Abstract
Background: The link between longitudinal cognitive change and polymorphisms in the vitamin D receptor (VDR) and MEGALIN [or LDL receptor–related protein 2 (LRP2)] genes remains unclear, particularly among African-American (AA) adults.
Objectives: We aimed to evaluate associations of single nucleotide polymorphisms (SNPs) for VDR [rs11568820 (Cdx-2:T/C), rs1544410 (BsmI:G/A), rs7975232 (ApaI:A/C), rs731236 (TaqI:G/A)] and LRP2 [rs3755166:G/A,rs2075252:C/T, rs2228171:C/T] genes with longitudinal cognitive performance change in various domains of cognition.
Methods: Data from 1024 AA urban adult participants in the Healthy Aging in Neighborhoods of Diversity Across the Life Span (Baltimore, Maryland) with complete genetic data were used, of whom 660–797 had complete data on 9 cognitive test scores at baseline and/or the first follow-up examination and complete covariate data (∼52% female; mean age: ∼52 y; mean years of education: 12.6 y). Time between examination visits 1 (2004–2009) and 2 (2009–2013) ranged from <1 y to ∼8 y, with a mean ± SD of 4.64 ± 0.93 y. Latent class and haplotype analyses were conducted by creating gene polymorphism groups that were related to longitudinal annual rate of cognitive change predicted from mixed-effects regression models.
Results: Among key findings, the rs3755166:G/A MEGALIN SNP was associated with faster decline on the Mini-Mental State Examination overall (β = −0.002, P = 0.018) and among women. VDR2 (BsmI/ApaI/TaqI: G−/A−/A−) SNP latent class [SNPLC; compared with VDR1 (ApaI: “AA”)] was linked to faster decline on the Verbal Fluency Test, Categorical, in women, among whom the MEGALIN2 (rs2228171: “TT”) SNPLC (compared with MEGALIN1:rs2228171: “CC”) was also associated with a faster decline on the Trailmaking Test, Part B (Trails B), but with a slower decline on the Digit Span Backward (DS-B). Moreover, among men, the VDR1 SNP haplotype (SNPHAP; GCA:baT) was associated with a slower decline on the Trails B, whereas the MEGALIN1 SNPHAP (GCC) was associated with a faster decline on the DS-B, reflected as a faster decline on cognitive domain 2 (“visual/working memory”).
Conclusion: VDR and MEGALIN gene variations can alter age-related cognitive trajectories differentially between men and women among AA urban adults, specifically in global mental status and domains of verbal fluency, visual/working memory, and executive function.
Keywords: VDR, MEGALIN, single nucleotide polymorphism, cognitive change, aging
Introduction
Vitamin D is a hormone that maintains and stabilizes intracellular signaling pathways involved in memory and cognitive function (1, 2). 25-Hydroxyvitamin D deficiency (≤20 compared with >20 ng/mL) may double the risk of incident Alzheimer disease (AD)7 and age-related cognitive decline (3–5). We and others have shown that genetic polymorphisms in the vitamin D receptor (VDR) and those in its endocytic binding protein MEGALIN were associated with age-related cognitive decline, including AD and Parkinson disease (6–12). VDR is strongly expressed in neurons of the human cortex and hippocampus, which are key areas for cognition (13). Dysfunctional VDR−/− mice have anxiety-like behavior (14, 15) but no other features of AD such as memory deficits (16). In contrast, mice lacking MEGALIN, also known as LDL receptor–related protein 2 (LRP2), which is expressed in multiple epithelial cells including those of the choroid plexus (i.e., blood-brain barrier) and mediates vitamin D transport (10, 17), develop increased anxiety and impaired learning ability and memory recognition along with neuronal degeneration, similar to symptoms described in AD (18). MEGALIN also binds apoE (19), a protein involved in the redistribution of cholesterol for nerve repair (20). In fact, the APOE genotype is associated with cognitive impairment, decline, and dementia, particularly in AD (21, 22), as well as a number of neurobiological factors implicated in dementia: β-amyloid deposition, tangle formation, oxidative stress, lipid homeostasis dysregulation, synaptic plasticity loss, and cholinergic dysfunction (23). Importantly, 1,25-dihydroxycholecalciferol, the active form of vitamin D, increases VDR (24–26) and LRP2 expression in the choroid plexus and directly participates in the clearance of neurotoxic β-amyloids (19, 27–30), which are involved in the pathogenesis of AD (31). Few current studies thus far have examined the relation between VDR and MEGALIN gene polymorphisms and incident AD (6, 10, 11).
This study will further test the associations of VDR and MEGALIN single nucleotide polymorphisms (SNPs), SNP latent classes (SNPLCs), and SNP haplotypes (SNPHAPs) with longitudinal changes in cognitive function with the use of a large long-term study in African-American (AA) urban adults.
Methods
Database.
Initiated in 2004, the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study is a prospective cohort study that used area probability sampling to recruit a representative sample of AAs and whites (30–64 y old) living in Baltimore, Maryland (32). Written informed consent was obtained from participants provided with protocol booklets and a video explaining the study procedures, including future re-contacts. The study protocol was reviewed and approved by the National Institute on Environmental Health Sciences Institutional Review Board of the NIH. This study analyzes longitudinal HANDLS data from baseline and first follow-up examinations among a sample of AAs with complete genetic and cognitive data, among others. Time between examination visits 1 (wave 1: 2004–2009) and 2 [also known as wave 3: 2009–2013 (33)] ranged from <1 to ∼8 y, with a mean of 4.64 ± 0.93 y.
Study participants.
A total of 3720 participants were recruited (mean ± SD age: 48.3 ± 9.4 y; 45.3% men; 59.1% AA and 40.9% white). Genetic data were available for 1024 AA participants of 2198 included in the original sample. Cognitive testing was done at waves 1 and 3 for several tests. Nine test scores previously selected in a previous analysis of data from the Baltimore Longitudinal Study of Aging were also selected in our current study in an attempt to replicate findings as closely as possible (6). Complete data on those tests at either visit among AAs ranged from n = 1634 for California Verbal Learning Test (CVLT)–List A and Delayed Free Recall (DFR; n′ = 2588 observations, k = 1.6 visits/person) to n = 1738 for the Benton Visual Retention Test (BVRT; n′ = 2918 observations, k = 1.7 visits/person). Mixed-effects regression models for predicting the annual rate of cognitive change assumed data to be missing at random (34) and used data on cognitive test scores available at either visit. The final sample size ranged from n = 660 for CVLT-DFR to n = 797 for the Verbal Fluency Test, Categorical (VFT-C), as shown in Supplemental Methods 1. As discussed in further detail in the “Statistical analysis” section, possible sample selectivity was corrected by using a 2-stage Heckman selection approach (35).
Cognitive assessment.
Cognitive assessment included 6 tests with 9 test scores covering 7 domains (mental status, attention, learning and memory, executive function, visuo-spatial and visuo-construction ability, psychomotor speed, language and verbal): the Mini-Mental State Examination (MMSE; mental status); the CVLT immediate (List A) and DFR (learning and memory, language and verbal domains); Digit Span Forward and Backward tests (DS-F and DS-B; attention and working memory); the BVRT (figural memory and visuo-constructional abilities); the VFT-C (semantic verbal fluency); and the Trailmaking Test, Parts A and B (Trails A and B; attention and executive functioning). It is worth noting that the BVRT and Trails A and B were coded in the direction of a higher score → poorer performance (Supplemental Methods 2). Linear mixed models with quadratic age terms were used for estimating cognitive scores at specific ages, as detailed in a previous study (6), to estimate the slope for annual cognitive change at that particular age. The latter, termed the longitudinal annual rate of cognitive change (LARCC), which is the main outcome of interest, can be interpreted as the annual rate of change in cognitive scores between ages 50 y and the mean age at follow-up per individual and cognitive test. The LARCC for each cognitive test score was entered into a factor analytic model as a measured variable (36), with factors extracted on the basis of common variance, factor loadings, and residual variance. The common factor model is shown below:
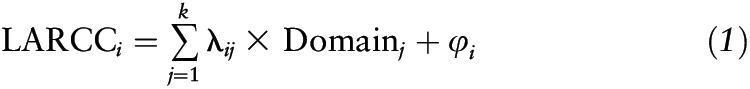 |
where LARCCi is the standardized z score for each cognitive test LARCC, λij is the factor loading for each LARCC and each factor, Domainj is the standardized z score for each factor j, and φi is the residual error. By using an eigenvalue >1 rule, 2 factors were extracted and rotated with the use of varimax orthogonal rotation. Those 2 factors were interpreted and the 2 underlying cognitive domains were labeled based on significant loadings, with a criterion of 0.40. Domains were labeled as follows: Domain 1 (“Verbal memory and fluency”) and Domain 2 (“Visual/working memory”). With the exception of Trails B, all LARCCi factor loadings were significant for 1 of the 2 domains, creating a relatively simple structure that is easy to label and interpret (see Supplemental Methods 1).
All of the participants were judged to be capable of informed consent and were probed for understanding of the protocol. Although no formal dementia diagnoses were made, all participants were administered mental status tests, which they completed successfully. In every case, low mental status performance was due to poor literacy skills with no other signs of dementia.
VDR and MEGALIN (LRP2) SNPs, SNPLCs, and SNPHAPs.
HANDLS participants were genotyped by using the Illumina 1 M genotyping arrays (Illumina Inc., San Diego, California). A total of 1024 individuals were successfully genotyped and passed genotype quality-control criteria. Details are provided in Supplemental Methods 3.
