Abstract
The patterns of diversity and abundance of the carrion insect species in the different habitats of the Natural Park “Hoces del Río Riaza” (central Spain) were studied with the use of carrion-baited traps. Representativeness of the inventories was assessed with the calculation of randomized species richness curves and nonparametric estimators. Coleoptera families, Silphidae and Dermestidae, and Diptera families, Calliphoridae and Muscidae, were dominant in every sampling habitat, but differences in the patterns of diversity and abundance were found. Lusitanian oakwood and riparian forest were the most diverse habitats with high abundance of saprophagous species, whereas more open (i.e., exposed to continuous sunlight during the day) habitats showed lower diversity values and a different species composition and distribution of species abundance, favoring thermophilous species and necrophagous species with high tolerance to different environmental conditions. Differences in the bioclimatical features of the sampled habitats are suggested to explain the composition and diversity of the carrion insect assemblages in different environments.
Keywords: Carrion insect, diversity, assemblage, Spain
Every natural environment contains three kinds of organisms whose interactions keep the ecosystem in working order: producers, consumers, and decomposers. The last group includes those organisms that feed on organic fecal matter as well as decaying plant and animal remains. Decomposing organic matter such as carrion is an ephemeral but valuable food source for different organisms ( Barton et al. 2013 ), among which arthropods represent the most diverse and abundant group associated with this kind of resource ( Braack 1987 ). Carrion supports high-diverse communities of coexisting insect species, which exploit the same resource ( Woodcock et al. 2002 ); nevertheless, insects visiting carrion can exploit this resource in many different ways. In this sense, Leclercq (1978) and Braack (1987) distinguished four categories of carrion-attendant arthropods according to their kind of use of such resource: 1) necrophagous, i.e., those species that feed directly on carcasses and usually complete their life cycle on them, including both sarcophagous (those consuming flesh and soft tissues) and dermatophagous species (those consuming skin and dry tissues); 2) necrophilous, i.e., those species that are predators of necrophagous insects (mainly Diptera larvae), including also parasitoid species; 3) saprophagous, i.e., those feeding on decomposing organic matter but usually not completing their life cycle on a carcass; and 4) opportunistic or casual, i.e., those using the carcass as a refuge or those having a nonnecrophagous diet but occasionally obtaining nutrients from carrion as a food supplement. Moreover, several studies have shown habitat to affect the species composition and succession patterns of insects associated with carrion ( Goff 1991 , Anderson and VanLaerhoven 1996 ). Also, species abundances differ among habitats ( Martín-Vega and Baz 2012 , 2013 ), so each habitat can be typified by different dominant species.
A correct management of natural resources involves their study to obtain a better knowledge of their dynamics and composition. This article assesses the species richness and diversity patterns of carrion insects in a protected natural area in central Spain. The representativeness of the inventories, the species composition, and the observed diversity patterns within the different habitats of the study area are considered and discussed.
Materials and Methods
The aim of this study was to invent the sarcosaprophagous insect fauna of the Natural Park “Hoces del Río Riaza” (Segovia Province, central Spain), a protected area of 5,185 Ha around the Riaza river. The main vegetation of the park consists in riparian forests, although it can also be found in other types of forests. Concretely, there are five main representative habitat types in the park: 1) herbaceous and shrub vegetation, 2) juniper forest ( Juniperus thurifera L. ), 3) holm oakwood ( Quercus ilex L. ssp. ballota (Desf.)Samp ), 4) Lusitanian oakwood ( Quercus faginea Lamarck ), and 5) riparian forest ( Populus nigra L. ). Two sites for each habitat were selected, resulting in a total of 10 sampling sites ( Fig. 1 ). Detailed information on the locations and bioclimatical features of the sampling sites are listed in Table 1 . Climatic data were obtained from Ninyerola et al. (2005) , and they represent the annual maximum, minimum, and mean temperature, as well as the total rainfall ( Table 1 ). Moreover, a thermometer was installed next to each trap, registering minimum and maximum temperatures between surveys. Average minimum and maximum temperature and the mean temperature during the whole sampling period as registered by thermometers are listed in Table 1 .
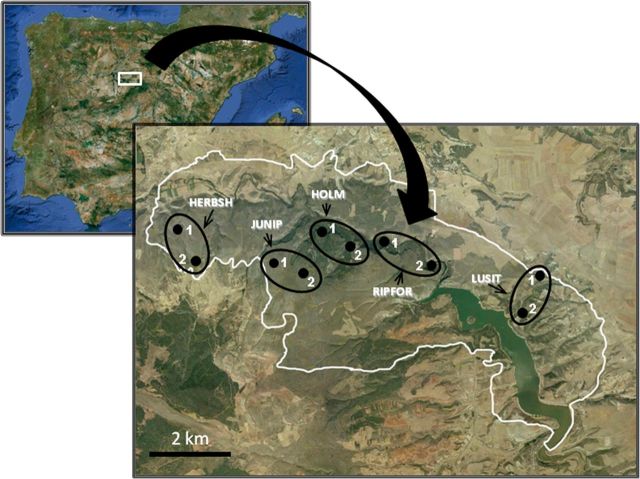
Fig. 1.
Map showing the study area with an indication of the sampling sites. HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
Table 1.
