Abstract
The cerebral vasculature incorporates several fail-safes that must be breached before an irreversible ischemic event takes place. In particular, when autoregulatory vasodilatation fails secondary to falling cerebral perfusion pressure (CPP; stage I hemodynamic failure), increases in the oxygen extraction fraction work to maintain the cerebral metabolic rate of oxygen. Previously, failure of this mechanism, stage II hemodynamic failure, or misery perfusion, has been imaged via positron emission tomography/computed tomography (PET/CT). Current susceptibility-weighted sequences (SWI) allow for more efficient imaging of this physiology. In this case, we identify an incident of reversible ischemia caused by spontaneous carotid artery dissection using a combination of diffusion weighted imaging (DWI) and SWI. The level of hemodynamic failure identified by the imaging sequences elevated the urgency of neurointervention, expediting the patient’s arrival to the neurointerventional table and thus avoiding impending irreversible ischemia.
Keywords: cerebral misery perfusion, susceptibility-weighted imaging, susceptibility weighted imaging, SWI, carotid artery dissection, vertebral artery dissection, cerebral autoregulation, hemodynamic failure, neurointerventional radiology, neuroradiology, neuroanatomy, stroke intervention, therapeutic cerebral angioplasty, therapeutic cerebral stenting, misery perfusion, cerebral angiography
CASE REPORT
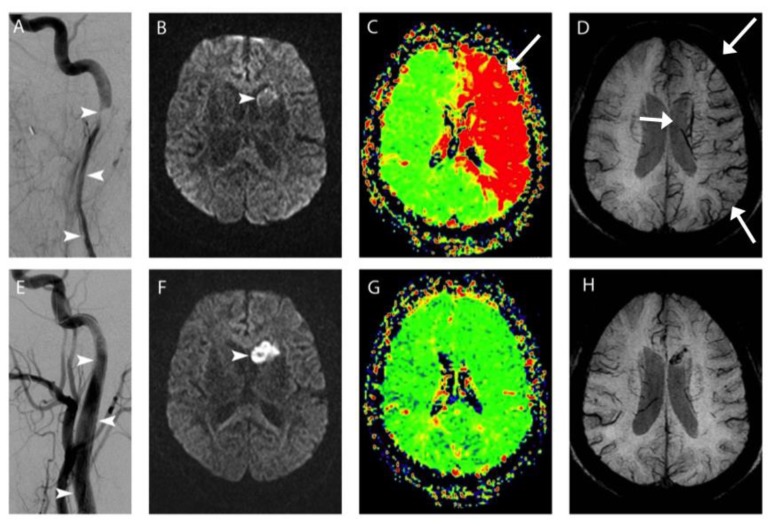
A 57-year-old female presented to the emergency room approximately four hours after the onset of right-sided weakness and slurred speech. On physical examination, the patient was dysarthric with right-sided hemiparesis and facial droop. Magnetic resonance imaging (MRI) of the brain showed major mismatch between a 2.0 cc area of subtle diffusion restriction in the left caudate head (arrowhead, Figure 1, Panel B) and a 500 cc area of prolonged mean transit time involving the left anterior and middle cerebral artery territories (Figure 1, Panel C), consistent with a small core infarct and large ischemic penumbra. On SWI the cortical, deep medullary, and subependymal veins showed marked prominence in the left cerebral hemisphere (Figure 1, Panel D); this may represent stage II hemodynamic failure, or misery perfusion, as increased oxygen extraction leads to elevated concentration of venous deoxyhemoglobin, which appears dark on SWI. Emergency cerebral angiography revealed a long segment of severe narrowing in the cervical left internal carotid artery caused by acute dissection (arrowheads, Figure 1, Panel A). After stenting, the artery was restored to wide patency (Figure 1, Panel E) and repeat MRI 26 hours later showed resolution of the prior left cerebral misery perfusion (Figure 1, Panels G, H) and minimal enlargement of the left caudate infarct to involve the anterior putamen (Figure 1, Panel F). The patient was discharged home three days later with trace right-sided weakness that fully resolved before her 2-month clinic visit.
