Abstract
Introduction
Improved HIV outcomes as a result of expanded antiretroviral therapy (ART) access is threatened by increasing rates of loss to follow up (LTFU) among those on ART, largely reported in urban populations. Some reports suggest that LTFU rates are overestimated due to patient movement to other facilities and inadequate medical records.
Study objective
To define the proportion disengaging from HIV care as well as the characteristics of those LTFU in order to design and implement appropriate interventions to increase retention.
Methods
We performed a retrospective review of patients who discontinued ART at a central hospital ART clinic in rural South Africa and compared with patients receiving care at the 15 primary health clinics (PHCs) to determine the true proportion of those who were LTFU. We also compared those who discontinued ART with those who did not at the central hospital ART clinic to determine predictors of loss to follow up.
Results
Among 3242 patients on ART, 820 were originally marked as LTFU. Among all patients, 272 (8.4%) were found at a clinic on treatment, 56 (1.7%) were found at a clinic from which they had since discontinued treatment, and 10 (0.3%) returned to care between June and July 2016, leaving 475 (14.7%) unaccounted for and thus categorized as ‘true’ LTFU. Factors found to be associated with discontinuation include being male, age 18–35, having a CD4 count under 200 cells/μL, and being on ART for under six months.
Conclusions
Young men with low CD4 counts early after ART initiation are at highest risk of ART disengagement in this rural South African HIV clinic. Novel interventions targeting this group are needed to improve retention in care.
Background
Substantial progress has been made globally in the fight against HIV. The number of new infections has decreased from a peak of 2.7 million per year in 2005 to 2.0 million per year in 2014 11], accompanied by an increase in access to antiretroviral therapy (ART). In 2000, only 700,000 people were on ART globally compared to 15.8 million in mid-2015 [1]. Approximately 7 million people are currently living with HIV in South Africa, with a 19.2% prevalence rate among adults age 15–49 [1]. In 2014, 180,000 South African deaths were attributable to AIDS [1]. ART access has increased dramatically over the past 15 years in South Africa, from only 4,000 patients on treatment in 2000 to over 3.1 million on treatment in 2014 [1].
Surveillance studies in South Africa estimate that 62.2% of male and 45% of female persons living with HIV are unaware of their HIV+ status [2]. Until recently, HIV+ individuals were only eligible for ART in South Africa if they met CD4 count criteria. Under previous criteria restricting ART to those with CD4<350, 59% of those eligible were not receiving ART [2]. Since September 2016, South Africa has progressively implemented a test-and-treat policy in which every individual with a positive HIV test result would be eligible to receive ART immediately, regardless of CD4 count. Thus, strategies are needed to ensure that more individuals are aware of their HIV status and are linked to and retained in care. Currently, approximately 65–80% of patients are retained in HIV care in resource-limited settings (RLS) [3, 4, 5, 6]. Factors associated with loss to follow-up among HIV+ patients on ART include dissatisfaction with health services, lack of financial resources to access treatment, stigma, improving health condition, male gender, baseline CD4 count greater than 200 cells/ml, follow-up CD4 count less than 200 cells/ml, and confidentiality concerns [7, 8, 9, 10, 11, 12, 13].
Rates of loss to follow up among those on ART are approximately 10–20% [14, 15, 16]. However, some reports suggest that loss to follow up rates are overestimated due to patient movement to other facilities and inadequate medical records [14, 17, 18]. Without more precise data about the rate of LTFU as well as the characteristics of those who disengage from treatment, appropriate interventions to increase ART adherence cannot be designed and implemented. While previous studies have investigated characteristics of those who are LTFU in South Africa, questions still remain about the dynamics of LTFU in rural settings. Accordingly, we sought to more accurately characterize movement between a central hospital ART clinic and its associated primary health clinics (PHCs) in a rural region of South Africa to determine the proportion and characteristics of patients who were no longer on ART and therefore considered “true” loss to follow up.
