Abstract
IMPORTANCE
Soy isoflavone supplements are used to treat several chronic diseases, although the data supporting their use are limited. Some data suggest that supplementation with soy isoflavone may be an effective treatment for patients with poor asthma control.
OBJECTIVE
To determine whether a soy isoflavone supplement improves asthma control in adolescent and adult patients with poorly controlled disease.
DESIGN, SETTING, AND PARTICIPANTS
Multicenter, randomized, double-blind, placebo-controlled trial conducted between May 2010 and August 2012 at 19 adult and pediatric pulmonary and allergy centers in the American Lung Association Asthma Clinical Research Centers network. Three hundred eighty-six adults and children aged 12 years or older with symptomatic asthma while taking a controller medicine and low dietary soy intake were randomized, and 345 (89%) completed spirometry at week 24.
INTERVENTIONS
Participants were randomly assigned to receive soy isoflavone supplement containing 100 mg of total isoflavones (n=193) or matching placebo (n=193) in 2 divided doses administered daily for 24 weeks.
MAIN OUTCOMES AND MEASURES
The primary outcome measure was change in forced expiratory volume in the first second (FEV1) at 24 weeks. Secondary outcome measures were symptoms, episodes of poor asthma control, Asthma Control Test score (range, 5–25; higher scores indicate better control), and systemic and airway biomarkers of inflammation.
RESULTS
Mean changes in prebronchodilator FEV1 over 24 weeks were 0.03 L (95% CI, −0.01 to 0.08 L) in the placebo group and 0.01 L (95% CI, −0.07 to 0.07 L) in the soy isoflavone group, which were not significantly different (P = .36). Mean changes in symptom scores on the Asthma Control Test (placebo, 1.98 [95% CI, 1.42–2.54] vs soy isoflavones, 2.20 [95% CI, 1.53–2.87]; positive values indicate a reduction in symptoms), number of episodes of poor asthma control (placebo, 3.3 [95% CI, 2.7–4.1] vs soy isoflavones, 3.0 [95% CI, 2.4–3.7]), and changes in exhaled nitric oxide (placebo, −3.48 ppb [95% CI, −5.99 to −0.97 ppb] vs soy isoflavones, 1.39 ppb [95% CI, −1.73 to 4.51 ppb]) did not significantly improve more with the soy isoflavone supplement than with placebo. Mean plasma genistein level increased from 4.87 ng/mL to 37.67 ng/mL (P < .001) in participants receiving the supplement.
CONCLUSIONS AND RELEVANCE
Among adults and children aged 12 years or older with poorly controlled asthma while taking a controller medication, use of a soy isoflavone supplement, compared with placebo, did not result in improved lung function or clinical outcomes. These findings suggest that this supplement should not be used for patients with poorly controlled asthma.
Asthma is a complex disease whose prevalence and severity are determined by genetic and environmental factors. Increases in asthma prevalence and severity over the last several decades1 are likely due at least in part to environmental factors. Diet is one environmental factor that is associated with asthma prevalence and severity.2 During an evaluation of the link between diet and asthma, we found an association between dietary intake of the soy isoflavone genistein and forced expiratory volume in the first second (FEV1), a marker of asthma severity.3 We subsequently confirmed the association in an independent asthma population4 and explored the mechanistic basis for this finding. We found that genistein inhibits a key pathway that may contribute to asthma severity, eosinophil leukotriene C4 synthesis. We also found that administration of a soy isoflavone supplement containing genistein reduces exhaled nitric oxide and ex vivo leukotriene C4 synthesis in a small group of patients with inadequately controlled asthma.5
With the increasing cost of prescription drugs for asthma, it is important to identify effective, safe, and less expensive therapies than those currently available. Patients with asthma frequently seek alternative therapies in the belief that they are less toxic. Soy isoflavones clearly fit this role. However, previous reports of an association between dietary intake of individual nutrients and asthma prevalence and severity have not been confirmed in adequately powered intervention studies.6–8 To determine whether this novel treatment is effective in patients with asthma, we conducted a 6-month randomized, double-blind, placebo-controlled, parallel-group clinical trial of soy isoflavones among individuals aged 12 years or older with symptomatic, poorly controlled asthma who were receiving at least 1 controller medication.
Methods
Study Design
The Study of Soy Isoflavones in Asthma was a multisite randomized clinical trial conducted at 19 clinical centers in the United States from May 2010 through August 2012. Most clinical centers were specialty care clinics associated with academic medical centers. All study centers received approval from their respective institutional review boards. All participants or their legal guardians provided written informed consent. Participants younger than 18 years signed assent forms according to local regulatory policies. The trial protocol is available in Supplement 1.
Participants were randomly assigned in a 1:1 allocation ratio to receive either a soy isoflavone supplement or a matching placebo twice daily for 6 months (Figure 1). Each isoflavone tablet contained 49 mg of soy isoflavones (genistein, daidzein, and glycitein), approximately 32 mg as the aglycone form (nearly evenly distributed between genistein and daidzein). The soy isoflavone and placebo tablets were reanalyzed twice during the study. On each occasion, the isoflavone content was between 48 and 49 mg, while the isoflavone content of the placebo tablets was consistently less than 0.05 mg. The treatment assignment schedule was created by the coordinating center using a documented, auditable SAS program and was stratified by center with randomly permuted blocks of varying size. Unique treatment assignment numbers were issued via an online randomization system for each participant after all eligibility criteria were evaluated. The assignment number was used to distribute and track study treatment with soy isoflavone supplement or placebo. Personnel at the coordinating center involved in creating the randomization system or in treatment packaging and distribution had access to treatment identification information. No personnel at clinical centers had access to treatment assignments. Analysts looked at treatment identity after data collection was complete and were aware of the treatment assignment when performing the analyses of the completed data set.