Figure 1.
57-year old female with left internal carotid artery dissection, and stroke symptoms including slurred speech and right-sided weakness.
FINDINGS: MRI prior to emergency neurointervention demonstrates mild diffusion restriction (arrowhead, Panel B), with a large area of perfusion defect as evidenced by increased Mean Transit Time (MTT; arrow, Panel C) and prominent hypodensities on SWI throughout the left hemisphere (arrows, Panel D). At emergency neurointervention, carotid angiography revealed a long segment of severe narrowing in the cervical left internal carotid artery caused by acute dissection (arrowheads, Panel A). After stenting, the artery was restored to wide patency (arrowheads, Panel E) and repeat MRI 26 hours later showed resolution of the prior left cerebral misery perfusion (Panels G, H) and minimal enlargement of the left caudate infarct to involve the anterior putamen (arrowhead, Panel F).
TECHNIQUE:
PANEL A AND F, left carotid angiographic images obtained during bolus injection of Iohexol iodinated contrast before (A) and after (F) stenting.
REMAINING PANELS IMAGED USING A Siemens TrioTim 3T MRI SCANNER, USING A 12-CHANNEL HEAD COIL. PANEL B, DWI images obtained using the following parameters: TR 5800, TE 102, 3mm slice thickness with 3.9mm spacing, FOV 192 × 192. PANEL C, MTT images obtained using the following parameters: TR 2850, TE 32, 3mm slice thickness with 3.9mm spacing, FOV 128 × 128, after 17.2cc Gadodiamide gadolinium-based contrast agent. PANEL D, SWI images obtained using the following parameters: TR 29, TE 20, 2mm slice thickness with no spacing, FOV 448 × 336. PANEL F, DWI images obtained using the following parameters: TR 5800, TE 102, 3mm slice thickness with 3.9mm spacing, FOV 192 × 192. PANEL G, MTT images obtained using the following parameters: TR 2850, TE 32, 3mm slice thickness with 3.9mm spacing, FOV 128 × 128, after 15.0cc Gadodiamide gadolinium-based contrast agent. PANEL H, SWI images obtained using the following parameters: TR 29, TE 20, 2mm slice thickness with no spacing, FOV 448 × 336.
DISCUSSION
Overview of Cerebral Ischemia
Cerebral ischemia is an important clinical pathology commonly encountered in the hospital setting. At presentation, patients undergoing an ischemic event will have two types of affected tissues in the brain. First, the infarcted core represents the tissue that has undergone irreversible ischemia, tissue that cannot be revived. However, surrounding this region is a region of at-risk tissue, the ischemic penumbra. The ischemic penumbra is commonly identified by MRI using perfusion weighted sequences. The goal of neurointerventional therapy is to revascularize at-risk tissues, including those in Type I and Type II hemodynamic failure, in which the ischemia can be reversed, before they progress to an irreversible infarction.
Etiology
The etiology of spontaneous carotid artery dissection can be multifactorial, related to mechanical factors[1], genetic disease (<5% [2]), connective tissues diseases, or other causes[3]. However, the mechanism of injury is the same, with a tear in the tunica intima within the media of the carotid, with the vasa vasorum as the potential origin. Then, blood dissects along the artery, leading to intramural hematoma which then condenses into a thrombus, thus narrowing the lumen of the artery and potentially embolizing into the brain.
Demographics
Spontaneous carotid artery dissections, while occurring rarely (2.5–3 per 100,000)[4], are increasingly found to be the cause of ischemic events like transient ischemic attack or stroke[5]. Given the rare occurrence of spontaneous carotid artery dissections, it is important to be aware of the potential for occlusive ischemia. As such, the use of SWI in concert with MR perfusion and DWI can be used to identify patients with the most limited metabolic reserve. Identifying such patients will support the use of urgent intervention to prevent acute stroke [6,7,8].
The condition is more common in men than in women, although there is no difference in outcome or mortality for sexes [9]. One study investigating a group of 135 patients with known symptomatic internal carotid artery dissection found a mean age of carotid artery dissection to be 47.0 years [10].
