Abstract
Background: Peptidomics research has demonstrated that protease activity is higher in breast milk from preterm-delivering mothers than from term-delivering mothers. However, to our knowledge, the effect of the degree of prematurity and postnatal age on proteases and protease inhibitors in human milk remains unknown.
Objective: We aimed to determine the change of proteases and protease inhibitors in milk from mothers who delivered prematurely across gestational age (GA) and postnatal age.
Methods: Milk samples were collected from 18 mothers aged 26–40 y who delivered preterm infants and who lacked mastitis. For analysis, samples were separated into 2 groups: 9 from early GA (EGA) (24–26 wk GA)-delivering mothers and 9 from late GA (LGA) (27–32 wk GA)-delivering mothers. Within the 9 samples in each group, the collection time ranged from postnatal days 2 to 47. The activity and predicted activity of proteases in preterm milk were determined with the use of fluorometric and spectrophotometric assays and peptidomics, respectively. Protease and protease inhibitor concentrations were determined with the use of ELISA. Linear mixed models were applied to compare enzymes across GA and postnatal age.
Results: Carboxypeptidase B2, kallikrein, plasmin, elastase, thrombin, and cytosol aminopeptidase were present and active in the milk of preterm-delivering mothers. Most milk protease and antiprotease concentrations did not change with GA or postnatal age. However, the concentration and activity of kallikrein, the most abundant and active protease in preterm milk, increased by 25.4 ng · mL−1 · d−1 and 0.454 μg · mL−1 · d−1 postnatally, respectively, in EGA milk samples while remaining stable in LGA milk samples.
Conclusions: This research demonstrates that proteases are active in human milk and begin to degrade milk protein within the mammary gland before consumption by infants. Proteases and protease inhibitors in milk from mothers of premature infants mostly did not vary substantially across GA and postnatal age.
Keywords: plasmin, elastase, thrombin, cathepsin D, carboxypeptidase B2, cytosol aminopeptidase, α1-antitrypsin, α2-antiplasmin, antithrombin III, α1-antichymotrypsin
Introduction
In addition to meeting the basic nutritional requirements of rapidly growing neonates, human milk provides biologically active molecules that protect against infection and promote gut and immune system development. Part of this bioactivity derives from milk’s intact proteins. However, research is now revealing that some milk proteins serve as precursors to encrypted protein fragments—peptides—that become active only after being released by proteases. These bioactive peptides have an array of biological activities, including antimicrobial, antihypertensive, and antifungal activities and calcium absorption and immunomodulation enhancement (1). Previous research with the use of peptidomics-based enzyme predictions has revealed that milk proteases begin hydrolyzing milk proteins inside the mother’s mammary gland and continue to act in the stomach of term infants, releasing bioactive peptides (2–7). The peptidomic analysis and bioinformatics predicted that plasmin, carboxypeptidase B2, cytosol aminopeptidase, cathepsin D, and elastase were actively digesting proteins within the mammary gland (6, 7) and these milk proteases were active in preterm and term milk (3).These findings led to the hypothesis that the activity of proteases within human milk is an important component of the infant’s digestive capacity and influences the development of the intestinal microbiota and the innate and adaptive immune systems.
Previous research has demonstrated that human milk contains numerous inactive protease precursors (zymogens), active proteases, protease activators, and protease inhibitors (8). Several proteases have been identified in human milk via qualitative proteomic analyses, namely carboxypeptidase B2, plasmin, kallikrein, thrombin, cathepsin D, and cytosol aminopeptidase (9–11). Because proteomic analysis depends on identifying sequence fragments, this evidence does not distinguish between the presence of active proteases and inactive zymogens. Except for plasmin activity (12, 13), nonquantitative elastase activity detection (14), procathepsin D concentration (15), and the qualitative immunodetection of elastase (16), the activities and concentrations of these proteases in human milk have not previously been measured to our knowledge. In bovine milk, the activity of plasmin (17) and elastase (18) has been measured, and the concentration of plasmin (17), cathepsin D (19), and elastase (16) has been measured with the use of ELISA. Kallikrein has also been identified in bovine milk but was not quantified (20). Previous work with bovine milk has demonstrated the presence of numerous inactive (zymogen) and active forms of proteases in bovine milk, including plasminogen and plasmin (20). To our knowledge, only the zymogen forms of plasmin (plasminogen) have been found in human (21) and bovine milk (20, 21). The protease inhibitors α1-antitrypsin, α1-antichymotrypsin, α2-antiplasmin, plasma Ser protease inhibitor, and antithrombin III have been identified in human milk by proteomic analysis (9–11), but only α1-antitrypsin and α1-antichymotrypsin to our knowledge have been measured for concentration. α1-Antitrypsin (22) has been identified in bovine milk, whereas α2-antiplasmin, plasma Ser protease inhibitor, and antithrombin III have been measured with the use of ELISA in bovine colostrum (23). Protease activators found in milk include the tissue-type plasminogen activator and the urokinase-type plasminogen activator for human milk (24) and bovine milk (25), which allow the activation of plasminogen to plasmin. Based on our previous peptidomics data, these systems of zymogens, active proteases, inhibitors, and activators appear to provide a controlled and protein-selective proteolytic digestion in the mammary gland and infant stomach (3). Although protease activities and protease inhibitor concentrations in human milk likely differ across the degree of prematurity because of the physiologic maturation differences, these specific factors remained unexplored. We aimed in this study to evaluate the effects of the degree of prematurity and postnatal age on protease activities and inhibitor concentrations in human milk. Synthetic substrate assays were performed to determine which of the proteases predicted to be active were indeed active in human milk. ELISAs were used to determine the concentration of proteases (which may include both active and inactive zymogens depending on the antibody) in preterm milk. Peptides identified in peptidomic analyses were mapped to known protease cleavage specificities to predict which proteases were active. These predicted activities were compared with the activities determined with the use of substrate assay measurements.
