Abstract
Enterocytozoon bieneusi microsporidiosis is an emerging disease in immunocompromised patients. We report 2 cases of this disease in allogeneic hematopoietic stem cell transplant patients successfully treated with fumagillin. Thrombocytopenia occurred but without major adverse events. Modifications of immunosuppression could be avoided when E. bieneusi is rapidly identified and fumagillin therapy is started promptly.
Keywords: Enterocytozoon bieneusi, protozoa, microsporidia, microsporidiosis, parasites, obligate intracellular parasite, transplantation, allogeneic, hematopoietic stem cell transplant recipients, immunocompromised patients, antiprotozoal drugs, fumagillin
Enterocytozoon bieneusi, the most common cause of microsporidiosis in humans (1), causes chronic diarrhea and severe wasting syndrome in immunocompromised patients (2). In 2002, oral fumagillin was established as an effective treatment for E. bieneusi microsporidiosis in HIV-infected and solid organ transplant (SOT) patients (3). In contrast to previous treatments that did not result in parasitologic clearance or clinical remission, fumagillin showed a cure rate of 100%, even for severely immunocompromised patients (2,4–10).
Thrombocytopenia is the main adverse event of fumagillin therapy, occurring in up to 33% of patients (3) and raising concerns about fumagillin use in patients with hematologic disorders. We report 2 cases of E. bieneusi microsporidiosis in allogeneic hematopoietic stem cell transplant (HSCT) recipients who were treated with fumagillin and experienced thrombocytopenia.
Patient 1 was a 50-year-old woman admitted to Centre Hospitalier Universitaire de Caen (Caen, France) after profuse watery diarrhea and abdominal discomfort for 3 weeks. She had not traveled abroad. Three years earlier, she received a genoidentical allogeneic HSCT for myeloid leukemia. She recently had cutaneous chronic graft-versus-host disease. Her immunosuppression regimen used was prednisone and mycophenolate mofetil.
At admission, the patient was dehydrated and had a weight loss of 3 kg. Laboratory analyses showed lymphocytopenia (960 lymphocytes/mm3), reference neutrophil (5,100 cells/mm3) and platelet (408,000 platelets/mm3) counts, and a C-reactive protein level <5 mg/L.
Results of fecal sample analyses were negative for pathogenic bacteria and viruses. Microscopic examination of fecal smears stained with Weber-Green–modified trichrome showed microsporidia. E. bieneusi was identified by using monoclonal antibodies (IFA-MAbs; Bordier Affinity Products, Crissier, Switzerland).
The mycophenolate mofetil dose was reduced by 50% for 8 days but no benefit was shown. Resolution of symptoms occurred <5 days after initiating fumagillin therapy (60 mg/d for 14 d); fecal smears were negative for microsporidia on day 9, and transient thrombocytopenia (131,000 platelets/mm3) was observed on day 18 (Figure). Fecal smears remained negative for E. bieneusi during the 6-month follow-up. No clinical relapse occurred.
Figure.
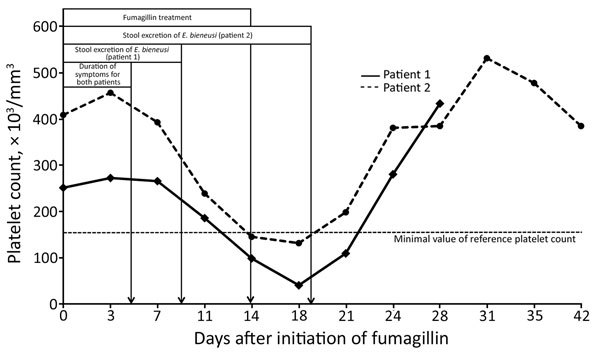
Platelet counts and clinical and parasitologic characteristics during fumagillin therapy and a 1-month follow-up of 2 allogeneic hematopoietic stem cell recipients with Enterocytozoon bieneusi microsporidiosis.
Patient 2 was a 42-year-old man referred to Centre Hospitalier Universitaire de Bicêtre (Le Kremlin-Bicêtre, France) after profuse acute diarrhea for 2 weeks and a weight loss of 10 kg. He had not traveled abroad. Four years earlier, he received a genoidentical allogeneic HSCT for acute leukemia. During the follow-up period, he was given a diagnosis of chronic graft-versus-host disease. He was given extracorporeal phototherapy with mycophenolate mofetil, sirolimus, and prednisolone.
At admission, the patient was afebrile and dehydrated. Blood analyses showed severe lymphocytopenia (400 lymphocytes/mm3), reference neutrophil (4,680 cells/mm3) and platelet (251,000 platelets/mm3) counts, and a C-reactive protein level <5 mg/L. Results of microbiological analyses of fecal samples were negative for viruses and pathogenic bacteria. Microscopic examination of fecal smears stained with Weber-Green–modified trichrome showed microsporidia. E. bieneusi was identified by using monoclonal antibodies.
The patient was treated with fumagillin (60 mg/d for 14 d) (Figure). Immunosuppressive therapy was not modified. Clinical symptoms resolved within 5 days. Platelet counts progressively decreased. Fumagillin was withdrawn on day 14, but thrombocytopenia worsened (40,000 platelets/mm3) by day 18. However, the patient spontaneously recovered in 10 days without any bleeding. No relapses were observed. Microsporidia were not detected in fecal samples during the 6-month follow-up.
E. bieneusi is an emerging pathogen in immunocompromised patients (1). Increasing numbers of cases have been reported in SOT patients. We report 2 cases of E. bieneusi microsporidiosis in allogeneic HSCT recipients who were treated with fumagillin without modifying the immunosuppressive regimen for 1 recipient. In France, fumagillin can be obtained from the French National Agency for Medicines and Health Products Safety (Saint-Denis, France) after an individual patient expanded-access request is submitted.