Methods
Setting
The study was conducted at the Church of Scotland Hospital (COSH) HIV clinic in Tugela Ferry, South Africa. COSH is a 350-bed district hospital which serves Msinga sub-district of KwaZulu-Natal, South Africa and receives referrals from 15 PHCs. Msinga has a population of approximately 180,000 and is characterized by extreme poverty. Unemployment rates are approximately 85%, 70% of people have no access to electricity, and 70% have no access to clean water [19]. At the time of this study, adults were considered eligible for ART following HIV+ diagnosis with CD4 count of <500 cells/μL. At COSH, patients are typically given 1-month ART prescriptions in which they have to physically come to the clinic each month to receive their medication refills [20]. However, once stable on ART for 12 months, patients can be enrolled in Medipost, signifying monthly refills from designated ART dispensing stations and clinic visits once every six months [20, 21].
Study design
We performed a retrospective review of patients who discontinued ART at the central hospital HIV clinic and compared with patients receiving care at the 15 PHCs to determine the true proportion of LTFU. We also compared those who discontinued ART with those who did not at the central hospital clinic to determine characteristics associated with LTFU.
Data collection and measures
Data on patients initiating HIV care at COSH is recorded in an electronic database called tier.net [22]. Doctors and nurses record patient information at each visit into a paper file which is then entered into the computer system by a data capturer. Each of the 15 PHCs has a tier.net database, but they are not linked to each other or to COSH. We reviewed the tier.net database from COSH and generated a list of patients who discontinued ART from January 2015-June 2016, defined as patients who had not collected ART for a minimum of 90 days.
Patients located at the PHCs were categorized as either “found on treatment,” defined as actively receiving treatment at that clinic or having transferred out while actively taking the treatment, or as “found not on treatment,” defined as patients who were listed in the PHC database but who had since become LTFU from that clinic. Outcomes were censored as of July 1, 2016. A comparison list of those who were retained in care was generated from COSH’s tier.net database. Patients retained in care were defined as patients who were on ART and collecting medication without LTFU for at least three months from January 2015 to June 2016. Abstracted data included age, gender, months on ART, and most recent CD4 count and viral load, for both those who were LTFU and those who were retained in care. Double data entry was used to ensure data quality and patients categorized as lost to follow up were confirmed with staff at each clinic.
Data analysis
Chi-square and Kruskal-Wallis analyses were performed to determine predictors of LTFU. Using a threshold of p<0.1, we conducted a multivariable regression analysis to determine independent predictors of LTFU. All analyses were performed using SAS Version 9.4.
The study was approved by the South African Medical Association Research Ethics Committee (#1401013255) and Yale University Human Investigations Committee (#1401013255). The data reviewed and presented in this study was originally collected in medical records as part of routine clinical care. Informed consent was not requested of patients since recording of data into the medical charts was for the purpose of regular medical care.
Results
Sample characteristics
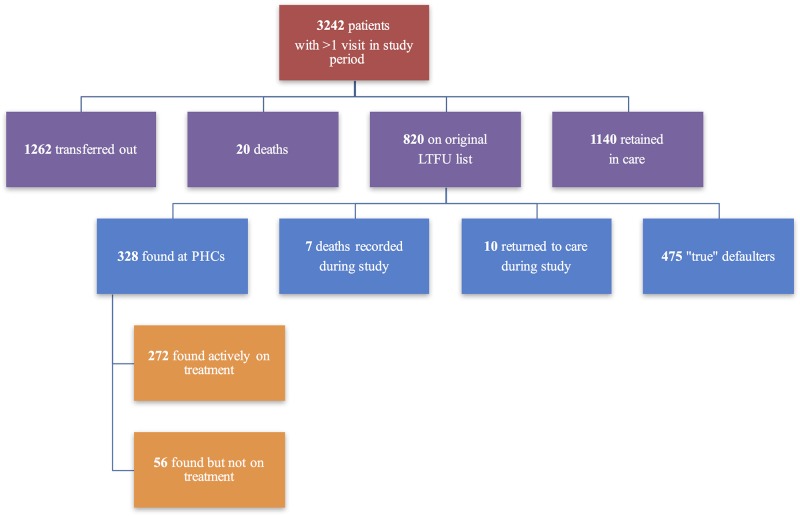
Of all 3242 patients who visited the COSH central hospital HIV clinic at least once from January 2015 to June 2016 (Table 1), the majority were female (57.6%) and between 18–35 years old (33.4%). Among all patients with at least one visit, 1140 (35.1%) remained on treatment, 1262 (38.9%) transferred out to another clinic, 20 (0.6%) died, and 820 (25.2%) were recorded as LTFU (Fig 1).