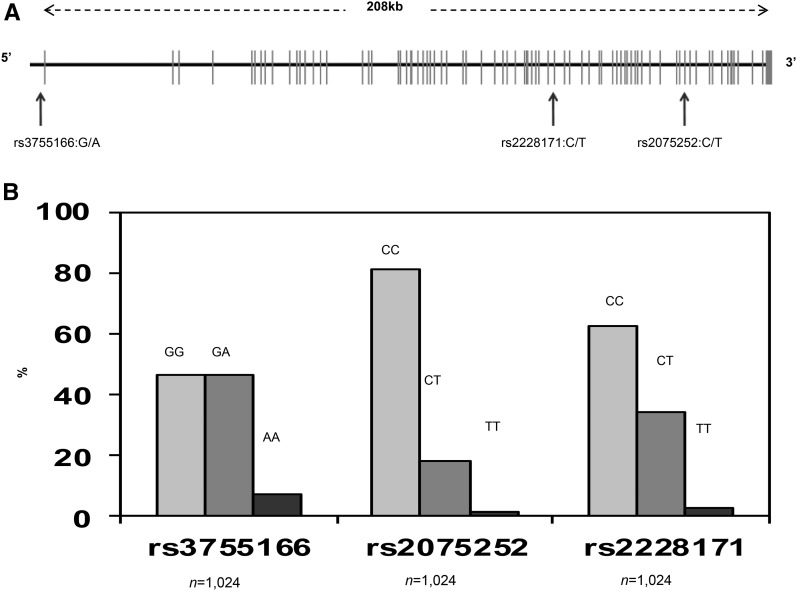
SNP selection was based on previously published genomewide association studies relating cognitive function, decline, or dementia to VDR (8, 37) and MEGALIN (10, 11) gene polymorphisms and as an attempt to replicate a previous study in whites participating in the Baltimore Longitudinal Study of Aging (6). Most of those selected SNPs were available in our database, with few exceptions (e.g., VDR SNP rs10735810, FokI: G/A). Four VDR SNPs [rs11568820 (Cdx-2: T/C), rs1544410 (BsmI:G/A), rs7975232 (ApaI: A/C), and rs731236 (TaqI: G/A)] and 3 MEGALIN SNPs (rs3755166: G/A, rs2075252: C/T, and rs2228171: C/T) were chosen. Figure 1 lists selected SNPs and shows their gene locations and frequency distributions.
FIGURE 1.
(A) Schematic representation of the VDR gene. The SNP and gene coordinates are based on NCBI build 36 (hg18, March 2006) with the use of RefSeq gene prediction. The VDR gene on chromosome 12 was composed of ≤11 exons spanning ∼63 kb. (B) Genotype frequencies of selected VDR SNPs of the original sample with complete genetic data (n = 1024). hg, human genome; NCBI, National Center for Biotechnology Information; RefSeq, Reference Sequence; SNP, single nucleotide polymorphism; VDR, vitamin D receptor gene.
VDR and MEGALIN SNPLCs were obtained by using latent class analysis (PROC LCA in SAS version 9.3) (38, 39), in which sex and first-visit age were introduced as potential covariates and each selected SNP per gene was entered into that model (1 gene/model) in additive mode of inheritance (i.e., 0/1/2). Model fit was determined on the basis of Akaike Information Criterion and Bayesian Information Criterion, which led to deciding the appropriate number of latent classes. The method is detailed in our previous study in whites who participated in the Baltimore Longitudinal Study of Aging (6).
SNPHAPs were considered as main predictors in our analysis for each of the 2 genes. For the VDR gene, the BsmI, ApaI, and TaqI SNPs were combined together to form SNPHAPs and their proportions in the population were found to be similar to ≥1 previous study (8). Three SNPHAPs were found in this population with the SNP combinations being either 1 of the 3—VDR1: GCA (baT), VDR2: AAG (BAt), or VDR3: GAA (bAT)—for 1 or 2 alleles. Participants were coded as 0 = having no VDRx haplotype, 1 = having 1 allele carrying the VDRx haplotype, or 2 = having 2 alleles carrying the VDRx haplotype. This approach was also applied to the 3 MEGALIN SNPs and 8 haplotypes were found. However, only 3 were considered in the main analysis because their proportion in the population (with 1 or 2 copies) was >10%. Those findings in terms of the most common SNPHAPs are comparable to our previous study in whites participating in the Baltimore Longitudinal Study of Aging (6). Detailed descriptions of SNPLCs and SNPHAPs are found in Table 1.
TABLE 1.
Findings from latent class analysis and haplotype analysis: definitions and distributions of SNPLCs and SNPHAPs for the selected VDR and LRP2 (MEGALIN) SNPs1
| SNPHAPs |
SNPLCs |
|||
| Definitions | Distributions, % | Definitions | Distributions, % | |
| VDR | (BsmI/ApaI/TaqI) | |||
| Overall | VDR1: GCA: baT | 36.5 | VDR1: ApaI: AA | 12.1 |
| VDR2: AAG: BAt | 19.1 | VDR2: BsmI/ApaI/TaqI: G−/A−/A− | 49.0 | |
| VDR3: GAA: bAT | 25.2 | VDR3: BsmI/TaqI: GG/AA | 38.9 | |
| VDR4: AAA: BAT | 10.1 | |||
| Allelic copies | ||||
| VDR1 | VDR1: GCA: baT | |||
| 0 | 68.0 | |||
| 1 | 18.5 | |||
| 2 | 13.6 | |||
| VDR2 | VDR2: AAG: BAt | |||
| 0 | 78.5 | |||
| 1 | 17.3 | |||
| 2 | 4.2 | |||
| VDR3 | VDR3: GAA: bAT | |||
| 0 | 27.3 | |||
| 1 | 65.8 | |||
| 2 | 6.8 | |||
| VDR4 | VDR4: AAA: BAT | |||
| 0 | 90.2 | |||
| 1 | 8.8 | |||
| 2 | 1.0 | |||
| MEGALIN | (rs3755166, rs2075252, rs2228171) | (rs2228171) | ||
| Overall | ||||
| MEGALIN1:GCC | 53.3 | MEGALIN1:CC | 62.6 | |
| MEGALIN2:ACC | 24.3 | MEGALIN2:TT | 2.1 | |
| MEGALIN3:CT | 35.3 | |||
| Allelic copies | ||||
| MEGALIN1 | MEGALIN1:GCC | |||
| 0 | 10.1 | |||
| 1 | 63.5 | |||
| 2 | 26.5 | |||
| MEGALIN2 | MEGALIN1:GCC | |||
| 0 | 64.9 | |||
| 1 | 30.8 | |||
| 2 | 4.3 | |||
n = 1024. Most eligible participants (>99%) had well-defined SNPLCs that could be summarized by BsmI, ApaI, and TaqI SNP combinations. SNPHAPs were defined on the basis of 3 VDR SNP combinations—BsmI, ApaI, and Taq—and were expressed as dosages (0 = none, 1 = 1 copy, 2 = 2 copies) in the main analysis. Most eligible participants (>99%) had well-defined SNPLCs that could be summarized by the genotype of rs2228171. SNPHAPs were defined on the basis of all 3 MEGALIN SNP combinations—rs3755166, rs2075252, and rs2228171—and were expressed as dosages (0 = none, 1 = 1 copy, 2 = 2 copies) in the main analysis. LRP2, LDL receptor–related protein 2; SNP, single nucleotide polymorphism; SNPHAP, single nucleotide polymorphism haplotype; SNPLC, single nucleotide polymorphism latent class; VDR, vitamin D receptor gene.
Covariates.
Three sets of covariates were assessed as potential confounders, including the following: 1) sociodemographic factors, namely baseline age, sex, educational attainment (years of schooling), and 1 lifestyle-related factor, namely smoking status (never, former, or current smoker); 2) self-reported history of type 2 diabetes, hypertension, cardiovascular disease (stroke, congestive heart failure, nonfatal myocardial infarction, or atrial fibrillation), and dyslipidemia at first visit; and 3) measured first-visit BMI (in kg/m2). Right and left sitting systolic and diastolic blood pressure levels were averaged. Blood pressure was measured noninvasively by using brachial artery auscultation with an aneroid manometer, a stethoscope, and an inflatable cuff. After an overnight fast (8–12 h) and consent, blood was drawn and collected from an antecubital vein. Serum total cholesterol, HDL cholesterol, and glucose were assessed by using a spectrophotometer (Olympus 5400). First-visit blood pressure (systolic and diastolic in millimeters of mercury), plasma total and HDL cholesterol, and fasting blood glucose (in milligrams per deciliter) were only analyzed in relation to the availability of genetic data for descriptive purposes, as was done in our previous study (6).
Statistical analysis.
For each SNP that was included in our analyses, Hardy-Weinberg equilibrium was examined by using an exact test, and pairwise linkage disequilibrium was calculated and visualized by using the Haploview version 4.2 package (40, 41) (Supplemental Figures 1 and 2). To describe study participant characteristics and compare them by genetic data availability, 1-factor ANOVA, t test, and chi-square test were used.
Furthermore, ordinary least square (OLS) models were carried out to examine the association of VDR and MEGALIN SNPs, SNPLCs, and SNPHAPs as predictors of LARCC for each cognitive test, controlling for potential confounding variables including baseline age, sex, education, baseline smoking status, self-reported comorbid conditions, and measured BMI. SNPs (wild-type with variant v) were examined in terms of genotypes, comparing the 2 variant genotypes (wv, vv) with wild-type genotype (ww) and in terms of dosage of the variant allele (v) by using an additive mode of inheritance model. P-trend was also computed when testing associations between haplotype dosage (0, 1, and 2 copies) and cognitive outcomes.
To account for selection bias in OLS models (due to the nonrandom selection of participants with genetic data from the target population), a 2-stage Heckman selection model was constructed (35) by using a probit model to obtain an inverse mills ratio at the first stage (derived from the predicted probability of being selected, conditional on covariates in the probit model), as was done in an earlier study (23). The inverse mills ratio was included in the main OLS models at a second stage to adjust for selection bias. Stratification was done, and effect modification was tested (by adding interaction terms) by sex for the analysis when SNP, SNPLC, and SNPHAP were the main predictors. In fact, sex differences in the association between the MEGALIN gene polymorphism and cognitive outcomes were hypothesized a priori, as discussed later (42–44).
A type I error of 0.05 was considered for all analyses, and P values between 0.05 and 0.10 were considered to be borderline significant for main effects, whereas a P value <0.10 was considered significant for interaction terms (45), before correction for multiple testing. Correction for multiple testing was done by using a familywise Bonferroni procedure whereby a family was defined by a cognitive test or a cognitive domain, assuming that they are independent content-wise, although not necessarily in their degree of correlation (46). Within each cognitive test, there were generally 2 test scores to take into account for correction. This was the case for CVLT-DFR and CVLT-List A, Trails A and Trails B, and DS-F and DS-B. For these cognitive tests, the significance criterion for P values and P values for trend was reduced to P = 0.05/2 = 0.025 (marginal significance: P = 0.10/2 = 0.05). In the case of MMSE (a measure of global cognition), BVRT, and VFT-C, no correction was needed, an approach taken in our previous study (6). All of the analyses (except for latent class analysis, which was conducted in SAS version 9.3) were performed by using Stata version 14.0 (47).
Results
Study sample characteristics.