Figure 1. Flow of Participants in the Study of Soy Isoflavones in Asthma Randomized Clinical Trial.
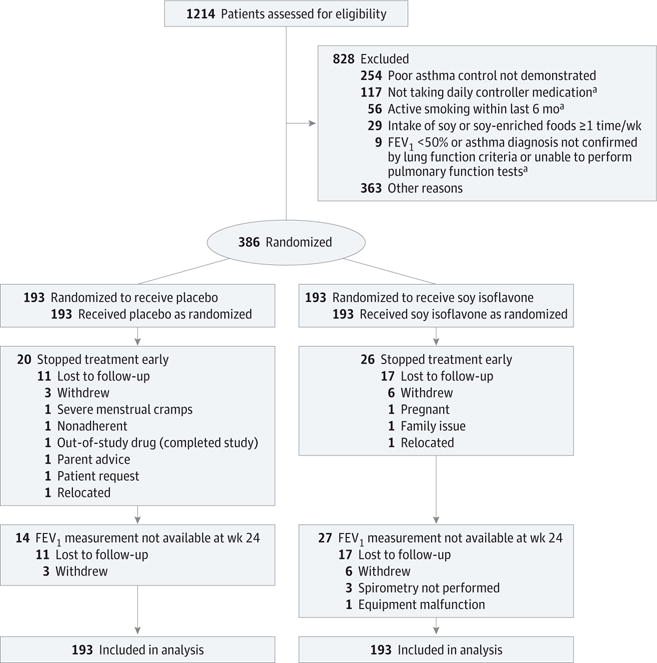
FEV1 indicates forced expiratory volume in the first second.
aFor 1 individual in this group, it was also reported that poor asthma control was not demonstrated.
After randomization, participants kept daily diaries to record morning peak expiratory flow, medication use, and asthma symptoms. They returned for assessment every 4 weeks for 24 weeks. Procedures performed at each visit included an interval medical history, spirometry (Koko Spirometer, Ferris Respiratory) performed according to American Thoracic Society standards,9 exhaled nitric oxide measurement (NIOX MINO, Aerocrine) following American Thoracic Society/European Respiratory Society guidelines,10 and asthma control and asthma quality-of-life questionnaires (described below). At randomization (visit 2) and select follow-up visits, urine and blood were collected (visits 4 and 9). The Block 2005 Food Frequency Questionnaire for adults, Block Kids 2004 Food Frequency Questionnaire for children aged 12 to 17 years,11,12 and Block Soy Foods Screener (NutritionQuest) were administered at randomization and at visit 9. Adverse and toxic effects were assessed by questionnaire and open-ended questions at each visit. Race and ethnicity were self-reported by participants at baseline and at each spirometry test.
Participant Selection
Inclusion criteria were age 12 years or older; physician diagnosis of asthma; current or previous (within 2 years) evidence of at least a 12% increase in FEV1 after inhaling 2 to 4 puffs of albuterol or a positive methacholine challenge (20% decrease in FEV1 at <16 mg/mL); FEV1 equal to or greater than 50% predicted prebronchodilator; currently prescribed daily controller asthma medication(s) (eg, inhaled corticosteroids and/or leukotriene modifier); nonsmoker for 6 months or longer and less than 10 pack-year smoking history; and evidence of poor asthma control. Poor asthma control was defined as having 1 or more of the following: a score of 19 or less on the ACT,13 use of β-agonist for asthma symptoms 2 or more times per week, nocturnal awakening with asthma symptoms more than once per week, and 2 or more episodes of asthma symptoms in the past 12 months, with each requiring at least 1 of the following: emergency department visit, unscheduled physician visit, prednisone course, or hospitalization.
Patients were excluded if they had chronic illness that in the judgment of the physician would interfere with study participation; history of physician diagnosis of chronic bronchitis, emphysema, or chronic obstructive pulmonary disease; oral corticosteroid use within the past 6 weeks; current consumption of soy isoflavone supplements; intake of soy or soy-containing foods 1 or more times a week; use of an investigational treatment in the previous 30 days; known adverse reaction to genistein, other phytoestrogens, or soy products; pregnancy or lactation; asthma exacerbation within 6 weeks; upper respiratory tract infection within 2 weeks; body weight less than 77 lb (35 kg); or change in diet over the past month or expected change in diet (eg, initiation of weight loss diet) during the study.