Description of the localities sampled
| Habitat | UTM coordinates | Elevation (m) | Temp (°C) mean | Temp (°C) max | Temp (°C) min. | Total rainfall | Temp T (°C) mean | Temp T (°C) max | Temp T (°C) min. |
|---|---|---|---|---|---|---|---|---|---|
| HERBSH-1 | 30T 446440, 4599328 | 996 | 12 | 18 | 5 | 521 | 21.77 | 38.77 | 4.78 |
| HERBSH-2 | 30T 446756, 4598380 | 1,050 | 11 | 18 | 5 | 451 | 21.85 | 39.71 | 4 |
| JUNIP-1 | 30T 449536, 4598040 | 1,081 | 11 | 17 | 5 | 517 | 22 | 40.7 | 3.3 |
| JUNIP-2 | 30T 449212, 4598128 | 1,057 | 11 | 17 | 5 | 499 | 22.32 | 40.07 | 4.57 |
| HOLM-1 | 30T 450328, 4599426 | 901 | 12 | 18 | 6 | 520 | 24.4 | 43 | 5.8 |
| HOLM-2 | 30T 451196, 4599034 | 902 | 12 | 18 | 6 | 521 | 22.8 | 40.1 | 5.5 |
| LUSIT-1 | 30T 456652, 4598028 | 973 | 12 | 18 | 5 | 454 | 22.69 | 40.38 | 5 |
| LUSIT-2 | 30T 456171, 4597179 | 930 | 12 | 18 | 5 | 453 | 21.33 | 37.75 | 4.92 |
| RIPFOR-1 | 30T 452051, 4598944 | 903 | 12 | 18 | 5 | 476 | 21.88 | 38 | 5.77 |
| RIPFOR-2 | 30T 453316, 4598375 | 899 | 12 | 18 | 6 | 468 | 22.32 | 38.21 | 6.43 |
Average minimum and maximum temperature and the mean temperature registered by installed thermometers (temp T ) are also included. The numbers 1 and 2 indicate the two sites selected for each habitat. HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
Sarcosaprophagous insects were collected using carrion-baited traps, modified from the design of Morón and Terrón (1984) as described by Baz et al. (2007) . Traps were baited with squid, which has been shown to be very effective in attracting necrophagous insects ( Newton and Peck 1975 ), semiburied in the ground, and protected from vertebrate scavengers with a perimeter of stones. One carrion-baited trap was installed at each site. Traps were operated 15 d each month from June to September 2007; thus, a total of 36 samples were obtained at the end of the survey as 4 samples were destroyed, probably by wild animals. Moreover, to determine whether some species were accidentally collected by the traps and not attracted to carrion, an identical but unbaited trap was installed next to each carrion-baited trap. The collected specimens were either preserved in 80% ethanol or oven dried and pinned, and deposited in the collection of the Department of Life Sciences of the University of Alcalá. Collected nonsarcosaprophagous insects (i.e., opportunistic or casual species) have not been considered in this study, but their captures were discussed in a previous article ( Baz et al. 2010 ).
Species richness was estimated in every sampling site to determine whether the observed species richness was representative enough of the total number of species. Species richness was estimated from the samples by nonparametric methods ( Colwell and Coddington 1994 ). Six nonparametric estimators were used: abundance-based coverage estimator (ACE), incidence-based coverage estimator (ICE), jackknife 1, jackknife 2, Chao 1, and Chao 2. ACE is based on the proportion of abundant species (those with >10 collected individuals) and rare species (those with <10 collected individuals), whereas ICE is based on the proportion of frequent species (those collected in >10 sampling units) and infrequent species (those collected in <10 sampling units; Magurran 2004 ). Jackknife 1 estimator is based on the number of species that occur in only one sample (uniques), whereas jackknife 2 and Chao 2 estimators are based on the number of species that occur in only one sample (uniques), as well as the number that occur in exactly two samples (duplicates; Colwell and Coddington 1994 ). On the other hand, Chao 1 estimator is based on the number of observed species that are represented by only one individual (singletons) and the number that occur with exactly two individuals (doubletons; Colwell and Coddington 1994 ). Randomized accumulation curves of observed species richness using the Mao Tau estimator and different estimators were calculated for each sampling site using EstimateS software ( Colwell 2005 ). One hundred randomizations were always used. Inventory completeness, defined as observed species richness in relation with estimated richness ( Cardoso et al. 2009 ), was calculated using nonparametric estimations.
In addition to species richness, species diversity was also measured using Shannon diversity index and Simpson diversity index. Both indices combine information on richness and relative abundance in different ways, and they were also computed using EstimateS software ( Colwell 2005 ). The distribution of the abundances of collected species was logarithmically depicted. A cluster analysis was performed to study the degree of association among habitats according to their species composition and the average number of collected individuals in each survey. Data were transformed using the log ( x + 1) prior to analysis, and a dendrogram was computed using the Squared Euclidean distance and the complete linkage method. Cluster analysis was performed using the software Statgraphics Plus 5.1 (Statistical Graphic Corp. 1994–2000 Princeton, New Jersey).
Results
In total, 25,033 individuals belonging to 112 carrion insect species from orders Coleoptera and Diptera were collected in carrion-baited traps throughout the sampling period. A complete list of these Coleoptera and Diptera species and numbers collected in each sampling site are presented in Tables 2 and 3 , respectively. Among Coleoptera, the most abundant species were the silphids Thanatophilus rugosus (7,333 individuals) and Thanatophilus ruficornis (1,885 individuals), and the dermestid Dermestes frischi (2,252 individuals; Table 2 ). Among Diptera, the most abundant species were the muscids Hydrotaea armipes (1,753 individuals) and Hydrotaea ignava (1,394 individuals), and the calliphorid Chrysomya albiceps (1,707 individuals; Table 3 ). As mentioned earlier, opportunistic or casual insect species has not been included in this study, but details on their captures can be found in Baz et al. (2010) . Unbaited traps virtually captured no insects, and in any case, only accidental species were collected in these traps.
Table 2.
List of carrion Coleoptera species collected and abundances by sampling site and habitat
| Coleoptera species | HERBSH-1 | HERBSH-2 | Total HERBSH | JUNIP-1 | JUNIP-2 | Total JUNIP | HOLM-1 | HOLM-2 | Total HOLM | LUSIT-1 | LUSIT-2 | Total LUSIT | RIPFOR-1 | RIPFOR-2 | Total RIPFOR | Total Captures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silphidae | ||||||||||||||||
| T. rugosus (L. 1758) | 662 | 688 | 1,350 | 1,268 | 792 | 2,060 | 1,387 | 1,183 | 2,570 | 135 | 37 | 172 | 584 | 597 | 1,181 | 7,333 |
| T. ruficornis (Küster 1851) | 359 | 236 | 595 | 368 | 248 | 616 | 237 | 275 | 512 | 16 | 19 | 35 | 64 | 63 | 127 | 1,885 |
