Abstract
This study was conducted to investigate the effects of dietary protein levels on honey bee colonies, specifically the population growth, physiology, and longevity of honey bee workers during early spring. Diets containing four different levels of crude protein (25.0, 29.5, 34.0, or 38.5%) and pure pollen (control) were evaluated. Twenty-five colonies of honey bees with sister queens were used in the study. We compared the effects of the different bee diets by measuring population growth, emergent worker weight, midgut proteolytic enzyme activity, hypopharyngeal gland development, and survival. After 48 d, the cumulative number of workers produced by the colonies ranged from 22,420 to 29,519, providing a significant fit to a quadratic equation that predicts the maximum population growth when the diet contains 31.7% crude protein. Significantly greater emergent worker weight, midgut proteolytic enzyme activity, hypopharyngeal gland acini, and survival were observed in the colonies that were fed diets containing 34.0% crude protein compared with the other crude protein levels. Although higher emergent worker weight and survival were observed in the colonies that were fed the control diet, there were no significant differences between the control colonies and the colonies that were fed 34.0% crude protein. Based on these results, we concluded that a dietary crude protein content of 29.5–34.0% is recommended to maximize the reproduction rate of honey bee colonies in early spring.
Keywords: Apis mellifera , dietary protein, population growth, worker quality, survival
Every year in early spring in northern regions, honey bee colonies are broodless, containing populations of long-lived workers ( Fukuda and Sekiguchi 1966 , Mattila et al. 2001 ). Brooding activity commences before the foraging season begins ( Winston 1987 , Mattila and Otis 2006 ). The main focus of the colony shifts from survival to growth to rebuild the aging winter population ( Johnson 2010 ). Protein is the most important nutrient for population recovery. Protein is essential for the rapid growth of larvae and the satisfactory development of worker bees ( Crailsheim et al. 1992 , Pernal and Currie 2000 , Zerbo et al. 2001 , Hoover et al. 2006 ).
Honey bees rely on pollen as their only natural source of protein ( Grogan and Hunt 1979 , Loper et al. 1980 ). However, not all pollen contains adequate nutrition for colony development ( Pernal and Currie 2000 ). In addition, honey bees have limited foraging opportunities due to inclement weather in early spring, and they thus have fewer chances to gather resources ( Farrar 1934 ). Hence, supplementary diets including pollen or pollen substitutes are sometimes necessary to provide the nutrients required for colonies to rear brood, increase their populations, overwinter, and produce honey ( Herbert 1992 , Goodwin et al. 1994 ).
In general, the most rapid population growth results in increased probabilities for colonies to obtain the abundant products when the resource of nectar and pollen is available in natural environment. The mechanism for population growth is increased brood rearing ( Sagili and Pankiw 2007 ). Ordinarily, nurse bees tend the young larvae within the nest. It has been established that the digestion and absorption of protein mainly occurs in the midgut of bees ( Crailsheim 1990 ), where protein is hydrolyzed by proteolytic enzymes. Subsequently, the nutrients are converted into worker jelly by a twinned pair of food glands together referred to as the hypopharyngeal gland (HPG; Hrassnigg and Crailsheim 1998b ). The jelly, which is rich in protein, is fed to young larvae and to the queen. Brood rearing is therefore reduced during times of low nutritional reserves in a colony ( Keller et al. 2005 , Mattila and Otis 2007 ).
The nutrient reserves of newly emerged workers constitute an accurate indicator of nutritional investment during the larval stage ( Mattila and Otis 2006 ). At emergence, the long-lived winter bees have greater weights and protein contents than the short-lived workers reared during other seasons ( Kunert and Crailsheim 1988 ). Hence, the nutritional status of newly emerged workers is relatively stable in contrast to the performance of workers as adults ( Mattila and Otis 2006 ). In addition, newly emerged bees consume a great deal of protein to support tissue differentiation.
Much research has concentrated on producing an ideal supplemental protein diet for bees that is healthy, rich in the required nutrients, and readily accepted. The effects of such protein-rich diets on colony development, longevity, and honey bee physiology have been studied previously ( Doull 1980 , Herbert 1992 , Cremonez et al. 1998 , Mattila and Otis 2006 , Dastouri and Maheri-Sis 2007 ). Importantly, Herbert et al. (1977) found that caged adult bees required an optimal level of 23–30% protein for brood rearing. However, this study was not performed in a natural environment but rather under brood rearing conditions. Therefore, further studies are needed to investigate the effects of dietary protein contents on the reproductive output of honey bees under natural conditions. This study was conducted to determine the optimal dietary protein level for winter bees for rebuilding colony populations during early spring. Herein, we report the results of the effects of different dietary protein levels on population growth and worker quality.
Materials and Methods
The study was conducted from February to April 2011 in an apiary at Shandong Agricultural University, Tai′an, Shandong, China (36° 15′N, 117° 01′E, 310 m above sea level). The study was terminated when flowering plants became available for the bees and supplemental feeding was no longer necessary. A set of equalized honey bee ( Apis mellifera ligustica Spinola) colonies were selected during the previous fall. All the colonies lived through the winter under the same conditions. During early spring, the colonies were randomly assigned to different protein-level treatments. Prior to the experiment, each colony consisted of a 6-mo-old queen that had been reared and mated naturally in the laboratory and the same quantity of overwintered bees (15,000–15,500 bees). Before the experiment, the old comb frames were replaced with new ones of the same standards. Three empty comb frames were used for each brood chamber, and two comb frames were full of honey. The population of winter bees was estimated according to Burgett and Burikam (1985) .