Outcome Measures
The primary outcome measure was FEV1. Secondary outcomes included the ACT (score range, 5–25; higher scores indicate better control),13 the Asthma Symptoms Utility Index (score range, 0–1.0; higher scores indicate fewer symptoms),14,15 and the Marks Asthma Quality of Life Questionnaire (score range, 0–80; higher scores indicate worse quality of life) for participants aged 17 years or older16 or the Children’s Health Survey for Asthma (score range, 0–100; higher scores indicate better quality of life) for participants aged 12 to 16 years.17 Other outcomes included peak expiratory flow; symptom-free days (defined as days with no asthma episodes reported on diary card); and rates of episodes of poor asthma control defined from diary cards by 1 of the following: 30% or greater decrease in morning peak expiratory flow (from personal best) for 2 consecutive days (definite yellow zone event according to the National Heart, Lung, and Blood Institute’s Asthma Action Plan46), addition of oral corticosteroid to treat asthma symptoms, unscheduled contact with a health care practitioner (emergency department, physician office, hospital) for asthma symptoms, and increased use of bronchodilator rescue medication since baseline by 4 or more puffs of metered dose inhaler or 2 or more nebulizer treatments on 1 day.18
Additional outcomes included exhaled nitric oxide, peripheral blood eosinophil count, serum interleukin 6 (Quantikine HS ELISA kit, R&D Systems), serum C-reactive protein (DSL ultrasensitive coated-well CRP ELISA kit, Diagnostic Systems Laboratories), and urinary leukotriene E4 measured by high-performance liquid chromatography.19
Quantification of Total Blood Genistein
Blood for total genistein concentration (unconjugated genistein plus genistein conjugated to glucuronide and sulfate) was collected in 10-mL heparinized Vacutainer tubes (BD, Franklin Lakes), centrifuged, and the plasma collected and stored at −70°C. A 200-μL aliquot of plasma was incubated in a solution contain β-glucuronidase and sulfatase overnight to de-conjugate genistein and extracted with diethyl ether as previously described.20 The mixture was evaporated to dryness and the dry residues were quantified by time-resolved fluoroimmunoassay (TR-FIA Genistein kits, Labmaster) as previously described.21
Sample Size
The planned sample size of 380 participants provided 80% power to detect a difference in the change in prebronchodilator FEV1 of 0.134 L or greater based on a 2-sample t test assuming a standard deviation for the 24-week change in FEV1 of 0.400 L, a cumulative 2-sided type I error rate of 2.5% (adjusting for 2 interim analyses based on O’Brien-Fleming boundaries), and 10% inflation to account for missing data and loss to follow-up. The 0.134-L difference approximated the 4% to 5% change in percentage predicted FEV1 observed between those with the highest and lowest consumption of soy genistein in our 2 previous analyses3,4 and is similar to the lower bound for clinically important changes (0.100–0.200 L).22 The study also provided 80% power to detect clinically meaningful differences for changes in exhaled nitric oxide (8 ppb, which translates to a 20% change)23 and the asthma control test (3 units)24 assuming a 2-sided type I error rate of 0.0125 for each.
Data Analysis
The data were analyzed at the Data Coordinating Center at Johns Hopkins University. All analyses were performed according to treatment assignment, and all available data from all patients were included in the analyses, following the intention-to-treat principle. The primary analysis was performed using a linear mixed-effects model incorporating all available longitudinal patient data on FEV1 unadjusted for additional covariates. The fixed effects included indicator variables for genistein treatment (placebo = 0; genistein = 1), visit time indicators (baseline and 4, 8,12,16, 20, and 24 weeks), and treatment × time interaction terms. The random effects included random intercepts for clinics as well as an adjustment for the correlation between repeated measures. An unstructured covariance structure was used. Prespecified subgroup analyses relied on the same approach by adding appropriate covariates and treatment group interaction terms into the models.
Analyses for continuous outcomes related to secondary hypotheses followed the same analytic approach proposed for the primary outcome. Laboratory values were censored at the lowest level of detection to allow for log-transformations to address skewness in the data. Random effects for batch replaced the clinic-level random effects for the analysis of plasma genistein levels. Rates of episodes of poor asthma control were evaluated using negative binomial models to allow for over-dispersion with random intercepts for clinics.25 Kaplan-Meier estimates of the survival function were used to estimate the proportion of individuals who developed a particular symptom after being free of that symptom at randomization. Frailty models with a random effect for clinic were used to compare the risk of developing each symptom.
Data from daily diaries and pill counts were used to evaluate adherence to the treatment protocol. Each participant’s overall diet and dietary soy intake at baseline and at the end of the study were analyzed using the Block Food Frequency Questionnaire and Block Soy Foods Screener to assess stability of diet and nutrient intake during the study.
Data were assumed to be missing at random. Mixed-effects models were fit using residual maximum likelihood to accommodate data missing at random in a manner equivalent to multiple imputation. Best- and worst-case scenarios were used to quantify the robustness of our findings to the missing-at-random assumption. Robust standard error estimates were used for all regression models. All tests were 2-sided. The type I error was split between the primary outcome (FEV1; α = .025) and the 2 most important secondary outcomes (exhaled nitric oxide and ACT score; α = .0125 each). All other tests were performed at the α = .05 level without adjustment for multiple comparisons. Analyses were performed using SAS version 9.1 (SAS Institute Inc), STATA release 13 (Stata Corp) and R version 2.11.1 (R Project for Statistical Computing; http://www.r-project.org/).
Results
Participant Characteristics
A total of 1214 individuals were assessed for eligibility (Figure 1); 828 were excluded either before enrollment or during the run-in period. Three hundred eighty-six adults and children aged 12 years or older with symptomatic asthma and low dietary soy intake were randomized. The baseline characteristics of the participants are shown in Table 1. Of the 386 participants, 345 (89%) completed the study. A completer was defined as an individual who had an evaluable primary outcome measurement at 24 weeks. Participant characteristics were similar in the 2 groups. The median age was 36 years, a majority were women, 59% were from minority groups, and most were taking a combination inhaled corticosteroid/long-acting β-agonist. Participants had reduced FEV1 (82% of predicted), substantial symptom burden (mean ACT score, 17), and frequent use of health care resources. Exhaled nitric oxide and peripheral blood eosinophil counts were mildly increased; serum interleukin 6 and C-reactive protein levels were normal. Baseline dietary genistein intake was low. Dietary intake of vitamins A, C, D, and E were in the low to normal range (eTable 1 in Supplement 2).