| Thanatophilus sinuatus (F. 1775) | 3 | 9 | 12 | 1 | 1 | 2 | 6 | 19 | 25 | 11 | 0 | 11 | 45 | 24 | 69 | 119 |
| Nicrophorus interruptus Stephens 1830 | 1 | 0 | 1 | 0 | 2 | 2 | 6 | 17 | 23 | 1 | 1 | 2 | 13 | 21 | 34 | 62 |
| Nicrophorus vestigator Herschel 1807 | 3 | 8 | 11 | 3 | 5 | 8 | 7 | 3 | 10 | 9 | 4 | 13 | 22 | 10 | 32 | 74 |
| Nicrophorus humator (Gleditsch 1767) | 0 | 7 | 7 | 3 | 2 | 5 | 5 | 15 | 20 | 0 | 1 | 1 | 3 | 2 | 5 | 38 |
| Nicrophorus vespillo (L. 1758) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 6 | 6 | 0 | 0 | 0 | 3 | 1 | 4 | 12 |
| Necrodes littoralis (L. 1758) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Silpha tristis Illiger 1798 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 9 | 9 |
| Silpha puncticollis Lucas 1846 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 5 |
| Dermestidae | ||||||||||||||||
| D. frischi Kugelann 1792 | 212 | 664 | 876 | 379 | 451 | 830 | 105 | 139 | 244 | 104 | 78 | 182 | 74 | 46 | 120 | 2,252 |
| D. undulatus Brahm 1790 | 7 | 50 | 57 | 17 | 15 | 32 | 55 | 82 | 137 | 84 | 316 | 400 | 165 | 164 | 329 | 955 |
| Dermestes mustelinus Erichson 1846 | 0 | 3 | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 3 | 3 | 9 | 0 | 9 | 18 |
| Dermestes hispanicus Kalik 1952 | 2 | 2 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 1 | 8 |
| Dermestes olivieri Lepesme 1939 | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Nitidulidae | ||||||||||||||||
| Nitidula flavomaculata Rossi 1790 | 0 | 10 | 10 | 7 | 7 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 26 |
| Nitidula carnaria (Schaller 1783) | 28 | 31 | 59 | 10 | 20 | 30 | 60 | 54 | 114 | 38 | 19 | 57 | 37 | 23 | 60 | 310 |
| Omosita discoidea (F. 1775) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 3 |
| Soronia oblonga Brisout de Barneville 1863 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Catopidae | ||||||||||||||||
| Catops coracinus Kellner 1846 | 0 | 0 | 0 | 4 | 1 | 5 | 4 | 18 | 22 | 41 | 5 | 46 | 15 | 186 | 201 | 274 |
| Catops fuliginosus Erichson 1837 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 0 | 2 | 2 | 0 | 0 | 0 | 7 |
| Catops nitidicollis Kraatz 1856 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Ptomaphagus subvillosus (Goeze 1777) | 0 | 2 | 2 | 1 | 0 | 1 | 13 | 1 | 14 | 9 | 13 | 22 | 37 | 3 | 40 | 79 |
| Ptomaphagus tenuicornis (Rosenhauer 1856) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 25 | 27 | 29 |
| Ptinidae | ||||||||||||||||
| Ptinus ( Cyphoderes ) hirticornis (Kiesenwetter 1867) | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 3 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Ptinus sp (♀ no identificable) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Histeridae | ||||||||||||||||
| Saprinus acuminatus (F. 1798) | 3 | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Saprinus caerulescens (Hoffmann 1803) | 0 | 2 | 2 | 7 | 5 | 12 | 1 | 0 | 1 | 1 | 3 | 4 | 7 | 0 | 7 | 26 |
| Saprinus detersus (Illiger 1807) | 27 | 50 | 77 | 41 | 15 | 56 | 24 | 49 | 73 | 8 | 8 | 16 | 47 | 24 | 71 | 293 |
| Saprinus figuratus Marseul 1855 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 |
| S. furvus Erichson 1834 | 40 | 76 | 116 | 33 | 21 | 54 | 8 | 8 | 16 | 6 | 1 | 7 | 0 | 1 | 1 | 194 |
| Saprinus georgicus Marseul 1862 | 11 | 10 | 21 | 12 | 5 | 17 | 11 | 13 | 24 | 5 | 0 | 5 | 3 | 4 | 7 | 74 |
| Saprinus inmundus (Gyllenhal 1827) | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 6 |
| Saprinus lautus Erichson 1839 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 4 | 1 | 0 | 1 | 1 | 0 | 1 | 8 |
| Saprinus maculatus (Rossi 1792) | 1 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Saprinus melas Küster 1849 | 9 | 21 | 30 | 5 | 2 | 7 | 1 | 2 | 3 | 3 | 5 | 8 | 0 | 1 | 1 | 49 |
| Saprinus politus (Brahm 1790) | 10 | 4 | 14 | 11 | 0 | 11 | 5 | 3 | 8 | 1 | 2 | 3 | 15 | 3 | 18 | 54 |
| Saprinus subnitescens Bickhardt 1909 | 25 | 18 | 43 | 8 | 18 | 26 | 19 | 17 | 36 | 10 | 9 | 19 | 31 | 19 | 50 | 174 |
| Saprinus tenuistrius Marseul 1855 | 6 | 1 | 7 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Margarinotus brunneus (F. 1775) | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 3 | 3 | 6 | 9 |
| Margarinotus ignobilis (Marseul 1854) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 |
| Atholus duodecimstriatus (Schrank 1781) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 | 4 |
| Chalcionellus decemstriatus (Rossi 1792) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Onthophilus striatus (Forster 1771) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 |
| Staphylinidae | ||||||||||||||||
| Creophillus maxillosus (L. 1758) | 16 | 4 | 20 | 7 | 4 | 11 | 6 | 9 | 15 | 7 | 4 | 11 | 64 | 20 | 84 | 141 |
| Ontholestes tessellatus (Geoffroy 1785) | 0 | 0 | 0 | 1 | 2 | 3 | 2 | 3 | 5 | 0 | 0 | 0 | 37 | 16 | 53 | 61 |
| Platydracus meridionalis (Rosenhauer 1847) | 1 | 6 | 7 | 0 | 7 | 7 | 0 | 0 | 0 | 15 | 19 | 34 | 2 | 5 | 7 | 55 |
| Philonthus intermedius (Lacordaire 1835) | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 5 | 0 | 0 | 0 | 100 | 1 | 101 | 106 |
| Philonthus politus (L. 1758) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| Quedius latinus Gridelli 1938 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 7 | 10 | 10 |
| Scarabaeidae | ||||||||||||||||
| Scarabaeus laticollis L. 1767 | 3 | 3 | 6 | 37 | 1 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Onthophagus (Palaeonthophagus) lemur (F. 1781) | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 4 | 3 | 2 | 5 | 4 | 2 | 6 | 17 |
| Onthophagus (Palaeonthophagus) coenobita (Herbst 1783) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 10 | 10 |
| Onthophagus (Palaeonthophagus) similis (Scriba 1790) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 3 | 4 |
| Onthophagus (Trichonthophagus) maki (Illiger 1803) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 |
| Onthophagus (Furconthophagus) furcatus (F. 1781) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Trogiidae | ||||||||||||||||
| Trox perlatus Goeze 1777 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Trox cricetulus Ádám 1994 | 0 | 0 | 0 | 3 | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| Carabidae | ||||||||||||||||
| Calathus granatensis Vuillefroy 1866 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 30 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 34 |
| Calathus ambiguus (Paykull 1790) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Calathus fuscipes (Goeze 1777) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Calathus cinctus Motschulsky 1850 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ocys harpaloides (Audinet-Serville 1821) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Trechus obtusus Erichson 1837 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
Table 3.