Methods
Participants and sample collection
Informed consent was obtained from all mothers participating in the study, which was approved by the UC Davis Institutional Review Board. Human milk samples were collected from 18 mothers who delivered preterm infants [24–32 wk gestational age (GA6)] enrolled in the UC Davis Children’s Hospital Neonatal Intensive Care Unit in Sacramento, California. None of the mothers demonstrated any signs of mastitis. Preterm milks were separated into 2 groups of GA: 9 early GA (EGA) (24–26 wk GA) and 9 late GA (LGA) (27–32 wk GA) (Table 1). A single sample was collected from each mother. These samples were collected from postnatal days 2–47 by pumping on-site or at home with clean electric breast pumps into sterile plastic containers and stored immediately at –20°C. The breast was cleaned with water on a washcloth (no soap or alcohol) before pumping. Samples were transported to Oregon State University on dry ice and thawed on ice, and five 0.5-mL aliquots were placed into vials that were then stored at –80°C.
TABLE 1.
Characteristics of mothers who provided milk samples and their infants in EGA and LGA groups1
| GA group | GA, wk | Postnatal age, d | Birth weight, g | Mother's age, y | Infant sex |
| EGA | 24.9 ± 0.7 (24–26) | 27 ± 3 (2–47) | 683 ± 19 (620–770) | 29 ± 2 (18–37) | 5 males; 4 females |
| LGA | 29.9 ± 0.6 (27–32) | 21 ± 5 (5–44) | 1246 ± 140 (890–2210) | 30 ± 2 (26–40) | 4 males; 5 females |
All values are means ± SEMs (ranges), n = 9, unless otherwise indicated. EGA, early gestational age; GA, gestational age; LGA, late gestational age.
General sample preparation
Milk samples were thawed at 4°C. Samples were centrifuged at 4226 × g for 10 min at 4°C, and the infranate was collected with the use of a pipette from below the upper fat layer. Aliquots of 50 μL of this solution were placed into 20 vials and stored at –80°C until use for each assay. Samples were thawed only once to avoid the possible degradation of enzymes during thawing and freezing.
pH
The pH of the human milk samples were measured with an S220 SevenCompact pH/Ion meter (Mettler-Toledo) equipped with a combined sealed glass electrode. Samples (2 vials of 50 μL for each sample) were thawed at room temperature, and the electrode was equilibrated in the sample before the pH value was recorded.
Spectrophotometric and fluorometric assays
The spectrophotometric and fluorometric assays were performed according to the methods described by the manufacturers. Measurements were collected with a microplate reader (Spectramax M2; Molecular Devices) with SoftMax Pro 4.8 (Molecular Devices) and with 2 replicates of blanks, standards, and samples. For each assay (ELISA and fluorometric and spectrophotometric assays), sample concentrations or activities were interpolated from standard calibration curves with the use of the 4-parameter logistic curve fit, as recommended by the assay manufacturers.
ELISA
All ELISAs were carried out as described by the manufacturer (Innovative Research Inc. or MyBioSource) (Supplemental Table 1).
Protease activity assays
Enzyme activity assays were carried out according to the manufacturer’s instructions. The specific assay and manufacturer, amount of milk sample used, dilution factor, and range of the standard curve are shown for each protease in Supplemental Table 2. The peptide substrate assays were selected based on the cleavage specificity from Merops (26) and preference for specific amino acids (27) for each identified protease (Supplemental Table 3). In each case, the standard curve was based on varying concentrations of the isolated active form of the enzyme provided (either in a kit or purchased separately).
Plasmin.
Plasmin activity was determined with the use of the substrate assay detailed in Supplemental Tables 2 and 3. As the synthetic substrate for plasmin [Asp-Val-Leu-Lys-|-7-amino-4-methylcoumarin (AMC)] (“|” specifies the cleavage site) (28) is cleaved C-terminal to lysine, trypsin, thrombin, and kallikrein may also be able to cleave this substrate (26) (Supplemental Table 3). Plasmin and trypsin have a higher preference for Lys than do thrombin and kallikrein. Trypsin is present in human milk but was not found to be active in Monti et al. (29). Therefore, we assigned the release of AMC from Asp-Val-Leu-Lys-|-7-AMC to plasmin, but thrombin and kallikrein may slightly contribute to the release of the substrate.
Elastase.
Elastase activity was measured as described in Supplemental Tables 2 and 3. The substrate used—methoxy-Suc-Ala-Ala-Pro-Val-|-antibody-fluorophore conjugate—is specific to elastase because it is the only milk protease with a preference for Val at the P1 position (27) (Supplemental Table 3).
Thrombin.
Thrombin activity was measured according to Supplemental Tables 2 and 3. The key cleavage site used was Pro-Arg-|-Ser-Phe-far-red fluorogen, which matches known thrombin cleavage specificity. Plasmin, kallikrein, and trypsin can cleave Arg at the P1 position but only thrombin as a preference for Ser at the P1′ position. Therefore, we assigned the release of far-red fluorogen to thrombin (although plasmin and kallikrein might contribute to its release).
Cathepsin D.
Cathepsin D activity was determined according to Supplemental Tables 2 and 3 with the use of a cathepsin d-specific synthetic substrate [7-methoxycoumarin-4-yl acetyl-Gly-Lys-Pro-Ile-Leu-Phe-|-Phe-Arg-Leu-Lys-dinitrophenol-d-Arg-NH2 (30)]. The kit-provided buffer was adjusted to pH 6.5 with 6 M sodium hydroxide to match the milk sample pH.
Kallikrein.
Kallikrein activity was determined with the use of the His-Asp-Pro-Phe-Arg-|-p-nitroaniline (pNA) (Bachem Americas, Inc.) spectrophotometric assay as described previously (31), with minor modifications (Supplemental Tables 2 and 3). Fifty microliters of diluted samples, kallikrein standards (human plasma kallikrein; EMD Millipore Corp.), or blanks (distilled water) were added to 150 μL of the substrate solution in microplate wells and incubated with mixing at 300 rpm for 1 h at 37°C in a thermomixer.
Of the many known kallikreins, only kallikreins 11 and 6 to our knowledge have been identified with the use of proteomics (9) in human milk. Plasmin, thrombin and trypsin can cleave after Arg at the P1 position, but only kallikreins 6 and 11 have a preference for Phe at the P2 position (27). Because trypsin has not been found to be active in milk, we did not consider it as responsible for any part of this activity. Therefore, we have assigned all activity from the degradation of this substrate to kallikrein (although thrombin and plasmin could participate in its release).
Carboxypeptidase B.