Study sample characteristics (Table 2) are presented for participants with genetic data available and compared with eligible AA participants who were excluded due to unavailable genetic data, both of whom had complete cognitive score data at baseline and/or follow-up and other covariate data at baseline. MMSE LARCC was used as the criterion to describe sociodemographic, lifestyle, and health-related factors by genetic data availability, whereas cognitive test score–specific LARCCs were used otherwise, with sample sizes ranging from 648 to 797. Generally, participants from both groups had comparable distributions in terms of sociodemographic, lifestyle, and health-related characteristics, with the exception of a higher mean systolic blood pressure among those included. Moreover, a significantly faster decline on the BVRT and DS-B tests was noted among those with complete genetic data as opposed to those who were excluded from the main analyses.
TABLE 2.
Study sample characteristics by availability of genetic data: HANDLS study1
| Genetic data available |
Genetic data not available |
||||
| n | Value | n | Value | P2 | |
| Female, % | 788 | 52.1 | 482 | 61.5 | 0.06 |
| Baseline age, y | 788 | 47.8 ± 0.573 | 482 | 46.8 ± 0.66 | 0.27 |
| Education at first visit, y | 788 | 12.60 ± 0.16 | 482 | 12.68 ± 0.23 | 0.78 |
| Smoking status at first visit, % | 788 | 482 | 0.68 | ||
| Never/former | 49.6 | 47.5 | |||
| Current | 50.4 | 52.5 | |||
| Type 2 diabetes at first visit, % | 788 | 14.3 | 482 | 13.9 | 0.89 |
| Hypertension at first visit, % | 788 | 42.3 | 482 | 41.2 | 0.82 |
| Cardiovascular disease at first visit,4 % | 788 | 14.8 | 482 | 9.1 | 0.05 |
| Dyslipidemia at first visit, % | 788 | 23.9 | 482 | 16.6 | 0.06 |
| BMI at first visit, kg/m2 | 788 | 29.6 ± 0.5 | 482 | 29.7 ± 0.6 | 0.90 |
| Systolic blood pressure, mm Hg | 768 | 122.1 ± 1.1 | 471 | 118.0 ± 1.2 | 0.011 |
| Diastolic blood pressure, mm Hg | 755 | 77.7 ± 0.8 | 461 | 76.1 ± 0.7 | 0.12 |
| Serum total cholesterol, mg/dL | 760 | 186.5 ± 3.0 | 425 | 180.3 ± 3.7 | 0.19 |
| Serum HDL cholesterol, mg/dL | 760 | 53.7 ± 1.3 | 424 | 55.9 ± 2.1 | 0.37 |
| Fasting plasma glucose, mg/dL | 760 | 104.2 ± 2.3 | 426 | 109.6 ± 7.9 | 0.51 |
| Predicted annual rate of cognitive change between age 50 y and mean age of follow-up5 | |||||
| MMSE | 788 | −0.040 ± 0.001 | 482 | −0.039 ± 0.002 | 0.62 |
| BVRT | 782 | +0.195 ± 0.003 | 468 | +0.185 ± 0.003 | 0.029 |
| CVLT-List A | 680 | −0.280 ± 0.001 | 392 | −0.278 ± 0.002 | 0.20 |
| CVLT-DFR | 662 | −0.128 ± 0.001 | 383 | −0.127 ± 0.001 | 0.44 |
| VFT-C | 797 | −0.056 ± 0.002 | 476 | −0.054 ± 0.002 | 0.63 |
| Trails A | 745 | +0.803 ± 0.071 | 460 | +0.769 ± 0.046 | 0.68 |
| Trails B | 745 | +4.480 ± 0.163 | 460 | +4.193 ± 0.192 | 0.25 |
| DS-F | 782 | −0.022 ± 0.001 | 470 | −0.021 ± 0.001 | 0.31 |
| DS-B | 775 | −0.022 ± 0.001 | 466 | −0.018 ± 0.001 | 0.016 |
| Cognitive domain 1 | 648 | −0.03 ± 0.07 | 376 | +0.09 ± 0.07 | 0.24 |
| Cognitive domain 2 | 648 | −0.07 ± 0.06 | 376 | −0.20 ± 0.05 | 0.11 |
n = 1024. Sociodemographic, lifestyle, and health-related factors are presented for participants with complete data on those variables as well as complete data on the MMSE LARCC. LARCC measures are presented for eligible participants with complete data on covariates entered into subsequent models as well as complete data on each of the cognitive test scores at either baseline or the follow-up wave. Unreliable data from each cognitive test score were excluded. BVRT, Benton Visual Retention Test; CVLT-DFR, California Verbal Learning Test, Delayed Free Recall; CVLT-List A, California Verbal Learning Test, List A; DS-B, Digit Span Backward; DS-F, Digit Span Forward; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; LARCC, longitudinal annual rate of cognitive change; MMSE, Mini-Mental State Examination; Trails A, Trailmaking Test, Part A; Trails B, Trailmaking Test, Part B; VFT-C, Verbal Fluency Test, Categorical.
P value for null hypothesis of no difference between those with and those without genetic data. Note that this analysis was conducted in African-American participants with complete baseline covariates, including baseline MMSE scores.
Mean ± SE (all such values).
Reported any of the following conditions at first visit: stroke, congestive heart failure, nonfatal myocardial infarction, or atrial fibrillation.
Cognitive scores were predicted at the mean age at follow-up before onset of dementia or for all time points by using a linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed-effects variables to allow for quadratic nonlinear change. The slope or annual rate of change was predicted from these models at the mean age at follow-up (i.e., between age 50 y and the individual mean age at follow-up for each cognitive test). By using factor analysis, 2 factor scores were estimated and were labeled as LARCC in the following domains: Domain 1 (“Verbal memory and fluency”) and Domain 2 (“Visual/working memory”) (see Supplemental Methods 2).
All of the examined SNPs were in Hardy-Weinberg equilibrium (P > 0.002). Variants within each VDR and MEGALIN (LRP2) gene were deemed in low linkage equilibrium (r2 < 0.30; Supplemental Figure 2). Genotypic frequencies suggested that 1 genotype in each SNP had a prevalence of >45% and thus was dominant compared with the other genotypes (Figure 1, Figure 2). Table 1 shows the percentage distributions of VDR and MEGALIN SNPLCs (determined by latent class analysis) and SNPHAPs (0, 1, or 2 copies). Note that SNPHAP distribution is nonmutually exclusive because it reflects allelic combinations for each individual. The description and labeling of the SNPLCs and SNPHAPs are presented in detail as well.
FIGURE 2.
(A) Schematic representation of the MEGALIN (LRP2) gene. The SNP and gene coordinates are based on NCBI build 36 (hg18, March 2006) with the use of RefSeq gene prediction. The MEGALIN gene on chromosome 2 has 79 exons and is ∼235 kb in size. (B) Genotype frequencies of selected MEGALIN SNPs of the original sample with complete genetic data (n = 1024). hg, human genome; LRP2, LDL receptor–related protein 2; NCBI, National Center for Biotechnology Information; RefSeq, Reference Sequence; SNP, single nucleotide polymorphism.
VDR SNPs and LARCCs.
Supplemental Table 1 presents findings from multiple OLS models examining the association between VDR SNPs (entered alternatively, models A–D) and LARCCs, stratifying by sex. After correction for multiple testing, TaqI:G/A was associated with a slower decline on a test of executive function, specifically visuo-motor scanning (Trails B) among men, an effect that differed significantly between sexes. Moreover, TaqI:G/A was also associated with a slower decline on cognitive domain 2 among women, which was mostly driven by a slower decline on both the DS-F and DS-B, which were previously described as tests of attention and working memory. The effect of TaqI:G/A on cognitive domain 2 also differed significantly between sexes (P-interaction < 0.05 for sex × SNP interaction).
MEGALIN SNPs and LARCCs: sex-stratified findings.
Similarly, in OLS models that included only MEGALIN SNPs (Table 3), significant associations were found between the rs3755166:G/A MEGALIN SNP and LARCC on MMSE, whereby an increasing dose of the A allele was associated with a faster decline in both sexes combined and among women (P = 0.024), an association deemed significant after correction for main effects of multiple testing (P < 0.025). Moreover, a decline on the DS-B (a test of working memory) overall and among men was faster with each T allele for the third MEGALIN SNP (rs2228171:C/T), an association deemed significant even after correction for multiple testing (P < 0.025).
TABLE 3.