List of carrion Diptera species collected and abundances by sampling site and habitat
| Diptera species | HERBSH-1 | HERBSH-2 | Total HERBSH | JUNIP-1 | JUNIP-2 | Total JUNIP | HOLM-1 | HOLM-2 | Total HOLM | LUSIT-1 | LUSIT-2 | Total LUSIT | RIPFOR-1 | RIPFOR-2 | Total RIPFOR | Total captures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcophagidae | ||||||||||||||||
| Sarcophaga lehmanni (Müeller 1922) | 10 | 5 | 15 | 12 | 16 | 28 | 8 | 10 | 18 | 22 | 9 | 31 | 51 | 13 | 64 | 156 |
| Sarcophaga portschinskyi (Rohdendorf 1937) | 0 | 4 | 4 | 6 | 7 | 13 | 3 | 4 | 7 | 17 | 25 | 42 | 0 | 0 | 0 | 66 |
| Sarcophaga argyrostoma Robineau-Desvoidy 1830 | 1 | 2 | 3 | 1 | 9 | 10 | 3 | 4 | 7 | 8 | 0 | 8 | 0 | 0 | 0 | 28 |
| Sarcophaga africa (Wiedemann 1824) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 3 |
| Sarcophaga belgiana Lehrer 1976 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 |
| Sarcophaga variegata Scopoli 1763 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 3 |
| Sarcophaga subvicina Rohdendorf 1937 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Sarcophaga teretirostris Pandellé 1896 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Sarcophaga cultellata (Pandelle 1896) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ravinia pernix (Harris 1780) | 1 | 0 | 1 | 3 | 3 | 6 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Sarcophila latifrons (Fallén 1817) | 5 | 5 | 10 | 36 | 20 | 56 | 0 | 10 | 10 | 27 | 0 | 27 | 10 | 0 | 10 | 113 |
| Calliphoridae | ||||||||||||||||
| Calliphora vomitoria (L. 1758) | 1 | 0 | 1 | 0 | 0 | 0 | 15 | 14 | 29 | 1 | 0 | 1 | 9 | 62 | 71 | 102 |
| Calliphora vicina Robineau-Desvoidy 1830 | 6 | 28 | 34 | 16 | 28 | 44 | 37 | 53 | 90 | 96 | 27 | 123 | 35 | 101 | 136 | 427 |
| C. albiceps (Wiedemann 1819) | 15 | 107 | 122 | 266 | 187 | 453 | 77 | 82 | 159 | 185 | 19 | 204 | 555 | 214 | 769 | 1,707 |
| Lucilia sericata (Meigen 1826) | 25 | 16 | 41 | 11 | 14 | 25 | 17 | 8 | 25 | 20 | 10 | 30 | 19 | 25 | 44 | 165 |
| Lucilia caesar (L. 1758) | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 12 | 26 | 1 | 1 | 2 | 15 | 21 | 36 | 64 |
| Lucilia richardsi (Collin 1926) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Pollenia rudis (F. 1794) | 4 | 32 | 36 | 6 | 25 | 31 | 52 | 50 | 102 | 55 | 5 | 60 | 445 | 63 | 508 | 737 |
| Pollenia labialis Robineau-Desvoidy 1863 | 0 | 4 | 4 | 3 | 32 | 35 | 3 | 4 | 7 | 7 | 0 | 7 | 69 | 16 | 85 | 138 |
| Pollenia fulvipalpis Macquart 1835 | 0 | 0 | 0 | 0 | 10 | 10 | 0 | 0 | 0 | 7 | 6 | 13 | 0 | 0 | 0 | 23 |
| Pollenia bicolor Robineau-Desvoidy 1830 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 5 |
| Pollenia ponti Rognes 1991 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 3 |
| Stomorhina lunata (F. 1805) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Muscidae | ||||||||||||||||
| Muscina levida (Harris ×) | 2 | 7 | 9 | 24 | 35 | 59 | 353 | 325 | 678 | 153 | 67 | 220 | 250 | 50 | 300 | 1,266 |
| Muscina prolapsa (Harris 1780) | 0 | 2 | 2 | 6 | 35 | 41 | 31 | 40 | 71 | 82 | 97 | 179 | 61 | 160 | 221 | 514 |
| Muscina stabulans (Fallén 1817) | 0 | 1 | 1 | 0 | 7 | 7 | 2 | 2 | 4 | 1 | 1 | 2 | 7 | 3 | 10 | 24 |
| Hydrotaea aenescens (Wiedemann 1830) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 |
| H. armipes (Fallén 1825) | 0 | 19 | 19 | 145 | 24 | 169 | 582 | 228 | 810 | 259 | 19 | 278 | 178 | 299 | 477 | 1,753 |
| Hydrotaea capensis (Wiedemann 1818) | 0 | 1 | 1 | 3 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 18 | 23 |
| Hydrotaea cyrtoneurina (Zetterstedt 1845) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 10 | 18 | 36 | 54 | 64 |
| Hydrotaea dentipes (F. 1805) | 0 | 0 | 0 | 1 | 0 | 1 | 9 | 8 | 17 | 0 | 0 | 0 | 29 | 129 | 158 | 176 |
| H. ignava (Harris 1780) | 7 | 9 | 16 | 40 | 8 | 48 | 194 | 209 | 403 | 59 | 7 | 66 | 380 | 481 | 861 | 1,394 |
| M. domestica L. 1758 | 24 | 109 | 133 | 180 | 59 | 239 | 0 | 1 | 1 | 5 | 1 | 6 | 90 | 3 | 93 | 472 |
| Helina reversio (Harris 1780) | 0 | 1 | 1 | 3 | 19 | 22 | 0 | 0 | 0 | 6 | 1 | 7 | 0 | 0 | 0 | 30 |
| Helina pubescens (Stein 1893) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Helina impuncta (Fallén 1825) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 2 |
| Helina depuncta (Fallén 1825) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 4 | 6 |
| Polietes lardarius (F. 1751) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Phaonia mediterranea Hennig 1963 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Phaonia subventa (Harris 1780) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 1 | 2 | 3 | 1 | 0 | 1 | 7 |
| Phaonia pallida (F. 1787) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 3 | 4 |
| Eudasyphora cyanella (Meigen 1826) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Tachinidae | ||||||||||||||||
| Tachina magnicornis (Zetterstedt 1844) | 5 | 2 | 7 | 14 | 4 | 18 | 1 | 2 | 3 | 5 | 0 | 5 | 8 | 3 | 11 | 44 |
| Peleteria rubescens (Robineau-Desvoidy 1830) | 0 | 13 | 13 | 6 | 23 | 29 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 3 | 46 |
| Peleteria varia (F. 1794) | 0 | 0 | 0 | 1 | 3 | 4 | 2 | 9 | 11 | 13 | 0 | 13 | 24 | 7 | 31 | 59 |
| Heleomyzidae | ||||||||||||||||
| Suillia affinis (Meigen 1830) | 0 | 17 | 17 | 10 | 40 | 50 | 85 | 36 | 121 | 81 | 78 | 159 | 2 | 0 | 2 | 349 |
| Suillia tuberiperda (Rondani 1867) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 3 |
| Suillia flagripes (Czerny 1904) | 0 | 1 | 1 | 5 | 4 | 9 | 43 | 2 | 45 | 11 | 18 | 29 | 12 | 5 | 17 | 101 |
The sampling habitat with the highest number of inventoried species was the riparian forest (80 species), while the rest of sampling habitats showed 60–66 inventoried species ( Table 4 ). The species accumulation curves for each sampling habitat were not quite reaching the asymptote by the end of the sampling process; in the curve for the total of inventoried species in the study area, the asymptotic tendency was clearer ( Fig. 2 ). All the nonparametric estimators showed inventory completeness >70% in every sampling habitat, with the exception of the herbaceous and shrub vegetation where the inventory completeness was 68.8 and 67.33% by Chao 1 and jackknife 2 estimators, respectively ( Table 4 ). Inventory completeness was >80% for the total of inventoried species in the study area, according to all the nonparametric estimators ( Table 4 ).