Table 1. Characteristics of all HIV infected patients on ART at the hospital HIV clinic from January 2015-June 2016.
| n = 3242 | n | (%) |
|---|---|---|
| Outcome | ||
| Retained in care | 1140 | (35.2%) |
| Transferred out | 1262 | (38.9%) |
| Died | 27 | (0.8%) |
| Lost to follow-up | 475 | (14.7%) |
| Found at clinics on treatment | 272 | (8.4%) |
| Found at clinic not on treatment | 56 | (1.7%) |
| Returned to care during study | 10 | (0.3%) |
| Sex | ||
| Male | 1374 | (42.4%) |
| Female | 1868 | (57.6%) |
| Age | ||
| <18 | 526 | (16.2%) |
| 18–35 | 1082 | (33.4%) |
| 35–45 | 962 | (29.7%) |
| ≥45 | 668 | (20.6%) |
| Unknown | 4 | (0.1%) |
| Most Recent CD4 Count | ||
| <200 | 636 | (19.6%) |
| 200–500 | 859 | (26.5%) |
| ≥500 | 761 | (23.5%) |
| Unknown | 986 | (30.4%) |
| Most Recent Viral Load | ||
| <200 | 984 | (30.4%) |
| 200–1000 | 277 | (8.5%) |
| ≥1000 | 397 | (12.2%) |
| Unknown | 1584 | (48.9%) |
| Months on ART | ||
| <6 | 129 | (4.0%) |
| 6–11 | 269 | (8.3%) |
| 12–23 | 504 | (15.5%) |
| ≥24 | 1951 | (60.2%) |
| Unknown | 389 | (12.0%) |
Fig 1. Patients with ≥1 Visit to COSH HIV clinic from January 2015-June 2016.
Finding LTFU at primary health clinics
Among the 820 individuals originally marked as LTFU, 272 (8.4%) were found at a clinic on treatment, 56 (1.7%) were found at a clinic from which they had since become LTFU, and 10 (0.3%) returned to care between June and July 2016, leaving 475 (14.7%) unaccounted for and thus categorized as ‘true’ LTFU (Fig 1).
With regards to those LTFU (n = 475), the majority (51.4%) were male (Table 2). A majority of those LTFU were between the ages of 18 to 45 (70.3%). This group of patients who became LTFU had a median time on ART of 20 months (IQR 10–42). The clinics at which patients were located varied in the number of patients found and the ratio of patients found on treatment to found not on treatment. The highest number of patients were located at Gateway (73), Pomeroy (50), and Mhlangana (31) clinics (Table 3). Only 75% of the patients found at Gateway were still on treatment, compared to 82% at Pomeroy and 97% at Mhlangana, suggesting potential differences in retention in care at the clinic level.
Table 2. Characteristics of patients who were LTFU at the hospital HIV clinic from January 2015-June 2016.
| n = 475 | n | (%) |
|---|---|---|
| Sex | ||
| Male | 244 | (51.4%) |
| Female | 231 | (48.6%) |
| Age | ||
| <18 | 69 | (14.5%) |
| 18–35 | 188 | (39.6%) |
| 35–45 | 146 | (30.7%) |
| ≥45 | 72 | (15.2%) |
| Most Recent CD4 Count | ||
| <200 | 142 | (30.0%) |
| 200–500 | 118 | (24.8%) |
| ≥500 | 76 | (16.0%) |
| Unknown | 139 | (29.3%) |
| Most Recent Viral Load | ||
| <200 | 89 | (18.7%) |
| 200–1000 | 28 | (5.9%) |
| ≥1000 | 48 | (10.1%) |
| Unknown | 310 | (65.3%) |
| Months on ART | ||
| <6 | 62 | (13.1%) |
| 6–11 | 67 | (14.1%) |
| 12–23 | 105 | (22.1%) |
| ≥24 | 190 | (40.0%) |
| Unknown | 51 | (10.7%) |
Table 3. Number and proportion of patients located at primary care clinics.
