Abstract
Alexander disease, also known as fibrinoid leukodystrophy, is a rare leukoencephalopathy which occurs due to a mutation in the glial fibrillary acid protein (GFAP) gene. Magnetic resonance imaging (MRI) has proven to be highly sensitive in making the diagnosis. Typical MRI findings, in combination with positive genetic blood analysis, confirm the diagnosis.
Keywords: Alexander disease, Fibrinoid leukodystrophy, Infantile, Van der Knaap MRI diagnostic criteria, Frontal lobe predominance
CASE REPORT
A 14 month old African boy was referred to our MRI department with a history of seizures which were increasing in frequency despite oral treatment with phenobarbital. The first reported episode of seizures was at 14 weeks of age. Clinically, the child had psychomotor developmental delay, but no spasticity. The head circumference was within normal limits for the child’s age, and he was feeding well. There was no family history of similar symptoms in other siblings, or of previous child loss.
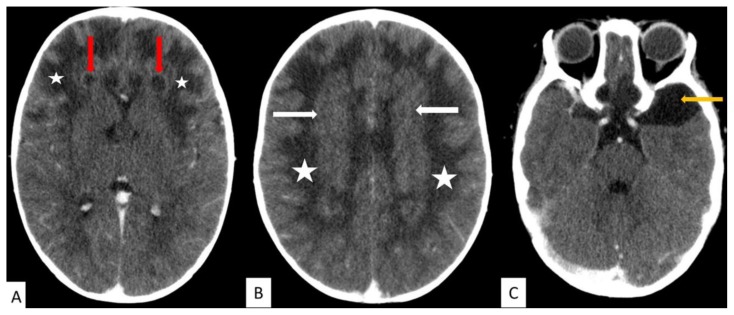
A computer tomography (CT) scan of the brain performed at the base hospital with a Toshiba CT scan, demonstrated diffuse white matter hypodensity predominantly involving the fronto-parietal lobes, rim-enhancing cysts in the frontal lobes, as well as thick nodular minimally enhancing periventricular soft tissue density (Fig. 1). An incidental left temporal arachnoid cyst was also present. The patient was referred to the neurosurgery department at a quaternary hospital who suggested further investigation with MRI.
Figure 1.
14 month old male with Alexander disease.
FINDINGS: Diffuse white matter hypodensity involving the fronto-parietal lobes bilaterally (white stars in image A and B).
A. Rim enhancing cysts in the frontal lobes bilaterally (red arrows).
B. Minimally enhancing thick periventricular soft tissue density (white arrows).
C. Incidental left anterior temporal lobe arachnoid cyst (yellow arrow).
TECHNIQUE: Toshiba CT scanner, post contrast CT brain, 10mls Omnipaque (300), 100kV, 167mAs, 1.5mm slice thickness.
MRI was performed using a Philips Intera, 1.5 Tesla machine. Imaging demonstrated diffuse, symmetrical, subcortical and deep white matter T2-weighted (T2W) hyperintensity, predominantly involving the fronto-parietal regions with extension to the anterior temporal lobes and occipital lobes (Figs. 2 and 3). This signal abnormality extended to the external and extreme capsules. There was a thick periventricular rim which was predominantly T2W hypointense and T1-weighted (T1W) hyperintense (Figs. 2 and 3) with no diffusion restriction on diffusion weighted images (DWI) and apparent diffusion coefficient (ADC) (Figs. 3). This showed heterogenous, nodular enhancement on post contrast images (Fig. 4). There was swelling and hyperintense T2W signal intensity involving the basal ganglia (caudate and lentiform nuclei) bilaterally (Fig. 2). This demonstrated no restricted diffusion on ADC and DWI (Fig. 5), and enhancement of the caudate nucleus was demonstrated post contrast (Fig. 4). There were symmetrical, round, rim-enhancing cysts anterior to the frontal horns, and further, smaller non-enhancing cysts inferiorly in the frontal lobes (Fig. 2, 4 and 5). Nodular parenchymal enhancement was seen inferiorly in the frontal lobes, and there was also enhancement of the fornices (Fig. 4). The brainstem was normal. MRS was non-contributory as all the parameters were low in the affected white matter in the fronto-parietal lobes. The MRI findings were suggestive of leukoencephalopathy and the pattern of abnormality fulfilled the MRI diagnostic criteria for Alexander disease. Incidental arachnoid cysts were seen in the anterior left temporal and inferior frontal lobes (Fig. 2).