Table 4.
Number of observed species and number of species calculated from each estimator for each sampling habitat
| Habitat | Observed species | ACE | ICE | Chao 1 | Chao 2 | Jackknife 1 | Jackknife 2 |
|---|---|---|---|---|---|---|---|
| HERBSH | 60 | 82.4 | 82.38 | 87.2 | 82.14 | 80.13 | 89.11 |
| 72.81% | 72.83% | 68.80% | 73.04% | 74.88% | 67.33% | ||
| JUNIP | 66 | 76.34 | 78.05 | 74.25 | 75.71 | 80.57 | 86.17 |
| 86.45% | 84.56% | 88.88% | 87.17% | 81.92% | 76.59% | ||
| HOLM | 63 | 73.47 | 74.38 | 79.5 | 72.89 | 76.6 | 82.3 |
| 85.75% | 84.70% | 79.24% | 86.43% | 82.24% | 76.55% | ||
| LUSIT | 66 | 79.41 | 77.84 | 74.27 | 78.25 | 84.38 | 91.13 |
| 83.11% | 78.74% | 88.86% | 84.34% | 78.21% | 72.42% | ||
| RIPFOR | 80 | 96.29 | 100.64 | 102.67 | 96.1 | 101 | 110 |
| 83.08% | 79.50% | 77.92% | 83.25% | 79.21% | 72.72% | ||
| Total | 112 | 125.3 | 127.06 | 125.6 | 121.72 | 131.44 | 133.82 |
| 89.38% | 87.77% | 89.17% | 92.01% | 85.21% | 83.69% |
HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
Fig. 2.
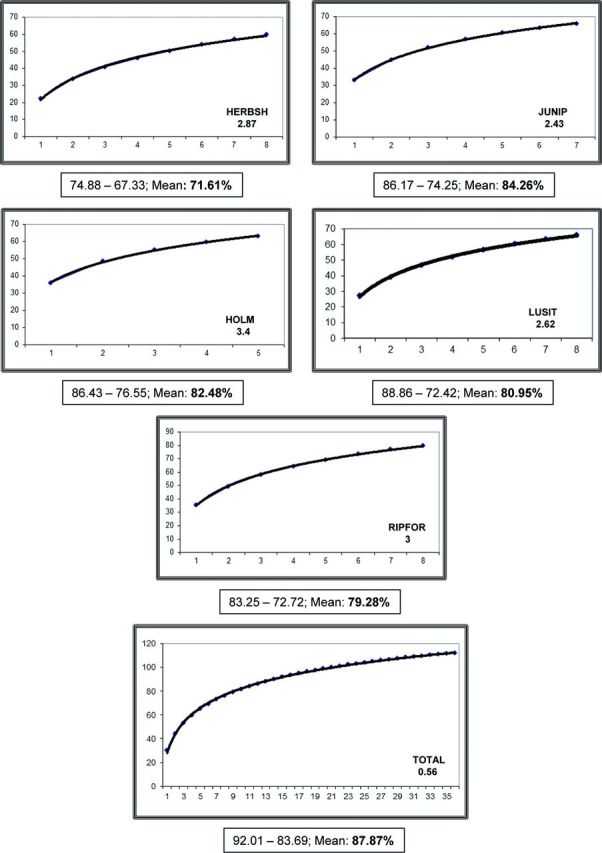
Randomized accumulation curves for observed species richness in each sampling habitat and for the total captures. The rate of appearance of new inventoried species is included below the abbreviation for each habitat. Maximum, minimum, and mean values of inventory completeness according to the different estimators are also included. HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
Both Shannon and Simpson diversity indices showed Lusitanian oakwood and riparian forest as the most diverse habitats; the rest of sampling habitats showed lower but similar diversity values ( Table 5 ). The logarithmical distribution of species abundance ( Figs. 3–5 ) in two of the less diverse habitats, herbaceous and shrub vegetation and juniper forest, showed that only six species were represented by the collection of more than 100 specimens. The silphid beetles T. rugosus and T. ruficornis , the dermestid beetle D.frischi , the calliphorid fly C. albiceps, and the muscid fly Musca domestica were the most abundant species in both habitats, together with the histerid beetle Saprinus furvus in herbaceous and shrub vegetation and the muscid fly H.armipes in juniper forest ( Fig. 3 ). On the other hand, holm oakwood, Lusitanian oakwood, and riparian forest showed 11, 9, and 14 species represented by the collection of more than 100 specimens, respectively ( Figs. 4 and 5 ). The silphid T. rugosus was also the most abundant species in holm oakwood and riparian forest, whereas in Lusitanian oakwood, the most abundant species was the dermestid beetle Dermestes undulatus ( Figs. 4 and 5 ). Among others, the muscid flies H. ignava , H. armipes , and Muscina levida , the blow fly C. albiceps, and the dermestid D. frischi were also abundant in these habitats ( Figs. 4 and 5 ).
Table 5.