Carboxypeptidase B activity was measured via the spectrophotometric assay as described by others (21, 22), with some modifications, using parameters as in Supplemental Table 2). The assay measures the release of hippuric acid from a carboxypeptidase-specific synthetic substrate (N-benzoyl-Gly-|-Arg, H-2508; Sigma-Aldrich) (Supplemental Table 3). Carboxypeptidase N could also cleave this synthetic substrate, but no study to our knowledge has found it in human milk. Therefore, we assigned the results from this substrate to carboxypeptidase B. Twenty microliters of diluted samples, standards (carboxypeptidase B, porcine pancreas; Sigma-Aldrich), or blanks (distilled water) were added to 200 μL of the substrate solution in the microplate and mixed and incubated for 1 h at 25°C in a thermomixer.
Cytosol aminopeptidase.
Cytosol aminopeptidase activity was determined with the use of a spectrophotometric assay (23) with some modifications with the use of the parameters shown in Supplemental Table 2. Cytosol aminopeptidase activity catalyzes the splitting of pNA from the substrate Leu-|-pNA (l-Leu; Sigma-Aldrich) (Supplemental Table 3). The only aminopeptidases identified with the use of proteomics in human milk to our knowledge thus far are cytosol aminopeptidase and aminopeptidase N (9). Only cytosol aminopeptidase to our knowledge has been identified as active in human milk via peptidomics (3). Therefore, we assigned the release of pNA to cytosol aminopeptidase. Ten microliters of diluted samples, standards (Aeromonas aminopeptidase recombinant protein; ProSci), or blanks (tricine buffer) were added to 90 μL of the substrate solution in microplate wells and incubated at 25°C for 2 min.
Peptidomic analysis
Peptide extraction from milk samples was performed as described previously (3). After the Folch extraction, trichloroacetic acid protein precipitation and 96-well plate C18 microextraction, the eluted peptide solutions were dried by centrifugal evaporation and solubilized in 50 μL 0.1% trifluoroacetic acid for MS analysis. MS parameters were as described previously (32).
Spectra were analyzed by searching databases in Proteome Discoverer version 2.1.0.81 (Thermo Fisher Scientific) with the use of an in-house human milk protein sequence database. Potential modifications allowed included the phosphorylation of Ser and Thr and the oxidation of Met. Only peptides identified with high confidence were included (P < 0.01).
Computational enzymatic analysis
Peptide sequences and cleavage information were analyzed with an in-house program to estimate the enzyme activity as described previously (5). Briefly, the software locates the position of each peptide in the parent protein and assigns the cleavage to enzymes according to a selected set of protease specificity rules derived from Merops (26). The cleavage sites for cytosol aminopeptidase and carboxypeptidase B2 were determined by manually examining the peptide data as described previously (3). The cleavage specificities used for this analysis are shown in Supplemental Table 4.
Statistical analysis
Conclusions were drawn from linear mixed models fit in SAS version 9.4 (SAS Institute) with the use of PROC GLM. GA, EGA, LGA, and postnatal age (continuous) were modeled as fixed effects and preterm milks from each mother as random effects. This design was used to determine the effect of GA and postnatal age on human milk proteases and protease inhibitors. Least-squares means and estimated differences were computed for each group at postnatal ages 11 and 31 d, the first and third quartiles of postnatal age in the data set. The Tukey-Kramer method was used to adjust for multiple comparisons. All statistical conclusions that do not mention a variable assume that the variable was held constant. Differences were designated significant at P < 0.05. The slopes for each GA group across postnatal age in preterm milk and their P value were determined to interpret how postnatal age influenced GA. The slopes with P > 0.05 indicate that data plot remained constant.
Results
Proteases and protease inhibitors in human milk
From the highest to the lowest, protease concentrations (active and inactive zymogen forms) in the milk of preterm-delivering mothers identified with the use of ELISA were carboxypeptidase B2, plasmin, kallikrein, elastase, thrombin, cathepsin D, and cytosol aminopeptidase (Table 2). Activities of proteases (active form) in the milk of preterm-delivering mothers were measured with the use of fluorogenic or chromogenic substrate assays and reported in concentrations based on the standard curve. Protease activities, from highest to lowest, were kallikrein, carboxypeptidase, plasmin, thrombin, elastase, and cytosol aminopeptidase, whereas cathepsin D activity was undetectable (Table 2). The predicted active proteases in the milk of preterm-delivering mothers based on the peptidomics data, from highest to lowest activity, were plasmin, kallikrein, and thrombin; elastase; cathepsin D; cytosol aminopeptidase; and carboxypeptidase B2 (Table 2). Concentrations of 5 protease inhibitors were measured with the use of ELISA and compared with values reported in previous studies (Table 3). Identified protease inhibitors, from highest to lowest concentration, were α1-antitrypsin, antithrombin III, α1-antichymotrypsin, α2-antiplasmin, and the plasma Ser protease inhibitor (Table 3). The overall mean ± SD pH for all samples was 6.35 ± 0.03.
TABLE 2.
Concentration, activity, and predicted activity of proteases in human milk from mothers who delivered preterm infants (24–32 wk GA) during the first 7 wk of postnatal ages 2–47 d1
| Protease | ELISA-based concentration,2 ng/mL | Activity,3 ng/mL | Predicted activity (peptide abundance)4 |
| Carboxypeptidase B2 | 1574 ± 3 | 1900 ± 500 | 3.4 ± 0.4 × 1010 |
| Plasmin | 836 ± 128 | 146 ± 20 | 1.1 ± 0.1 × 1011 |
| Kallikrein | 700 ± 100 | 16,000 ± 2000 | 1.1 ± 0.1 × 1011 |
| Elastase | 382 ± 10 | 2.1 ± 0.3 | 6.5 ± 0.6 × 1010 |
| Thrombin | 25 ± 7 | 71 ± 13 | 1.1 ± 0.1 × 1011 |
| Cathepsin D | 0.8 ± 0.2 | ND | 5.3 ± 0.6 × 1010 |
| Cytosol aminopeptidase | 1.1 ± 0.1 | 1.0 ± 0.1 | 4.9 ± 0.5 × 1010 |
Values are overall means ± SEMs of the combined EGA and LGA preterm milks, n = 18. EGA, early gestational age; GA, gestational age; LGA, late gestational age; ND, not detected.
Concentration of the active enzyme and zymogen.
