness (VE) have been reported using different methodologies in different countries including Japan.12,52-54 After reviewing the data, we set the VE in decreasing hospitalisation at 90%, while VE in decreasing GP visit were at 85% and 82% for children who are less than 2 y.o. and those over 2 y.o., respectively.12,52-54 These variables were based on Soares-Weiser et al.'s report, wherein countries with low-mortality rates, RV1 prevents severe rotavirus diarrhea by 86% and 85%, while RV5 is at 87% and 82% in the first and second years, respectively.54 VE between doses was not considered in our study, because it was observed to be high and roughly comparable to VE after a complete vaccination course, and it has little impact on results of both vaccines' cost-effectiveness analyses.28 We conducted sensitivity analyses, which assumed VE to decrease by 10% every year from the base-case.12,52-54
Costing
Direct medical costs
Vaccination costs per full course was assumed at ¥30,000 (US$283), which included doctor's fee for medical advice and technical fee for administering (around ¥ 3500 or US$33 per shot). Average treatment cost, /15,000 (US$142) per outpatient episode, was from a study which reported the economic effects of RVGE on families with rotavirus infected infants.55 /221,000 (US$2,085) per hospitalisation episode was from the largest multi-hospital study across Japan, which captures approximately 0.5% of the annual RVGE hospitalisations in children less than 6 y.o.6 Meningitis' treatment costs by Iwata et al. at ¥852,642 (US$8,044) per episode, was used for patients who developed encephalitis.56 Long-term treatment costs for a child suffering from neurological sequelae at ¥420,464 (US$3,967) per year was also from the same study.56 We incorporated those costs reported before 2014 with no adjustment because the variation of consumer price index of services related to medical care were less than 0.1% during these 10 years, while the sensitivity analyses were conducted on each cost-related data.
Productivity loss by care-giver
Under the context of this study, productivity loss per disease episode or per shot was valued as a product of care-giver's absent working hours from paid employment and an average hourly wage, ¥1,402 (US$13), of Japanese women labourers.57 Productivity loss of a care-giver to accompany a child for one vaccine uptake was assumed for a half-day (4h) when uptaking RVs alone, while no incremental productivity loss occurs when uptaking RVs simultaneously with one of the other listed vaccines. Care-giver's absent working days for outpatient episode was assumed seven days (8 working hours per day), for hospitalised episode was 5 hospital days (2 hours per day to visit and comfort the patient) plus one absent working day before and after hospitalisation, respectively.6,58 The productivity loss per sequelae episode was from Yamanaka et al.59 We assumed that the absent working hours of a care-giver to take care of one child with neurological sequelae is 8 hours per day.56 Productivity loss due to mortality was not included because it can result to double counting if survived cases were to be incorporated in utility weights and disease duration in calculating QALYs.60
Discounting
Costs and outcomes were discounted at an annual rate of 3%.60
One-way sensitivity analyses and probabilistic analyses
One-way sensitivity analyses were performed on costs, probabilities, VE, and utility weights. The ranges were varied by ±50% of base-case for cost-related data and ±20% for other variables. Sensitivity analysis which allowed VE to decrease by 10% every year from the base-case was also performed. Furthermore, we varied the costs per course vaccination to find out how ICER changes with the costs. In addition to the above sensitivity analyses, 3 sets of 1,000 Monte Carlo simulations, i.e., probabilistic analyses were also conducted. The costs of vaccination per course were at ¥30,000 (US$283), ¥25,000 (US$236), and ¥20,000 (US$189). Gamma distribution is used for costs items,61 uniform distribution for probabilities, and normal distribution for utility weights (Table 3). All the sensitivity analyses were performed from payers' perspective.
Abbreviations
- CEAC
cost-effectiveness acceptability curves
- Hib
Haemophilus influenzae type b
- ICER
incremental cost-effectiveness ratio
- MHLW
the Ministry of Health, Labor and Welfare
- QALY
quality adjusted life year
- PCV−13
13-valent pneumococcal conjugate vaccine
- RV
rotavirus vaccine
- RV1
monovalent rotavirus vaccine
- RV5
pentavalent rotavirus vaccine
- RVGE
rotavirus gastroenteritis
- VE
vaccine effectiveness
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by a research grant for Research on Emerging and Re-emerging Infectious Diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare, Japan. We would also like to acknowledge the help of Xerxes Seposo with regard to the language check of this manuscript.