Number of observed species and Shannon and Simpson diversity indices values for each sampling habitat
| Habitat | Observed species | Shannon | Simpson |
|---|---|---|---|
| HERBSH | 60 | 2.18 | 4.91 |
| JUNIP | 66 | 2.29 | 4.97 |
| HOLM | 63 | 2.35 | 5.23 |
| LUSIT | 66 | 3.03 | 14.05 |
| RIPFOR | 80 | 3.01 | 12.36 |
HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
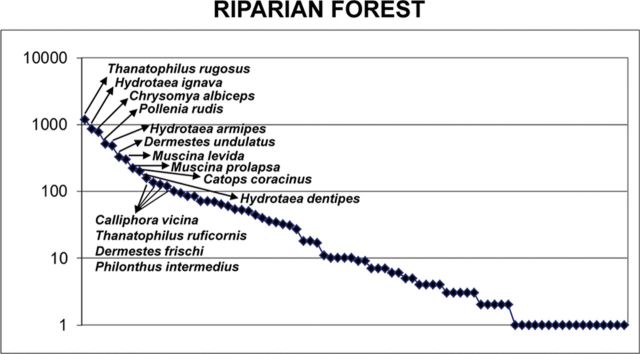
Fig. 3.
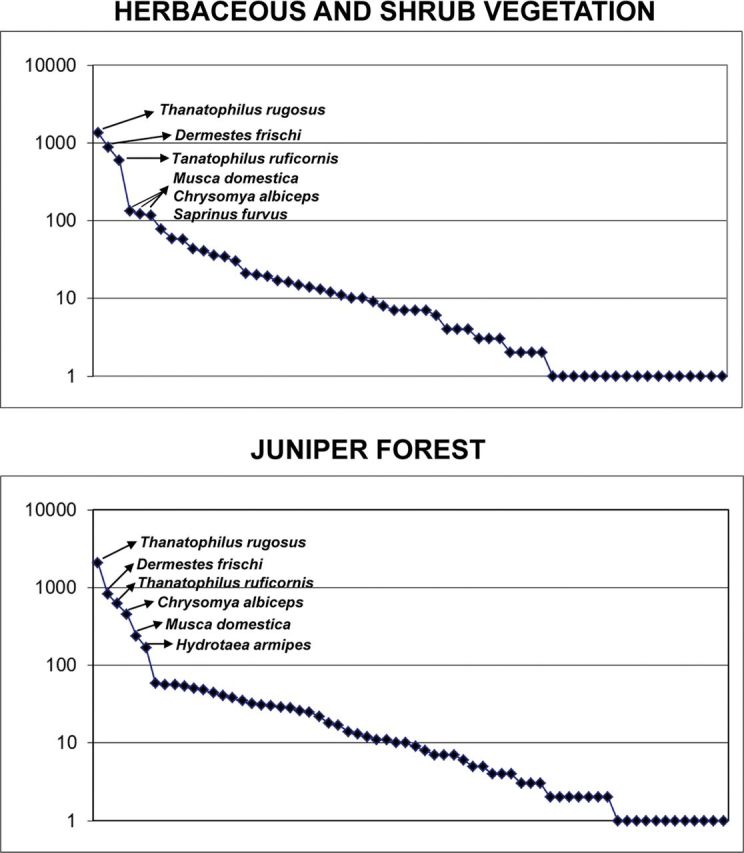
Distribution of the abundances of the inventoried species in herbaceous and shrub vegetation and juniper forest habitats. Species names of those represented by the collection of >100 specimens are shown.
Fig. 5.
Distribution of the abundances of the inventoried species in riparian forest habitat. Species names of those represented by the collection of >100 specimens are shown.
Fig. 4.
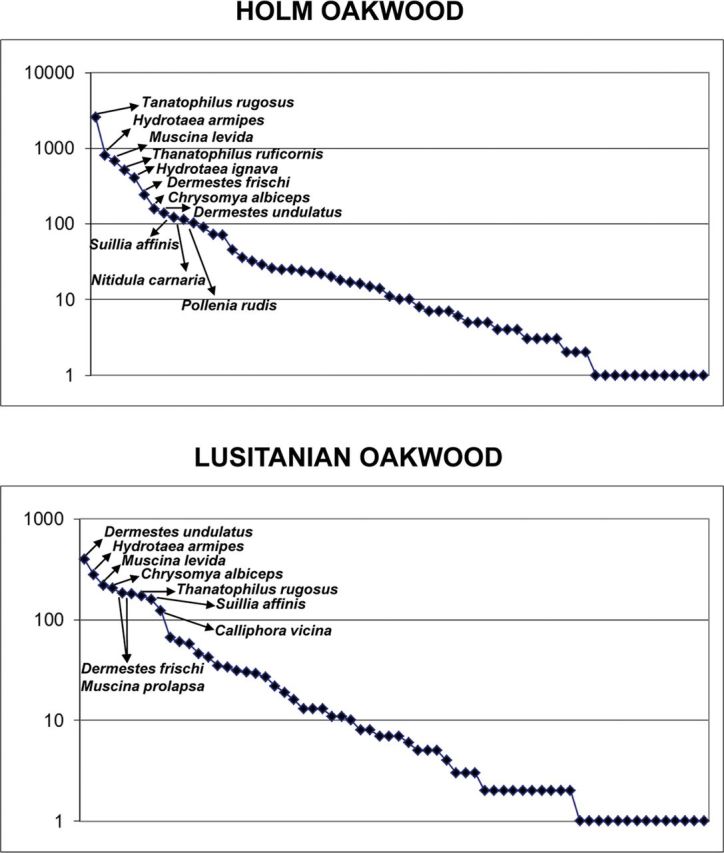
Distribution of the abundances of the inventoried species in holm oakwood and Lusitanian oakwood habitats. Species names of those represented by the collection of >100 specimens are shown.
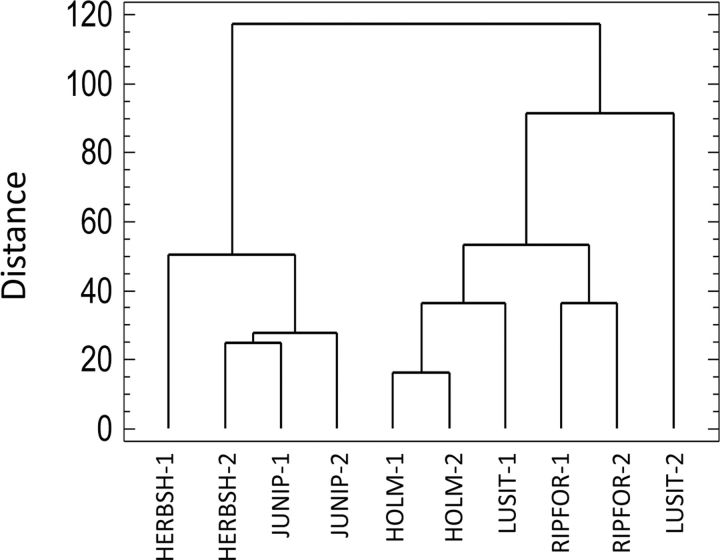
Finally, the cluster analysis showed two groups of habitats clearly separated ( Fig. 6 ): one corresponding to more open habitats (herbaceous and shrub vegetation and juniper forest) and the other corresponding to more wooded areas (holm oakwood, Lusitanian oakwood, and riparian forest). Interestingly, the two habitats included in the first group were also the two least diverse habitats ( Table 5 ).