Clinical & Imaging Findings
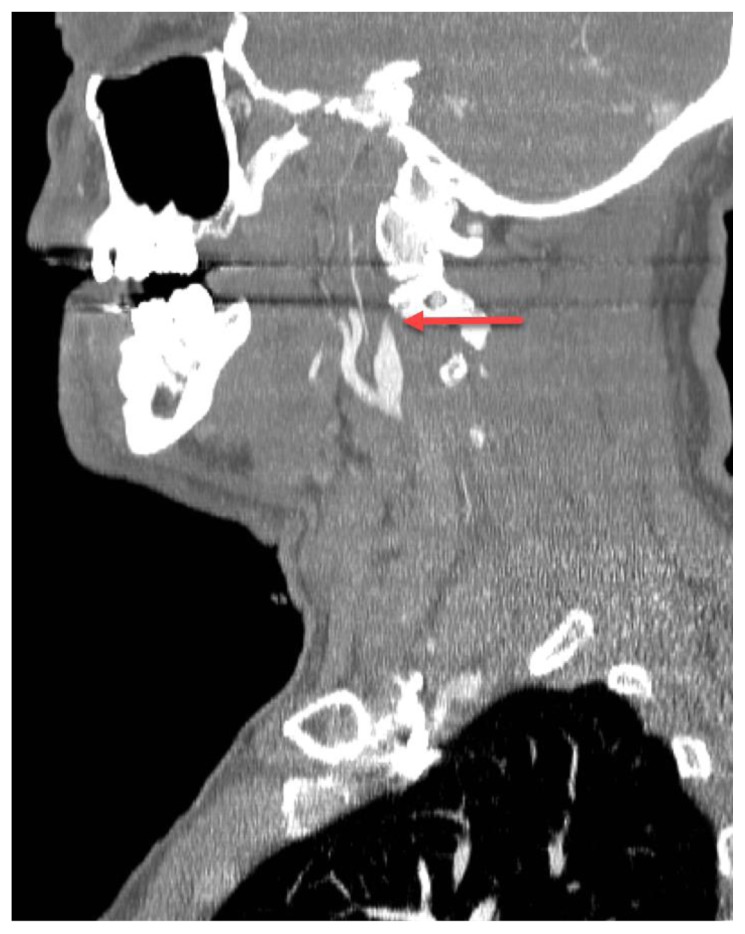
Spontaneous internal carotid artery dissection can be imaged on CT angiography, showing tapering of the dissected vessel wall (Figure 2). CT angiography, however, is not sufficient for visualization of cerebral metabolism.
Figure 2.
63 year old male with internal carotid artery dissection.
FINDINGS: A single sagittal slice from CT angiogram (arterial phase post-contrast images) on a different patient than that in the test case demonstrates tapering of a dissected internal carotid artery. This is the classical “flame-shaped” appearance of a carotid artery dissection (arrow).
TECHNIQUE: Using a 128-slice GE Discovery CT 750 HD CT scanner, 120kV, 350mAs, following injection of 120cc Iohexol iodinated contrast, helical images were obtained and reconstructed into the sagittal plane.
In the past, the visualization of cerebral metabolism was limited to techniques requiring ionizing radiation, such as PET/CT [11]. The development of MRI techniques, such as DWI and MR perfusion, now allows visualization of physiologic changes without exposing patients to ionizing radiation. However, DWI and MR perfusion are limited in how they visualize cerebral metabolism in stroke and reversible ischemia. In DWI, we observe bright signal in areas of restricted water diffusion, which in the case of a stroke is caused by cytotoxic edema leading to high intracellular water content and ultimately apoptosis. MR perfusion imaging visualizes altered regional blood flow parameters by recording decreased signal loss as the paramagnetic tracer flows more slowly through affected tissues than non-affected tissues. This allows for the extraction of transit time and blood volume information, from which ischemic and infarcted characteristics may be inferred.