Experimental Diets
We simultaneously tested the effects of four diets containing different levels of crude protein (CP) and bee-collected rape pollen, which is commonly fed to honey bees in China. The dietary formulas and the approximate compositions of four isocaloric test diets (diet 1–diet 4) and rape pollen (control diet) are shown in Table 1 . The CP of diets was estimated using the Kjeldahl nitrogen method. The experiment consisted of testing 25 colonies (5 diets by 5 colonies). The defatted soybean meal (Shandong, China) and corn flour (Shandong, China) components of the diet were sieved through a #120 mesh. The diets were supplemented by providing the colonies with prepared feed patties weighing 400 g each, consisting of the dry feeds or pollen mixed with liquid sucrose at a 2:3 ratio by weight. The patties were wrapped in ordinary waxed paper and placed on top of the frames over the brood clusters. In addition, the honey and patties were checked every 3–4 d, and new honey and patties were supplied when the levels were insufficient.
Table 1.
Ingredients and chemical compositions of the experimental diets (on dry matter basis)
|
Dietary protein levels
|
|||||
|---|---|---|---|---|---|
| 25.00% | 29.50% | 34.00% | 38.50% | Control diet | |
| (Diet 1) | (Diet 2) | (Diet 3) | (Diet 4) | (Control) | |
| Ingredients (%) | |||||
| Rape pollen | 25 | 25 | 25 | 25 | 99.5 |
| Defatted soybean meal | 16.4 | 28.4 | 43.4 | 55.4 | 0 |
| Corn flour | 51.4 | 39.4 | 25.9 | 13.9 | 0 |
| Peanut protein isolate | 6 | 6 | 4.5 | 4.5 | 0 |
| Calcium hydrogen phosphate | 0.4 | 0.4 | 0.4 | 0.4 | 0 |
| Vitamin premix a | 0.3 | 0.3 | 0.3 | 0.3 | 0 |
| Antioxidant b | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Proximate analysis | |||||
| Dry matter (%) | 90.42 | 90.28 | 90.3 | 90.11 | 90.03 |
| Crude protein (%) | 24.88 | 29.43 | 33.7 | 38.35 | 26.88 |
| Gross energy (MJ/kg) c | 17.38 | 17.54 | 17.65 | 17.79 | 17.86 |
a Vitamin premix contained the following vitamins per kilogram of feed: vitamin A, 5,000 IU; thiamin, 5.4 mg; riboflavin, 6 mg; vitamin C, 100 mg; vitamin D3, 2,000 IU; vitamin E, 240 mg; vitamin B6, 7 mg; folic acid, 20 mg; niacin, 18 mg; and inositol, 318 mg.
b Antioxidant is used to prevent the powder from deteriorating.
c Gross energy (kJ/g diet) = (%Crude protein × 23.6) + (%Crude lipids × 39.5) + (%Carbohydrates × 17.3).
Coding and Sampling the Bees
To obtain bees of defined ages, emerging brood combs were collected from the source colonies and incubated at 34°C and 60% RH. Two hundred newly emerged adult worker bees were collected per colony within 12 h, and their thoraxes were marked with shellac paint ( von Frisch 1965 ). All the newly emerged bees were then introduced into their mother colonies to avoid impacting the age structures of the colonies. To minimally disturb the colonies, the sampling was performed without the use of smoke, which induces workers to feed on honey ( Free 1968 , Hrassnigg and Crailsheim 1998a , b ) and affects the trophallactic behavior of the workers ( Farina and Nunez 1991 ).
Monitoring Brood Rearing Activity in the Colonies
The number of capped broods was estimated to determine the number of workers reared in each colony. Assessments were made at 12-d intervals, once before and four times after treatment. The pupating workers remained in the sealed cells for 12 d ( Winston 1987 ), and the mortality of the sealed broods was very low (the mortality of workers in sealed broods is typically <3%; Fukuda and Sakagami 1968 ). Thus, a new set of pupating workers was counted in each assessment. The number of capped broods was monitored using a modified square grid system ( Mattila and Otis 2007 ). The grid consisted of 24 squares, each with an area of 35 cm 2 . The grids were placed over the brood frames, and the comb area occupied by the capped brood was measured. The total number of bees reared in each colony was estimated using a factor of 4.29 worker cells per square centimeter ( Seeley and Visscher 1985 ).
Newly Emerged Workers
At the end of the study, the wet weight of 20 bees per colony was obtained within 2 h after emergence. Three bees were randomly selected from the 20 bee groups and analyzed for protein concentration. The heads and thoraxes of the three workers were removed and homogenized individually in 1.5-ml Eppendorf microcentrifuge tubes containing a 75-mM phosphate buffer solution (pH 7.4). The samples were centrifuged and analyzed for protein concentration using a PA102 Bradford protein assay kit (Tiangen Biotech Co. Ltd, Beijing, China). Standard curves for estimating protein concentration were prepared using bovine serum albumin. The protein absorbance was measured at 595 nm versus a blank reagent using a Shimadzu spectrophotometer (Model #UV-2450, Shimadzu Corporation, Kyoto, Japan).
Nurse Bees
Hemolymph Protein Concentration
The 6-d-old bees were captured directly from the comb surface, placed inside centrifuge tubes, and anesthetized by burying the centrifuge tubes in ice. After 5 min, hemolymph was collected from each bee by making a small dorsal incision in the intersegmental membrane between abdominal segments 5 and 6 and pressing a capillary tube against it ( Wegener et al. 2009a ). The total protein content in the hemolymph was estimated according to Wegener et al. (2009b) .