Table 1.
Baseline Characteristics of Participants in the Study of Soy Isoflavones in Asthma
| Characteristics | All (n=386) |
Placebo (n=193) |
Soy Isoflavone (n=193) |
|---|---|---|---|
| Age, median (IQR), y | 36 (18–49) | 38 (17–49) | 34 (20–49) |
| Female, No. (%) | 254 (66) | 125 (65) | 129 (67) |
| Race or ethnic group, No. (%) | |||
| White | 147 (38) | 75 (39) | 72 (38) |
| Black | 179 (47) | 90 (44) | 89 (47) |
| Hispanic | 47 (12) | 23 (12) | 24 (13) |
| Other | 10(3) | 4(2) | 6 (3) |
| Body mass index, median (IQR)a | 28 (24–35) | 27 (23–34) | 29 (24−35) |
| Age at asthma onset, median (IQR), y | 8(2−22) | 6 (1−20) | 9 (2−23) |
| Emergency visit in past 12 mo, No. (%) | 296 (77) | 148 (77) | 148 (77) |
| Steroid burst in past 12 mo, No. (%) | 195 (51) | 94 (49) | 101 (53) |
| Controller medications, No. (%) | |||
| Inhaled corticosteroids alone | 89 (23) | 49 (25) | 40 (21) |
| Inhaled corticosteroids + long-acting β-agonists | 283 (74) | 135 (70) | 148 (77) |
| Oral antileukotrienes | 129 (34) | 64 (33) | 65 (34) |
| Other | 10(3) | 3 (2) | 7 (4) |
| Asthma score, median (IQR) | |||
| Asthma Control Test (range, 5–25)b | 17 (14–19) | 17 (14–20) | 17 (14–19) |
| Asthma Symptoms Utility Index (range, 0–1)b | 0.80 (0.67–0.89) | 0.80 (0.67–0.89) | 0.82 (0.69–0.89) |
| Quality-of-life scores, median (IQR) | |||
| Marks Asthma Quality of Life Questionnaire (range, 0–80)c,d | 15 (9–27) | 16 (8–26) | 15 (10–28) |
| Children’s Health Survey for Asthma (range, 0–100)b,e | |||
| Physical health | 89 (75–95) | 89 (75–95) | 89 (76–94) |
| Activity, child | 90 (75–100) | 95 (75–100) | 90 (75–100) |
| Activity, family | 100 (91–100) | 100 (91–100) | 100 (91–100) |
| Emotion, child | 85 (50–100) | 90 (55–100) | 82 (50–95) |
| Emotion, family | 79 (64–89) | 80 (63–92) | 76 (64–86) |
| Lung function, median (IQR) | |||
| FEV1, L | 2.43 (1.99–2.91) | 2.44 (1.96–2.93) | 2.40 (2.01–2.91) |
| FEV1, % predicted | 82.1 (69.8–92.6) | 83.4 (71.9–92.1) | 80.1 (68.3–93.3) |
| FEV1 bronchodilator response, %f | 8.77 (3.94–14.29) | 7.90 (3.44–14.26) | 9.04 (4.57–14.49) |
| Forced vital capacity, L | 3.39 (2.77–4.09) | 3.40 (2.77–3.99) | 3.39 (2.78–4.16) |
| Forced vital capacity, % predicted | 93.7 (83.8–103.8) | 93.7 (83.5–103.7) | 94.0 (84.0–104.0) |
| Peak flow, L/min | 350 (300–420) | 360 (300–423) | 350 (300–420) |
| Peak flow, % predicted | 83.8 (69.3–95.1) | 85.8 (69.7–96.2) | 82.4 (68.6–94.7) |
| Biomarkers, median (IQR)g | |||
| Exhaled nitric oxide, ppb | 25 (15–46) | 25 (17–52) | 25 (15–43) |
| Eosinophil count, /μL | 235 (130–430) | 230 (122–410) | 240 (140–450) |
| Interleukin 6, serum, pg/mL | 1.4 (0.9–2.6) | 1.4 (0.9–2.3) | 1.4 (0.9–2.7) |
| Serum C-reactive protein, mg/L | 1.8 (0.6–4.9) | 1.7 (0.6–4.3) | 2.1 (0.6–5.4) |
| Urinary leukotriene E4/creatinine, nmol/mol | 12.1 (7.2–20.7) | 12.3 (7.2–20.3) | 11.9 (7.2–21.0) |
Abbreviations: FEV,, forced expiratory volume in the first second; IQR, interquartile range.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Higher scores indicate less severe disease.
Lower scores indicate less severe disease.
The Marks Asthma Quality of Life Questionnaire was assessed in participants aged 17 years or older.
The Children’s Health Survey for Asthma was assessed in participants aged 12 to 16 years.
FEV1 bronchodilator response is calculated as 100 × (postbronchodilator FEV1 - prebronchodilator FEV1)/prebronchodilator FEV1.
Normal ranges for biomarkers are as follows: exhaled nitric oxide, 2–25 ppb; eosinophil count, 0–600/μL; interleukin 6, 0.31–5.0 pg/mL; C-reactive protein, 0–10 mg/L; urinary leukotriene E4/creatinine, 0.7–28.9 nmol/mol.
Adherence to Study Treatment and Stable Diet
Participants reported taking at least 1 dose of the study treatment on more than 90% of follow-up days. Analysis of dietary nutrient intake after study completion showed that there were no significant changes in overall diet or in dietary intake of genistein and vitamins A, C, D, and E in either group over the 24-week study period.