We would like to emphasize the utility of a newer MRI technique, SWI, which presents additional physiologic data by showing the paramagnetic metabolic byproduct, deoxyhemoglobin, in concentration-dependent fashion [12]. As the oxygen extraction fraction increases as a response to decreased CPP, SWI sequences demonstrate darker and thicker venous structures compared to normally-perfused tissues. As a result, there may be visualization of stage II hemodynamic failure, which was previously only possible with PET/CT [6,7,8].
Treatment & Prognosis
The available therapies for spontaneous carotid artery dissection include therapeutic anticoagulation/antiplatelet agents and endovascular stenting. While medical management is preferred, there are several indications for progressing to endovascular therapy, such as TIA or evidence of ischemia despite medical therapy, poor collateral circulation, or aneurysms of either the contralateral vessel or anterior communicating artery [13].
The prognosis of spontaneous carotid artery dissection is generally good, with <5% of cases ending in death, and of those who progress to stroke, approximately 75% make a good functional recovery [3]. While there is limited data on cases that are specifically found to be in the misery perfusion phase of ischemia, these patients will most likely progress to stroke without urgent diagnosis and therapy.
This case illustrates the utility of combining SWI as it was used to guide medical decision making in this case of ischemia due to a carotid artery dissection. Specifically, the addition of SWI sequences helped differentiate type II ischemia (increased oxygen extraction) from the milder type I ischemia (autoregulatory vasodilatation), affording a better risk estimate of progression to infarct, a discrimination that could not be made solely with the DWI and perfusion data alone. SWI is a relatively short MRI sequence that is readily available at many imaging centers, and provides a more time-efficient and radiation-safe method of imaging compared to the current standard of PET/CT.
Differential Diagnoses
Spontaneous carotid artery dissection can be occlusive or non-occlusive. In the case of this patient, the differential also includes acute intracranial vasculature thromboembolic occlusion. The differentiation of the three depends on the imaging modality used to elucidate the cause of the patient’s symptoms.
Angiographically, an acute intracranial vasculature thromboembolic occlusion would present with an arterial filling defect without any signs of vascular defect indicating a dissection. In contrast, a dissection would be itself visualized, and the occlusive nature of the dissection would be revealed by an arterial filling defect. Both would exhibit a loss opacification of distal vasculature.
On CT angiography, an acute thromboembolic occlusion would present differently based on the timing of the insult. In the immediate stage (<1 hour), there may be no findings, though a hyperdense vessel may be identified on a non-contrast CT study, corresponding to an intravascular thromboembolism. Thereafter, in the hyperacute phase (1–3 hours), a non-contrast CT study would show hypodensity in the affected deep grey nuclei with loss of grey-white differentiation, and a CT angiography study would show hypodensity in the area of the affected vessel as a filling defect. A dissection, on the other hand, would show the same findings on CT angiography as an acute thromboembolic occlusion if it is occlusive, but would also present with a vascular wall abnormality in the area of the carotid with the dissection whether it is occlusive or non-occlusive. On sagittal imaging, this occlusion is classically flame-shaped in the acute phase.
Both CT and MR perfusion imaging would show decreased cerebral blood flow and increased time to peak flow in an acute cerebral infarction, with similar findings of decreased cerebral blood flow and increased time to peak flow in the ischemic penumbra. MRI allows the added benefit of DWI with apparent diffusion coefficient (ADC) maps, where diffusion restriction is visualized in the infarcted tissue, and confirmed with ADC maps, which eliminate false positives caused by T2 shinethrough. Inconsistency between the area of infarct (as defined by DWI/ADC map) and delayed perfusion (as defined by perfusion imaging) indicates a perfusion/diffusion mismatch in the at-risk tissue, or penumbra. In an occlusive carotid artery dissection, the CT and MR perfusion findings describe above would be the same, except that there would also be an area of vascular filling defect in the affected portion of the carotid artery on CT, MR, or diagnostic angiography. If the dissection is not hemodynamically significant, there is a visualized vascular filling defect in the area of the dissection on CT, MR, or diagnostic angiography, but preserved cerebral blood flow and no diffusion restriction.