Midgut Proteolytic Enzyme Activity
The midguts of the 6-d-old bees were excised carefully, placed in Eppendorf tubes containing 100 µl of Tris–HCl (pH 7.9) each, and stored at −80°C for further processing. The total midgut protease activity was measured as described by Sagili et al. (2005) . Briefly, frozen guts were crushed using a glass pestle, homogenized in Tris–HCl buffer (pH 7.9), and centrifuged at 10,000 rpm at 4°C for 5 min. The supernatants were used to determine the total midgut proteolytic enzyme activity ( Michaud et al. 1995 ).
Twenty microliters of assay buffer (0.1 M Tris–HCl, pH 7.9) and 60 μl of 2% (w/v) azocasein diluted in assay buffer were added to 5 μl of supernatant. After 6 h of incubation at 37°C, the reaction was stopped by adding 300 μl of 10% (w/v) trichloroacetic acid to each mixture. The tubes were centrifuged for 5 min at 10,000 rpm, and 350 μl of the supernatant was added to 200 μl of 50% ethanol in water. The absorbance of this mixture was measured at 440 nm using a Shimadzu spectrophotometer (Model #UV-2450, Shimadzu Corporation). The total midgut proteolytic activity was expressed as OD 440 .
Hypopharyngeal Gland Measurements
Five honey bee workers from each colony were selected at 6 and 12 d of age to assess HPG development. The HPGs were removed and placed in a Petri dish with wax depressions each containing a droplet of ice-cold sodium chloride solution (0.85%, isotonic to the hemolymph). Micrographs of the HPGs were taken using a microscope equipped with a camera. For calibration, an image of a 1-mm scale bar was obtained at the same magnification. The analysis of HPG acini was performed by measuring the areas of five acini cells selected at random for each bee using the Photoshop (Adobe) pixel counting routine. Thus, 25 acini cells were examined for each colony for a total of 125 acini cells per treatment.
Estimating Longevity of Spring-Reared Workers
Forty newly emerged adult worker bees (<12 h old) were obtained from each colony by placing brood frames overnight in an incubator (33°C and 50% RH) and randomly assigned to a wooden cage (200 bees per treatment). Each experimental cage had a movable plastic slide on top and contained a single diet plus water, a 50% sucrose solution and a piece of beeswax foundation. The bees were fed with the same diets, which were replaced with a fresh mixture daily, and incubated at 33°C with 45–50% RH for 30 d. Dead bees were removed from each cage and counted daily. The longevity and survivorship of the workers since the initiation of the experiment were determined for each treatment group.
Statistical Analysis
SAS version 9.1 was used for all statistical analyses ( SAS Institute 2004 ). The data were analyzed using a one-way analysis of variance to determine the effects of the four experimental diets. The significance level for all tests was set at α = 0.05, and Duncan's new multiple range test was used to rank the groups. All data are presented as means ± SEM.
Results
Population Development During Early Spring
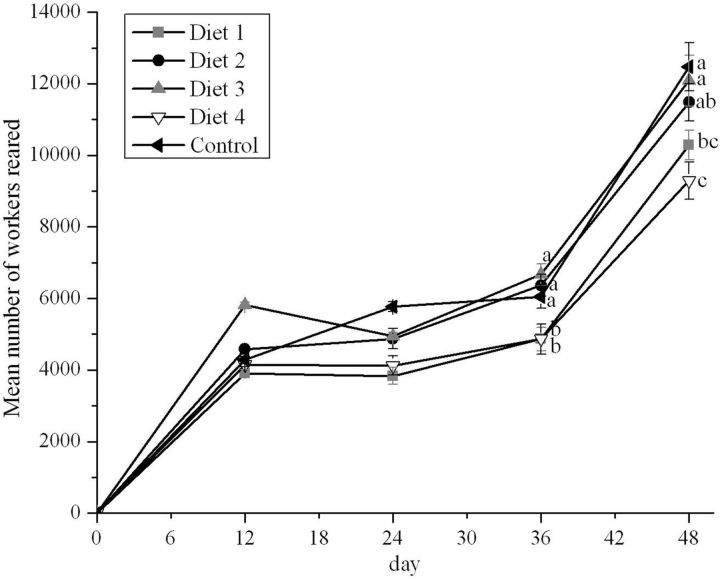
The population of workers in sealed broods during the experiment is shown in Fig. 1 . Furnishing diets with different CP levels to the experimental colonies during early spring had a significant effect on the timing of the increase in brood rearing activity as the season progressed ( P < 0.05). The mean number of workers produced by the colonies increased quickly during early spring. At the beginning of the experiment, all the colonies were broodless. When pollen supplements were used, 34% CP colonies tended to have the greatest number of workers in sealed broods at each colony census, and brood rearing activity was not significantly different from that observed in the control colonies ( P > 0.05). A mean of 28,583 ± 936 workers per colony were reared in the control colonies during the entire experiment. This number represents 25–27% more workers than those reared in the 25% CP colonies (mean 22,885 ± 961 workers per colony) or in the 38.5% CP colonies (mean 22,420 ± 828 workers per colony) over the same period.
Fig. 1.
Mean number of workers (±SE) reared by colonies fed rape pollen and diets with different protein levels (25.0, 29.5, 34.0, and 38.5%) during early spring ( N = 5 colonies per treatment). Different letters signify significant differences at P < 0.05.
Newly Emerged Workers—Emergent Worker Weight and Protein Concentration
The average emergent worker weight and the protein concentrations of the head and thorax capsules were significantly affected by dietary protein levels ( P < 0.05; Table 2 ). The greatest emergent worker weights were obtained with honey bees fed the control diet, but there was no significant difference between the weights of the bees fed the 34% CP diet and the control diet. The highest concentrations of protein in the head and thorax capsules were obtained using the 38.5% CP diet ( P > 0.05). The protein concentrations of the head and thorax capsules increased significantly with increasing dietary protein levels ( P < 0.05).