Primary Outcome
The mean changes in prebronchodilator FEV1 over 24 weeks were 0.03 L (95% CI, −0.01 to 0.08 L) in the placebo group and −0.001 L (95% CI, −0.07 to 0.07 L) in the soy isoflavone group, which were not significantly different (Table 2 and Figure 2). The distribution of FEV1 responses was the same in the 2 groups (eFigure in Supplement 2). These results are robust to all but the most extreme forms of informative missingness (eg, imputation of values on the order of 1.1 L and −1.32 L).
Table 2.
Model-Based Estimates of Mean Change From Baseline to 24 Weeks for Lung Function, Asthma Scores, and Laboratory Markers
| Outcomes | Mean Difference, 24 Weeks - Baseline (95% CI) | P Value | |
|---|---|---|---|
| Placebo (n = 193) |
Soy Isoflavone (n = 193) |
||
| FEV1, L | 0.03 (−0.01 to 0.08) | −0.001 (−0.07 to 0.07) | .36 |
| FEV1 bronchodilator response, %a | −1.24 (−3.35 to 0.88) | −0.05 (−1.71 to 1.61) | .49 |
| Forced vital capacity, L | 0.03 (−0.01 to 0.08) | −0.03 (−0.08 to 0.02) | .04 |
| Peak flow, L/min | 15.8 (4.4 to 27.2) | 9.6 (−0.4 to 19.6) | .34 |
| Asthma Control Test score (range, 5–25)b | 1.98 (1.42 to 2.54) | 2.20 (1.53 to 2.87) | .53 |
| Asthma Symptoms Utility Index score (range, 0–1)b | 0.06 (0.03 to 0.09) | 0.06 (0.04 to 0.09) | .79 |
| Marks Asthma Quality of Life Questionnaire score (range, 0–80)c,d | −4.30 (−6.07 to −2.54) | −2.99 (−4.73 to −1.24) | .25 |
| Children’s Health Survey for Asthma score (range, 0–100)b,e | |||
| Physical healthf | 4.77 (−0.29 to 9.82) | 7.52 (1.92 to 13.13) | .49 |
| Activity, child | 4.65 (1.59 to 7.72) | 5.53 (0.78 to 10.28) | .69 |
| Activity, family | 2.49 (0.62 to 4.36) | −0.37 (−1.54 to 0.79) | .03 |
| Emotion, child | 4.34 (−1.78 to 10.47) | 7.13 (3.17 to 11.09) | .44 |
| Emotion, family | 3.50 (0.95 to 6.06) | 1.23 (−1.68 to 4.14) | .29 |
| Exhaled nitric oxide, ppb | −3.48 (−5.99 to −0.97) | 1.39 (−1.73 to 4.51) | .007 |
| Eosinophil count, /μLg | 1.09 (0.71 to 1.66) | 1.06 (0.72 to 1.56) | .91 |
| Interleukin 6, pg/mLg | 0.98 (0.90 to 1.09) | 0.98 (0.90 to 1.06) | .98 |
| Serum C-reactive protein, mg/Lg | 1.03 (0.91 to 1.15) | 0.98 (0.86 to 1.12) | .61 |
| Urinary leukotriene E4/creatinine, nmol/molg | 1.01 (0.86 to 1.18) | 1.04 (0.89 to 1.22) | .81 |
Abbreviation: FEV1, forced expiratory volume in the first second.
FEV1 bronchodilator response is calculated as 100 × (postbronchodilator FEV1 - prebronchodilator FEV1)/prebronchodilator FEV1.
Higher scores indicate less severe disease.
Lower scores indicate less severe disease.
The Marks Asthma Quality of Life questionnaire was assessed in participants aged 17years or older.
The Children’s Health Survey for Asthma was assessed in participants aged 12 to 16 years.
No random intercept for clinic was included in the model for Children’s Health Survey for Asthma physical health score because of lack of convergence.
Differences were calculated on a log scale and transformed to give the ratio of the estimates of the week 24 value divided by the baseline value.
Figure 2. Change in Prebronchodilator FEV1 During 24 Weeks of Treatment.
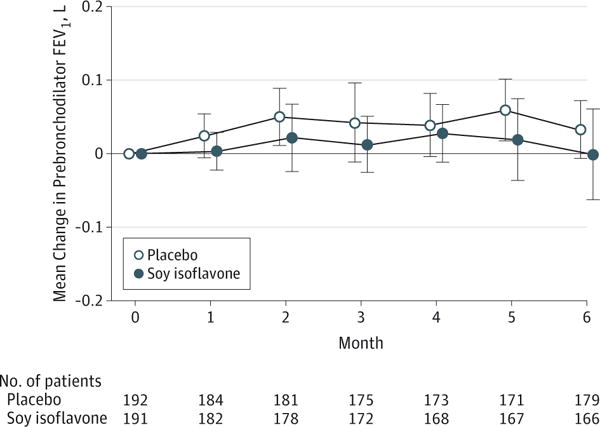
Model-based mean change in prebronchodilator forced expiratory volume in the first second (FEV1) at each study visit compared with baseline values. Error bars indicate with 95% confidence intervals. Evaluable FEV1 results were not available at the randomization visit for 1 participant in the placebo group and 2 participants in the soy isoflavone group.