Determined with the use of fluorometric or spectrophotometric substrate assays and represents only the active enzyme.
Based on the total ion intensity ascribed to the protease (unitless).
TABLE 3.
Comparison of protease inhibitor concentrations in human milk from mothers who delivered preterm infants (24–32 wk GA) during the first 7 wk of postnatal ages 2–47 d and previous studies of preterm and term milks1
| Protease inhibitor | This study | Previous studies |
| α1-Antitrypsin, μg/mL | 70 ± 6 | 210 (1 d PNA) and 13 (14 d PNA) in term and preterm mother’s milks (30, 31, 35, and 36 wk GA) (27) |
| α1-Antichymotrypsin, μg/mL | 1.10 ± 0.07 | 670 (1 d PNA) and 12 (14 d PNA) in term and preterm mother’s milks (30, 31, 35, and 36 wk GA) (27) |
| α2-Antiplasmin, ng/mL | 417 ± 35 | Undetectable (1–14 d PNA) in term and preterm mother’s milks (27); undetectable (<30,000) (32); 230,000 in bovine colostrum (12) |
| Antithrombin III, ng/mL | 1272 ± 148 | Undetectable (1–14 d PNA) in term and preterm mother’s milks (27); undetectable (<30,000) (32); 40,700 in bovine colostrum (12) |
| Plasma Ser protease inhibitor, ng/mL | 326 ± 26 | 29,000 in bovine colostrum (12) |
Values are overall means ± SEMs of the combined EGA and LGA preterm milks, n = 18. EGA, early gestational age; GA, gestational age; LGA, late gestational age; PNA, postnatal age.
Changes in proteases and protease inhibitors in milk across GA and postnatal age.
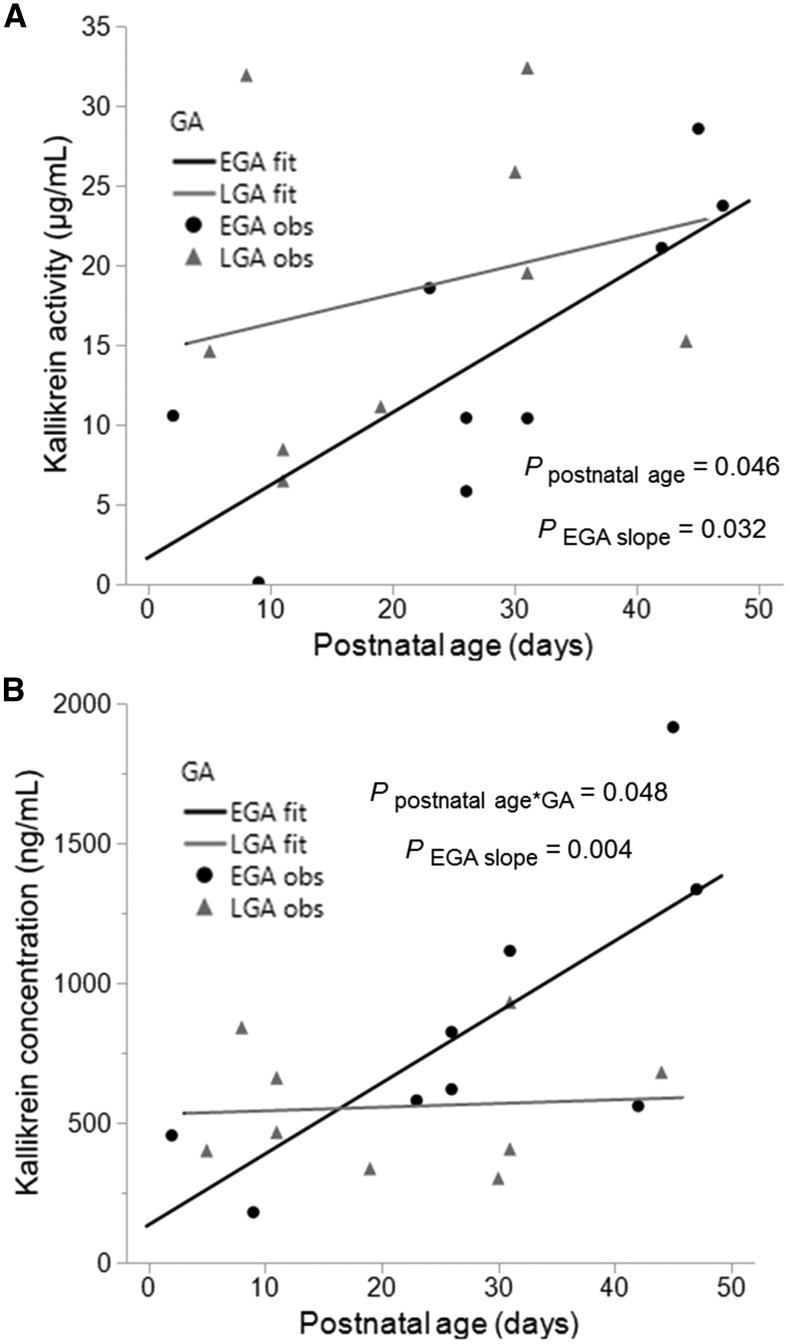
Kallikrein activity in the milk of preterm-delivering mothers was affected by postnatal age (P = 0.046). Kallikrein activity was increased across postnatal age in EGA milk (slope = 0.454 μg · mL−1 · d−1; P = 0.032) (Figure 1A) but remained stable in LGA milk (slope = 0.183 μg · mL−1 · d−1; P = 0.42). GA and postnatal age had a combined effect on the kallikrein concentration (P = 0.048) in the milk of preterm-delivering mothers. Kallikrein concentration increased across postnatal age in EGA milk (slope = 25.4 ng · mL−1 · d−1; P = 0.004), whereas its concentration remained constant in LGA milk (slope = 1.31 ng · mL−1 · d−1; P = 0.88) (Figure 1B).
FIGURE 1.