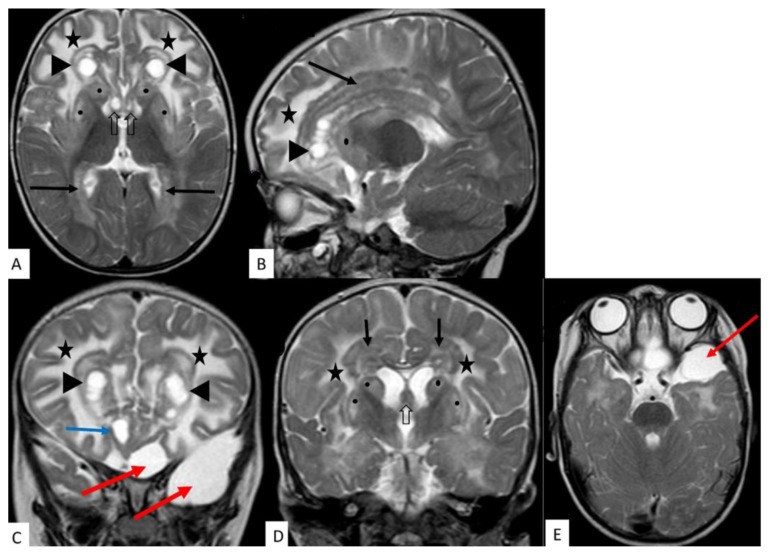
Figure 2.
14 month old male with Alexander disease.
FINDINGS: A, B, C and D demonstrate diffuse white matter hyperintensity (stars), predominantly involving the fronto-parietal and anterior temporal lobes with extension to the occipital lobes.
Round cysts in the frontal lobes with thick hypointense rim (A, B, C - arrowheads).
Further smaller cysts in the inferior frontal lobes (C - blue arrow).
Thick, nodular, predominantly hypointense periventricular tissue (A, B, D - black arrows).
Hyperintensity and swelling involving the caudate nucleus and lentiform nucleus (A, B, D - black dots).
Hyperintensity and swelling involving fornices (A & D - black open arrows)
Incidental arachnoid cysts in the left anterior temporal and inferior frontal regions (C and E- red arrows).
TECHNIQUE: MRI 1,5T; T2W (TE = 100ms, TR= 4509ms) ; 5mm slice thickness
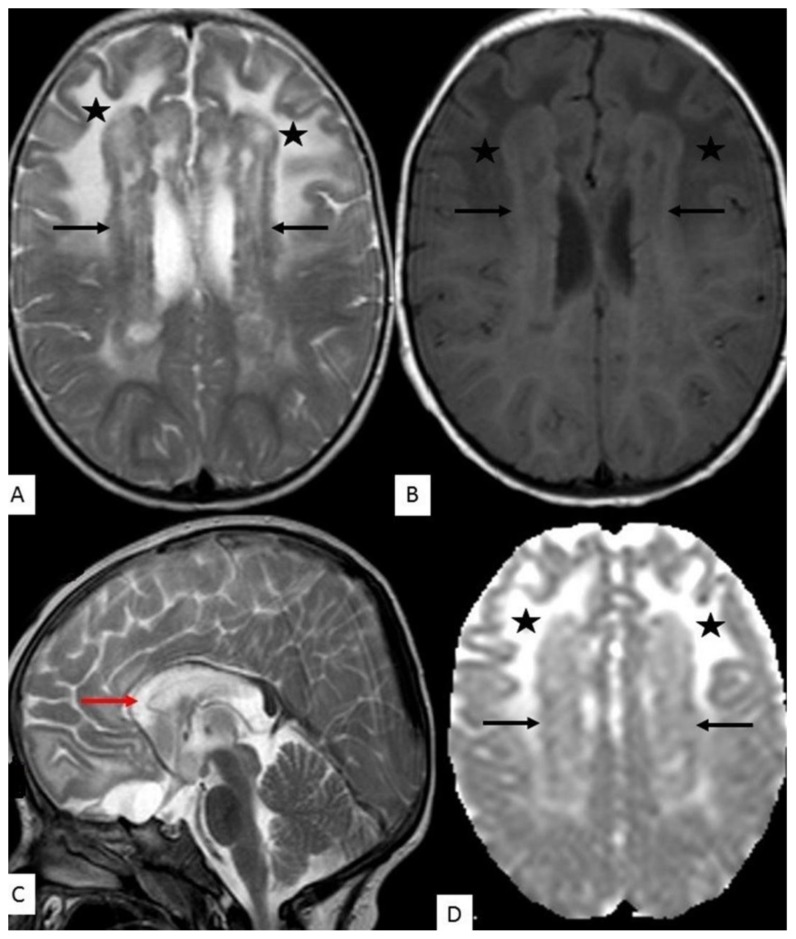
Figure 3.