Authors' contributions
SLH participated in the concept and design of the study, performed the literature searches, acquired the data, participated in the analysis and interpretation of the data, and wrote the manuscript. MK and IO participated in the concept and design of the study, and in the interpretation of the data and results.
References
- [1].Rotavirus vaccines WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88(5):49-64; PMID:23424730 [PubMed] [Google Scholar]
- [2].Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12(2):304-6; PMID:16494759; http://dx.doi.org/ 10.3201/eid1202.050006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Payne DC, Staat MA, Edwards KM, Szilagyi PG, Gentsch JR, Stockman LJ, Curns AT, Griffin M, Weinberg GA, Hall CB, et al.. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics 2008; 122(6):1235-43; PMID:19047240; http://dx.doi.org/ 10.1542/peds.2007-3378 [DOI] [PubMed] [Google Scholar]
- [4].Newall AT, MacIntyre R, Wang H, Hull B, Macartney K. Burden of severe rotavirus disease in Australia. J Paediatr Child Health 2006; 42(9):521-7; PMID:16925538; http://dx.doi.org/ 10.1111/j.1440-1754.2006.00915.x [DOI] [PubMed] [Google Scholar]
- [5].Van Damme P, Giaquinto C, Maxwell M, Todd P, Van der Wielen M; REVEAL Study Group . Distribution of rotavirus genotypes in Europe, 2004-2005: the REVEAL Study. J Infect Dis 2007; 195(Suppl 1):S17-25; PMID:17387648; http://dx.doi.org/ 10.1086/516715 [DOI] [PubMed] [Google Scholar]
- [6].Tajiri H, Takeuchi Y, Takano T, Ohura T, Inui A, Yamamoto K, Higashidate Y, Kawashima H, Toyoda S, Ushijima K, et al.. The burden of rotavirus gastroenteritis and hospital-acquired rotavirus gastroenteritis among children aged less than 6 years in Japan: a retrospective, multicenter epidemiological survey. BMC Pediatr 2013; 13:83; PMID:23697664; http://dx.doi.org/ 10.1186/1471-2431-13-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le Saux N, Bettinger JA, Halperin SA, Vaudry W, Scheifele DW. Canadian immunization monitoring program, Active (IMPACT). Substantial morbidity for hospitalized children with community-acquired rotavirus infections: 2005-2007 IMPACT surveillance in Canadian hospitals. Pediatr Infect Dis J 2010; 29:879-82; PMID:20467353; http://dx.doi.org/ 10.1097/INF.0b013e3181e20c94 [DOI] [PubMed] [Google Scholar]
- [8].Payne DC, Baggs J, Zerr DM, Klein NP, Yih K, Glanz J, Curns AT, Weintraub E, Parashar UD. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clin Infect Dis 2014; 58(2):173-7; PMID:24265355; http://dx.doi.org/ 10.1093/cid/cit671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, Takanashi J, Hirose S, Yamagata T, Yamanouchi H, et al.. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev 2012; 34(5):337-43; PMID:21924570; http://dx.doi.org/ 10.1016/j.braindev.2011.07.012 [DOI] [PubMed] [Google Scholar]
- [10].Kawamura Y, Ohashi M, Ihira M, Hashimoto S, Taniguchi K, Yoshikawa T. Nationwide survey of rotavirus-associated encephalopathy and sudden unexpected death in Japan. Brain Dev 2014; 36(7):601-7; PMID:23972382; http://dx.doi.org/ 10.1016/j.braindev.2013.07.013 [DOI] [PubMed] [Google Scholar]
- [11].Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29(37):6335-41; PMID:21640780; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.017 [DOI] [PubMed] [Google Scholar]
- [12].Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370(9601):1757-63; PMID:18037080; http://dx.doi.org/ 10.1016/S0140-6736(07)61744-9 [DOI] [PubMed] [Google Scholar]
- [13].Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq®, in Finnish infants up to 3 years of age: the Finnish Extension Study. Eur J Pediatr 2010; 169(11):1379-86; PMID:20559656; http://dx.doi.org/ 10.1007/s00431-010-1242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine RotarixTM co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine 2010; 28(32):5272-9; PMID:20538094; http://dx.doi.org/ 10.1016/j.vaccine.2010.05.057 [DOI] [PubMed] [Google Scholar]