Secondary Outcomes
The only lung function measure that was significantly different between the 2 treatment groups was forced vital capacity, which had a slightly greater but not clinically meaningful improvement after 24 weeks in the placebo-treated group (Table 2). Mean changes also were not significantly different between the groups for ACT symptom scores (placebo, 1.98 [95% CI, 1.42–2.54] vs soy isoflavones, 2.20 [95% CI, 1.53–2.87]), Marks Asthma Quality of Life Questionnaire scores (placebo, −4.30 [95% CI, −6.07 to −2.54] vs soy isoflavones, −2.99 [95% CI, −4.73 to −1.24]), number of episodes of poor asthma control (placebo, 3.3 [95% CI, 2.7–4.1] vs soy isoflavones, 3.0 [95% CI, 2.4–3.7]), and measures of systemic inflammation (se-rum interleukin 6 and C-reactive protein, peripheral blood eosinophil counts, urinary leukotriene E4) (Table 2 and Table 3). The biomarker that differed between the 2 treatment groups was exhaled nitric oxide, which had a small, statistically significant improvement after 24 weeks in the placebo-treated group. This was not seen in the soy isoflavone group.
Table 3.
Secondary Outcomes Based on Participant Diary Cards
| Outcomes | Treatment Group | P Value | ||
|---|---|---|---|---|
| Placebo (n=185) |
Soy Isoflavone (n=182) |
Relative Risk (95% CI) |
||
| No. of person-years of follow-up | 80.3 | 78.6 | ||
| Episodes of poor asthma controla | ||||
| No. of events | 269 | 235 | ||
| No. of individuals with >1 event | 102 | 93 | ||
| No. of events/ person-year (95% CI) | 3.3 (2.7–4.1) | 3.0 (2.4–3.7) | 0.89 (0.66–1.21) | .46 |
| Exacerbation components | ||||
| Peak flow, 30% decrease | ||||
| No. of events | 123 | 117 | ||
| No. of individuals with >1 event | 45 | 43 | ||
| No. of events/ person-year (95% CI) | 1.5 (1.0–2.2) | 1.4 (0.9–2.2) | 0.98 (0.57–1.68) | .94 |
| Urgent care | ||||
| No. of events | 29 | 27 | ||
| No. of individuals with >1 event | 25 | 22 | ||
| No. of events/ person-year (95% CI) | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) | 0.95 (0.54–1.66) | .85 |
| New use of oral steroids | ||||
| No. of events | 35 | 30 | ||
| No. of individuals with >1 event | 31 | 28 | ||
| No. of events/ person-year (95% CI) | 0.4 (0.3–0.6) | 0.4 (0.2–0.5) | 0.87 (0.54–1.43) | .59 |
| Rescue medications | ||||
| No. of events | 147 | 124 | ||
| No. of individuals with >1 event | 60 | 60 | ||
| No. of events/ person-year (95%CI) | 1.8 (1.3–2.5) | 1.6 (1.1–2.4) | 0.92 (0.61–1.39) | .69 |
| Symptom-free days, median (interquartile range), % | 65 (17–84) | 60 (2–86) | .41 | |
Episodes of poor asthma control are defined by one of the following: ≥30% decrease in morning peak expiratory flow (from personal best) for 2 consecutive days (definite yellow zone event according to the National Heart, Lung, and Blood Institute’s Asthma Action Plan), addition of oral corticosteroid to treat asthma symptoms, unscheduled contact with a health care practitioner (emergency department, physician office, hospital) for asthma symptoms, increased use of bronchodilator rescue medication from baseline by 4 or more puffs of metered-dose inhaler or 2 or more nebulizer treatments on 1 day.
Association Between Patient Characteristics and Response
We sought to determine whether specific characteristics influenced the response to the soy isoflavone supplement. In prespecified subgroup analyses, we looked at the effect on change in prebronchodilator FEV1 of the following measures: higher vs lower lung function (percentage predicted FEV1 ≤80% vs >80%), symptom burden (ACT score >19 vs ≥19), race (non-African American vs African American), sex (male vs female), body mass index (<30 vs ≥30; calculated as weight in kilograms divided by height in meters squared), and exhaled nitric oxide (≤25 ppb vs >25 ppb). We found no significant difference in the effect of treatment over 6 months when stratifying by any patient or disease characteristics or inflammatory markers including peripheral blood eosinophil counts and urinary leukotriene E4 (P=.31 to P>.99 by test for interaction).
Association Between Genistein Intake and Levels and Asthma Outcomes
Plasma genistein levels were analyzed at baseline and 24 weeks for a subset of 143 patients (37%; n=83 in the placebo group and n=60 in the soy isoflavone group). In the group that received the soy isoflavone supplement, the mean plasma genistein level increased during the study from 4.87 ng/mL to 37.67 ng/mL (P < .001) (eTable 2 in Supplement 2), although individual responses were highly variable. Mean plasma genistein increased slightly in the placebo group (from 5.10 ng/mL to 7.11 ng/mL; P = .22). Overall, the ratio of the estimate of mean week 24 plasma genistein divided by mean baseline plasma genistein for those receiving soy isoflavone compared with placebo was 5.5 (95% CI, 2.70–11.39; P < .001). In this subset, there was no association between the increase in plasma genistein achieved during treatment and the change in FEV1.
Treatment-Related Adverse Events and Symptoms
There were few serious adverse events in either treatment group and no statistically significant differences between the groups (Table 4). Multiple symptoms were reported by both groups during the study (Table 5), but there were no statistically significant differences in any of them, including breast tenderness. In the subset of participants who were menstruating women (46%), the number reporting a change in menstrual symptoms or menstrual cycle was not statistically different between the 2 treatment groups.
Table 4.