MEGALIN SNP associations with predicted annual rates of cognitive change between age 50 y and the mean age at follow-up: multiple OLS regression analysis—HANDLS study1
| Predicted annual rate of cognitive change between age 50 y and mean age at follow-up2 |
|||
| n | β ± SE3 | P-trend | |
| MMSE | |||
| MEGALIN: rs3755166: G/A | 788 | −0.002 ± 0.001 | 0.0184 |
| Men | 349 | −0.001 ± 0.002 | 0.35 |
| Women | 439 | −0.002 ± 0.001 | 0.0244 |
| MEGALIN: rs2075252: C/T | 788 | +0.0005 ± 0.0014 | 0.74 |
| Men | 349 | +0.0001 ± 0.003 | 0.99 |
| Women | 439 | +0.0008 ± 0.016 | 0.56 |
| MEGALIN: rs2228171: C/T | 788 | +0.0003 ± 0.0011 | 0.76 |
| Men | 349 | −0.0001 ± 0.0020 | 0.97 |
| Women | 439 | −0.0006 ± 0.0129 | 0.63 |
| BVRT | |||
| MEGALIN: rs3755166: G/A | 782 | +0.000 ± 0.002 | 0.985 |
| Men | 350 | +0.004 ± 0.003 | 0.12 |
| Women | 432 | −0.003 ± 0.002 | 0.25 |
| MEGALIN: rs2075252: C/T | 782 | −0.0046 ± 0.0028 | 0.09 |
| Men | 350 | −0.0010 ± 0.0043 | 0.82 |
| Women | 432 | −0.007 ± 0.004 | 0.06 |
| MEGALIN: rs2228171: C/T | 782 | +0.002 ± 0.002 | 0.40 |
| Men | 350 | +0.0048 ± 0.0034 | 0.16 |
| Women | 432 | +0.001 ± 0.003 | 0.81 |
| CVLT-List A | |||
| MEGALIN: rs3755166: G/A | 680 | −0.0001 ± 0.0002 | 0.74 |
| Men | 295 | +0.0001 ± 0.0003 | 0.73 |
| Women | 385 | −0.0002 ± 0.0003 | 0.56 |
| MEGALIN: rs2075252: C/T | 680 | −0.0003 ± 0.004 | 0.33 |
| Men | 295 | +0.0003 ± 0.0005 | 0.62 |
| Women | 385 | −0.0007 ± 0.0005 | 0.12 |
| MEGALIN: rs2228171: C/T | 680 | −0.0000 ± 0.003 | 0.97 |
| Men | 295 | +0.0000 ± 0.0004 | 0.97 |
| Women | 385 | −0.0000 ± 0.0004 | 0.94 |
| CVLT-DFR | |||
| MEGALIN: rs3755166: G/A | 660 | +0.0001 ± 0.0004 | 0.73 |
| Men | 284 | −0.0004 ± 0.0010 | 0.51 |
| Women | 376 | +0.0003 ± 0.0005 | 0.56 |
| MEGALIN: rs2075252: C/T | 660 | +0.0008 ± 0.0006 | 0.20 |
| Men | 284 | +0.0004 ± 0.0009 | 0.65 |
| Women | 376 | +0.0011 ± 0.0009 | 0.21 |
| MEGALIN: rs2228171: C/T | 660 | +0.0001 ± 0.005 | 0.79 |
| Men | 284 | −0.0006 ± 0.008 | 0.44 |
| Women | 376 | +0.0007 ± 0.0007 | 0.32 |
| VFT-C | |||
| MEGALIN: rs3755166: G/A | 797 | +0.0004 ± 0.0114 | 0.74 |
| Men | 356 | −0.0002 ± 0.0020 | 0.89 |
| Women | 441 | +0.0011 ± 0.0014 | 0.45 |
| MEGALIN: rs2075252: C/T | 797 | +0.002 ± 0.002 | 0.25 |
| Men | 356 | +0.0024 ± 0.0031 | 0.42 |
| Women | 441 | +0.0019 ± 0.0023 | 0.43 |
| MEGALIN: rs2228171: C/T | 797 | +0.0009 ± 0.0019 | 0.55 |
| Men | 356 | +0.004 ± 0.002 | 0.14 |
| Women | 441 | −0.0012 ± 0.019 | 0.49 |
| Trails A | |||
| MEGALIN: rs3755166: G/A | 745 | −0.056 ± 0.067 | 0.41 |
| Men | 326 | +0.111 ± 0.160 | 0.49 |
| Women | 419 | −0.140 ± 0.097 | 0.15 |
| MEGALIN: rs2075252: C/T | 745 | −0.015 ± 0.112 | 0.89 |
| Men | 326 | −0.111 ± 0.160 | 0.49 |
| Women | 419 | +0.032 ± 0.155 | 0.83 |
| MEGALIN: rs2228171: C/T | 745 | −0.049 ± 0.088 | 0.58 |
| Men | 326 | +0.058 ± 0.125 | 0.65 |
| Women | 419 | +0.090 ± 0.125 | 0.47 |
| Trails B | |||
| MEGALIN: rs3755166: G/A | 745 | −0.012 ± 0.124 | 0.92 |
| Men | 326 | +0.209 ± 0.195 | 0.66 |
| Women | 419 | −0.142 ± 0.166 | 0.39 |
| MEGALIN: rs2075252: C/T | 745 | −0.141 ± 0.206 | 0.49 |
| Men | 326 | −0.144 ± 0.328 | 0.66 |
| Women | 419 | −0.159 ± 0.267 | 0.55 |
| MEGALIN: rs2228171: C/T | 745 | +0.155 ± 0.164 | 0.34 |
| Men | 326 | +0.065 ± 0.257 | 0.80 |
| Women | 419 | +0.296 ± 0.217 | 0.17 |
| DS-F | |||
| MEGALIN: rs3755166: G/A | 773 | −0.0002 ± 0.0005 | 0.66 |
| Men | 349 | +0.0001 ± 0.0008 | 0.86 |
| Women | 424 | −0.0002 ± 0.0007 | 0.73 |
| MEGALIN: rs2075252: C/T | 773 | −0.0001 ± 0.0008 | 0.905 |
| Men | 349 | +0.0013 ± 0.0013 | 0.33 |
| Women | 424 | −0.0012 ± 0.0011 | 0.28 |
| MEGALIN: rs2228171: C/T | 773 | +0.0006 ± 0.0007 | 0.40 |
| Men | 349 | +0.0010 ± 0.0010 | 0.34 |
| Women | 424 | +0.0005 ± 0.0009 | 0.56 |
| DS-B | |||
| MEGALIN: rs3755166: G/A | 775 | +0.0007 ± 0.0008 | 0.42 |
| Men | 351 | +0.0017 ± 0.0012 | 0.18 |
| Women | 424 | +0.0004 ± 0.0012 | 0.71 |
| MEGALIN: rs2075252: C/T | 775 | −0.0010 ± 0.0014 | 0.47 |
| Men | 351 | −0.0015 ± 0.0021 | 0.47 |
| Women | 424 | −0.0009 ± 0.0020 | 0.64 |
| MEGALIN: rs2228171: C/T | 775 | +0.003 ± 0.001 | 0.0204 |
| Men | 351 | +0.004 ± 0.002 | 0.0204 |
| Women | 424 | +0.0023 ± 0.0015 | 0.13 |
| Cognitive domain 1 | |||
| MEGALIN: rs3755166: G/A | 648 | +0.0071 ± 0.0176 | 0.45 |
| Men | 277 | −0.0212 ± 0.0474 | 0.45 |
| Women | 371 | +0.0260 ± 0.0234 | 0.27 |
| MEGALIN: rs2075252: C/T | 648 | +0.0368 ± 0.0293 | 0.21 |
| Men | 277 | +0.0666 ± 0.0474 | 0.16 |
| Women | 371 | +0.0179 ± 0.0383 | 0.64 |
| MEGALIN: rs2228171: C/T | 648 | +0.0092 ± 0.0232 | 0.69 |
| Men | 277 | −0.0103 ± 0.0378 | 0.79 |
| Women | 371 | +0.0216 ± 0.0299 | 0.47 |
| Cognitive domain 2 | |||
| MEGALIN: rs3755166: G/A | 648 | +0.0424 ± 0.0389 | 0.28 |
| Men | 277 | +0.0851 ± 0.0583 | 0.96 |
| Women | 371 | +0.0147 ± 0.053 | 0.78 |
| MEGALIN: rs2075252: C/T | 648 | −0.0710 ± 0.0649 | 0.27 |
| Men | 277 | +0.0050 ± 0.0990 | 0.96 |
| Women | 371 | −0.1323 ± 0.0873 | 0.13 |
| MEGALIN: rs2228171: C/T | 648 | +0.0866 ± 0.0513 | 0.09 |
| Men | 277 | +0.1136 ± 0.0791 | 0.15 |
| Women | 371 | +0.0836 ± 0.0681 | 0.22 |
n = 648–788. Note that each SNP is denoted by an rs number followed by the polymorphism in which one nucleotide is replaced by another (e.g., C/T or G/A). BVRT, Benton Visual Retention Test; CVLT-List A, California Verbal Learning Test, List A; CVLT-DFR, California Verbal Learning Test, Delayed Free Recall; DS-B, Digit Span Backward; DS-F, Digit Span Forward; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; MMSE, Mini-Mental State Examination; OLS, ordinary least square; SNP, single nucleotide polymorphism; Trails A, Trailmaking Test, Part A; Trails B, Trailmaking Test, Part B; VFT-C, Verbal Fluency Test, Categorical.
Cognitive scores were predicted at the mean age at follow-up before onset of dementia or for all time points by using a linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed-effects variables to allow for quadratic nonlinear change. The slope or annual rate of change was predicted from these models at the mean age at follow-up (i.e., between age 50 y and individual mean age at follow-up for each cognitive test). With the use of factor analysis, 2 factor scores were estimated and were labeled as the longitudinal annual rate of cognitive change in the following domains: Domain 1 (“Verbal memory and fluency”) and Domain 2 (“Visual/working memory”) (Supplemental Methods 1).
Based on multiple OLS regression models with the outcome being cognitive annual rate of change and main exposures being the 3 MEGALIN SNPs. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease, and BMI. The 10 principal components obtained from the genotype data with multidimensional scaling analysis (Supplemental Methods 3) were also added in a separate sensitivity analysis.
Significant main effects after family-wise Bonferroni correction: P < 0.05 for MMSE, BVRT, VFT-C, and cognitive domains and P < 0.025 for other cognitive tests.
P < 0.05 for the null hypothesis that sex × SNP interaction term = 0 in a model where the main effect of sex was added.
VDR and MEGALIN SNPLC associations with LARCC: sex-stratified findings.
In Table 4, we conducted OLS regression models whereby SNPLC predicted LARCC among men and women, separately. After correction for multiple testing, VDR2 (compared with VDR1) was linked to a faster rate of decline on the VFT-C in women only (P-interaction < 0.05 for sex × SNPLC interaction). Moreover, the MEGALIN2 SNPLC (compared with MEGALIN1) was associated with a faster rate of decline on Trails B among women (P-interaction < 0.05 for sex × SNPLC interaction), coupled with a slower decline on the DS-B among women as well. None of the other sex-specific associations retained their significance after correction for multiple testing.
TABLE 4.