Changes of kallikrein activity (A) and concentration (B) in milk from mothers who delivered at an EGA (24–26 wk GA) and LGA (27–32 wk GA) across postnatal ages 2–47 d. Line plots were used to estimate the slopes of EGA and LGA milk samples across postnatal ages (n = 9 per group). EGA and LGA fit represent the line plots for each group, and EGA and LGA obs represent each data point within each group. For panel A, Ppostnatal age represents the P value of the effect of postnatal age on kallikrein activity for EGA and LGA samples combined, and PEGA slope represents the P value of postnatal age on kallikrein activity for EGA samples alone; for panel B, Ppostnatal age*GA represents the P value of the interaction between postnatal age and GA on kallikrein concentration, and PEGA slope represents the P value of postnatal age on kallikrein concentration for EGA samples alone. EGA, early gestational age; GA, gestational age; LGA, late gestational age; obs, observation.
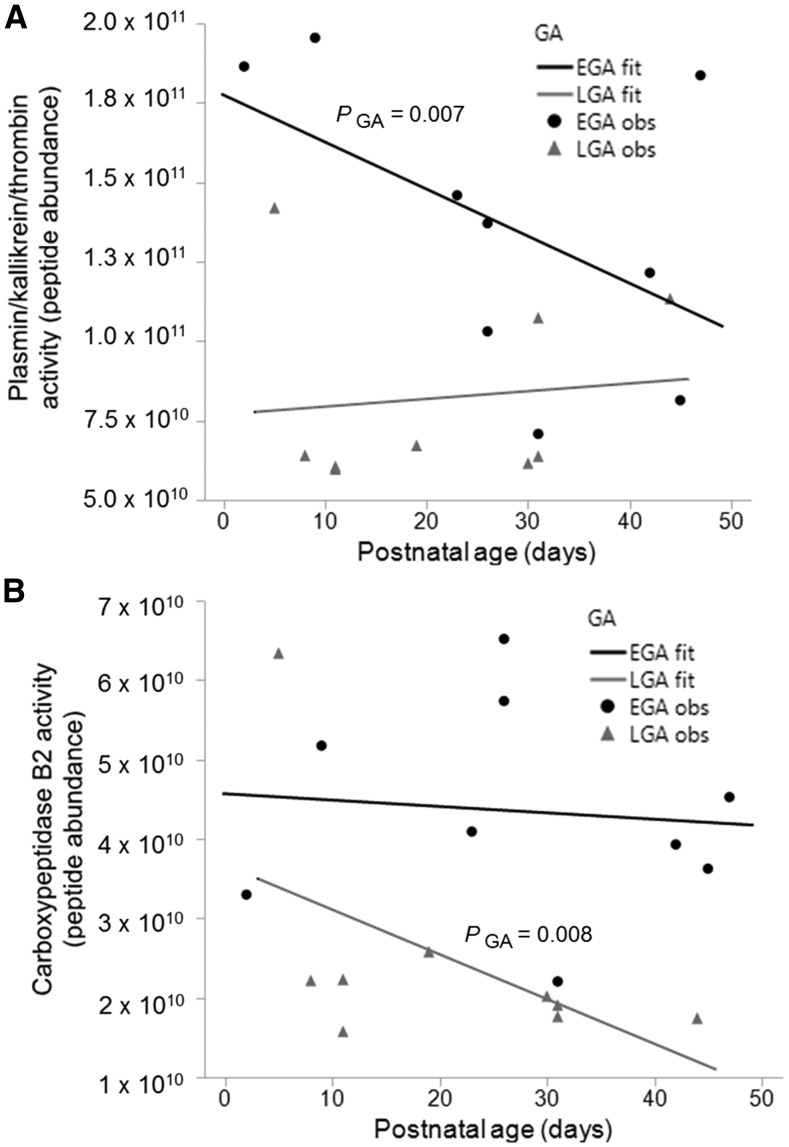
Plasmin activity in the milk of preterm-delivering mothers was not influenced by GA, but EGA and LGA milks tended to decrease across postnatal age (P = 0.090) (Supplemental Figure 1). Predicted plasmin, kallikrein, and thrombin activity in the milk of preterm-delivering mothers was affected by GA (P = 0.016). The overall estimated mean for the predicted activity of plasmin, kallikrein, and thrombin (peptide abundance) in EGA milk was 6 × 1010 peptides (95% CI: 2 × 1010, 10 × 1010; P = 0.007) higher than in LGA milk (Figure 2A). At postnatal days 11 and 31, the estimated mean of the predicted active plasmin, kallikrein, and thrombin in EGA milk was 8 × 1010 (95% CI: 3 × 1010, 13 × 1010; P = 0.006) and 4.7 × 1010 (95% CI: 0.3 × 1010, 9.1 × 1010; P = 0.039) peptides higher than in LGA milk, respectively.
FIGURE 2.
Changes of predicted (peptide abundance) of plasmin, kallikrein, and thrombin activity (A) and carboxypeptidase B2 activity (B) in milk from mothers who delivered at an EGA (24–26 wk GA) and LGA (27–32 wk GA) across postnatal ages 2–47 d. Line plots were used to estimate the slopes of EGA and LGA milk samples across postnatal ages (n = 9 per group). EGA and LGA fit represent the line plots for each group, and EGA and LGA obs represent each data point within each group. PGA represents the P value of the effect of GA on the predicted plasmin, kallikrein, and thrombin activity. EGA, early gestational age; GA, gestational age; LGA, late gestational age; obs, observation.
Predicted carboxypeptidase B activity was influenced by GA in the milk of preterm-delivering mothers (P = 0.042). The overall estimated mean of predicted carboxypeptidase B activity (peptide abundance) in EGA milk was 2.1 × 1010 (95% CI: 0.6 × 1010, 3.5 × 1010; P = 0.008) higher than LGA milk (Figure 2B). At postnatal day 31, the predicted active carboxypeptidase B in EGA milk was 2.4 × 1010 (95% CI: 0.8 × 1010, 2.4 × 1010; P = 0.006) higher than in LGA milk, but there was no significant difference at 11 d. There were no significant effects of GA or postnatal age in the milk of preterm-delivering mothers for other protease activities, predicted activities, or concentrations.