With the addition of SWI, MR imaging can be used to further characterize the at-risk tissue by imaging venous deoxyhemoglobin, allowing for the correlation to cerebral misery perfusion. This, therefore, may allow for the distinction between stage I and stage II hemodynamic failure and demonstration of critically at-risk tissue necessitating urgent neurointervention, as in this case [6,7,8].
TEACHING POINT
Susceptibility weighted imaging may allow for distinguishing between type II ischemia (increased oxygen extraction) from type I ischemia (autoregulatory vasodilatation) in patients with cerebrovascular accident without exposure to ionizing radiation.
Table 1.
Summary table for Spontaneous Carotid Artery Dissection.
| Etiology | Tear in the intima along vasa vasorum |
| Incidence | 2.5–3 per 100,000 |
| Gender ratio | M>F |
| Age predilection | 40s |
| Risk factors | Hyperhomocysteinemia, manipulative therapy of the neck, α-1 antitrypsin deficiency, connective tissue disease, genetic abnormalities |
| Treatment | Antithrombotics/anticoagulation Endovascular stenting |
| Prognosis | <5% mortality 75% recovery of function if progression to stroke |
| Imaging findings |
Catheter Angiography: Vascular wall abnormality with arterial filling defect, classically flame shaped and absent distal vessels CT Angiography: Filling defect in the affected vessel, classically flame shaped, with distal hypodensity MR Perfusion (PWI) + Diffusion-weighted imaging (DWI) Reversible ischemia: DWI/PWI mismatch; restriction of cerebral blood flow, cerebral blood volume, and increase time to peak flow in the area of reversible ischemia without diffusion restriction Irreversible ischemia: No DWI/PWI mismatch; restriction of cerebral blood flow, cerebral blood volume, and increase time to peak flow in the area of reversible ischemia with diffusion restriction MR Perfusion + DWI + Susceptibility-weighted imaging (SWI) Stage I hemodynamic failure: DWI/PWI mismatch without increased prominence of the venous vasculature Stage II hemodynamic failure (misery perfusion): Increased prominence of the venous vasculature in the area of DWI/PWI mismatch Irreversible ischemia: Diffusion restriction with decreased blood flow (no DWI/PWI mismatch), and prominence of the venous vasculature on SWI |
Table 2.
Differential diagnosis of Acute-Onset Slurred Speech and Weakness.
| Diagnosis | Catheter Angiography | CT Angiography | MR Perfusion + DWI | MR Perfusion + DWI + SWI |
|---|---|---|---|---|
| Occlusive Carotid Artery Dissection | Vascular wall abnormality with arterial filling defect, classically flame shaped, and absent distal vessels | Filling defect in the affected vessel, classically flame shaped, with distal hypodensity |
Reversible ischemia: DWI/PWI mismatch; restriction of cerebral blood flow, cerebral blood volume, and increase time to peak flow in the area of reversible ischemia without diffusion restriction Irreversible ischemia: No DWI/PWI mismatch; restriction of cerebral blood flow, cerebral blood volume, and increase time to peak flow in the area of reversible ischemia with diffusion restriction |
Stage I hemodynamic failure: DWI/PWI mismatch without increased prominence of the venous vasculature Stage II hemodynamic failure (misery perfusion): Increased prominence of the venous vasculature in the area of DWI/PWI mismatch Irreversible ischemia: Diffusion restriction with decreased blood flow (no DWI/PWI mismatch), and prominence of the venous vasculature on SWI |
| Non-Hemodynamically Significant (<50–70% Occlusive) Carotid Artery Dissection† | Vascular wall abnormality with arterial filling defect and normally visualized vessels distally | Vascular wall abnormality in the affected vessel with distal contrast opacification | Normal cerebral blood flow, cerebral blood volume, and time to peak flow, without diffusion restriction | No diffusion restriction or decreased cerebral blood flow |
| Acute intracranial vasculature thromboembolic occlusion | No vascular wall abnormality proximally, with arterial filling defect with absent distal vessels at the site of thromboembolic occlusion. |
Early (<1hr): normal findings Late (>1hr): CT Angiogram: hypodense findings in the distribution of the affected vessel Noncontrast CT: Hyperdense lesion in affected artery representing acute thrombus |
Decreased cerebral blood flow, variable cerebral blood volume, and increased time to peak flow on PWI, with diffusion restriction in the area of the infarct and DWI/PWI mismatch in the at-risk tissue of the penumbra |
Stage I hemodynamic failure: DWI/PWI mismatch without increased prominence of the venous vasculature Stage II hemodynamic failure (misery perfusion): Increased prominence of the venous vasculature in the area of DWI/PWI mismatch Irreversible ischemia: Diffusion restriction with decreased blood flow (no DWI/PWI mismatch), and prominence of the venous vasculature on SWI |
This assumes poor collateral flow in the setting of an incomplete circle of Willis. In the setting of adequate collateral flow, no change in perfusion parameters or diffusion characteristics will be seen on MRI.