Clinical and microbiological responses for the 2 case-patients were similar to those reported for other immunocompromised patients (3). No relapses were observed for 4 HIV-infected patients whose CD4 cell counts remained low, or for 2 SOT recipients who did not receive tapering immunosuppressive therapy (3). In other studies, 15 (70%) of 21 patients treated with fumagillin were cured without modifying immunosuppression regimens (2,6,10); for 6 other patients, immunosuppressive therapy was tapered (n = 4) or withdrawn (n = 2), but reasons for modifying immunosuppression were not specified. For 1 of our patients, the mycophenolate mofetil dose was reduced by 50% to decrease the immunosuppression level. However, no benefit was observed. In contrast, fumagillin led to clinical remission within 5 days.
We observed thrombocytopenia (platelet count <40,000/mm3) in both patients but no evidence of bleeding. In other non-AIDS patients, thrombocytopenia was reported in 11 (52%) SOT patients receiving fumagillin, including 4 patients with severely low platelet counts (<25,000/mm3) (1,4,6). For these patients, including those we report, thrombocytopenia occurred during the second week of treatment; a minimum value was observed a few days after completing fumagillin therapy. Spontaneous recovery occurred within 2 weeks. Bleeding, hematoma, or requirements for platelet transfusions were not reported. For both patients we report, microsporidia were not detected in fecal samples of both patients during the 6-month follow-up.
In conclusion, fumagillin was highly efficient in curing E. bieneusi microsporidiosis in 2 allogeneic HSCT recipients. Thrombocytopenia occurred but without major adverse events. Modifications to immunosuppression could be avoided when E. bieneusi is rapidly identified and fumagillin therapy is started promptly.
Biography
Dr. Bukreyeva is a physician in the Infectious and Tropical Diseases Unit at the Bicêtre University Hospital, Kremlin-Bicêtre, France. Her research interests include prevention and treatment of infections in immunocompromised patients.
Footnotes
Suggested citation for this article: Bukreyeva I, Angoulvant A, Bendib I, Gagnard J-C, Bourhis J-H, Dargère S, et al. Enterocytozoon bieneusi microsporidiosis in stem cell transplant recipients treated with fumagillin. Emerg Infect Dis. 2017 Jun [date cited]. https://dx.doi.org/10.3201/eid2306.161825
Results from this study were presented at the 26th European Congress of Clinical Microbiology and Infection Diseases; April 9–12, 2016; Amsterdam, the Netherlands.
References
- 1.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19:485–92. 10.1097/01.qco.0000244055.46382.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanternier F, Boutboul D, Menotti J, Chandesris MO, Sarfati C, Mamzer Bruneel MF, et al. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl Infect Dis. 2009;11:83–8. 10.1111/j.1399-3062.2008.00347.x [DOI] [PubMed] [Google Scholar]
- 3.Molina JM, Tourneur M, Sarfati C, Chevret S, de Gouvello A, Gobert JG, et al. ; Agence Nationale de Recherches sur le SIDA 090 Study Group. Fumagillin treatment of intestinal microsporidiosis. N Engl J Med. 2002;346:1963–9. 10.1056/NEJMoa012924 [DOI] [PubMed] [Google Scholar]
- 4.Desoubeaux G, Maakaroun-Vermesse Z, Lier C, Bailly E, Morio F, Labarthe F, et al. Successful treatment with fumagillin of the first pediatric case of digestive microsporidiosis in a liver-kidney transplant. Transpl Infect Dis. 2013;15:E250–9. 10.1111/tid.12158 [DOI] [PubMed] [Google Scholar]
- 5.Pomares C, Santín M, Miegeville M, Espern A, Albano L, Marty P, et al. A new and highly divergent Enterocytozoon bieneusi genotype isolated from a renal transplant recipient. J Clin Microbiol. 2012;50:2176–8. 10.1128/JCM.06791-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godron A, Accoceberry I, Couret A, Llanas B, Harambat J. Intestinal microsporidiosis due to Enterocytozoon bieneusi in a pediatric kidney transplant recipient successfully treated with fumagillin. Transplantation. 2013;96:e66–7. 10.1097/TP.0b013e3182a902e7 [DOI] [PubMed] [Google Scholar]
- 7.Kicia M, Wesolowska M, Jakuszko K, Kopacz Z, Sak B, Květonova D, et al. Concurrent infection of the urinary tract with Encephalitozoon cuniculi and Enterocytozoon bieneusi in a renal transplant recipient. J Clin Microbiol. 2014;52:1780–2. 10.1128/JCM.03328-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghoshal U, Khanduja S, Pant P, Prasad KN, Dhole TN, Sharma RK, et al. Intestinal microsporidiosis in renal transplant recipients: Prevalence, predictors of occurrence and genetic characterization. Indian J Med Microbiol. 2015;33:357–63. 10.4103/0255-0857.158551 [DOI] [PubMed] [Google Scholar]
- 9.Bednarska M, Bajer A, Welc-Faleciak R, Czubkowski P, Teisseyre M, Graczyk TK, et al. The first case of Enterocytozoon bieneusi infection in Poland. Ann Agric Environ Med. 2013;20:287–8. [PubMed] [Google Scholar]
- 10.Champion L, Durrbach A, Lang P, Delahousse M, Chauvet C, Sarfati C, et al. Fumagillin for treatment of intestinal microsporidiosis in renal transplant recipients. Am J Transplant. 2010;10:1925–30. 10.1111/j.1600-6143.2010.03166.x [DOI] [PubMed] [Google Scholar]