Discussion
Our study showed that numerous proteases (kallikrein, carboxypeptidase, plasmin, thrombin, elastase, and cytosol aminopeptidase) were present and active in the milk of mothers who delivered prematurely. Cathepsin D was present but not active. We showed that most protease activities and concentrations and antiprotease concentrations were not substantially altered by the degree of prematurity or postnatal age in these milks. Although to our knowledge only procathepsin D and elastase have previously been measured with the use of immunodetection in human milk (15, 16), many proteases have been measured with the use of ELISA in bovine milk, including plasmin (17), cathepsin D (19), and elastase (16). Only plasmin activity has to our knowledge hbeen measured quantitatively in human milk (12, 13); elastase activity has been identified in human milk but not quantified (14). Both plasmin (17) and elastase (18) activities have been measured in bovine milk. The plasmin activities measured in this study were in the same range as those found previously in the milk of mothers who delivered at term (12). Plasmin activities were also measured in pre- and term milk in another study (13); however, the data were provided in units per milliliter without a conversion factor to enable a comparison with our study. Because the inactive zymogen (plasminogen), the active form (plasmin), and plasmin activators (urokinase- and tissue-type plasminogen activators) and inhibitors have been found in human milk (24), we plan to examine the complete plasminogen system in human milk in the future.
Among the proteases, kallikrein was the most abundant and active in the milk of mothers who delivered prematurely. This finding was surprising because plasmin, among human milk proteases, has received most of the research attention. The focus on plasmin likely derives from the fact that plasmin-induced proteolysis is one of the most important contributors to the quality of milk and dairy products, especially cheese, because plasmin activity generates flavor and texture development during ripening (33). Plasmin has also been an important concern because milk plasmin activity increases during mastitis (34), leading to increased protein degradation and poor product quality. No study, to our knowledge, has investigated the biological and technological effects of kallikrein in milk. Indeed, this Ser protease, which is abundant in the blood, was identified in human milk by proteomics (9), but its physiologic role in milk remains unknown to our knowledge. In the blood, kallikrein plays an important role in the activation of blood coagulation, fibrinolysis, and kinin formation (35). A transcriptomic analysis has revealed that bovine mammary epithelial cells can secrete kallikrein into milk (36). Therefore, kallikrein in milk may derive from either secretion by the mammary cells or from absorption into milk from the blood.
Kallikrein activity and concentration increased considerably in the EGA but not LGA milks across postnatal age. This difference could have derived from changes in the mammary cell’s prekallikrein and kallikrein production, the permeability of the mammary gland cells that allows blood proteins to enter the milk, or differences in protease inhibitor production. We did not detect any differences in antiproteases based on GA or postnatal age in this study. However, one additional antiprotease has been identified in milk that was not measured in this study. α2-Macroglobulin, which can inhibit kallikrein (37), has been identified in human milk with the use of proteomics (11) but was below the limit of detection for previous ELISA measurements in term human milk (14). Raw bovine milk is known to contain substantial amounts of this antiprotease (126.8 mg/L) (23). Because the ELISAs used in this study were more sensitive than those used previously (14), we believe that we will be able to determine the concentration of this inhibitor in future studies.
Our previous studies based on peptidomic data (comparing the cleavage-site specificity of known milk enzymes to the sites of cleavages for identified peptides) predicted that the following proteases, from highest to lowest activity, were active in preterm and term human milk: plasmin, carboxypeptidase B2, cathepsin D, elastase, and cytosol aminopeptidase (4, 6, 7). Plasmin, kallikrein and thrombin cleavages were considered in our in-house predictions to be indistinguishable [all cleavages C-terminal to P1 lysine or arginine (38) were assigned to plasmin, kallikrein, and thrombin]. Predicted proteases, from the highest to lowest, were plasmin, kallikrein, and thrombin; elastase; cathepsin D; cytosol aminopeptidase; and carboxypeptidase B2. An enzyme analysis was performed via substrate assays as well as the peptidomics-based protease prediction models to determine the extent to which they produce matching results. With the use of specific peptide substrate assays, we confirmed that human milk proteases predicted to be active based on peptidomics analysis, including kallikrein, carboxypeptidase B2, plasmin, thrombin, and elastase, are indeed active in human milk (Table 2). However, cathepsin D was not active in human milk. Cleavages assigned to cathepsin D were likely caused by other milk proteases. The difference between protease activity rankings for the prediction software and peptide substrate could have been caused by the poorer accounting for more subtle protease specificity in the peptidomics-based prediction model. The peptidomics-based prediction examines the sum of what has happened in the milk—the end result of protease activity within the mammary gland—whereas the enzyme substrate assays examine the instantaneous activity of milk proteases in the test buffer.
Predicted plasmin, kallikrein, and thrombin activity was higher in EGA than in LGA milk, especially at an early postnatal age. These findings partially match with those of Armaforte et al. (13), who showed that preterm milk has higher plasmin activity than term milk, and our previous peptidomics-based prediction that plasmin activity was greater in preterm milk than in term milk ≤29–41 d of postnatal age (4).
α1-Antitrypsin concentrations were within the range of those previously reported for human milk (Table 3). Similar to previous studies, α1-antitrypsin was the most abundant protease inhibitor in human milk. α1-Antitrypsin remained stable across postnatal age in preterm milks, which differed from Lindberg et al. (14), who reported a decrease across time (from 0.21 to 0.013 g/L from 1 to 14 d of postnatal age) in 90 term and 4 preterm milks combined. This difference may have been caused by the use of combined term and preterm mother’s milk samples.
α1-Antichymotrypsin detected in the preterm milk (Table 3) was 991% lower than previously detected in milk from full- and preterm mothers (14). The lower concentration measured in this study could have resulted from the fact that the preterm mothers in Lindberg et al. (14) delivered at a later mean GA (4 infants: 30, 31, 35, and 36 wk GA) than those used in our study (24–32 wk GA). Lindberg et al. (14) also observed a decrease in α1-antichymotrypsin over time postpartum, but we did not observe such a trend. This difference could have resulted from the higher number of preterm milks as well as the longer time postpartum (until 47 d of postnatal age) of our milk samples compared with the samples examined in Lindberg et al. (14).
Antithrombin III and α2-antiplasmin were detected in this study but not in full-term and LGA preterm milks examined in previous studies (14, 39). This difference is likely because of our use of a more sensitive ELISA. To our knowledge, no previous study has measured plasma Ser protease inhibitor concentration in human milk. In bovine colostrum, antithrombin III, α2-antiplasmin, and plasma Ser protease inhibitor concentrations were 552-, 34-, and 96-fold higher, respectively (23), than in this study of preterm human milk. Milk proteomic studies have also qualitatively identified the presence of α1-antitrypsin, α1-antichymotrypsin, α2-antiplasmin, antithrombin III, and the plasma Ser protease inhibitor in human milk (9, 10).