VDR and MEGALIN SNPLC associations with predicted annual rates of cognitive change between age 50 y and the mean age at follow-up: multiple OLS regression analysis—HANDLS study1
| Men |
Women |
|||||
| n | β ± SE2 | P | n | β ± SE2 | P | |
| MMSE | 349 | 439 | ||||
| VDR2 vs. VDR1 | −0.0012 ± 0.003 | 0.68 | −0.002 ± 0.002 | 0.40 | ||
| VDR3 vs. VDR1 | −0.0001 ± 0.003 | 0.96 | −0.003 ± 0.002 | 0.16 | ||
| MEGALIN2 vs. MEGALIN1 | +0.005 ± 0.007 | 0.44 | −0.007 ± 0.004 | 0.12 | ||
| MEGALIN3 vs. MEGALIN1 | −0.000 ± 0.002 | 0.94 | +0.001 ± 0.001 | 0.47 | ||
| BVRT | 350 | 432 | ||||
| VDR2 vs. VDR1 | +0.005 ± 0.005 | 0.29 | +0.003 ± 0.004 | 0.50 | ||
| VDR3 vs. VDR1 | +0.001 ± 0.005 | 0.85 | +0.005 ± 0.005 | 0.31 | ||
| MEGALIN2 vs. MEGALIN1 | +0.001 ± 0.014 | 0.93 | +0.003 ± 0.010 | 0.73 | ||
| MEGALIN3 vs. MEGALIN1 | +0.003 ± 0.003 | 0.31 | −0.004 ± 0.003 | 0.16 | ||
| CVLT-List A | 295 | 385 | ||||
| VDR2 vs. VDR1 | −0.0006 ± 0.0006 | 0.28 | +0.0008 ± 0.0006 | 0.14 | ||
| VDR3 vs. VDR1 | −0.0007 ± 0.0006 | 0.29 | +0.0009 ± 0.0006 | 0.11 | ||
| MEGALIN2 vs. MEGALIN1 | −0.0015 ± 0.0015 | 0.32 | −0.0000 ± 0.0013 | 0.97 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0003 ± 0.0004 | 0.39 | −0.0004 ± 0.0004 | 0.23 | ||
| CVLT-DFR | 284 | 376 | ||||
| VDR2 vs. VDR1 | −0.0001 ± 0.0011 | 0.99 | −0.0006 ± 0.0010 | 0.56 | ||
| VDR3 vs. VDR1 | +0.0005 ± 0.0011 | 0.65 | +0.0003 ± 0.0010 | 0.79 | ||
| MEGALIN2 vs. MEGALIN1 | +0.0023 ± 0.0029 | 0.44 | +0.0041 ± 0.0026 | 0.11 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0005 ± 0.0007 | 0.49 | +0.0007 ± 0.0007 | 0.29 | ||
| VFT-C | 356 | 441 | ||||
| VDR2 vs. VDR1 | +0.0035 ± 0.0035 | 0.32 | −0.0075 ± 0.0029 | 0.0093,4 | ||
| VDR3 vs. VDR1 | +0.0011 ± 0.0036 | 0.76 | −0.0059 ± 0.0029 | 0.047 | ||
| MEGALIN2 vs. MEGALIN1 | +0.0129 ± 0.0082 | 0.12 | +0.0062 ± 0.0062 | 0.32 | ||
| MEGALIN3 vs. MEGALIN1 | +0.004 ± 0.002 | 0.08 | −0.0020 ± 0.0019 | 0.294 | ||
| Trails A | 326 | 419 | ||||
| VDR2 vs. VDR1 | −0.1003 ± 0.1804 | 0.58 | +0.1227 ± 0.1865 | 0.51 | ||
| VDR3 vs. VDR1 | +0.1454 ± 0.1843 | 0.43 | +0.0856 ± 0.1930 | 0.66 | ||
| MEGALIN2 vs. MEGALIN1 | +0.1273 ± 0.4784 | 0.79 | +0.2799 ± 0.4170 | 0.50 | ||
| MEGALIN3 vs. MEGALIN1 | −0.0156 ± 0.1208 | 0.90 | −0.1384 ± 0.1260 | 0.27 | ||
| Trails B | 326 | 419 | ||||
| VDR2 vs. VDR1 | −0.1019 ± 0.3707 | 0.78 | −0.3245 ± 0.3125 | 0.30 | ||
| VDR3 vs. VDR1 | −0.4595 ± 0.3794 | 0.23 | −0.0286 ± 0.3230 | 0.93 | ||
| MEGALIN2 vs. MEGALIN1 | −1.0571 ± 0.9839 | 0.28 | +1.8775 ± 0.6982 | 0.0073,4 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0505 ± 0.2487 | 0.84 | +0.0172 ± 0.2107 | 0.94 | ||
| DS-F | 349 | 424 | ||||
| VDR2 vs. VDR1 | −0.0010 ± 0.0015 | 0.53 | −0.0007 ± 0.0013 | 0.62 | ||
| VDR3 vs. VDR1 | +0.0001 ± 0.0016 | 0.94 | +0.0015 ± 0.0014 | 0.26 | ||
| MEGALIN2 vs. MEGALIN1 | +0.0046 ± 0.0038 | 0.23 | +0.0041 ± 0.0030 | 0.17 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0014 ± 0.0010 | 0.18 | −0.0007 ± 0.0009 | 0.46 | ||
| DS-B | 351 | 424 | ||||
| VDR2 vs. VDR1 | +0.0008 ± 0.0024 | 0.75 | +0.0003 ± 0.0022 | 0.89 | ||
| VDR3 vs. VDR1 | +0.0001 ± 0.0024 | 0.98 | +0.0033 ± 0.0023 | 0.15 | ||
| MEGALIN2 vs. MEGALIN1 | +0.0062 ± 0.0059 | 0.30 | +0.0124 ± 0.0051 | 0.0143 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0029 ± 0.0016 | 0.07 | +0.0005 ± 0.0015 | 0.72 | ||
| Cognitive domain 1 | 277 | 371 | ||||
| VDR2 vs. VDR1 | −0.0395 ± 0.0565 | 0.49 | −0.0767 ± 0.046 | 0.10 | ||
| VDR3 vs. VDR1 | −0.0206 ± 0.0577 | 0.72 | −0.0106 ± 0.0469 | 0.82 | ||
| MEGALIN2 vs. MEGALIN1 | +0.2000 ± 0.1464 | 0.17 | +0.1223 ± 0.1078 | 0.26 | ||
| MEGALIN3 vs. MEGALIN1 | +0.0112 ± 0.0356 | 0.75 | +0.0035 ± 0.0299 | 0.91 | ||
| Cognitive domain 2 | 277 | 371 | ||||
| VDR2 vs. VDR1 | +0.0466 ± 0.1177 | 0.69 | −0.0018 ± 0.1044 | 0.99 | ||
| VDR3 vs. VDR1 | −0.0576 ± 0.1202 | 0.63 | +0.1568 ± 0.1070 | 0.14 | ||
| MEGALIN2 vs. MEGALIN1 | +0.0519 ± 0.3049 | 0.87 | +0.2704 ± 0.2459 | 0.27 | ||
| MEGALIN3 vs. MEGALIN1 | +0.1005 ± 0.0740 | 0.18 | −0.0343 ± 0.068 | 0.61 | ||
n = 648–788. Cognitive scores were predicted at the mean age at follow-up before onset of dementia or for all time points by using a linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed-effects variables to allow for quadratic nonlinear change. The slope or annual rate of change was predicted from these models at the mean age at follow-up (i.e., between age 50 y and the individual mean age at follow-up for each cognitive test). By using factor analysis, 2 factor scores were estimated and were labeled as longitudinal annual rate of cognitive change in the following domains: Domain 1 (“Verbal memory and fluency”) and Domain 2 (“Visual/working memory”) (Supplemental Methods 1). See Table 1 for more details on definitions of the SNP latent classes. Note that VDR1, VDR2, and VDR3 denote VDR SNPLCs, whereas MEGALIN1, MEGALIN2, and MEGALIN3 denote MEGALIN SNPLCs. BVRT, Benton Visual Retention Test; CVLT-DFR, California Verbal Learning Test, Delayed Free Recall; CVLT-List A, California Verbal Learning Test, List A; DS-B, Digit Span Backward; DS-F, Digit Span Forward; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; MMSE, Mini-Mental State Examination; OLS, ordinary least square; SNP, single nucleotide polymorphism; SNPLC, single nucleotide polymorphism latent class; Trails A, Trailmaking Test, Part A; Trails B, Trailmaking Test, Part B; VDR, vitamin D receptor gene; VFT-C, Verbal Fluency Test, Categorical.
Based on multiple OLS regression models with the outcome being cognitive annual rate of change and main exposures being the 3 MEGALIN SNPs. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease, and BMI. The 10 principal components obtained with multidimensional scaling (see Supplemental Methods 3) were also added in a separate sensitivity analysis.
Significant main effects after family-wise Bonferroni correction: P < 0.05 for MMSE, BVRT, VFT-C, and cognitive domains and P < 0.025 for other cognitive tests.
P < 0.05 for the null hypothesis that sex × SNPLC interaction term = 0 in a model where the main effect of sex was added.
VDR and MEGALIN SNPHAP associations with LARCC: sex-stratified findings.
VDR SNPHAPs combined SNPs that were shown to be in low linkage disequilibrium [rs1544410 (BsmI:G/A), rs7975232 (ApaI:A/C), rs731236(TaqI:G/A)], as did MEGALIN SNPHAPs, which consisted of rs3755166:G/A, rs2075252:C/T, and rs2228171:C/T combinations. Those SNPHAPs were entered as haplotype dosages and examined separately in relation to LARCC among men and women (Table 5). After correction for multiple testing, among men only, the VDR1 (GCA) haplotype was associated with a slower decline on Trails B (P < 0.05 for sex × SNPHAP interaction). When MEGALIN SNPHAPs were examined in relation to LARCC, MEGALIN1 (GCC) was associated with a significantly faster decline on the DS-B among men, which was translated into a faster decline on cognitive domain 2. Finally, adding the 10 principal components as additional covariates (Supplemental Methods 1) did not alter the key findings.
TABLE 5.