| Found on treatment (n = 272) |
Found not on treatment (n = 56) |
|
|---|---|---|
| Collessie | 7 (2.6%) | 7 (12.5%) |
| Cwaka | 15 (5.5%) | 0 |
| Ethembeni | 29 (10.7%) | 3 (5.4%) |
| Gateway | 55 (20.2%) | 18 (32.1%) |
| Gunjana | 19 (7.0%) | 3 (5.4%) |
| Mandleni | 11 (4.0%) | 4 (7.1%) |
| Mawele | 2 (0.7%) | 1 (1.8%) |
| Mazabeku | 6 (2.2%) | 0 |
| Mbangweni | 22 (8.1%) | 2 (3.6%) |
| Mhlangana | 30 (11.0%) | 1 (1.8%) |
| Mumbe | 7 (2.6%) | 3 (5.4%) |
| Ngubevu | 14 (5.1%) | 2 (3.6%) |
| Nocomboshe | 7 (2.6%) | 2 (3.6%) |
| Pomeroy | 41 (15.0%) | 9 (16.1%) |
| Qinelani | 7 (2.6%) | 1 (1.8%) |
Comparing patients who became lost to follow-up and those retained in care
In total, we evaluated and compared 475 patients who became LTFU and 1140 who were retained in care (Table 4). Males accounted for 51% of LTFU compared to 40% of those retained in care (p = 0.001), the median number of months on ART for those LTFU was 20 (IQR 10–42) compared to 48 for those retained in care (IQR 26–70, p<0.0001), the most recent CD4 count for those LTFU was 248 (IQR 94–436) compared to 449 (IQR 260–693, p<0.0001) for those retained in care. The percentage of those LTFU virally suppressed was 54% compared to 64% of those retained in care (p = 0.01). There was a trend towards significance with regards to age, with a median age of those LTFU of 34 years (26.7–41) and of those retained in care 35.7 years (IQR 24.9–44, p = 0.08).
Table 4. Characteristics of patients on ART lost to follow-up and patients retained in care at the hospital HIV clinic from January 2015 –June 2016.
| Characteristics | n | LTFU (n = 475) |
Retained in care (n = 1140) |
p-value |
|---|---|---|---|---|
| Age, median years (IQR) | 1615 | 34.0 (26.7–41.0) | 35.7 (24.9–44.0) | 0.08 |
| <18 | 289 | 8.9 (2.5–14.5) | 10.5 (6.8–14.2) | 0.05 |
| 18–35 | 519 | 30.4 (26.4–32.6) | 29.7 (25.4–32.1) | 0.67 |
| 35–45 | 483 | 39.2 (37.4–42.1) | 40.3 (37.8–42.4) | 0.08 |
| >45 | 324 | 52.5 (48.1–59.3) | 51.2 (47.8–56.5) | 0.29 |
| Male gender (n, %) | 1615 | 244 (51%) | 685 (40%) | 0.001 |
| Months on ART total (median, IQR) | 1614 | 20 (10–42) | 48 (26–70) | <.0001 |
| Most recent CD4 count, cells/uL (median, IQR) | 1318 | 248 (94–436) | 449 (260.3–693) | <.0001 |
| Last VL <200 (n, %) | 1101 | 89 (54%) | 601 (64%) | 0.01 |
Lack of laboratory monitoring
Only 62.2% of patients LTFU and retained in care had complete data on all variables of interest. While 81.6% of individuals either LTFU or retained in care (n = 1615, Table 4) had at least one CD4 measurement recorded in tier.net (86.1% of those retained in care, 70.7% of patients LTFU), only 68.2% of those LTFU and retained in care had a VL measurement recorded (82.1% of those retained in care, 34.7% of those LTFU). Among those became LTFU, the median time from last CD4 measurement to LTFU was 8 months (IQR 4–18). Among those retained in care, the median time from last CD4 measurement to last visit was 6 months (IQR 1–18). The number and proportion of all individuals seen at COSH (n = 3242), including patients who officially transferred out of care, with missing VL and CD4 data can be seen in Table 1.