- [15].Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, Staat MA, Halasa NB, Weinberg GA, Szilagyi PG, Chappell J, et al.. Long-term Consistency in Rotavirus Vaccine Protection: RV5 and RV1 Vaccine Effectiveness in US Children, 2012-2013. Clin Infect Dis 2015; 61(12):1792-9; PMID:26449565; http://dx.doi.org/ 10.1093/cid/civ872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang WC, Yen C, Wu FT, Huang YC, Lin JS, Huang FC, Yu HT, Chi CL, Lin HY, Tate JE, et al.. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J 2001; 33(3):e81-6; http://dx.doi.org/ 10.1097/INF.0000000000000105 [DOI] [PubMed] [Google Scholar]
- [17].Lo Vecchio A, Liguoro I, Dias JA, Berkley JA, Boey C, Cohen MB, Cruchet S, Salazar-Lindo E, Podder S, Sandhu B, et al. Rotavirus immunization: Global coverage and local barriers for implementation. Vaccine 2017; 35(12):1637-44; http://dx.doi.org/ 10.1016/j.vaccine.2017.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakagomi T, Nakagomi O, Takahashi Y, Enoki M, Suzuki T, Kilgore PE. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis 2005; 192(Suppl 1):S106-10; PMID:16088792; http://dx.doi.org/ 10.1086/431503 [DOI] [PubMed] [Google Scholar]
- [19].Ministry of Health, Labour and Welfare. Vital statistics of Japan. Tokyo: Ministry of Health, Labour and Welfare; 1997 [in Japanese]. [Google Scholar]
- [20].Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunisation program in Japan. Jpn J Infect Dis 2011; 64(4):277-83; PMID:21788701 [PubMed] [Google Scholar]
- [21].Itzler R, O'Brien MA, Yamabe K, Abe M, Dhankhar P. Cost-effectiveness of a pentavalent rotavirus vaccine in Japan. J Med Econ 2013; 16(10):216-27; http://dx.doi.org/ 10.3111/13696998.2013.831869 [DOI] [PubMed] [Google Scholar]
- [22].Record of proceedings of the committee of Immunisation and Vaccine Health Science Council, Ministry of Health, Welfare and Labour of Japan. 2013, April ∼2016, Feb (1st meeting∼8th meeting). [Accessed 2016June 15, in Japanese] http://www.mhlw.go.jp/stf/shingi/shingi-kousei.html?tid=127713 [Google Scholar]
- [23].Rotavirus working group, Infectious Disease Sectional Committee, Ministry of Health, Welfare and Labour of Japan. Interim report. 2013. [accessed 2016 15 Jun, in Japanese]. Available at: http://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000029637.pdf [Google Scholar]
- [24].Committee to Study Priorities for Vaccine Development Institute of Medicine. Vaccines for the 21st century: a tool for decision making. Washington, DC: National Academy Press; 2000 [Google Scholar]
- [25].Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine 2010; 28(11):2356-9; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.035 [DOI] [PubMed] [Google Scholar]
- [26].Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010; 19(4):422-37; PMID:19382128; http://dx.doi.org/ 10.1002/hec.1481 [DOI] [PubMed] [Google Scholar]
- [27].Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 7-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine 2012; 30(22):3320-8; PMID:22386745; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.033 [DOI] [PubMed] [Google Scholar]
- [28].Rozenbaum MH, Mangen MJ, Giaquinto C, Wilschut JC, Hak E, Postma MJ, Consensus Group on Dutch Rotavirus Vaccination (CoRoVa-Group) . Cost-effectiveness of rotavirus vaccination in the Netherlands; the results of a consensus model. BMC Public Health 2011;11:462; PMID:21663620; http://dx.doi.org/ 10.1186/1471-2458-11-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goossens LM, Standaert B, Hartwig N, Hövels AM, Al MJ. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine 2008; 26(8):1118-27; PMID:18215445; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.070 [DOI] [PubMed] [Google Scholar]