Table 2.
Mean values (±SE) for the protein concentrations in the head and thorax and the wet weights of the emergent worker bees fed with different test diets ( N = 5 colonies per treatment)
| Emergent worker weight (mg) | Protein concentration of head (µg protein/mg) | Protein concentration of thorax (µg protein/mg) | |
|---|---|---|---|
| Diet 1 | 105.4 ± 3.4c | 149.8 ± 12.0b | 163.7 ± 11.5b |
| Diet 2 | 108.0 ± 1.0bc | 148.0 ± 8.7b | 171.4 ± 3.7b |
| Diet 3 | 114.7 ± 1.2ab | 198.9 ± 9.6a | 233.5 ± 6.1a |
| Diet 4 | 107.6 ± 4.1bc | 223.8 ± 8.5a | 248.6 ± 8.8a |
| Control diet | 116.0 ± 1.7a | 222.4 ± 4.6a | 232.5 ± 7.0a |
Values in the same row with different letters are significantly different ( P < 0.05).
Nurse Bees—Midgut Proteolytic Enzyme Activity, Hemolymph Protein Concentration, and HPG Development
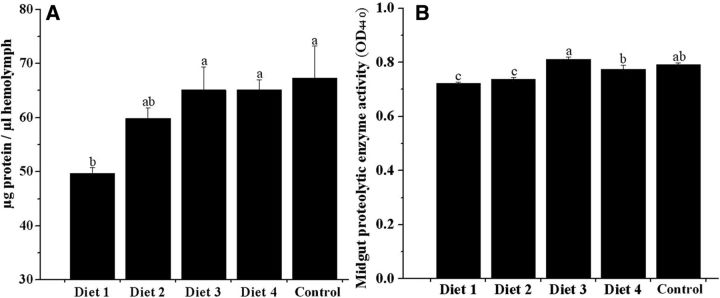
Figure 2 a shows that the mean hemolymph protein concentration increased with increasing protein levels. However, no significant differences were observed in the bees fed with diets ranging from 29.5% CP to 38.5% CP ( P > 0.05). Only in the 25% CP colonies were the hemolymph protein concentrations significantly lower than those in the control colonies ( P < 0.05).
Fig. 2.
Mean hemolymph protein concentrations (A, ±SE) and mean midgut proteolytic enzyme activities (B, ±SE) of 6-d-old bees fed rape pollen and diets containing different protein levels (25.0, 29.5, 34.0, and 38.5%; N = 5 colonies per treatment). Different letters signify significant differences at P < 0.05.
The results indicated that the dietary protein levels significantly affected midgut proteolytic enzyme activity ( P < 0.05; Fig. 2 b). The highest midgut proteolytic enzyme activity of the workers was obtained with the 34% CP diet. However, there was no significant difference between the hemolymph protein concentrations in the control colony and the 34% CP diet colonies. Bees fed the diets containing 25.0% CP and 29.5% CP had significantly lower midgut protease activity than the other treatments ( P < 0.05).
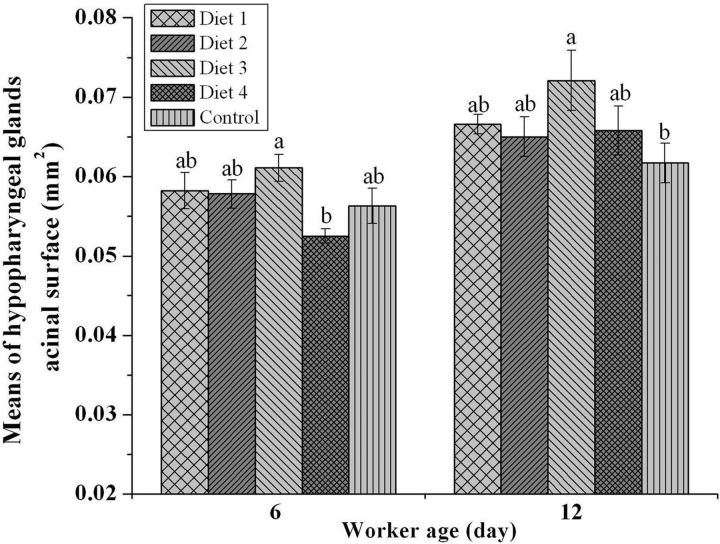
The largest HPG acini in the nurse bees were obtained at 34.0% CP ( Fig. 3 ; 6 and 12 d old). There were no significant differences in HPG development in 6-d-old bees fed pollen supplements versus those receiving pure pollen ( P > 0.05). However, the mean area of the HPG acini was significantly greater in bees fed the 34.0% CP diet compared with bees fed the 38.5% CP diet. The 12-d-old bees fed the 34.0% CP diet had significantly larger HPG acini than the bees fed the control diet ( Fig. 3 ; P < 0.05). However, there were no significant differences between the other groups that were fed protein supplements versus those receiving natural pollen.
Fig. 3.
Development of hypopharyngeal glands during the nursing period of honey bee workers (at 6 and 12 d; N = 5 colonies per treatment). The control diet is rape pollen, and the dietary protein levels in the diets are as follows: diet 1, 25.0% CP; diet 2, 29.5% CP; diet 3, 34.0% CP; and diet 4, 38.5% CP. Different letters signify significant differences at P < 0.05.