Fig. 6.
Dendrogram from cluster analysis for the sampling sites according to their species composition and the average number of collected individuals in each survey. The numbers 1 and 2 indicate the two sites selected for each habitat. HERBSH, herbaceous and shrub vegetation; JUNIP, juniper forest; HOLM, holm oakwood; LUSIT, Lusitanian oakwood; RIPFOR, riparian forest.
Discussion
Faunistic inventories are generally incomplete due to the fact that the number of inventoried species continues increasing with sampling effort ( Gotelli and Colwell 2001 , Jiménez-Valverde and Hortal 2003 ). In accordance with it, in this study, none of the randomized accumulation curves reached the asymptote by the end of the sampling process ( Fig. 2 ). However, estimations of species richness become stable when inventories cover at least 70% of the total number of species ( Jiménez-Valverde and Hortal 2003 ). Thus, nonparametric estimators showed that the inventory completeness of this study was representative enough of the species richness in the study area ( Table 4 ).
Riparian forest was the sampling habitat with the greatest species richness ( Table 5 ), whereas both Shannon and Simpson indices showed not only riparian forest but also Lusitanian oakwood as the most diverse habitats ( Table 5 ). Diversity is actually a set of concepts ( Peet 1974 ), and it is not a synonym of number of species. For example, both juniper forest and Lusitanian oakwood showed the same number of inventoried species, but juniper forest was clearly a less diverse habitat in accordance with Shannon and Simpson indices values ( Table 5 ). The explanation lies in the combination of the number of species and their relative abundance. In juniper forest, fewer species dominate in terms of number of individuals than in Lusitanian oakwood, where the number of collected specimens appears to be more equally distributed among the inventoried species ( Figs. 3 and 4 ). Among the most abundant species, the silphids T. rugosus and T. ruficornis and the dermestids D. undulatus and D. frischi clearly stood out ( Figs. 3–5 ). Those species are well represented in every type of natural habitats in central Spain, dominating the necrophagous beetles assemblages ( Martín-Vega and Baz 2012 ). On the other hand, the blow fly C. albiceps and Muscidae species from genera Muscina and Hydrotaea have also been found among the dominant species in the sarcosaprophagous Diptera assemblages in summer months in the different natural habitats of central Spain ( Martínez-Sánchez et al. 2000 , Martín-Vega and Baz 2013 ), in accordance with the present results ( Figs. 3–5 ). Carrion sustains a high diverse insect community exploiting the same resource at the same time, although calliphorid flies and silphid and dermestid beetles usually monopolize the different carcass tissues along the insect succession ( Braack 1987 , Matuszewski et al. 2010 ).
The cluster analysis divided the sampled habitats into two well-differentiated groups ( Fig. 6 ), which corresponded to the least diverse habitats (herbaceous and shrub vegetation and juniper forest), and, on the other hand, the most diverse (holm oakwood, Lusitanian oakwood, and riparian forest; Table 5 ). The fact that these two groups also coincide with the more open (i.e., less wooded) and the more forest habitats allow speculating on the reasons for the different diversity values. Thus, in deciduous and sclerophyllous forests, the ground usually accumulates great quantities of fallen leaves, which may favor the proliferation of saprophagous species. Furthermore, forest habitats may also contain a higher diversity of small vertebrates than more open, homogeneous habitats ( Tews et al. 2004 ), thus providing carrion insects with higher numbers of animal carcasses. It may explain the high abundance of Muscina and Suillia species in wooded habitats ( Figs. 4 and 5 ; Table 3 ). Despite muscid flies of genus Muscina breed frequently on carrion, they are also common on fungus and other types of decaying organic matter ( Gregor et al. 2002 ). Also, heleomyzid flies of genus Suillia can be collected abundantly in association with carrion (e.g., Martín-Vega and Baz 2013 ), but they are mainly considered as saprophagous ( Séguy 1934 ). Both Muscina and Suillia species are among the dominant species, which typify the sarcosaprophagous fly assemblages of wooded habitats in central Spain ( Martín-Vega and Baz 2013 ). Moreover, the accumulated fallen leaves in wooded habitats may favor the presence of invertebrates, which can be parasitized by some fly species that are also attracted to carrion. This is the case of Pollenia species ( Figs. 4 and 5 ; Table 3 ), which are primarily considered as parasites or predators of earthworms, but they have also been collected in association with carrion ( Baz et al. 2007 , Martín-Vega and Baz 2013 ). Also, Muscina species can act as parasites of larvae of other insects ( Gregor et al. 2002 ). On the other hand, the traps installed in more open habitats appeared to be subjected to higher temperatures ( Table 1 ) as they were more exposed to continuous sunlight during the day. Such situation may have favored the dominance of the silphids T. rugosus and T. ruficornis , and the dermestid D. frischi ( Fig. 3 ), which are tolerant to different environmental conditions ( Martín-Vega and Baz 2012 ). Particularly, D. frischi appears to be more dominant in open habitats ( Fig. 3 ); it may be due to its food preferences. As dermestid species breed on carcasses in advanced stages of decomposition, they are usually more abundant in those habitats with low humidity and high temperatures, where carrion desiccates quickly ( Sánchez Piñero 1997 , Martín-Vega and Baz 2012 ). In this sense, open habitats may have also favored the dominance of thermophilous species like C. albiceps and M. domestica , which show a preference for warm conditions in its spatial distribution ( Baz et al. 2007 , Martín-Vega and Baz 2013 ); moreover, C. albiceps have shown to be more abundant on carrion under sunny conditions than shaded conditions ( Prado e Castro et al. 2011 ).
In conclusion, the different characteristics of the studied habitats and their climatic conditions may be determinant explaining the composition and diversity of their typical carrion insect assemblages. From an applied point of view, differences in habitat preferences and species composition of carrion insects may provide forensic investigators with useful information indicating possible postmortem movements of a corpse ( Amendt et al. 2011 ). Further studies on the diversity and composition of carrion insect species in different habitats and environments within a same region need to be done.