Predictors of LTFU
Upon univariate analysis, we found that ages 18–45, male gender, CD4 <500, and ART <24 months were significantly associated with ART disengagement (Table 5). Males were 1.6 (95% CI 1.3–2.0) times more likely to become LTFU than females. Those in the age 18 to 35 group were 2.0 (1.5–2.7) times more likely to disengage compared to those over age 45. Patients with a most recent CD4 count of under 200 were 4.3 (3.1–6.0) times more likely to become LTFU than those with a CD4 count of above 500. Those with a most recent viral load of above 1000 were 1.5 (1.0–2.3) times more likely to disengage than those with a most recent viral load of less than 200. Patients on ART for less than six months had a 10.3 (6.4–16.5) times higher likelihood of disengagement.
Table 5. Predictors of LTFU.
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Sex | ||
| Male | 1.59 (1.28–1.97)* | 1.31 (.91–1.89) |
| Female | Reference | Reference |
| Age | ||
| <18 | 1.10 (.75–1.60) | 1.65 (.89–3.07) |
| 18–35 | 2.00 (1.45–2.73)* | 1.79 (1.05–3.07)* |
| 35–45 | 1.52 (1.09–2.10)* | 1.57 (.92–2.67) |
| ≥45 | Reference | Reference |
| CD4 Count | ||
| <200 | 4.34 (3.12–6.03)* | 2.64 (1.61–4.35)* |
| 200–500 | 1.69 (1.23–2.33)* | 1.24 (.80–1.88) |
| ≥500 | Reference | Reference |
| Viral Load | ||
| <200 | Reference | Reference |
| 200–1000 | 1.54 (.96–2.45) | 1.37 (.84–2.24) |
| ≥1000 | 1.53 (1.04–2.25)* | .99 (.63–1.55) |
| Months on ART | ||
| <6 | 10.30 (6.42–16.53)* | 2.83 (1.02–7.82)* |
| 6–11 | 3.06 (2.16–4.32)* | .45 (.20–1.02) |
| 12–23 | 3.88 (2.87–5.25)* | 1.35 (.80–2.25) |
| ≥24 | Reference | Reference |
*significant at p≤0.05
However, on multivariate analyses, the only factors that remained independent predictors of ART disengagement were age 18–35, CD4 count under 200, and ART for less than 6 months (Table 5). Those aged 18–35 were at a 1.8 (1.1–3.1) times higher risk of disengaging compared to those over age 45. Patients with a CD4 count below 200 were at a 2.6 (1.6–4.4) higher risk of LTFU compared to those with a CD4 count of over 500, and those on ART for under 6 months had a 2.8 (1.0–7.8) higher risk of LTFU compared to those on ART for over 2 years.
Discussion
This study characterized disengagement from a central hospital ART clinic in rural KwaZulu-Natal, South Africa. The overall “true” LTFU rate from the central hospital HIV clinic was 14.7% within the Msinga sub-district. Significant differences between those who became LTFU and those who were retained in care exist with respect to gender, time on ART, most recent CD4 count, and proportion virally suppressed (defined as having a VL of under 200). Independent correlates of disengagement include male gender, age 18–35, lower CD4 count (<200) and shorter duration on ART (<6 months). While male gender was only borderline significant, the higher risk for younger males may partially be explained by the mobile nature of the population in Tugela Ferry. Employment opportunities are limited in this rural impoverished region and thus males of working age frequently travel long distances to work in other areas such as Johannesburg for months at a time. If these patients did not request a formal transfer of care, they would be recorded as LTFU. Though some of these patients likely attended another clinic and continued ART, others simply stopped ART while away from home. [23, 24]
Additionally, patients with more advanced HIV disease were most likely to disengage. In particular, those with CD4 counts of below 200 cells/mm3 were at highest risk, nearly three times more likely to stop ART. Previous studies have found mixed results on the impact of CD4 count, with evidence for both higher and lower CD4 counts increasing the risk of LTFU [8, 9, 11, 12]. There are multiple possible explanations for our findings. First, patients with CD4 counts of below 200 cells/mm3 may have died within three months of their last clinic visit, an outcome we were unable to confirm in this study. A previous South African study found that among 23% of patients who were LTFU, 48.8% had died [8], though was unable to ascertain date of death and thus could not determine whether death occurred within three months of their last clinic visit. A meta-analysis of ART programs found a combined mortality rate of 46% among patients lost to follow-up, though there were significant differences between studies [25]. In our study as well, dates of death were not available, so the impact of mortality is unknown. However, these findings suggest that the significant association of low CD4 count with disengagement may at least partially be attributed to unreported patient death and not LTFU.