14 month old male with Alexander disease.
FINDINGS:
A: T2W axial demonstrates thick, nodular periventricular tissue which is predominantly hypointense on T2W images (black arrows). Diffuse white matter hyperintensity (stars) involving subcortical and deep white matter with frontal predominance.
B: T1W axial demonstrates thick nodular periventricular tissue which is predominantly hyperintense on T1W images (black arrows). Diffuse T2W white matter hyperintensity is hypointense on T1W images (stars).
C: T2W mid-sagittal slice demonstrates extension of the hyperintense white matter signal abnormality across the corpus callosum (red arrow).
D: ADC map demonstrates that the periventricular tissue and the white matter signal abnormality has no restricted diffusion (black arrows and black stars, respectively).
TECHNIQUE: MRI 1,5T; T1W (TE = 633ms, TR =15ms), T2W (TE = 100ms, TR = 4509ms), ADC (TE = 3426ms, TR = 89ms), 5mm slice thickness
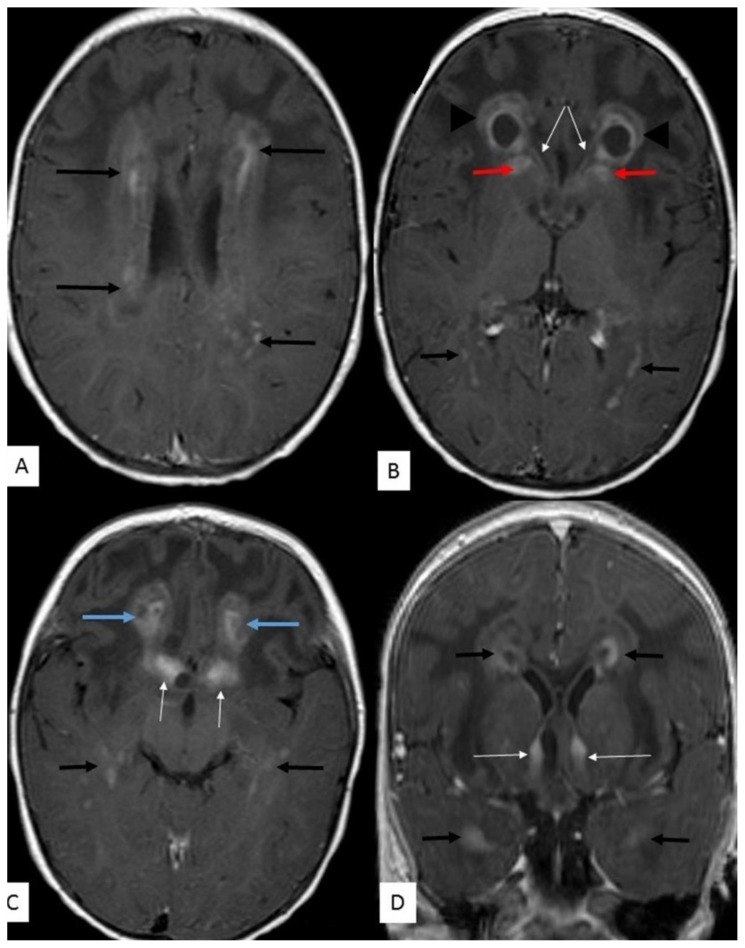
Figure 4.