Survival
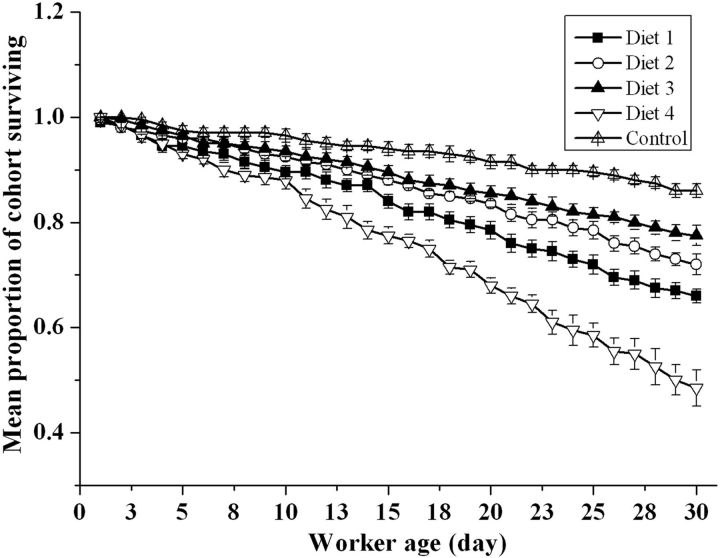
The importance of dietary protein for the survival of honey bees is illustrated in Fig. 4 . The overall survival of bees from the different groups in the study averaged 70.0% after feeding for 30 d. The survivorship curve indicates that bees that were fed with the diet containing 38.5% CP had the lowest survival rate, followed by the groups fed the 25.0, 29.5, and 34.0% CP diets. Among the bees fed the 38.5% CP diet, 40% died by the 24th day of the trial, and less than 48% survived the complete 30-day trial. In contrast, at least 66% of the bees in the other treatments survived the complete trial—a significantly higher survival rate than those fed the 38.5% CP diet. The highest survival rate was obtained with the control diet.
Fig. 4.
The mean proportions (±SE) of workers ( N = 5 colonies per treatment) that survived over time when fed rape pollen and the various dietary protein levels (25.0, 29.5, 34.0, and 38.5%).
Discussion
The results of this study demonstrate that dietary protein levels strongly affect the population growth, performance, and physiological status of spring-reared worker bees. Mattila and Otis (2006) found that the quality of spring-reared workers is controlled by the nutritional state of the colony when the workers are reared. Protein is a structural and functional constituent of tissues and thus plays an important role in brood rearing and in the satisfactory development of adult bees ( Crailsheim 1990 , Pernal and Currie 2000 , Zerbo et al. 2001 , Hoover et al. 2006 ). This study indicates that honey bee ( Apis mellifera L.) colonies require 29.5–34.0% dietary CP (before mixing with sucrose and water) for maximum population growth and maximum worker quality.
In this study, the highest dietary protein level resulted in lower survival and reduced population growth. Excess protein ingested by honey bees is responsible for the accumulation of undigested material in the gut ( DeGroot 1953 ). Herbert et al. (1977) reported that pollen substitutes containing 50% protein depressed brood rearing. Although the 38.5% CP diet is rich in protein, the extra protein may inhibit the absorption of other nutrients or otherwise result in fitness costs. Raubenheimer and Simpson (1997) suggested that the ability to ingest excess nutrients may significantly limit nutritional regulation in many insects. In the natural environment, it is well known that the protein content of bee-collected pollen varies considerably among species ( Bell et al. 1983 , Roulston and Cane 2000 , Keller et al. 2005 ). However, in response to increasing demands for protein, bee colonies regulate the quantity of pollen collected rather than select pollen with a higher protein content ( Pernal and Currie 2000 , Keller et al. 2005 ). In addition, there is no evidence that bees prefer to collect pollen containing high levels of protein ( Roulston et al. 2000 ).
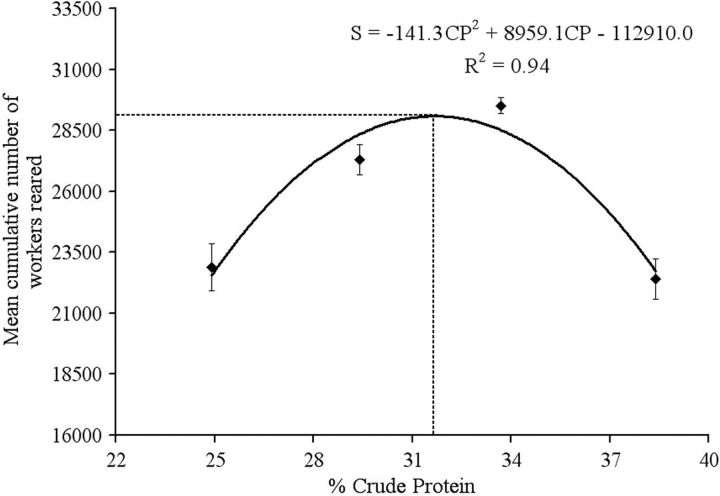
A significant regression analysis was constructed, which displays a satisfactory fit to a quadratic equation representing the mean cumulative number of spring-reared workers ( Fig. 5 ). Based on this regression, a dietary protein level of approximately 31.7% is predicted to maximize the cumulative number of workers produced by the colonies. This study demonstrates that such a protein level is optimal for meeting the nutritional requirements for brood rearing during early spring. Kleinschmidt and Kondos (1976) reported that honey bees require a minimum level of 20% CP (dry mass) in pollen for brood rearing, and Herbert et al. (1977) reported that caged honey bees require a diet containing 23–30% CP for optimal brood rearing. These optimal protein levels are lower than the levels proposed in our study, perhaps because the protein requirements of honey bees reared under laboratory conditions are lower than those of bees reared under natural conditions.
Fig. 5.
The mean cumulative numbers (±SE) of workers produced by the colonies fed with different protein levels (25.0, 29.5, 34.0, and 38.5%) for 48 d ( N = 5 colonies per treatment). The crude protein content yielding the maximum number of brood was calculated from the regression coefficients as follows: 8 959.1/(2 by 141.3) = 31.7 (dashed line).