Another possible explanation is that patients with low CD4 counts who are on ART may not be taking their medication as prescribed. Lack of clinical response to treatment may reinforce poor adherence and result in stopping ART altogether. Patients may also have baseline resistance to first line ART or develop resistance to ART, further decreasing the effectiveness of therapy and resulting in poor clinical response and bolstering patient notions of lack of ART effectiveness. Furthermore, individuals on ART for less than six months were also significantly more likely to disengage than those on treatment for longer lengths of time. This high initial attrition rate, demonstrated in other studies [3, 26], indicates that more targeted interventions early after ART initiation may be necessary to assist patients on treatment through the first six months. Moreover, though not measured in this retrospective record review, stigma remains a powerful contributor to lost to follow up, especially where patients may be viewed receiving treatment in their community [27]. In addition to the factors discussed above, this may explain the findings that patients disengaged from HIV care after transferring to their local PHC. Future studies, particularly among those in RLS who are LTFU soon after ART initiation, should examine reasons for treatment discontinuation [10, 28, 29, 30, 31] to inform targeted adherence interventions that are feasible [32, 33, 34, 35] in these settings.
Overall, a substantial proportion of patients on ART lacked a documented follow up CD4 and VL. South African guidelines stipulate that all HIV+ patients should have a CD4 count done upon ART initiation and every six months subsequently, and have a VL test done every six months post-ART initiation. However, in our rural South African primary health clinics, documentation was lacking and disproportionately affected those who became LTFU. It is unclear from this retrospective study whether this reflects inadequate systems to perform the test or record test results, or whether this is truly a marker for LTFU [36, 37, 38]. If the latter, lack of follow up testing could serve to identify patients at high risk of disengagement who need intervention.
Our data also suggest that there may be differences in retention according to primary care clinic. We speculate that clinic workload may play a role in differences in retention in care, though this data was not available in our review. These potential differences need to be evaluated and confirmed in future studies and the reasons for the disparities in retention among primary care clinics need to be explored.
We recognize several limitations to this study. First, national death registries were not able to be checked due to the lack of national ID numbers on many patient files. It is likely some patients on the true LTFU list have died. Secondly, we were only able to cross-reference the COSH LTFU list with the PHCs within the sub-district. We cannot account for patients who may have moved to other regions outside of Msinga. Third, our review focused on data recorded in the tier.net database, which unlike paper records, had a limited number of variables recorded. Data on clinical status of individual patients was not available. Fourth, the database recorded only the most recent CD4 count, precluding measuring impact of the nadir or baseline CD4 count. Furthermore, most patients are initiated on ART by nurses at primary care clinics in Msinga sub-district; the central hospital HIV clinic initiates patients who may have been recently admitted to the hospital or are otherwise more complicated and referred by the primary care clinics. Therefore, the study population may be different than the general population of ART initiators in this region and the results may not be generalizable. Further comparisons with those discontinuing ART at the primary care clinics are needed. Additionally, despite thorough efforts to account for duplication, it is possible that some duplicate files remained in the dataset. Lastly, this is a retrospective review of routine medical records and subject to missing data.
Conclusion
Though we identified a slightly lower LTFU rate than in other studies, this study confirmed that retention in care is emerging as a major problem in under-resourced settings, similar to developed nations. Increased efforts to target those at risk, in particular men, those age 18–35, those having lower CD4 count (<200), and those on ART for a shorter period of time (<6 months), are needed to prevent LTFU and improve overall VL suppression, reduce transmission, and decrease HIV incidence. Increased emphasis is also needed on ensuring the performance and recording of indicated lab tests and improving linkage to death registry data. Future research that develops and evaluates strategies for reducing disengagement, particularly in the first six months after ART initiation, improving medication adherence, and reengaging those who have discontinued ART is warranted.