Treatment-Related Serious Adverse Events by Treatment Group
| Placebo (n=193) |
Soy Isoflavone (n=193) |
|
|---|---|---|
| Follow-up time, person-years | 85.61 | 83.79 |
| Events, No. (%) | ||
| Pulmonary, including asthma exacerbations | 8(4) | 3(2)a,b |
| Cardiovascular, circulatory, and lymphatic | 0 | 1 (0.5)b |
| Renal/urinary | 1 (0.5) | 0 |
| Gastrointestinal | 0 | 2(1) |
| Neuropsychiatric | 0 | 4(2) |
| Musculoskeletal | 1 (0.5) | 3 (2) |
| Reproductive | 1 (0.5) | 1 (0.5) |
| Integumentary | 1 (0.5) | 0 |
| Total | 12 | 14 |
One patient had both a pulmonary and a gastrointestinal serious adverse event.
One patient had both a pulmonary and a cardiovascular serious adverse event.
Table 5.
Treatment-Related Symptoms by Treatment Group
| Symptoms | No. at Riska | With Symptoms at 24 wk, %b | Hazard Ratio (95% CI)c | P Valuec | |
|---|---|---|---|---|---|
| Placebo | Soy Isoflavone | ||||
| Rash | 327 | 25 | 23 | 0.97 (0.62–1.51) | .91 |
| Itching | 286 | 32 | 32 | 1.00 (0.66–1.51) | >.99 |
| Difficulty breathing | 158 | 61 | 57 | 0.94 (0.61–1.43) | .77 |
| Difficulty swallowing | 350 | 21 | 18 | 0.85 (0.52–1.37) | .50 |
| Hypotension | 362 | 5 | 7 | 1.42 (0.63–3.20) | .40 |
| Breast tenderness | 355 | 13 | 12 | 0.91 (0.51–1.65) | .78 |
| Change in menstrual symptoms/cycled | 133 | 55 | 59 | 1.23 (0.78–1.94) | .37 |
Thirteen individuals were excluded because of lack of follow-up. The number at risk includes all individuals who did not have the particular symptom at baseline.
Estimates of the percentage with a symptom are based on Kaplan-Meier estimates of the survival function.
Hazard ratios were computed using a frailty model with a random effect for clinic and robust confidence limits.
Analysis limited to women with a menstrual period within the last 12 months. Changes were defined as experiencing a heavier or lighter flow, spotting between periods, or more or less discomfort or cramping than usual.
Discussion
Providing a soy isoflavone supplement twice daily to patients with poorly controlled asthma who were already taking a controller medicine did not improve their FEV1 The supplement also did not improve additional aspects of asthma control, including other measures of lung function, symptoms, quality of life, and airway and systemic inflammation. This finding is despite evidence that plasma genistein increased to levels that inhibit eosinophil cysteinyl leukotriene synthesis in vitro and ex vivo. Although the study results are disappointing in view of the preclinical, epidemiologic, and pilot clinical data, they illustrate the limitations of cross-sectional, population-based studies of dietary nutrient intake and the use of surrogate markers of disease to predict clinically relevant outcomes of well-designed, carefully performed, and adequately powered intervention studies in patients with asthma.
Of the several mechanisms proposed to explain the increased prevalence and severity of asthma over the last several decades,1,2,26 change in diet is a likely candidate. In the general population, decreased consumption of fresh fruits, green vegetables, potatoes, and fresh fish, important sources of antioxidants and essential nutrients, has been associated with decreased lung function,27 a characteristic feature of asthma. Both epidemiologic and mechanistic studies support a role for diet as a risk factor for asthma.28 Yet the exact contribution of specific nutrients and antioxidant vitamins remains controversial and at times confusing.6–8,28–31 This could be due at least in part to the studies being underpowered, enrolling patients with asthma whose disease was well controlled and unlikely to show improvement, and choosing interventions for which there was limited preclinical support.
We tested a soy isoflavone mix consisting primarily of genistein and daidzein because of the strong epidemiologic and preclinical data supporting a role for genistein in health and disease32–34 and asthma in particular. Genistein is a small-molecule (molecular weight, 270 g/mol), broad-spectrum inhibitor of tyrosine kinases35 with biological effects that one might predict would have beneficial effects in patients with asthma. For example, genistein reduces antigen-induced guinea pig airway inflammation and airway hyperresponsive ness in vivo,36 facilitates bronchial vascular smooth muscle relaxation,37 and, in combination with daidzein, inhibits antigen-induced eosinophilia in guinea pigs.38 At the cellular level, genistein exerts potent antioxidant effects that are equal to or greater than those of vitamin C.35,39 These molecular, cellular, and physiological properties and others make genistein a seemingly attractive agent for treating asthma.
Previous studies in 2 broadly representative groups of patients with asthma demonstrated that those who consume soy isoflavones have better lung function than those who do not.3,4 A report from Japan identified an association between high intake of soy isoflavones and a lower prevalence of allergic rhinitis,40 a disease linked to asthma. To our knowledge, only 1 other study has explored the association between dietary intake of flavonoids and asthma in adults.32 That study failed to find an association between flavonoid intake and asthma prevalence or severity, but it did not specifically examine the effect of soy isoflavones. Recent in vitro results from our group showed that genistein, at levels achievable in plasma, inhibits synthesis of human peripheral blood eosinophil cysteinyl leukotrienes, important mediators of asthma. This effect occurred via inhibition of 5-lipoxygenase.5
In view of these preclinical and clinical findings and the low baseline intake and plasma levels of genistein and inadequately controlled asthma in the current study population, why did this study not demonstrate a positive effect? There are several possible explanations. First, the dosage administered (49 mg/d of soy isoflavones) may have been too low. We chose a dosage that was deemed physiologically relevant3,4 and supported by our in vitro eosinophil leukotriene C4 experiments,5 in which the IC50 was80nM(21 ng/mL). The dosage was approximately twice the genistein and daid-zein consumed by men and women in Japan (typically 25–50 mg/d),40,41 where the prevalence of asthma symptoms among children and adolescents is lower than in Western nations,42 and well above the median intake in our participants (eTable 1 in Supplement 2). The dosage was also chosen to avoid potential adverse effects.