VDR and MEGALIN SNPHAP associations with the predicted annual rate of cognitive change between age 50 y and the mean age at follow-up: multiple OLS regression analysis—HANDLS study1
| Predicted annual rate of cognitive change between age 50 y and mean age of follow-up2 |
||||||
| Men |
Women |
|||||
| n | β ± SE3 | P | n | β ± SE3 | P | |
| MMSE: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 349 | −0.0001 ± 0.0014 | 0.92 | 439 | −0.0001 ± 0.0008 | 0.91 |
| VDR2: AAG (0, 1, 2) | 349 | −0.0014 ± 0.0017 | 0.40 | 439 | +0.0018 ± 0.0012 | 0.14 |
| VDR3: GAA (0, 1, 2) | 349 | +0.0013 ± 0.0017 | 0.47 | 439 | −0.0012 ± 0.0011 | 0.27 |
| VDR4: AAA (0, 1, 2) | 349 | +0.0028 ± 0.0026 | 0.29 | 439 | +0.0019 ± 0.0018 | 0.29 |
| MEGALIN1: GCC (0, 1, 2) | 349 | +0.0018 ± 0.016 | 0.27 | 439 | +0.0017 ± 0.0011 | 0.11 |
| MEGALIN2: ACC (0, 1, 2) | 349 | −0.0021 ± 0.0016 | 0.19 | 439 | −0.00182 ± 0.0011 | 0.10 |
| BVRT: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 350 | −0.0028 ± 0.0023 | 0.22 | 432 | −0.0002 ± 0.0020 | 0.90 |
| VDR2: AAG (0, 1, 2) | 350 | +0.0014 ± 0.0028 | 0.61 | 432 | −0.0025 ± 0.0027 | 0.36 |
| VDR3: GAA (0, 1, 2) | 350 | −0.0028 ± 0.0023 | 0.22 | 432 | −0.0002 ± 0.0020 | 0.90 |
| VDR4: AAA (0, 1, 2) | 350 | −0.0012 ± 0.0046 | 0.79 | 432 | +0.0017 ± 0.0041 | 0.69 |
| MEGALIN1: GCC (0, 1, 2) | 350 | −0.0044 ± 0.0027 | 0.114 | 432 | +0.0027 ± 0.0025 | 0.27 |
| MEGALIN2: ACC (0, 1, 2) | 350 | +0.0023 ± 0.0027 | 0.41 | 432 | +0.0005 ± 0.0025 | 0.85 |
| CVLT-List A: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 295 | −0.0002 ± 0.0003 | 0.54 | 385 | −0.0000 ± 0.0002 | 0.92 |
| VDR2: AAG (0, 1, 2) | 295 | +0.0002 ± 0.0003 | 0.54 | 385 | −0.0003 ± 0.0003 | 0.35 |
| VDR3: GAA (0, 1, 2) | 295 | −0.0000 ± 0.0004 | 0.92 | 385 | +0.0005 ± 0.0003 | 0.15 |
| VDR4: AAA (0, 1, 2) | 295 | −0.0005 ± 0.0006 | 0.41 | 385 | −0.0004 ± 0.0005 | 0.40 |
| MEGALIN1: GCC (0, 1, 2) | 295 | −0.0002 ± 0.0003 | 0.40 | 385 | +0.0004 ± 0.0003 | 0.18 |
| MEGALIN2: ACC (0, 1, 2) | 295 | +0.0001 ± 0.0003 | 0.76 | 385 | +0.0000 ± 0.0003 | 0.98 |
| CVLT-DFR: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 284 | +0.0005 ± 0.0005 | 0.30 | 376 | +0.0004 ± 0.0004 | 0.33 |
| VDR2: AAG (0, 1, 2) | 284 | −0.0002 ± 0.0006 | 0.76 | 376 | −0.0002 ± 0.0006 | 0.71 |
| VDR3: GAA (0, 1, 2) | 284 | −0.0005 ± 0.0006 | 0.44 | 376 | −0.0003 ± 0.0006 | 0.57 |
| VDR4: AAA (0, 1, 2) | 284 | −0.0005 ± 0.0010 | 0.66 | 376 | +0.0002 ± 0.0009 | 0.82 |
| MEGALIN1: GCC (0, 1, 2) | 284 | +0.0006 ± 0.0006 | 0.31 | 376 | −0.0009 ± 0.0006 | 0.10 |
| MEGALIN2: ACC (0, 1, 2) | 284 | −0.0005 ± 0.0006 | 0.42 | 376 | −0.0002 ± 0.0006 | 0.71 |
| VFT-C: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 356 | −0.0026 ± 0.0016 | 0.12 | 441 | −0.0006 ± 0.0013 | 0.65 |
| VDR2: AAG (0, 1, 2) | 356 | +0.0003 ± 0.0020 | 0.89 | 441 | +0.0024 ± 0.0018 | 0.16 |
| VDR3: GAA (0, 1, 2) | 356 | +0.0038 ± 0.0021 | 0.08 | 441 | −0.0015 ± 0.0016 | 0.37 |
| VDR4: AAA (0, 1, 2) | 356 | −0.0013 ± 0.0033 | 0.71 | 441 | +0.0044 ± 0.0027 | 0.10 |
| MEGALIN1: GCC (0, 1, 2) | 356 | −0.0022 ± 0.0019 | 0.26 | 441 | −0.0018 ± 0.0016 | 0.26 |
| MEGALIN2: ACC (0, 1, 2) | 356 | −0.0002 ± 0.0020 | 0.31 | 441 | −0.0013 ± 0.002 | 0.49 |
| Trails A: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 326 | +0.112 ± 0.084 | 0.18 | 419 | +0.030 ± 0.082 | 0.71 |
| VDR2: AAG (0, 1, 2) | 326 | −0.003 ± 0.105 | 0.98 | 419 | −0.082 ± 0.114 | 0.47 |
| VDR3: GAA (0,1,2) | 326 | +0.002 ± 0.108 | 0.99 | 419 | −0.037 ± 0.108 | 0.73 |
| VDR4: AAA (0, 1, 2) | 326 | −0.162 ± 0.168 | 0.34 | 419 | −0.191 ± 0.180 | 0.29 |
| MEGALIN1: GCC (0, 1, 2) | 326 | −0.034 ± 0.101 | 0.74 | 419 | +0.193 ± 0.103 | 0.06 |
| MEGALIN2: ACC (0, 1, 2) | 326 | +0.033 ± 0.101 | 0.74 | 419 | −0.123 ± 0.107 | 0.25 |
| Trails B: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 326 | −0.402 ± 0.170 | 0.0184,5 | 419 | +0.041 ± 0.138 | 0.77 |
| VDR2: AAG (0, 1, 2) | 326 | +0.439 ± 0.214 | 0.041 | 419 | −0.005 ± 0.192 | 0.98 |
| VDR3: GAA (0, 1, 2) | 326 | +0.194 ± 0.222 | 0.38 | 419 | +0.006 ± 0.181 | 0.97 |
| VDR4: AAA (0, 1, 2) | 326 | −0.651 ± 0.350 | 0.06 | 419 | +0.195 ± 0.304 | 0.52 |
| MEGALIN1: GCC (0, 1, 2) | 326 | −0.195 ± 0.207 | 0.35 | 419 | −0.008 ± 0.176 | 0.96 |
| MEGALIN2: ACC (0, 1, 2) | 326 | +0.163 ± 0.206 | 0.43 | 419 | −0.080 ± 0.181 | 0.66 |
| DS-F: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 349 | +0.0006 ± 0.0007 | 0.37 | 424 | +0.0012 ± 0.0006 | 0.034 |
| VDR2: AAG (0, 1, 2) | 349 | +0.0001 ± 0.0009 | 0.90 | 424 | −0.0007 ± 0.0008 | 0.38 |
| VDR3: GAA (0, 1, 2) | 349 | −0.0004 ± 0.0009 | 0.61 | 424 | −0.0008 ± 0.0008 | 0.29 |
| VDR4: AAA (0, 1, 2) | 349 | −0.0014 ± 0.0015 | 0.36 | 424 | +0.0024 ± 0.0012 | 0.06 |
| MEGALIN1: GCC (0, 1, 2) | 349 | −0.0007 ± 0.0008 | 0.40 | 424 | +0.0001 ± 0.0007 | 0.92 |
| MEGALIN2: ACC (0, 1, 2) | 349 | −0.0006 ± 0.0008 | 0.42 | 424 | −0.0000 ± 0.0008 | 0.96 |
| DS-B: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 351 | −0.0002 ± 0.0011 | 0.86 | 424 | +0.0016 ± 0.0010 | 0.11 |
| VDR2: AAG (0, 1, 2) | 351 | +0.0000 ± 0.0014 | 0.96 | 424 | −0.0014 ± 0.0014 | 0.30 |
| VDR3: GAA (0, 1, 2) | 351 | +0.0001 ± 0.0014 | 0.96 | 424 | −0.0002 ± 0.0013 | 0.89 |
| VDR4: AAA (0, 1, 2) | 351 | −0.0028 ± 0.0023 | 0.23 | 424 | +0.0009 ± 0.0021 | 0.65 |
| MEGALIN1: GCC (0, 1, 2) | 351 | −0.0032 ± 0.0013 | 0.0185 | 424 | −0.0020 ± 0.0013 | 0.12 |
| MEGALIN2: ACC (0, 1, 2) | 351 | +0.0007 ± 0.0013 | 0.61 | 424 | +0.0008 ± 0.0013 | 0.54 |
| Cognitive domain 1: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 277 | +0.0064 ± 0.0249 | 0.80 | 371 | +0.0282 ± 0.0197 | 0.15 |
| VDR2: AAG (0, 1, 2) | 277 | +0.0252 ± 0.0324 | 0.44 | 371 | −0.0014 ± 0.0279 | 0.96 |
| VDR3: GAA (0, 1, 2) | 277 | −0.0117 ± 0.327 | 0.72 | 371 | −0.0359 ± 0.0265 | 0.18 |
| VDR4: AAA (0, 1, 2) | 277 | −0.0460 ± 0.0532 | 0.39 | 371 | +0.0615 ± 0.0422 | 0.15 |
| MEGALIN1: GCC (0, 1, 2) | 277 | +0.0106 ± 0.030 | 0.73 | 371 | −0.0391 ± 0.0250 | 0.12 |
| MEGALIN2: ACC (0, 1, 2) | 277 | −0.0333 ± 0.0289 | 0.25 | 371 | +0.0216 ± 0.0260 | 0.41 |
| Cognitive domain 2: models A–F | ||||||
| VDR1: GCA (0, 1, 2) | 277 | −0.0929 ± 0.0516 | 0.07 | 371 | +0.0593 ± 0.0450 | 0.19 |
| VDR2: AAG (0, 1, 2) | 277 | +0.0238 ± 0.0677 | 0.73 | 371 | −0.0578 ± 0.0636 | 0.36 |
| VDR3: GAA (0, 1, 2) | 277 | +0.0780 ± 0.0682 | 0.25 | 371 | +0.0330 ± 0.0606 | 0.59 |
| VDR4: AAA (0, 1, 2) | 277 | +0.0211 ± 0.0722 | 0.77 | 371 | −0.0566 ± 0.1113 | 0.61 |
| MEGALIN1: GCC (0, 1, 2) | 277 | −0.1255 ± 0.0627 | 0.0465 | 371 | −0.0243 ± 0.0572 | 0.67 |
| MEGALIN2: ACC (0, 1, 2) | 277 | +0.0256 ± 0.0605 | 0.67 | 371 | +0.0512 ± 0.0592 | 0.39 |
n = 648–788. Note that VDR1, VDR2, and VDR3 denote VDR SNPHAPs, whereas MEGALIN1, MEGALIN2, and MEGALIN3 denote MEGALIN SNPHAPs. “(0, 1, 2)” refers to ordinal coding with “0,” “1,” and “2” copies of each haplotype. Three VDR SNPs were combined to form the haplotypes, namely BsmI, ApaI, and TaqI. Only haplotypes 1–3 were selected for MEGALIN because their overall prevalence was >10%. BVRT, Benton Visual Retention Test; CVLT-DFR, California Verbal Learning Test, Delayed Free Recall; CVLT-List A, California Verbal Learning Test, List A; DS-B, Digit Span Backward; DS-F, Digit Span Forward; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; MMSE, Mini-Mental State Examination; OLS, ordinary least square; SNP, single nucleotide polymorphism; SNPHAP, single nucleotide polymorphism haplotype; Trails A, Trailmaking Test, Part A; Trails B, Trailmaking Test, Part B; VDR, vitamin D receptor gene; VFT-C, Verbal Fluency Test, Categorical.