The mean pH of preterm milk in this study was lower than the pH of term milk (pH: 7.45–7.04) previously reported (33). The pH of preterm milk has to our knowledge not been reported previously.
A limitation of our study is its modest number of milk from preterm-delivering mothers, which reduced our ability to detect significant differences between EGA and LGA milks across postnatal age. Moreover, the small sample size did not allow us to examine the effects of certain health conditions, such as gestational diabetes and obesity (present in some of the mothers), that could explain some of the variations in the results. Another limitation of the study is that milk samples were frozen and then thawed before measurement, which can cause the lysis of the somatic cells and the release of their cytosolic proteases. Although we included only mothers with no signs of mastitis, there was likely some variation in somatic cell count that could have affected the overall lysosomal protease measurements. Therefore, we plan to examine how these protease activity levels change with and without freezing and how this varies with somatic cell count in a future study.
Although cathepsin B has been identified in bovine milk (40), no previous work to our knowledge has identified procathepsin B or cathepsin B in human milk (including proteomic analyses). Because this protease has not been thoroughly examined in human milk, we plan to research it in the future.
The major finding from this study was that carboxypeptidase B2, kallikrein, plasmin, elastase, thrombin, and cytosol aminopeptidase were active in the milk of preterm-delivering mothers. Most milk protease activities and concentrations and antiprotease concentrations were not affected by either GA or postnatal age. However, the concentration and activity of kallikrein, the most abundant and active protease in preterm milk, increased in EGA milk samples across postnatal age while remaining stable in LGA milk samples. Future work will need to examine the extent to which human milk proteases continue to act within the infant stomach. Because preterm infants produce less gastric acid (41, 42), gastric pepsin (43, 44), and intestinal proteases than term infants (45), they likely have a lower digestive capacity than term infants. Therefore, the proteolytic actions of milk enzymes could be particularly important for their ability to break down and utilize breast milk proteins. Determining the difference in milk proteases between milk from mothers delivering at term and preterm and the contribution to these enzymes to infant digestion can help guide improved protein nutrition for preterm infants.
Acknowledgments
We thank Joseph Maurer for assistance with statistical analyses and Cora J Dillard for editing the manuscript. The authors’ responsibilities were as follows—VD-M: conducted the research, analyzed the data, and performed the statistical analysis; SDN: conducted the peptidomic analysis; MAU and RB: provided the milk samples; VD-M and DCD: designed the study, wrote the manuscript, and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: AMC, 7-amino-4-methylcoumarin; EGA, early gestational age; GA, gestational age; LGA, late gestational age; pNA, p-nitroaniline.
References
- 1.Clare DA, Swaisgood HE. Bioactive milk peptides: a prospectus. J Dairy Sci 2000;83:1187–95. [DOI] [PubMed] [Google Scholar]
- 2.Dallas DC, Guerrero A, Khaldi N, Castillo PA, Martin WF, Smilowitz JT, Bevins CL, Barile D, German JB, Lebrilla CB. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J Proteome Res 2013;12:2295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallas DC, Smink CJ, Robinson RC, Tian T, Guerrero A, Parker EA, Smilowitz JT, Hettinga KA, Underwood MA, Lebrilla CB, et al. . Endogenous human milk peptide release is greater after preterm birth than term birth. J Nutr 2015;145:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dallas DC, Guerrero A, Khaldi N, Borghese R, Bhandari A, Underwood MA, Lebrilla CB, German JB, Barile D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J Nutr 2014;144:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrero A, Dallas DC, Contreras S, Chee S, Parker EA, Sun X, Dimapasoc L, Barile D, German JB, Lebrilla CB. Mechanistic peptidomics: factors that dictate specificity in the formation of endogenous peptides in human milk. Mol Cell Proteomics 2014;13:3343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khaldi N, Vijayakumar V, Dallas DC, Guerrero A, Wickramasinghe S, Smilowitz JT, Medrano JF, Lebrilla CB, Shields DC, German JB. Predicting the important enzymes in human breast milk digestion. J Agric Food Chem 2014;62:7225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holton TA, Vijayakumar V, Dallas DC, Guerrero A, Borghese RA, Lebrilla CB, German JB, Barile D, Underwood MA, Shields DC, et al. . Following the digestion of milk proteins from mother to baby. J Proteome Res 2014;13:5777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallas DC, Murray NM, Gan J. Proteolytic systems in milk: perspectives on the evolutionary function within the mammary gland and the infant. J Mammary Gland Biol Neoplasia 2015;20:133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer DJ, Kelly VC, Smit A-M, Kuy S, Knight CG, Cooper GJ. Human colostrum: identification of minor proteins in the aqueous phase by proteomics. Proteomics 2006;6:2208–16. [DOI] [PubMed] [Google Scholar]
- 10.Picariello G, Ferranti P, Mamone G, Klouckova I, Mechref Y, Novotny MV, Addeo F. Gel-free shotgun proteomic analysis of human milk. J Chromatogr A 2012;1227:219–33. [DOI] [PubMed] [Google Scholar]
- 11.Molinari CE, Casadio YS, Hartmann BT, Livk A, Bringans S, Arthur PG, Hartmann PE. Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res 2012;11:1696–714. [DOI] [PubMed] [Google Scholar]
- 12.Korycha-Dahl M, Dumas BR, Chene N, Martal J. Plasmin activity in milk. J Dairy Sci 1983;66:704–11. [Google Scholar]