14 month old male with Alexander disease.
FINDINGS:
A, B, C - Periventricular tissue demonstrates nodular and patchy enhancement (black arrows - A, B, C & D). This is also seen around the occipital (B) and temporal (C & D) horns of the lateral ventricles.
Frontal lobe cysts anterior to the frontal horns demonstrate thick rim enhancement (B - arrowheads).
Enhancement of the caudate nucleus (B - red arrows) and fornices (B, C & D - thin white arrows).
Parenchymal enhancement in the inferior frontal lobes (C - blue arrows).
TECHNIQUE: MRI 1,5T, T1W (TR = 15ms, TE = 633ms) post contrast, gadolinium 2mls IVI; axial images (A, B & C) - 5mm slice thickness; coronal reconstructed images (D) - 1mm slice thickness.
Figure 5.
14 month old male with Alexander disease.
FINDINGS:
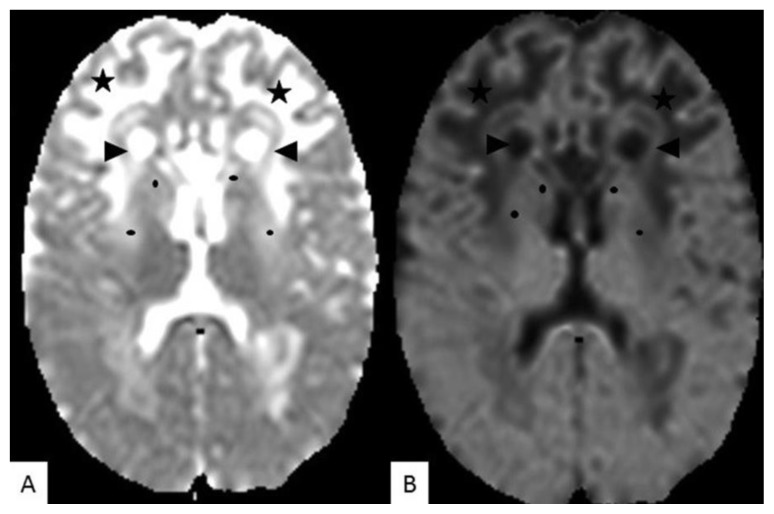
ADC map (A) and B1000 images (B), demonstrate no diffusion restriction in the pathological white matter signal intensity in the frontal lobes (stars), as well as the cysts anterior to the frontal horns (arrow heads). There is also no diffusion restriction in the caudate nucleus and lentiform (black dots).
TECHNIQUE: MRI 1,5 T, (A) - ADC (TE = 3426ms, TR = 89ms), (B) - DWI B 1000; 5mm slice thickness
The patient is currently being managed by neurosurgeons conservatively with antiepileptic treatment. The neurosurgeons have requested a repeat MRI scan in 1year to assess disease progression.
DISCUSSION
Etiology & Demographics
Alexander disease, also known as fibrinoid leukodystrophy, is a rare disease with several cases reported worldwide [1–5]. Larger case studies and research have been performed in Japan and China, where prevalence has been estimated to be 1 in 2.7 million individuals [3,6]. Alexander disease is a progressive leukoencephalopathy which typically shows frontal lobe predominance [1–5,7–11]. It was first described by Alexander in 1949 [4,6,8,9,12]. The disease is either inherited (autosomal dominant) or due to spontaneous mutations in the glial fibrillary acid protein (GFAP) gene. This results in the accumulation of Rosenthal fibres in astrocytes throughout the central nervous system (CNS), with subsequent astrocyte dysfunction [2,5]. Zang et al have demonstrated that 90% of de novo GFAP gene mutations were on the paternal allele, indicating male germ line transmission [5].