Cognitive scores were predicted at the mean age at follow-up before onset of dementia or for all time points by using a linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed-effects variables to allow for quadratic nonlinear change. The slope or annual rate of change was predicted from these models at the mean age at follow-up (i.e., between age 50 y and the individual mean age at follow-up for each cognitive test). By using factor analysis, 2 factor scores were estimated and were labeled as the longitudinal annual rate of cognitive change in the following domains: Domain 1 (“Verbal memory and fluency”) and Domain 2 (“Visual/working memory”) (Supplemental Methods 1). See Table 1 for more details on the definitions of the SNP haplotypes.
Based on multiple OLS regression models with the outcome being cognitive annual rate of change and main exposures being the 3 MEGALIN SNPs. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease, and BMI. The 10 principal components obtained with multidimensional scaling (Supplemental Methods 3) were also added in a separate sensitivity analysis.
P < 0.05 for the null hypothesis that sex × SNPHAP interaction term = 0 in a model where the main effect of sex was added.
Significant main effects after family-wise Bonferroni correction: P < 0.05 for MMSE, BVRT, VFT-C, and cognitive domains and P < 0.025 for other cognitive tests.
Discussion
This study examined associations of SNPs in VDR [rs11568820 (Cdx-2:T/C), rs1544410 (BsmI:G/A), rs7975232 (ApaI:A/C), rs731236 (TaqI:G/A)] and MEGALIN (rs3755166:G/A, rs2075252:C/T, rs2228171:C/T) genes with longitudinal cognitive performance changes among 660–797 AA HANDLS participants with complete genetic and cognitive data over the length of ∼5 y and 2 waves of data. Among key findings, the rs3755166:G/A MEGALIN SNP was associated with a faster decline on the MMSE overall, whereas the decline on the DS-B was faster with the rs2228171:C/T dosage among men. VDR TaqI:G/A was linked to slower decline on the Trails B among men and a slower decline on cognitive domain 2 (“visual/working memory”) among women. The VDR2 (BsMI/ApaI/TaqI: G−/A−/A−) SNPLC (compared with VDR1: ApaI:“AA”) was linked to a faster decline on the VFT-C in women, among whom the MEGALIN2 (rs2228171:“TT”) SNPLC (compared with MEGALIN1: rs2228171:“CC”) was also associated with a faster decline on Trails B but a slower decline on the DS-B. Moreover, among men, the VDR1 SNPHAP (GCA or baT) was associated with a slower decline on Trails B, whereas the MEGALIN1 SNHAP (GCC) was associated with a faster decline on the DS-B, translating into a faster decline on cognitive domain 2.
MEGALIN (10, 11) and VDR (8, 37) genetic polymorphisms were recently shown to be associated with cognitive impairment and AD. In fact, with respect to MEGALIN, in a case-control study in 1158 patients with sporadic AD and 1025 healthy controls, out of 3 tested SNPs (rs3755166, rs2075252, rs4668123), only 1 (rs3755166:G/A) was associated with increased AD risk. It is important to note that the A allele of rs3755166 had 20% less transcriptional activity for MEGALIN than did the G allele (10). Another case-control study in Chinese middle-aged and older adults (n = 361) was able to replicate those findings, with rs3755166 G/A associated with an OR of 1.38 (95% CI: 1.02, 1.87; P = 0.039) (11). Similarly, in our previous study in white adults residing in Baltimore, a marginally significant inverse relation was detected after adjustment for multiple testing, between rs3755166 G>A and MMSE LARCC, suggesting greater cognitive decline for participants with an “A” allele (6). This specific finding was replicated in our current study and was significant after correction for multiple testing. Moreover, our previous study in whites residing in Baltimore city indicated that this SNP was also significantly linked with a greater decline in verbal memory among men only, after adjusting for multiple testing (6). This finding was not replicated in our current study in AA urban adults. Moreover, our study indicated that the MEGALIN1 SNHAP “GCC” [1) rs3755166:G/A, 2) rs2075252:C/T, 3) rs2228171:C/T] was associated with a faster decline on the DS-B, reflected as a faster decline on cognitive domain 2 (“visual/working memory”). This appears to be a novel finding that has not been replicated elsewhere.
In our present study, the VDR2 (BsmI/ApaI/TaqI: G−/A−/A−) SNPLC [compared with VDR1 (ApaI:“AA”)] was linked to a faster decline on the VFT-C in women. This finding is comparable to our previous study in whites residing in Baltimore, whereby a marginally significant P-trend was detected indicating that “AA” for ApaI may be protective against cognitive decline on tests of global mental status and verbal memory, compared with “AC” or “CC” (6). Similarly, a current case-control study of late-onset AD cases compared with healthy age-matched controls found that the heterozygous ApaI genotype (“AC”) was associated with an increased risk of AD compared with the homozygous “AA” genotype (37). In contrast to the latter study and ours, the ApaI (A/C) variant allele (i.e., “CC” or “AC” compared with “AA”) was associated with better cognitive function at follow-up, particularly in immediate recall (8). In that prospective cohort study (Leiden 85-plus Study; n = 563), 3 of 5 VDR SNPs were deemed related to follow-up cognitive performance, namely BsmI, ApaI, and TaqI (8). Those 3 SNPs were combined in our study into haplotypes. We found that, among men, the VDR1 SNPHAP (GCA:baT) was associated with a slower decline on the Trails B. This is at odds with our finding in whites residing in Baltimore. In fact, in the latter study, after correction for multiple testing, VDR1 SNPHAP (GCA or baT) was associated with a greater decline on the VFT-C among women but not among men (6). On the other hand, Kuningas et al. (8) found that worse performance was ascribed to the VDR2 SNPHAP (AAG, or BAt). These findings indicate that there might be both race- and sex-specific associations between those haplotypes and cognitive performance or change over time. However, further studies are needed to replicate findings with the use of similar cognitive test batteries and domains.
More recent studies have been mixed. Gezen-Ak et al. (48), in a study in 108 patients with AD and 115 age-matched controls, found that the VDR (TaubF: TCAGC) SNPHAP was more prevalent in patients with AD than in the control group. In a sample of Uygur people (49) (n = 124 cases and n = 124 controls), the A allele of the VDR ApaI gene and the T allele of the VDR BsmI gene were related to an increased risk of mild cognitive impairment, with individuals with the VDR ApaI AA genotype at the highest risk of mild cognitive impairment. The latter result is at odds with both our current and past study among whites, possibly due to race-specific effects (6). Another study found the VDR FokI “FF” genotype to be related to a higher MMSE score, compared with the “ff” genotype group, in elderly (≥65 y) participants (50). FokI was not included among the VDR SNPs in our current or past study in whites (6). In contrast, 2 other studies found no association between the VDR ApaI and TaqI genes and late-onset AD in an Iranian sample (145 patients with AD and 162 age-matched controls) (51) or between VDR FokI and BsmI genes and AD in a Polish sample (108 patients with AD and 77 controls) (52). This may highlight the difference between the etiology of normal cognitive aging (e.g., cognitive change between early and midadulthood) as opposed to incident or prevalent AD.
Sex differences were detected in the association between MEGALIN SNPLCs and cognitive change, which may be ascribed to the interaction of MEGALIN with both estrogen, established to affect cognitive function (53), and with vitamin D, also known to affect cognitive performance (54–56). Notably, current experimental evidence indicates that vitamin D–binding protein (which binds, among others, 25-hydroxycholecalciferol and transports it to target tissues) and the estrogen receptor [sex hormone–binding globulin (SHBG)] share binding sites on MEGALIN, making them competitive ligands (42, 57, 58). Indeed, evidence is emerging that SHBG-bound estrogen and testosterone become biologically active via receptor-mediated endocytosis (42–44), mediated primarily via the MEGALIN receptor (42). In fact, MEGALIN gene knockout may induce both estrogen deficiency and vitamin D deficiency (42, 57), and the cross-effect modification of estrogen and vitamin D interventions was found for incident colorectal cancer by using data from the Women’s Health Initiative trial (59). Therefore, current evidence is suggestive of an interplay between estrogen and vitamin D via their shared receptor MEGALIN, which may explain the sex-specific role of MEGALIN gene polymorphism in cognitive performance and change over time. Our findings suggest that the majority of MEGALIN gene polymorphism putative effects on cognition were detected in 1 sex group but not the other. The same sex-specific findings applied to VDR polymorphisms, although no biological mechanism is available and further research in that area is needed.
Our study has several strengths, including a relatively large sample, a longitudinal study design, and the use of advanced statistical techniques by combining linear mixed-effects regression models with OLS multiple linear regression analyses to examine associations between gene polymorphisms and annual rates of change in cognitive performance. Although used less frequently than haplotype analysis, latent class analysis was conducted to examine clustering of genotypes within VDR and MEGALIN and the effect of that clustering on cognitive change over time.
Nevertheless, our study has notable limitations. First, the final analytic sample used in our analysis may have been selected in a nonrandom manner, whereby certain groups (e.g., age, sex, poverty status, education) may have been oversampled compared with the original selected sample of AAs in the HANDLS study. To diminish resulting biases, we used a 2-stage Heckman selection model (35). Second, baseline age and duration between visits varied between participants, rendering the data structure unbalanced. Mixed-effects regression models were therefore used to predict cognitive test scores and annual rates of change at specific ages at which data were most dense (mean age at follow-up for each subject; i.e., LARCC). In our main OLS regression models, we further controlled for both first visit and mean age at follow-up. Third, serum 25-hydroxyvitamin D was not available at the time of the analysis to examine vitamin D–gene interaction and its potential role in affecting age-related cognitive decline. Moreover, such interaction would only be possible to test in larger samples due to limited power. Finally, positive findings may have been due to chance, residual confounding by key unmeasured factors, or selection bias due to unequal probability of selection from the initial study sample of AAs, whereas negative findings may have been caused by lack of adequate power. Thus, until those findings are replicated elsewhere in comparable adult populations, they should be interpreted with caution.
In summary, sex-specific VDR and MEGALIN gene variations can alter age-related cognitive trajectories among AA urban adults, specifically in global mental status and domains of verbal fluency, visual and working memory, and executive function. Diet is an important modulator of the human metabolic phenotype, and studies addressing the interaction between gene polymorphisms of molecules responsible for the regulation of key metabolic nutrients such as vitamin D and cognitive function can be instrumental in driving the future of personalized nutritional medicine. Finally, future studies should attempt to examine associations of those SNPs, SNPLCs, and SNPHAPs with incident dementia, AD, and mild cognitive impairment in comparable populations.