- 13.Armaforte E, Curran E, Huppertz T, Ryan CA, Caboni MF, O’Connor PM, Ross RP, Hirtz C, Sommerer N, Chevalier F. Proteins and proteolysis in pre-term and term human milk and possible implications for infant formulae. Int Dairy J 2010;20:715–23. [Google Scholar]
- 14.Lindberg T, Ohlsson K, Westrom B. Protease inhibitors and their relation to protease activity in human milk. Pediatr Res 1982;16:479–83. [DOI] [PubMed] [Google Scholar]
- 15.Vĕtvicka V, Vagner J, Baudys M, Tang J, Foundling S, Fusek M. Human breast milk contains procathepsin—detection by specific antibodies. Biochem Mol Biol Int 1993;30:921–8. [PubMed] [Google Scholar]
- 16.Borulf S, Lindberg T, Mansson M. Immunoreactive anionic trypsin and anionic elastase in human milk. Acta Paediatr Scand 1987;76:11–5. [DOI] [PubMed] [Google Scholar]
- 17.Politis I, Lachance E, Block E, Turner J. Plasmin and plasminogen in bovine milk: a relationship with involution? J Dairy Sci 1989;72:900–6. [DOI] [PubMed] [Google Scholar]
- 18.Considine T, Healy A, Kelly A, McSweeney P. Proteolytic specificity of elastase on bovine α s1-casein. Food Chem 2000;69:19–26. [Google Scholar]
- 19.Larsen LB, Benfeldt C, Rasmussen LK, Petersen TE. Bovine milk procathepsin D and cathepsin D: coagulation and milk protein degradation. J Dairy Res 1996;63:119–30. [DOI] [PubMed] [Google Scholar]
- 20.Heegaard CW, Rasmussen LK, Andreasen PA. The plasminogen activation system in bovine milk: differential localization of tissue-type plasminogen activator and urokinase in milk fractions is caused by binding to casein and urokinase receptor. Biochim Biophys Acta 1994;1222:45–55. [DOI] [PubMed] [Google Scholar]
- 21.de Rham O, Andrews AT. The roles of native milk proteinase and its zymogen during proteolysis in normal bovine milk. J Dairy Res 1982;49:577–85. [DOI] [PubMed] [Google Scholar]
- 22.Weber B, Nielsen S. Isolation and partial characterization of a native serine-type protease inhibitor from bovine milk. J Dairy Sci 1991;74:764–71. [DOI] [PubMed] [Google Scholar]
- 23.Christensen S, Wiegers T, Hermansen J, Sottrup-Jensen L. Plasma-derived protease inhibitors in bovine milk. Int Dairy J 1995;5:439–49. [Google Scholar]
- 24.Heegaard CW, Larsen LB, Rasmussen LK, Højberg K-E, Petersen TE, Andreasen PA. Plasminogen activation system in human milk. J Pediatr Gastroenterol Nutr 1997;25:159–66. [DOI] [PubMed] [Google Scholar]
- 25.Lu DD, Suzanne Nielsen S. Isolation and characterization of native bovine milk plasminogen activators. J Dairy Sci 1993;76:3369–83. [DOI] [PubMed] [Google Scholar]
- 26.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 2014;42:D503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JL, Backes BJ, Leonetti F, Mahrus S, Ellman JA, Craik CS. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc Natl Acad Sci USA 2000;97:7754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato H, Adachi N, Ohno Y, Iwanaga S, Takada K, Sakakibara S. New fluorogenic peptide substrates for plasmin. J Biochem 1980;88:183–90. [PubMed] [Google Scholar]
- 29.Monti JC, Mermoud AF, Jollès P. Trypsin in human milk. Experientia 1986;42:39–41. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda Y, Kageyama T, Akamine A, Shibata M, Kominami E, Uchiyama Y, Yamamoto K. Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J Biochem 1999;125:1137–43. [DOI] [PubMed] [Google Scholar]
- 31.Gallimore MJ, Friberger P. Simple chromogenic peptide substrate assays for determining prekallikrein, kallikrein inhibition and kallikrein “like” activity in human plasma. Thromb Res 1982;25:293–8. [PubMed] [Google Scholar]
- 32.Dallas DC, Citerne F, Tian T, Silva VLM, Kalanetra KM, Frese SA, Robinson RC, Mills DA, Barile D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem 2016;197:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ismail B, Nielsen SS. Invited review: plasmin protease in milk: current knowledge and relevance to dairy industry. J Dairy Sci 2010;93:4999–5009. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Lacasse P. Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci 2008;86(13 Suppl):57–65. [DOI] [PubMed] [Google Scholar]
- 35.Schapira M, Scott CF, Colman RW. Contribution of plasma protease inhibitors to the inactivation of kallikrein in plasma. J Clin Invest 1982;69:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wickramasinghe S, Rincon G, Islas-Trejo A, Medrano JF. Transcriptional profiling of bovine milk using RNA sequencing. BMC Genomics 2012;13:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem 1983;52:655–709. [DOI] [PubMed] [Google Scholar]
- 38.Magklara A, Mellati AA, Wasney GA, Little SP, Sotiropoulou G, Becker GW, Diamandis EP. Characterization of the enzymatic activity of human kallikrein 6: autoactivation, substrate specificity, and regulation by inhibitors. Biochem Biophys Res Commun 2003;307:948–55. [DOI] [PubMed] [Google Scholar]
- 39.Urueña C, Telleria JJ, Blanco-Quiros A, Arranz E, Gomez-Carrasco JA. Alpha-1 antichymotrypsin levels are actively increased in normal colostrum. J Pediatr Gastroenterol Nutr 1998;26:376–9. [DOI] [PubMed] [Google Scholar]
- 40.Magboul AA, Larsen LB, McSweeney PL, Kelly AL. Cysteine protease activity in bovine milk. Int Dairy J 2001;11:865–72. [Google Scholar]
- 41.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev 1993;35:215–20. [DOI] [PubMed] [Google Scholar]
- 42.Hyman PE, Clarke DD, Everett SL, Sonne B, Stewart D, Harada T, Walsh JH, Taylor IL. Gastric acid secretory function in preterm infants. J Pediatr 1985;106:467–71. [DOI] [PubMed] [Google Scholar]
- 43.Henderson TR, Hamosh M, Armand M, Mehta NR, Hamosh P. Gastric proteolysis in preterm infants fed mother’s milk or formula. In: Newburg DS, editor. Bioactive components of human milk. New York: Springer; 2001. p. 403–8. [DOI] [PubMed] [Google Scholar]
- 44.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res 1996;40:429–37. [DOI] [PubMed] [Google Scholar]
- 45.Lindberg T, Engberg S, Jakobsson I, Lonnerdal B. Digestion of proteins in human milk, human milk fortifier, and preterm formula in infant rhesus monkeys. J Pediatr Gastroenterol Nutr 1997;24:537–43. [DOI] [PubMed] [Google Scholar]