Clinical & Imaging findings
Three subtypes have been described: infantile (onset before age 2), juvenile (onset 2–14 years) and adult (between 2nd and 7th decade) [1,5,7–12]. A neonatal variant has also been distinguished and is more rapidly fatal [8–10]. The infantile type is the most frequently seen and typically presents with macrocephaly, rapid neurological deterioration and seizures [1,3,8–10,12]. Patients with the infantile type usually present within the first year of life and have a poor prognosis. Early death ensues, usually within the first 2–3 years, however cases of patients who survive to adolescence have been reported [1–3,5,7,9]. The juvenile type presents later in childhood with neurologic deterioration which is less rapid and progressive bulbar symptoms and spasticity [8,10]. The adult type is the least frequent and the symptoms and disease course can be indistinguishable from multiple sclerosis [1].
Serological tests are generally unhelpful in making the diagnosis [8]. The presence of highly elevated levels of GFAP in the cerebrospinal fluid (CSF) has been reported [9]. De novo heterozygous mutation in the GFAP gene has been implicated on genetic studies [2–7,9,10]. Histology demonstrates the accumulation of Rosenthal fibres (which are composed of GFAP, heat shock protein 27 and αβ-crystalline) on the astrocytes throughout the CNS [1–3,5–9,11,12]. This occurs in the subependymal, subpial and perivascular regions [1,3,6,8,11]. Genetic studies and histology confirm the diagnosis, however brain biopsy is an invasive procedure and can usually only be performed at autopsy [1,3,8,9].
In our low-resource setting, a CT scan is usually the initial investigation of choice due to relatively easy access and low cost. CT demonstrates decreased attenuation in the involved frontal white matter that gradually extends posteriorly into the parietal region and internal capsule. Contrast enhancement may be seen near the tips of the frontal horns early on in the disease [1].
MRI has been proven to have high sensitivity and specificity in making the diagnosis [1,4,8,9], and is also crucial in the observation of patients with Alexander disease [1,2,9]. It demonstrates diffuse T2W hyperintense white matter signal in the frontal lobes, initially affecting the subcortical white matter, which later progresses posteriorly to the parietal lobes, internal and external capsules [1,2,8,10,11]. This correlates with areas of deposition of the Rosenthal fibres and demyelination [2,8]. Disruption of the blood-brain barrier results in parenchymal contrast enhancement in the involved white matter [2,8,11]. Cyst formation occurs in the affected regions at a later stage [1,2,11]. The periventricular T2W hypointense rim, which is hyperintense on T1W images, is thought to be due to the accumulation of dense Rosenthal fibres [8,11]. Magnetic resonance spectroscopy (MRS) demonstrates high myo-inositol (mI), low N-acetylaspartate (NAA), high lactate, increased choline (Cho) and Cho>NAA [13].
Van der Knaap et al describe five MRI criteria. Presence of four or more criteria indicates a high specificity for the diagnosis of Alexander disease [2–4,7–10]:
Extensive cerebral white matter abnormalities with frontal predominance.
Periventricular rim which is T1W hyperintense and T2W hypointense.
Abnormal signal in the brainstem (most frequently midbrain and medulla).
Abnormal signal with swelling or volume loss in the basal ganglia and thalami (most pronounced in the head of the caudate nucleus and putamen).
Contrast enhancement in one or more of the following structures: optic chiasm, fornix, ventricular lining, periventricular rim, white matter in the frontal lobes, basal ganglia, thalami, dentate nucleus and brainstem.
Studies performed by Van der Knaap and Yoshida et al concluded that if a patient fulfils the MRI diagnostic criteria, a presumed diagnosis of Alexander disease can be made without histological confirmation [3,8]. The case patient demonstrated four of the Van der Knaap criteria.