- [30].Jit M, Bilcke J, Mangen MJ, Salo H, Melliez H, Edmunds WJ, Yazdan Y, Beutels P. The cost-effectiveness of rotavirus vaccination: Comparative analyses for five European countries and transferability in Europe. Vaccine 2009;27(44):6121-8; PMID:19715781; http://dx.doi.org/ 10.1016/j.vaccine.2009.08.030 [DOI] [PubMed] [Google Scholar]
- [31].Mangen MJ, van Duynhoven YT, Vennema H, van Pelt W, Havelaar AH, de Melker HE. Is it cost-effective to introduce rotavirus vaccination in the Dutch national immunization program? Vaccine 2010; 28(14):2624-35; PMID:20109593; http://dx.doi.org/ 10.1016/j.vaccine.2010.01.014 [DOI] [PubMed] [Google Scholar]
- [32].Zomer TP, van Duynhoven YT, Mangen MJ, van der Maas NA, Vennema H, Boot H, de Melker HE. Assessing the introduction of universal rotavirus vaccination in the Netherlands. Vaccine 2008; 26(29-30):3757-64; PMID:18514975; http://dx.doi.org/ 10.1016/j.vaccine.2008.04.039 [DOI] [PubMed] [Google Scholar]
- [33].Tu HA, Rozenbaum MH, de Boer PT, Noort AC, Postma MJ. An update of “Cost-effectiveness of rotavirus vaccination in the Netherlands: the results of a Consensus Rotavirus Vaccine model”. BMC Infect Dis 2013; 13:54; PMID:23363553; http://dx.doi.org/ 10.1186/1471-2334-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Knoll S, Mair C, Benter U, Vouk K, Standaert B. Will vaccination against rotavirus infection with RIX4414 be cost-saving in Germany? Health Econ Rev 2013; 3(1):27; PMID:24246029; http://dx.doi.org/ 10.1186/2191-1991-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aidelsburger P, Grabein K, Böhm K, Dietl M, Wasem J, Koch J, Ultsch B, Weidemann F, Wichmann O. Cost-effectiveness of childhood rotavirus vaccination in Germany. Vaccine 2014; 32(17):1964-74; PMID:24561052; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.061 [DOI] [PubMed] [Google Scholar]
- [36].Giammanco MD, Coniglio MA, Pignato S, Giammanco G. An economic analysis of rotavirus vaccination in Italy. Vaccine 2009; 27(29):3904-11; PMID:19446934; http://dx.doi.org/ 10.1016/j.vaccine.2009.04.002 [DOI] [PubMed] [Google Scholar]
- [37].Martin A, Cottrell S, Standaert B. Estimating utility scores in young children with acute rotavirus gastroenteritis in the UK. J Med Econ 2008; 11(3):471-84; PMID:19450099; http://dx.doi.org/ 10.3111/13696990802321047 [DOI] [PubMed] [Google Scholar]
- [38].Postma MJ, Jit M, Rozenbaum MH, Standaert B, Tu HA, Hutubessy RC. Comparative review of three cost-effectiveness models for rotavirus vaccines in national immunization programs; a generic approach applied to various regions in the world. BMC Med 2011; 9:84; PMID:21740545; http://dx.doi.org/ 10.1186/1741-7015-9-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rautenberg TA, Zerwes U, Foerster D, Aultman R. Evaluating the cost utility of racecadotril for the treatment of acute watery diarrhea in children: the RAWD model. Clinicoecon Outcomes Res 2012; 4:109-16; PMID:22570557; http://dx.doi.org/ 10.2147/CEOR.S31238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martin A, Batty A, Roberts JA, Standaert B. Cost-effectiveness of infant vaccination with RIX4414 (Rotarix) in the UK. Vaccine 2009;27(33):4520-8; PMID:19446594; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.006 [DOI] [PubMed] [Google Scholar]
- [41].Pharmaceuticals and medical devices safety information No. 280. Ministry of Health, Labour and Welfare, Tokyo, June 2011 [access 2016 15 Jun]. Available at: https://www.pmda.go.jp/files/000153635.pdf [Google Scholar]
- [42].Nakagomi O, Nakagomi T. Rotavirus vaccine: Is there a need in Japan. Mordan Media 2008; 54(11):317-30. [Japanese] [Google Scholar]
- [43].Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996; 335(14):1022-8; PMID:8793926; http://dx.doi.org/ 10.1056/NEJM199610033351404 [DOI] [PubMed] [Google Scholar]