Supporting information
(XLSX)
Acknowledgments
We would like to acknowledge the South African Department of Health, in particular those at COSH and the PHCs in Msinga sub-district. We would also like to acknowledge Dr. Elizabeth Bradley for her advice and thoughtful review of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
RA received funding from Yale College, Program for Global Affairs, http://catalog.yale.edu/ycps/subjects-of-instruction/global-affairs/. SVS received funding from National Institutes of Allergy and Infectious Diseases, K23AI089260. The spouse of author Sheela V Shenoi works part time for Amgen Pharmaceuticals. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Global health sector response to HIV, 200–2015: Focus on innovations in Africa: Progress report. World Health Organization, 2015.
- 2.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [DOI] [PubMed]
- 3.Rosen S, Fox M, Gill C. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. Plos Med. 2007;4(10):e298 10.1371/journal.pmed.0040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 Suppl 1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kranzer K GD, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383 10.7448/IAS.15.2.17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budgell EP, Maskew M, Long L, Sanne I, Fox MP. Does most mortality in patients on ART occur in care or after lost to follow-up? Evidence from the Themba Lethu Clinic, South Africa. J Acquir Immune Defic Syndr. 2015;70(3):323–328. 10.1097/QAI.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantell JE, DiCarlo AL, Remien RH, Zerbe A, Morris D, Pitt B, et al. ‘There’s no place like home’: perceptions of home-based HIV testing in Lesotho. Health Educ Res. 2014;29(3):456–469. 10.1093/her/cyu004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mberi MN, Kuonza LR, Dube NM, Nattey C, Manda S, Summers R. Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004–2012: a cohort study. BMC Health Serv Res. 2015;15:259 10.1186/s12913-015-0912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790 10.1371/journal.pone.0005790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeliger KB, Niccolai LM, Mtungwa LN, Moll A, Shenoi SV. Antiretroviral therapy initiation and adherence in rural South Africa: community health workers' perspectives on barriers and facilitators. AIDS Care. 2016;28(8):982–993. 10.1080/09540121.2016.1164292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Glob Health Action. 2013;6(21682). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Cutsem G, Ford N, Hildebrand K, Goemaere E, Mathee S, Abrahams M, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6(2):e14684 10.1371/journal.pone.0014684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billong SC, Fokam J, Penda CI, Amadou S, Kob DS, Billong E, et al. Predictors of poor retention on antiretroviral early warning indicator in Cameroon: results from a nationwide systematic random sampling. BMC Infectious Diseases. 2016;16(678). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox M, Bor J, MacLeod W, Maskew M, Brennan A, Stevens W, Carmona S. Is Retention on ART Underestimated Due to Patient Transfers? Estimating System-Wide Retention Using a National Labs Database in South Africa. 21st International AIDS Conference, Durban, Abstract TUAB0205, 2016.
- 15.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12:687–694. 10.1111/j.1365-3156.2007.01830.x [DOI] [PubMed] [Google Scholar]
- 16.Dalal RP, MacPhail C, Mqhayi M, Wing J, Feldman C, Chersich MF, Venter WDF. Characteristics and Outcomes of Adult Patients Lost to Follow-Up at an Antiretroviral Treatment Clinic in Johannesburg, South Africa J Acquir Immune Defic Syndr. 2008;47(1):101–107. [DOI] [PubMed] [Google Scholar]
- 17.Yehia BR, Stephens-Shields AJ, Fleishman JA, Berry SA, Agwu AL, Metlay JP, et al. The HIV Care Continuum: Changes Over Time in Retention in Care and Viral Suppression. PLoS One. 2015;10(6):e0129376 10.1371/journal.pone.0129376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in Care and Connection to Care Among HIV-Infected Patients on Antiretroviral Therapy in Africa: Estimation via a Sampling-Based Approach. PLoS One. 2011;6(7):e21797 10.1371/journal.pone.0021797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massyn N, Day C, Peer N, Padarath A, Barron P, English R. District health barometer 2013/14. Durban, South Africa: Health Systems Trust, 2014. [Google Scholar]
- 20.Grey A, Conradie F, Crowley T, Gaede B, Gils T, Shroufi A, et al. Improving access to antiretrovirals in rural South Africa—A call to action. SAMJ: The South African Medical Journal. 2015;105(8). [DOI] [PubMed] [Google Scholar]