Diagnostic guidelines established in China by Yoshida et al, classify the disease into three types using the combination of neurological and MRI findings [2,3,5]. These are: type 1 – cerebral, type 2 – bulbospinal, type 3 – intermediate form [2–5]. Revised classification by Prust et al classifies the disease into types 1 and 2 based on age of onset, symptoms and MRI findings [5,10]. Type 1 classification is characterised by early onset of symptoms (<4 years), macrocephaly, seizures, encephalopathy, developmental delay, paroxysmal deterioration, failure to thrive and typical MRI findings [5,10]. Type 2 is characterised by later onset, autonomic dysfunction, ocular movement abnormalities, bulbar symptoms, palatal myoclonus and atypical MRI findings (abnormal signal and/or atrophy in the medulla oblongata and cervical cord) [5,10]. The established prevalence is 60% for type 1 and 40% for type 2 classification [5,10]. According to these two classifications, the case patient had type 1 Alexander disease; however there was no macrocephaly at the time of MRI diagnosis.
Treatment & Prognosis
Patients with early onset of Alexander disease (type 1) have rapid progression of the disease with death occurring within 2–3 years; however Prust et al has demonstrated a mean survival of 14±1.8 years [1,5,9,10]. The juvenile and adult types have a slow progression. Treatment is supportive, including seizure control.
Differential Diagnoses
The differential diagnoses include other causes of megalencephalic leukodystrophy; namely Canavan disease and megalencephalic leukoencephalopathy with subcortical cysts (MLC or Van der Knaap disease) [2,8]. Canavan disease is characterised by extensive white matter abnormality (T2W high signal, T1W low signal) without frontal predominance, a high NAA peak on MRS and no contrast enhancement [1,8,11,13]. Subcortical white matter is involved early in the disease and extension to the internal and external capsules may be present [1,11]. Basal ganglia abnormalities involve the thalamus and the globus pallidus, sparing the putamen and caudate nucleus [8,11]. MLC or Van der Knaap disease is characterised by extensive symmetrical white matter abnormality with slight swelling and subcortical cysts in the fronto-parietal border zone and anterior temporal lobes [7,8,11]. There is sparing of the basal ganglia and thalami [11].
X-linked adrenoleukodystrophy can have atypical frontal lobe predominance [8]. There may be involvement of the diencephalic nuclei and brainstem (corticospinal, corticobulbar and auditory tracts) [8,11]. Classic X-linked adrenoleukodystrophy involves the peritrigonal deep white matter with extension across the splenium of the corpus callosum. Subcortical white matter is initially spared [1,11]. Abnormal white matter is T2W hyperintense and peripheral contrast enhancement indicates areas of active inflammation and a break in the blood-brain barrier [8,11].
Metachromatic leukodystrophy is characterised by frontal white matter abnormality [2,8] and involvement of the long tracts in the brainstem [8]. Abnormal white matter demonstrates high signal on T2W images with sparing of the subcortical white matter and a tigroid or leopard skin pattern (indicating sparing of perivascular white matter). There is no contrast enhancement [1].
TEACHING POINT
Alexander disease is a rare leukodystrophy with a frontal predominance which occurs due to a mutation in the GFAP gene. MRI demonstrates diffuse white matter signal abnormality with frontal predominance. Cystic changes in the involved white matter are seen at a later stage. A periventricular T1W hyperintense, T2W hypointense rim, as well as contrast enhancement are also demonstrated. Atypical findings include signal abnormality in the brainstem and cervical spine.
Table 1.
Summary table for Alexander disease.
| Etiology | De novo, heterozygous, autosomal dominant or spontaneous mutation in the GFAP gene. |
| Incidence | Estimated 1 in 2,7 million in Japan |
| Gender ratio | Male more affected |
| Age predilection | More common in children, with infantile type being the most frequent. 4 types:
|
| Risk factors | Unknown |
| Treatment | Supportive Management of seizures |
| Prognosis | Poor Neonatal type is more rapidly fatal Infantile type – patients usually die within the first 2–3 years |
| Findings on imaging | CT: Diffuse white matter hypodensity with frontal predominance. Cysts in the involved frontal lobes, which may demonstrate rim enhancement. Enhancement in one or more of the following structures: tips of the frontal horns, frontal white matter, basal ganglia, thalami, dentate nucleus, brainstem, fornix, optic chiasm. MRI: Van der Knaap criteria: (4 out of 5 is diagnostic of Alexander disease)
|
Table 2.