Acknowledgments
We thank Ola S Rostant and Nicolle Mode (National Institute on Aging/NIH/Intramural Research Program) for internally reviewing our manuscript. The authors’ responsibilities were as follows—MAB: worked on the conceptualization, plan of analysis, data management, statistical analysis, and literature review, and wrote the manuscript; SMT: worked on the plan of analysis and data management, provided assistance with the statistical analysis, wrote parts of the manuscript, and revised the manuscript; GAD and J-AC: worked on the literature review, wrote parts of the manuscript, and revised the manuscript; HAB: helped to plan the analysis and literature review, wrote parts of the manuscript, and revised the manuscript; MKE: worked on data acquisition, wrote parts of the manuscript, and revised the manuscript; ABZ: worked on data acquisition and plan of analysis, wrote parts of the manuscript, and revised the manuscript; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: AA, African-American; AD, Alzheimer disease; BVRT, Benton Visual Retention Test; CVLT, California Verbal Learning Test; DFR, Delayed Free Recall; DS-B, Digit Span Backward; DS-F, Digit Span Forward; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; LARCC, longitudinal annual rate of cognitive change; LRP2, LDL–related protein 2; MMSE, Mini-Mental State Examination; OLS, ordinary least square; SHBG, sex hormone–binding globulin; SNP, single nucleotide polymorphism; SNPHAP, single nucleotide polymorphism haplotype; SNPLC, single nucleotide polymorphism latent class; Trails A, Trailmaking Test, Part A; Trails B, Trailmaking Test, Part B; VDR, Vitamin D receptor; VFT-C, Verbal Fluency Test, Categorical.
References
- 1.Berridge MJ. Vitamin D cell signalling in health and disease. Biochem Biophys Res Commun 2015;460:53–71. [DOI] [PubMed] [Google Scholar]
- 2.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 2013;34:47–64. [DOI] [PubMed] [Google Scholar]
- 3.Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, Bergethon P, Rosenberg IH, Folstein MF, Patz S, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010;74:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone 1998;23:555–7. [DOI] [PubMed] [Google Scholar]
- 5.Jorde R, Mathiesen EB, Rogne S, Wilsgaard T, Kjaergaard M, Grimnes G, Schirmer H. Vitamin D and cognitive function: the Tromso study. J Neurol Sci 2015;355:155–61. [DOI] [PubMed] [Google Scholar]
- 6.Beydoun MA, Ding EL, Beydoun HA, Tanaka T, Ferrucci L, Zonderman AB. Vitamin D receptor and MEGALIN gene polymorphisms and their associations with longitudinal cognitive change in US adults. Am J Clin Nutr 2012;95:163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med 2008;29:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RG, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging 2009;30:466–73. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci Lett 2011;504:79–82. [DOI] [PubMed] [Google Scholar]
- 10.Vargas T, Bullido MJ, Martinez-Garcia A, Antequera D, Clarimon J, Rosich-Estrago M, Martin-Requero A, Mateo I, Rodriguez-Rodriguez E, Vilella-Cuadrada E, et al. A MEGALIN polymorphism associated with promoter activity and Alzheimer’s disease risk. Am J Med Genet B Neuropsychiatr Genet 2010;153B:895–902. [DOI] [PubMed] [Google Scholar]
- 11.Wang LL, Pan XL, Wang Y, Tang HD, Deng YL, Ren RJ, Xu W, Ma JF, Wang G, Chen SD. A single nucleotide polymorphism in LRP2 is associated with susceptibility to Alzheimer's disease in the Chinese population. Clin Chim Acta 2011;412:268–70. [DOI] [PubMed] [Google Scholar]
- 12.Gatto NM, Sinsheimer JS, Cockburn M, Escobedo LA, Bordelon Y, Ritz B. Vitamin D receptor gene polymorphisms and Parkinson’s disease in a population with high ultraviolet radiation exposure. J Neurol Sci 2015;352:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005;29:21–30. [DOI] [PubMed] [Google Scholar]
- 14.Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Miettinen S, Tuohimaa P. Behavioural anomalies in mice evoked by "Tokyo" disruption of the vitamin D receptor gene. Neurosci Res 2006;54:254–60. [DOI] [PubMed] [Google Scholar]
- 15.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport 2004;15:1271–4. [DOI] [PubMed] [Google Scholar]
- 16.Burne TH, McGrath JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res 2005;157:299–308. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging 2008;29:902–12. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich M, Antequera D, Pascual C, Castro N, Bolos M, Carro E. Alzheimer’s disease-like impaired cognition in endothelial-specific MEGALIN-null mice. J Alzheimers Dis 2014;39:711–7. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/MEGALIN: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA 1996;93:4229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci 1994;17:525–30. [DOI] [PubMed] [Google Scholar]
- 21.Small BJ, Graves AB, McEvoy CL, Crawford FC, Mullan M, Mortimer JA. Is APOE-epsilon4 a risk factor for cognitive impairment in normal aging? Neurology 2000;54:2082–8. [DOI] [PubMed] [Google Scholar]
- 22.Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O'Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging 2012;33:720–31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedazo-Minguez A. Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med 2007;11:1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durk MR, Han K, Chow EC, Ahrens R, Henderson JT, Fraser PE, Pang KS. 1alpha,25-Dihydroxyvitamin D3 reduces cerebral amyloid-beta accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neurosci 2014;34:7091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience 2016;322:28–38. [DOI] [PubMed] [Google Scholar]
- 26.Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. 1alpha,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-beta peptide (1–40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus MEGALIN is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci 2005;25:10884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 2004;43:333–44. [DOI] [PubMed] [Google Scholar]
- 29.Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/MEGALIN: a mechanism to prevent pathological accumulation of amyloid beta-peptide. J Biol Chem 1997;272:18644–9. [DOI] [PubMed] [Google Scholar]
- 30.Zlokovic BV. Cerebrovascular transport of Alzheimer's amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci 1996;59:1483–97. [DOI] [PubMed] [Google Scholar]
- 31.Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA 1993;90:10836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute on Aging. Healthy Aging in Neighborhoods of Diversity across the LifeSpan (HANDLS) protocol [Internet]. [cited 2016 Dec 10]. Baltimore (MD): National Institute on Aging, Intramural Research Program, NIH; 2004. Available from: https://handls.nih.gov/02Protocol.htm.
- 34.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. Test 2009;18:1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckman JJ. Sample selection bias as a specification error. Econometrica 1979;47:153–61. [Google Scholar]
- 36.Sharma S. Applied multivariate techniques. New York: Wiley; 1996. [Google Scholar]
- 37.Gezen-Ak D, Dursun E, Ertan T, Hanagasi H, Gurvit H, Emre M, Eker E, Ozturk M, Engin F, Yilmazer S.. Association between vitamin D receptor gene polymorphism and Alzheimer's disease. Tohoku J Exp Med 2007;212:275–82. [DOI] [PubMed] [Google Scholar]
- 38.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling 2007;14:671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iivonen S, Corder E, Lehtovirta M, Helisalmi S, Mannermaa A, Vepsalainen S, Hanninen T, Soininen H, Hiltunen M. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology 2004;62:1170–6. [DOI] [PubMed] [Google Scholar]
- 40.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 2005;76:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263–5. [DOI] [PubMed] [Google Scholar]
- 42.Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005;122:751–62. [DOI] [PubMed] [Google Scholar]
- 43.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol 1999;69:481–5. [DOI] [PubMed] [Google Scholar]
- 44.Porto CS, Lazari MF, Abreu LC, Bardin CW, Gunsalus GL. Receptors for androgen-binding proteins: internalization and intracellular signalling. J Steroid Biochem Mol Biol 1995;53:561–5. [DOI] [PubMed] [Google Scholar]
- 45.Selvin S. Statistical analysis of epidemiologic data. 3rd ed. New York: Oxford University Press; 2004. [Google Scholar]
- 46.Hochberg Y, Tamhane AC. Multiple comparison procedures. New York: Wiley; 1987. [Google Scholar]
- 47.STATA. Statistics/data analysis: release 14.0. College Station (TX): Stata Corporation; 2015. [Google Scholar]
- 48.Gezen-Ak D, Dursun E, Bilgic B, Hanagasi H, Ertan T, Gurvit H, Emre M, Eker E, Ulutin T, Uysal O, et al. Vitamin D receptor gene haplotype is associated with late-onset Alzheimer’s disease. Tohoku J Exp Med 2012;228:189–96. [DOI] [PubMed] [Google Scholar]
- 49.Keyimu K, Zhou XH, Miao HJ, Zou T. Relationship between vitamin D receptor gene polymorphism and mild cognitive impairment in elderly Uygur people. Int J Clin Exp Med 2014;7:5282–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Najmi Varzaneh F, Sharifi F, Hossein-Nezhad A, Mirarefin M, Maghbooli Z, Ghaderpanahi M, Larijani B, Fakhrzadeh H. Association of vitamin D receptor with longevity and healthy aging. Acta Med Iran 2013;51:236–41. [PubMed] [Google Scholar]
- 51.Khorram Khorshid HR, Gozalpour E, Saliminejad K, Karimloo M, Ohadi M, Kamali K, Vitamin D. Receptor (VDR) polymorphisms and late-onset Alzheimer’s disease: an association study. Iran J Public Health 2013;42:1253–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Łaczmański Ł, Jakubik M, Bednarek-Tupikowska G, Rymaszewska J, Sloka N, Lwow F. Vitamin D receptor gene polymorphisms in Alzheimer’s disease patients. Exp Gerontol 2015;69:142–7. [DOI] [PubMed] [Google Scholar]
- 53.Espeland MA, Brunner RL, Hogan PE, Rapp SR, Coker LH, Legault C, Granek I, Resnick SM. Long-term effects of conjugated equine estrogen therapies on domain-specific cognitive function: results from the Women's Health Initiative study of cognitive aging extension. J Am Geriatr Soc 2010;58:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology 2010;75:1810–6. [DOI] [PubMed] [Google Scholar]
- 55.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 2007;460:202–5. [DOI] [PubMed] [Google Scholar]
- 56.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 2006;14:1032–40. [DOI] [PubMed] [Google Scholar]
- 57.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999;96:507–15. [DOI] [PubMed] [Google Scholar]
- 58.Moestrup SK, Verroust PJ. MEGALIN- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annu Rev Nutr 2001;21:407–28. [DOI] [PubMed] [Google Scholar]
- 59.Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women’s Health Initiative randomized trial. Int J Cancer 2008;122:1690–4. [DOI] [PubMed] [Google Scholar]