- 21.Magadzire BP, Marchal B, Ward K. Improving access to medicines through centralised dispensing in the public sector: a case study of the Chronic Dispensing Unit in the Western Cape Province, South Africa. BMC Health Services Research. 2015;15:513 10.1186/s12913-015-1164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osler M, Hilderbrand K, Hennessey C, Arendse J, Goemaere E, Ford N, Boulle A. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. Journal of the International AIDS Society. 2013;17:18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marson KG, Tapia K, Kohler P, McGrath CJ, John-Stewart GC, Richardson BA, et al. Male, mobile, and moneyed: loss to follow-up vs. transfer of care in an urban African antiretroviral treatment clinic. PLoS One. 2013;8(10):e78900 10.1371/journal.pone.0078900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabatabai J, Namakhoma I, Tweya H, Phiri S, Schnitzler P, Neuhann F. Understanding reasons for treatment interruption amongst patients on antiretroviral therapy—a qualitative study at the Lighthouse Clinic, Lilongwe, Malawi. Global Health Action. 2014;7:24795 10.3402/gha.v7.24795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkhof M, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790 10.1371/journal.pone.0005790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimsrud A, Cornell M, Schomaker M, Fox MP, Orrell C, Prozesky H, et al. CD4 count at antiretroviral therapy initiation and the risk of loss to follow-up: results from a multicenter cohort study. Journal of Epidemiology & Community Health. 2016;70(6):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeliger KB, Niccolai LM, Mtungwa LN, Moll A, Shenoi SV. Antiretroviral therapy initiation in rural South Africa: community health workers’ perspectives on barriers and facilitators. AIDS Care. 2016;28(8):982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-reported barriers to adherence to antiretroviral therapy: A systematic review and meta-analysis. PLoS Medicine. 2016;13(11):e1002183 10.1371/journal.pmed.1002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM, Bimirew MA, Kassie DM. Barriers and facilitators of adherence to antiretroviral drug therapy and retention in care among adult HIV-positive patients: A qualitative study from Ethiopia. PLoS One. 2014;9(5):e97353 10.1371/journal.pone.0097353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musumari PM, Feldman MD, Techasrivichien T, Wouters E, Ono-Kihara M, Kihara M. “If I have nothing to eat, I get angry and push the pill bottle away from me”: A qualitative study of patient determinants of adherence to antiretroviral therapy in the Democratic Republic of Congo. AIDS Care. 2013;25(10):1271–1277. 10.1080/09540121.2013.764391 [DOI] [PubMed] [Google Scholar]
- 31.Loeliger KB, Niccolai LM, Mtungwa LN, Moll A, Shenoi SV. “I have to push him with a wheelbarrow to the clinic”: Community health workers’ roles, needs, and strategies to improve HIV care in rural South Africa. AIDS Patient Care & STDs. 2016;30(8):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Implementation and operational research: Community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: A cohort study. Journal of Acquired Immune Deficiency Syndromes. 2016;71(1):e16–23. [DOI] [PubMed] [Google Scholar]
- 33.Kanters S, Park JJ, Chan K, Ford N, Forrest J, Thorlund K, et al. Use of peers to improve adherence to antiretroviral therapy: A global network meta-analysis. Journal of the International AIDS Society. 2016;19(1):21141 10.7448/IAS.19.1.21141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): A randomised trial. Lancet. 2010;376(9755):1838–1845. 10.1016/S0140-6736(10)61997-6 [DOI] [PubMed] [Google Scholar]
- 35.Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: A systematic review and network meta-analysis. Lancet HIV. 2016;S2352-3018(16)30206-5. [DOI] [PubMed] [Google Scholar]
- 36.Rutstein SE, Golin CE, Wheeler SB, Kamwendo D, Hosseinipour MC, Weinberger M, et al. On the front line of HIV virological monitoring: Barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care, 28(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries, January 2015-June 2016. Morbidity and Mortality Weekly Report. 2016;65(47):1332–1335. 10.15585/mmwr.mm6547a2 [DOI] [PubMed] [Google Scholar]
- 38.Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: Current and future implementation challengers. Clinical Infectious Diseases. 2016;62(8):1043–10 10.1093/cid/ciw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.