During brood rearing, the HPGs of nurse workers produce a proteinic secretion that is used to feed the increasing larvae ( Michener 1974 , Huang and Otis 1989 , Crailsheim 1990 ). This proteinic secretion is easy to digest and thus supports rapid growth of the sealed brood. We confirmed that the superficial area of HPG acini increased with the age of the bees regardless of whether the colonies were fed pollen or pollen supplements. Furthermore, the nurse bees fed pollen supplements had significantly larger HPG acini than the control group, which was fed pure pollen. Similar results were obtained when comparing honey bees fed pollen patties versus MegaBee patties (a protein supplement that lacks pollen; DeGrandi-Hoffman et al. 2010 ). It appears that the pollen supplements activated HPG development similarly to that activated by bee bread. Our observations indicate that bees fed the 34.0% CP diet had the largest HPG acini and brood rearing activities. Furthermore, when the colonies were fed diets with different protein levels, the trend in HPG variation in the acini of 6-d-old bees was the same as that of 12-d-old bees. Thus, feeding bees with the 34.0% CP diet in early spring was optimal for HPG development.
Additionally, HPG protein biosynthesis is affected by midgut proteolytic enzyme activity ( Sagili et al. 2005 ), which converts the natural and artificial protein diets into usable amino acids. Sagili et al. (2005) reported similarly high levels of total midgut proteolytic enzyme activity in 7-d-old bees, but higher enzyme levels were reported by Sagili and Pankiw (2007) . Midgut proteolytic enzyme activity tended to increase when the bees were fed diets containing 25.0% CP to 34.0% CP, and enzyme activity then decreased when the bees were fed 38.5% CP diets. This increase in the activity of the midgut proteolytic enzymes may be due to the use of excess dietary amino acids for growth. When the amount of amino acids in a diet exceeds a certain level, the opposite tendencies are observed ( DeGroot 1953 ). Hence, the size of the HPG acini decreased with the diet containing 38.5% CP, corresponding to reduced midgut proteolytic enzyme activity.
Cremonez et al. (1998) proposed that measuring the protein content in the hemolymph of 6-d-old bees is a rapid and precise method for choosing the suitable protein diets for workers. In our previous studies, the total protein content in the hemolymph increased when the dietary protein content was increased. The food sources of bees therefore affect their hemolymph composition ( Cremonez et al. 1998 ). There were no significant differences among the hemolymph protein contents in the bees fed natural pollen and pollen substitutes except when the bees were given the 25.0% CP diet. Hence, if pollen substitutes contain adequate nutrition, they can be superior to bee-collected pollen.
Worker longevity is affected by the age at which foraging begins ( Guzman-Novoa et al. 1994 , Rueppell et al. 2007 ). However, our colonies had no access to foraged pollen, the bees’ natural source of protein, during the study period. Additionally, we provided the colonies with protein diets, honey, and water. Differences in dietary protein are therefore the main factors determining worker longevity. Furthermore, midgut proteolytic enzyme activity, which is an important factor for bee survival ( Sagili et al. 2005 ), was significantly affected by dietary CP levels.
The lifespan and nutrient reserves of adult emergence provide an accurate indicator of nutritional investment during the larval stage and can be influenced by the nutritional composition of the colony’s diet ( Janmaat and Winston 2000 , Mattila and Otis 2006 ). Schmickl and Crailsheim (2001) reported that colonies experiencing pollen shortages produced workers that had reduced protein levels. Lower protein contents in the workers’ bodies result in shorter life spans ( Eischen et al. 1982 , Kulinĉević et al. 1983 ). In our study, emergent worker bees with high weights and protein contents had low mortality rates. In addition, worker longevity is one of the most important factors determining the population growth rates of honey bee colonies ( Schmid-Hempel et al. 1993 ). The colonies fed the 38.5% CP diet had high mortality rates and low rates of population growth. However, the colonies fed diets containing the three other protein levels displayed improvements in survival as protein levels increased. Simultaneously, the protein content of pollen is generally accepted to reflect the nutritional value of the pollen for bee colonies ( Pernal and Currie 2000 , Roulston et al. 2000 , Somerville and Nicol 2006 , Mapalad et al. 2008 ). Consequently, diets with optimal protein levels have higher nutritional value for bee colonies.
In conclusion, population growth and worker quality were significantly affected by dietary composition and can be manipulated as metabolic tools to assess the optimal concentration of dietary protein in the feeding of honey bees. The optimum protein content in field applications may vary according to dietary ingredient compositions and feeding methodology. Based on the results of our study, a CP content of 29.5–34.0% is recommended for the diet of honey bees during early spring.
Acknowledgments
This research was financially supported by the earmarked fund for Modern Agro-Industrial Technology Research Systems (CARS-45) and the Special Fund for Agro-Scientific Research in the Public Interest (200903006).