Differential diagnosis for Alexander disease.
| Entity | CT | MRI |
|---|---|---|
| Alexander disease |
|
Van der Knaap criteria: (4 out of 5 is diagnostic of Alexander disease)
|
| Canavan disease |
|
|
| Megalencephalic leukoencephalopathy with subcortical cysts (MLC or Van der Knaap disease) |
|
|
| X-linked adrenoleukodystrophy |
|
|
| Metachromatic leukodystrophy |
|
|
ACKNOWLEDGEMENTS
Shereen Matthews for performing the MRI scan and helping to compile the images for the case report.
ABBREVIATIONS
- ADC
apparent diffusion coefficient
- Cho
choline
- CSF
cebrospinal fluid
- CT
computer tomography
- DWI
diffusion weighted imaging
- GFAP
glial fibrillary acidic protein
- mI
myo-inositol
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAA
N-acetylaspartate
- T1W
T1 weighted
- T2W
T2 weighted
REFERENCES
- 1.Cheon J, Kim I, Hwang YS, et al. Leukodystrophy in children: A pictorial reveiw of MR imaging features. Radiographics. 2002;22:461–76. doi: 10.1148/radiographics.22.3.g02ma01461. [DOI] [PubMed] [Google Scholar]
- 2.Nishibayashi F, Kawashima M, Katada Y, Murakami N, Nokazi M. Infantile-onset alexander disease in a child with long term follow-up by serial magnetic resonance. Journal of medical case reports. 2013;7:1–5. doi: 10.1186/1752-1947-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Sasaki M, Yoshida M, et al. Nationwide survey of alexander disease in japan and proposed new guidelines for diagnosis. Journal of Neurology. 2011;258:1998–2008. doi: 10.1007/s00415-011-6056-3. [DOI] [PubMed] [Google Scholar]
- 4.Muralidharan CG, Tomar RPS, HJM, RA Mri diagnosis of alexander disease. SA Journal of Radiology. 2012;16:116–17. [Google Scholar]
- 5.Zang L, Wang J, Jiang Y, et al. Follow up study of 22 Chinese children with Alexander disease and analysis of parental origin of de novo GFAP mutations. Journal of Human Genetics. 2013;58:183–88. doi: 10.1038/jhg.2012.152. [DOI] [PubMed] [Google Scholar]
- 6.Pekny T, Faiz M, Wilhelmsson U, et al. Synemin is expressed in reactive astrocytes and rosenthal fibres in alexander disease. ACTA Pathologica, microbiologica ET immunologica Scandinavica. 2014;122:76–80. doi: 10.1111/apm.12088. [DOI] [PubMed] [Google Scholar]
- 7.Labauge P. Magnetic resonance finding in leukodystrophies and ms. The international MS journal. 2009;16:47–56. [PubMed] [Google Scholar]
- 8.Van der Knaap MS, Naidu S, Breiter SN, et al. Alexander disease: Diagnosis with MR imaging. American Journal of Neuroradiology. 2001;22:541–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen A, Martin C, Meyer S, et al. A 2 month old with vomiting, seizures and progressive apathy. Europian journal of paediatrics. 2012;171:993–95. doi: 10.1007/s00431-012-1681-0. [DOI] [PubMed] [Google Scholar]
- 10.Prust M, Wag J, Morizono H, et al. Gfap mutations, age at onset, and clinical subtypes in alexander disease. Neurology. 2011;77:1287–94. doi: 10.1212/WNL.0b013e3182309f72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim M, Parmar HA, Hoefling N, Srinivasan A. Inborn errors of metabolism: Combining clinical and radiological clues to solve the mystery. American Journal of Radiology. 2014;203:W315–W27. doi: 10.2214/AJR.13.11154. [DOI] [PubMed] [Google Scholar]
- 12.Cole G, De Villiers F, Proctor NSF, Freiman I, Bill P. Alexander’s disease: Case report including histopathological and electron microscopic features. Journal of neurology, neurosurgery and psychiatry. 1979;42:619–24. doi: 10.1136/jnnp.42.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JC, Raul NU. Magnetic resonance spectroscopy in the brain. Massachusetts General Hospital Depatment of Radiology. 2012;10:1–6. [Google Scholar]