Acknowledgments
We are grateful to Txomin Gilgado, Jacinto Gamo, Jaime Potti, Jorge Vicente, and Gonzalo Baz for their company and their help during the sampling process. We are also grateful to the staff of the “House of the Park Hoces del Río Riaza” for their kindness and good disposal, and to the ground keepers of the Natural Park and especially to Luis Mira for sharing with us their knowledge about the study area. Finally, we acknowledge Francisco Sánchez-Aguado (Paco), Director-Conservator of the Natural Park (and nevertheless, friend), for his infinite patience, besides other regards. Two anonymous reviewers provided valuable comments and suggestions, which improved this manuscript. This work was supported by the Consejería de Medio Ambiente de la Junta de Castilla y León (Project SG-25/2007) within the framework of the development of catalogues programmed by the Natural Park “Hoces del Río Riaza,” intended for improving the knowledge about its natural resources. The authors are members of the University Institute of Research in Police Sciences (IUICP) of the University of Alcalá.
References Cited
- Amendt J., Richards C. S., Campobasso C. P., Zehner R., Hall M.J.R. . 2011. . Forensic entomology: applications and limitations . Forensic Sci. Med. Pathol. 7 : 379 – 392 . [DOI] [PubMed] [Google Scholar]
- Anderson G., VanLaerhoven S. L. . 1996. . Initial studies on insect succession on carrion in southwestern British Columbia . J. Forensic Sci. 41 : 617 – 625 . [PubMed] [Google Scholar]
- Barton P. S., Cunningham S. A., Lindenmayer D. B., Manning A. D. . 2013. . The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems . Oecologia 171 : 761 – 772 . [DOI] [PubMed] [Google Scholar]
- Baz A., Cifrián B., Martín-Vega D., Baena M. . 2010. . Phytophagous insects captured in carrion-baited traps . Bull. Insectol. 63 : 21 – 30 . [Google Scholar]
- Baz A., Cifrián B., Díaz-Aranda L. M., Martín-Vega D. . 2007. . The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in central Spain . Ann. Soc. Entomol. France 43 : 289 – 296 . [Google Scholar]
- Braack L.E.O. 1987. . Community dynamics of carrion-attendant arthropods in tropical African woodland . Oecologia 72 : 402 – 409 . [DOI] [PubMed] [Google Scholar]
- Cardoso P., Henriques S. S., Gaspar C., Crespo L. C., Carvalho R., Schmidt J. B., Sousa P., Szűts T. . 2009. . Species richness and composition assessment of spiders in a Mediterranean scrubland . J. Insect Conserv. 13 : 45 – 55 . [Google Scholar]
- Colwell R. K. 2005. . Estimates: statistical estimation of species richness and shared species from samples, version 7.5 . ( http://viceroy.eeb.ucom.edu/estimates ) (accessed 10 December 2012) . [Google Scholar]
- Colwell R. K., Coddington J. A. . 1994. . Estimating terrestrial biodiversity through extrapolation . Philos. Trans. R. Soc. Lond. B 345 : 101 – 118 . [DOI] [PubMed] [Google Scholar]
- Goff M. L. 1991. . Comparison of insect species associated with decomposing remains recovered inside dwellings and outdoors on the island of Oahu, Hawaii . J. Forensic Sci. 36 : 748 – 753 . [PubMed] [Google Scholar]
- Gotelli N. J., Colwell R. K. . 2001. . Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness . Ecol. Lett. 4 : 379 – 391 . [Google Scholar]
- Gregor F., Rozkošný R., Barták M., Vaňhara J. . 2002. . The Muscidae (Diptera) of central Europe . Masaryk University, Brno, Czech Republic; . [Google Scholar]
- Jiménez-Valverde A., Hortal J. . 2003. . Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos . Rev. Ibérica Aracnol. 8 : 151 – 161 . [Google Scholar]
- Leclercq M. 1978. . Entomologie et médecine légale: datation de la mort . Masson; , Paris, France: . [Google Scholar]
- Magurran A. E. 2004. . Measuring biological diversity . Wiley-Blackwell; , London, United Kingdom: . [Google Scholar]
- Martín-Vega D., Baz A. . 2012. . Spatiotemporal distribution of necrophagous beetles (Coleoptera: Dermestidae, Silphidae) assemblages in natural habitats of central Spain . Ann. Entomol. Soc. Am. 105 : 44 – 53 . [Google Scholar]
- Martín-Vega D., Baz A. . 2013. . Sarcosaprophagous Diptera assemblages in natural habitats in central Spain: spatial and seasonal changes in composition . Med. Vet. Entomol. 27 : 64 – 76 . [DOI] [PubMed] [Google Scholar]
- Martínez-Sánchez A., Rojo S., Marcos-García M. A. . 2000. . Annual and spatial activity of dung flies and carrion in a Mediterranean holmoak pasture ecosystem . Med. Vet. Entomol. 14 : 56 – 63 . [DOI] [PubMed] [Google Scholar]
- Matuszewski S., Bajerlein D., Konwerski S., Szpila K. . 2010. . Insect succession and carrion decomposition in selected forests of central Europe. Part 1: pattern and rate of decomposition . Forensic Sci. Int. 194 : 85 – 93 . [DOI] [PubMed] [Google Scholar]
- Morón M. A., Terrón R. A. . 1984. . Distribución altitudinal y estacional de los insectos necrófilos en la Sierra Norte de Hidalgo, México . Acta Zool. Mexicana 3 : 1 – 47 . [Google Scholar]
- Newton A., Peck S. B. . 1975. . Baited pitfall traps for beetles . Coleopt. Bull. 29 : 45 – 46 . [Google Scholar]
- Ninyerola M., Pons X., Roure J. M. . 2005. . Atlas climático digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica . Universidad Autónoma de Barcelona, Bellaterra; , Spain: . [Google Scholar]
- Peet R. K. 1974. . The measurement of species diversity . Annu. Rev. Ecol. Syst. 5 : 285 – 307 . [Google Scholar]
- Prado e Castro C., Sousa J. P., Arnaldos M. I., Gaspar J., García M. D. . 2011. . Blowflies (Diptera: Calliphoridae) activity in sun exposed and shaded carrion in Portugal . Ann. Soc. Entomol. France 47 : 128 – 139 . [Google Scholar]
- Sánchez Piñero F. 1997. . Analysis of spatial and seasonal variability of carrion beetle (Coleoptera) assemblages in two arid zones of Spain . Environ. Entomol. 26 : 805 – 814 . [Google Scholar]
- Séguy E. 1934. . Diptères (Brachycères) (Muscidae Acalypterae et Scatophagidae) . Faune France 28 : 1 – 832 . [Google Scholar]
- Tews J., Brose U., Grimm V., Tielbörger K., Wichmann M. C., Schwager M., Jeltsch F. . 2004. . Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures . J. Biogeogr. 31 : 79 – 92 . [Google Scholar]
- Woodcock B. A., Watt A. D., Leather S. R. . 2002. . Aggregation, habitat quality and coexistence: a case study on carrion fly communities in slug cadavers . J. Anim. Ecol. 71 : 131 – 140 . [Google Scholar]