References Cited
- Bell R. R., Thornber E. J., Seet J. L. L., Groves M. T., Ho N. P., Bell D. T. . 1983. . Composition and protein quality of honeybee-collected pollen of Eucalyptus marginata and Eucalyptus calophylla . J. Nutr. 113 : 2479 – 2484 . [DOI] [PubMed] [Google Scholar]
- Burgett M., Burikam I. . 1985. . Number of adult honey bees (Hymenoptera: Apidae) occupying a comb: a standard for estimating colony populations . J. Econ. Entomol. 78 : 1154 – 1156 . [Google Scholar]
- Crailsheim K. 1990. . The protein balance of the honey bee worker . Apidologie 21 : 417 – 429 . [Google Scholar]
- Crailsheim K., Schneider L. H. W., Hrassnigg N., Bühlmann G., Brosch U., Gmeinbauer R., Schõffmann B. . 1992. . Pollen consumption and utilization in worker honeybees ( Apis melliferacarnica ): dependence on individual age and function . J. Insect Physiol. 38 : 409 – 419 . [Google Scholar]
- Cremonez T. M., De Jong D., Bitondi M. M. G. . 1998. . Quantification of hemolymph proteins as a fast method for testing protein diets for honey bees (Hymenoptera: Apidae) . J. Econ. Entomol. 91 : 1284 – 1289 . [Google Scholar]
- Dastouri M. R., Maheri-Sis N. . 2007. . The effect of replacement feeding of some protein sources with pollen on honey bee population and colony performance . J. Anim. Vet. Adv. 6 : 1258 – 1261 . [Google Scholar]
- DeGrandi-Hoffman G., Chen Y., Huang E., Huang M. H. . 2010. . The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees ( Apis mellifera L.) . J. Insect Physiol. 56 : 1184 – 1191 . [DOI] [PubMed] [Google Scholar]
- DeGroot A. P. 1953. . Protein and amino acid requirements of the honeybee ( Apis mellifica L.) . Physiol. Comp. Oecol. 3 : 197 – 285 . [Google Scholar]
- Doull K. M. 1980. . Relationships between consumption of a pollen supplement, honey production and broodrearing in colonies of honeybees Apis mellifera L. II . Apidologie 11 : 367 – 374 . [Google Scholar]
- Eischen F. A., Rothenbuhler W. C., Kulinĉević J. M. . 1982. . Length of life and dry weight of worker honeybees reared in colonies with different worker-larva ratios . J. Apic. Res. 21 : 19 – 25 . [Google Scholar]
- Farina W. M., Nunez J. A. . 1991. . Trophallaxis in the honeybee, Apis mellifera (L.) as related to the profitability of food sources . Anim. Behav. 42: 389 – 394 . [Google Scholar]
- Farrar C. L. 1934. . Bees must have pollen, Glean . Bee Cult. 62 : 276 – 278 . [Google Scholar]
- Free J. B. 1968. . Engorging of honey by worker honeybees when their colony is smoked . J. Apicult. Res. 7 : 135 – 138 . [Google Scholar]
- Fukuda H., Sekiguchi K. . 1966. . Seasonal change of the honeybee worker longevity in Sapporo, North Japan, with notes on some factors affecting the life-span . Jpn. J. Ecol. 16 : 206 – 212 . [Google Scholar]
- Fukuda H., Sakagami S. . 1968. . Worker brood survival in honeybees . Res. Popul. Ecol. 10 : 31 – 39 . [Google Scholar]
- Goodwin R. M., Ten Houten A., Perry J. H. . 1994. . Effect of feeding pollen substitutes to honey bee colonies used for kiwifruit pollination and honey production, New Zeal . J. Crop. Hort. 22 : 459 – 462 . [Google Scholar]
- Grogan D. E., Hunt J. H. . 1979. . Pollen proteases: their potential role in insect digestion . Insect Biochem. 9 : 309 – 313 . [Google Scholar]
- Guzman-Novoa E., Page R. E., Gary N. E. . 1994. . Behavioral and life history components of division of labor in honey bees ( Apis mellifera L.) . Behav. Ecol. Sociobiol. 34 : 409 – 417 . [Google Scholar]
- Herbert E. W., Jr. 1992. . Honey bee nutrition , pp. 197 – 233 . InGraham J. M. (ed.), The hive and the honey bee . Dadant and Sons; , Hamilton, IL: . [Google Scholar]
- Herbert E. W., Jr., Shimanuki H., Caron D. . 1977. . Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing . Apidologie 8 : 141 – 146 . [Google Scholar]
- Hoover S. E. R., Higo H. A., Winston M. L. . 2006. . Worker honey bee ovarian development: seasonal variation and the influence of larval and adult nutrition . J. Comp. Physiol. B 176 : 55 – 63 . [DOI] [PubMed] [Google Scholar]
- Hrassnigg N., Crailsheim K. . 1998a. . The influence of brood on the pollen consumption of worker bees ( Apis mellifera L.) . J. Insect Physiol. 44 : 393 – 404 . [DOI] [PubMed] [Google Scholar]
- Hrassnigg N., Crailsheim K. . 1998b. . Adaptation of hypopharyngeal gland development to the brood status of honeybee ( Apis mellifera L.) colonies . J. Insect Physiol. 44 : 929 – 939 . [DOI] [PubMed] [Google Scholar]
- Huang Z. Y., Otis G. W. . 1989. . Factors determining hypopharyngeal gland activity of worker honey bees ( Apis mellifera L.) . Insect Soc. 36 : 264 – 276 . [Google Scholar]
- Janmaat A., Winston M. L. . 2000. . The influence of pollen storage area and Varroa jacobsoni Oudemans parasitism on temporal caste structure in honey bees ( Apis mellifera L.) . Insect Soc. 47 : 177 – 182 . [Google Scholar]
- Johnson B. 2010. . Division of labor in honeybees: form, function, and proximate mechanisms . Behav. Ecol. Sociobiol. 64 : 305 – 316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller I., Fluri P., Imdorf. A. 2005. . Pollen nutrition and colony development in honey bees: part I . Bee World 86 : 3 – 10 . [Google Scholar]