ABBREVIATIONS
- CT
Computed Tomography
- DWI
Diffusion Weighted Imaging
- MRI
Magnetic Resonance Imaging
- MTT
Mean Transit Time
- PWI
Perfusion Weighted Imaging
- SWI
Susceptibility Weighted Imaging
REFERENCES
- 1.Rubinstein SM1, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005 Jul;36(7):1575–80. doi: 10.1161/01.STR.0000169919.73219.30. Epub 2005 Jun 2. [DOI] [PubMed] [Google Scholar]
- 2.Shea K, Stahmer S. Carotid and vertebral arterial dissections in the emergency department. Emerg Med Pract. 2012 Apr;14(4):1–23. [PubMed] [Google Scholar]
- 3.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009 Jul;8(7):668–78. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 4.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001 Mar 22;344(12):898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 5.Redekop GJ. Extracranial carotid and vertebral artery dissection: a review. Can J Neurol Sci. 2008 May;35(2):146–52. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- 6.Kavec M, Gröhn OH, Kettunen MI, Silvennoinen MJ, Penttonen M, Kauppinen RA. Use of spin echo T(2) BOLD in assessment of cerebral misery perfusion at 1.5 T. MAGMA. 2001 Mar;12(1):32–9. doi: 10.1007/BF02678271. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka M1, Okuchi K, Iwamura A, Taoka T, Siesjö BK. A mismatch between the abnormalities in diffusion- and susceptibility-weighted magnetic resonance imaging may represent an acute ischemic penumbra with misery perfusion. J Stroke Cerebrovasc Dis. 2013 Nov;22(8):1428–31. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.009. Epub 2013 Feb 12. [DOI] [PubMed] [Google Scholar]
- 8.Hingwala D1, Kesavadas C, Thomas B, Kapilamoorthy TR. Clinical utility of susceptibility-weighted imaging in vascular diseases of the brain. Neurol India. 2010 Jul-Aug;58(4):602–7. doi: 10.4103/0028-3886.68667. [DOI] [PubMed] [Google Scholar]
- 9.Arnold M, Kappeler L, Georgiadis D, Berthet K, Keserue B, Bousser MG, Baumgartner RW. Gender differences in spontaneous cervical artery dissection. Neurology. 2006 Sep 26;67(6):1050–2. doi: 10.1212/01.wnl.0000237341.30854.6a. [DOI] [PubMed] [Google Scholar]
- 10.Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517–1522. doi: 10.1212/wnl.45.8.1517. [DOI] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002 Mar;125(Pt 3):595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 12.Kesavadas C, Santhosh K, Thomas B. Susceptibility weighted imaging in cerebral hypoperfusion-can we predict increased oxygen extraction fraction? Neuroradiology. 2010 Nov;52(11):1047–54. doi: 10.1007/s00234-010-0733-2. [DOI] [PubMed] [Google Scholar]
- 13.Malek AM, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000 Aug;21(7):1280–1092. [PMC free article] [PubMed] [Google Scholar]