- [44].Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1):11-22; PMID:16394298; http://dx.doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- [45].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354(1):23-33; PMID:16394299; http://dx.doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- [46].Centers for Disease Control and Prevention Postmarketing monitoring of intussusception after RotaTeq vaccination–United States, February 1, 2006-February 15, 2007. MMWR 2007; 56(10):218-22; PMID:17363890 [PubMed] [Google Scholar]
- [47].Yokoo M, Arisawa K, Nakagomi O. Estimation of annual incidence, age-specific incidence rate, and cumulative risk of rotavirus gastroenteritis among children in Japan. Jpn J Infect Dis 2004; 57(4):166-71; PMID:15329449 [PubMed] [Google Scholar]
- [48].Kinoshita S, Noguchi A, Miura S, Nakagomi T, Nakagomi O, Takahashi T. A retrospective, hospital-based study to determine the incidence of rotavirus hospitalizations among children less than 5 years of age over a 10-year period (2001-2011) in Akita prefecture, Japan. Jpn J Infect Dis 2014; 67(6):464-8; PMID:25410562; http://dx.doi.org/ 10.7883/yoken.67.464 [DOI] [PubMed] [Google Scholar]
- [49].Lynch M, Lee B, Azimi P, Gentsch J, Glaser C, Gilliam S, Chang HG, Ward R, Glass RI. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin Infect Dis 2001; 33(7):932-8; PMID:11528562; http://dx.doi.org/ 10.1086/322650 [DOI] [PubMed] [Google Scholar]
- [50].Bloch KC, Glaser CA. Encephalitis Surveillance through the Emerging Infections Program, 1997-2010. Emerg Infect Dis 2015; 21(9):1562-7; PMID:26295485; http://dx.doi.org/ 10.3201/eid2109.150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brisson M, Sénécal M, Drolet M, Mansi JA. Health-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. Pediatr Infect Dis J 2010; 29(1):73-5; PMID:19907361; http://dx.doi.org/ 10.1097/INF.0b013e3181b41506 [DOI] [PubMed] [Google Scholar]
- [52].Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schödel F, Brown ML, et al.. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother 2013; 9(8):1626-33; PMID:23732903; http://dx.doi.org/ 10.4161/hv.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cortese MM, Immergluck LC, Held M, Jain S, Chan T, Grizas AP, Khizer S, Barrett C, Quaye O, Mijatovic-Rustempasic S, et al.. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132(1):e25-33; PMID:23776114; http://dx.doi.org/ 10.1542/peds.2012-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2012; 11:Cd008521; PMID:23152260 [DOI] [PubMed] [Google Scholar]
- [55].Nomoto H, Konishi N, Moriya M, Iwamoto T, Aoki D, Noma Y, Kawamura . Economic effects of rotavirus gastroenteritis on families of affected infants. J Chugoku Rosai Hospital 2014; 23(1):6-9. [In Japanese] [Google Scholar]
- [56].Iwata S, Ihiwada N, Sakata H, Sakano T, Sato Y, Nakano T, Nishi J, Haruta T, Hoshino N, Kamiya S. Burden of illness of bacterial meningitis and bacteremia caused by Streptococcus pneumonia in children. Jpn J Pediatr 2008; 61:2206-20. [In Japanese] [Google Scholar]
- [57].Ministry of Health, Labour and Welfare Basic survey on wage structure 2013. Tokyo: Health and Welfare Statistics Association; 2014, [in Japanese] [Google Scholar]
- [58].Nakagomi T, Kato K, Tsutsumi H, Nakagomi O. The burden of rotavirus gastroenteritis among Japanese children during its peak months: an internet survey. Jpn J Infect Dis 2013; 66(4):269-75; PMID:23883835; http://dx.doi.org/ 10.7883/yoken.66.269 [DOI] [PubMed] [Google Scholar]
- [59].Yamanaka N, Hotomi M, Sugita R. Cost-effectiveness of pneumococcal conjugate vaccine in Japan: estimation on acute pneumococcal otitis media in children. Jpn J Pediatr 2008; 61:2221-32. [In Japanese] [Google Scholar]
- [60].Drummond MF, Sculper MJ, Claxton K, Stoddart G, Torrance GW. Methods for the economic evaluation of health care programmes, 4th ed. Oxford, UK: Oxford University Press; 2015 [Google Scholar]
- [61].Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006 [Google Scholar]