- Kleinschmidt G. J., Kondos A. C. . 1976. . The influence of crude protein levels on colony production . Aust. Beekeep. 78 : 36 – 39 . [Google Scholar]
- Kulinĉević J. M., Rothenbuhler W. C., Rinderer T. E. . 1983. . Disappearing disease—II. Effects of certain protein sources on brood rearing and length of life in the honey bee under laboratory conditions . Am. Bee J. 123 : 50 – 53 . [Google Scholar]
- Kunert K., Crailsheim K. . 1988. . Seasonal changes in carbohydrate, lipid and protein content in emerging worker honeybees and their mortality . J. Apic. Res. 27 : 13 – 21 . [Google Scholar]
- Loper G. M., Standifer L. N., Thompson M. J., Gilliam M. . 1980. . Biochemistry and microbiology of bee-collected almond ( Prunus dulcis ) pollen and bee bread I—fatty acids, vitamins and minerals . Apidologie 11 : 63 – 73 . [Google Scholar]
- Mapalad K. S., Leu D., Nieh J. C. . 2008. . Bumble bees heat up for high quality pollen . J. Exp. Biol. 211 : 2239 – 2242 . [DOI] [PubMed] [Google Scholar]
- Mattila H. R., Otis G. W. . 2006. . The effects of pollen availability during larval development on the behaviour and physiology of spring-reared honey bee workers . Apidologie 37 : 533 – 546 . [Google Scholar]
- Mattila H. R., Otis G. W. . 2007. . Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn . Ecol. Entomol. 32 : 496 – 505 . [Google Scholar]
- Mattila H. R., Harris J. L., Otis G. W. . 2001. . Timing of production of winter bees in honey bee ( Apis mellifera ) colonies . Insect Soc. 48 : 88 – 93 . [Google Scholar]
- Michaud D., Cantin L., Vrain T. C. . 1995. . Carboxy-terminal truncation of oryzacystatin II by oryzacystatin-insensitive insect digestive proteinases . Arch. Biochem. Biophys. 322 : 469 – 474 . [DOI] [PubMed] [Google Scholar]
- Michener C. D. 1974. . The social behavior of the bees . Harvard University Press; , Cambridge, MA: . [Google Scholar]
- Pernal S. F., Currie R. W. . 2000. . Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees ( Apis mellifera L.) . Apidologie 31 : 387 – 409 . [Google Scholar]
- Raubenheimer D., Simpson S. J. . 1997. . Integrative models of nutrient balancing: application to insects and vertebrates . Nutr. Res. Rev. 10 : 151 – 179 . [DOI] [PubMed] [Google Scholar]
- Roulston T., Cane J. H. . 2000. . Pollen nutritional content and digestibility for animals . Plant Syst. Evol. 222 : 187 – 209 . [Google Scholar]
- Roulston T. H., Cane J. H., Buchmann S. L. . 2000. . What governs protein content of pollen: pollinator preferences, pollen pistil interactions, or phylogeny? Ecol. Monogr. 70 : 617 – 643 . [Google Scholar]
- Rueppell O., Bachelier C., Fondrk M. K., Page R. E., Jr . 2007. . Regulation of life history determines lifespan of worker honeybees ( Apis mellifera L.) . Exp. Gerontol. 42 : 1020 – 1032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagili R. R., Pankiw T. . 2007. . Effect of protein-constrained brood food on honey bee ( Apis mellifera L.) pollen foraging and colony growth . Behav. Ecol. Sociobiol .61 : 1471 – 1478 . [Google Scholar]
- Sagili R. R., Pankiw T., Zhu-Salzman K. . 2005. . Effects of soybean trypsin inhibitor on hypopharyngeal gland protein content, total midgut protease activity and survival of the honey bee ( Apis mellifera L.) . J. Insect Physiol. 51 : 953 – 957 . [DOI] [PubMed] [Google Scholar]
- SAS Institute . 2004. . SAS/STAT 9.1 user’s guide . SAS Institute, Inc. , Cary, NC: . [Google Scholar]
- Schmickl T., Crailsheim K. . 2001. . Cannibalism and early capping: strategies of honeybee colonies in times of experimental pollen shortages . J. Comp. Physiol. A 187 : 541 – 547 . [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P., Winston M. L., Ydenberg R. C. . 1993. . Foraging of individual workers in relations to colony state in the social Hymenoptera . Can. Entomol. 125 : 129 – 160 . [Google Scholar]
- Seeley T. D., Visscher P. K. . 1985. . Survival of honeybees in cold climates: the critical timing of colony growth and reproduction . Ecol. Entomol. 10 : 81 – 88 . [Google Scholar]
- Somerville D. C., Nicol H. I. . 2006. . Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity . Aust. J. Exp. Agric. 46 : 141 – 149 . [Google Scholar]
- von Frisch K. 1965. . Tanzsprache und orientierung der bienen . Springer-Verlag, Berlin, Heidelberg; , New York: . [Google Scholar]
- Wegener J., Huang Z. Y., Lorenz M. W., Bienefeld K. . 2009a. . Regulation of hypopharyngeal gland activity and oogenesis in honey bee ( Apis mellifera ) workers . J. Insect Physiol. 55 : 716 – 725 . [DOI] [PubMed] [Google Scholar]
- Wegener J., Lorenz M. W., Bienefeld K. . 2009b. . Physiological consequences of prolonged nursing in the honey bee . Insect. Soc. 56: 85 – 93 . [Google Scholar]
- Winston M. L. 1987. . The biology of the honey bee . Harvard University Press; , Cambridge, MA: . [Google Scholar]
- Zerbo A. C., Moraes R. L. M. S., Brochetto-Braga M. R. . 2001. . Protein requirements in larvae and adults of Scaptotrigona postica (Hymenoptera: Apidia, Meliponinae): midgut proteolytic activity and pollen digestion . Comp. Biochem. Phys. B 129 : 139 – 147 . [DOI] [PubMed] [Google Scholar]