A second possibility is that this particular soy isoflavone supplement was not adequately absorbed. In our pilot study, we observed ex vivo inhibition of blood eosinophil leukotriene C4 synthesis after ingestion of the same dose. Trough plasma levels of genistein in the majority of participants receiving the supplement in the current study exceeded the IC50 identified in the in vitro study. Nonetheless, we found substantial variability in plasma genistein levels after administration of the supplement, consistent with previous studies demonstrating variable uptake of genistein in the general population.43
Third, the beneficial effects of soy isoflavones may be related to intestinal production of the estrogenic metabolite equol,44 and differences in equol production between racial/ethnic groups (about 80% of individuals in China and Japan are equol producers in contrast to 25% in the United States45) may explain the reported differences in benefits. Fourth, there may be confounding by intake of other nutrients; however, we found no differences in diet between the groups, nor did we observe changes in diet over time. Fifth, asthma is a heterogeneous disease, which may make it difficult to identify the effects of novel treatments if benefit is limited to a subgroup with specific phenotypic or genetypic characteristics.
This study has some limitations. One is the population chosen for the study. Although the participants had inadequately controlled asthma as defined by low ACT scores and reduced lung function, which increased the likelihood of seeing a beneficial effect, they had little evidence of airway or systemic inflammation at baseline. Although we did not have induced sputum samples to directly assess airway inflammation, it is possible that a group that may have benefited most, those with airway inflammation, was not adequately represented. Another limitation is that the 6-month treatment period was not long enough to see a beneficial effect on secondary outcomes such as episodes of poor asthma control. A third limitation is that, knowing the purpose of the study, participants may have altered their diet to include more soy isoflavones, including isoflavone supplements. We specifically addressed this possibility by having all individuals complete food frequency and soy intake questionnaires at the beginning and at the end of the treatment period, by measuring plasma genistein levels, and by counseling participants on maintaining their usual diet throughout the study.
Conclusions
Among adults and children aged 12 years or older with poorly controlled asthma while taking a controller medication, use of a soy isoflavone supplement, compared with placebo, did not result in improved lung function or clinical outcomes, including symptoms, episodes of poor asthma control, or systemic or airway inflammation. These findings suggest that this supplement should not be used for patients with poorly controlled asthma.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants from the National Institutes of Health (grants U01 HL087987, U01 HL 0088367, and U54TR001018) and the American Lung Association. Archer Daniels Midland (Decatur, Illinois) provided the soy isoflavone supplement and the matching placebo.
Role of the Funders/Sponsors: The Steering Committee of the American Lung Association Asthma Clinical Research Centers designed, oversaw, and approved the study implementation. The funders (including Archer Daniels Midland) otherwise had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. They did see a near-final version of the manuscript before its submission.
Group Information
The American Lung Association Asthma Clinical Research Centers are as follows: Baylor College of Medicine, Houston: Nicola Hanania, MD, FCCP (principal investigator), Marianna Sockrider, MD, DrPH (co-principal investigator), Laura Bertrand, RN, RPFT (principal clinic coordinator), Mustafa A. Atik, MD (coordinator); former member: Blanca A. Lopez, LVN. Columbia University-New York University Consortium, New York: Joan Reibman, MD(principal investigator), Emily DiMango, MD, Linda Rogers, MD (co-principal investigators), Karen Carapetyan, MA (principal clinic coordinator at New York University), Kristina Rivera, MPH, Newel Bryce-Robinson, Melissa Scheuerman (clinic coordinators at Columbia University); former member: Elizabeth Fiorino, MD. Duke University Medical Center, Durham, North Carolina: John Sundy, MD, PhD (principal investigator), Deanna Green, MD, Ankoor Shah, MD(co-principal investigators), Catherine Foss, BS, RRT, CCRC (principal clinic coordinator), Jessica Ghidorzi (regulatory coordinator), Stephanie Allen, Sabrena Mervin-Blake, MS, Elise Pangborn, BS, V. Susan Robertson, BSN, Nicholas Eberlein, BA (coordinators); former members: Michael Land, MD, Brian Vickery, MD, Eveline Wu, MD, Denise Jaggers, RN (coordinator). Illinois Consortium, Chicago: Lewis Smith, MD (principal investigator), Ravi Kalhan, MD, James Moy, MD, Edward Naureckas, MD, Mary Nevin, MD (co-principal investigators), Jenny Hixon, BS, CCRC (principal clinic coordinator), Abbi Brees, BA, CCRC, Zenobia Gonsalves, Virginia Zagaja, Jennifer Kustwin, Ben Xu, BS, CCRC, Thomas Matthews, MPH, RRT, Lucius Robinson III, Noopur Singh (coordinators). Indiana University Asthma Clinical Research Center, Indianapolis: Michael Busk, MD, MPH (principal investigator), Paula Puntenney, RN, MA (principal clinic coordinator), Nancy Busk, BS, MS, Janet Hutchins, BSN (coordinators). Louisiana State University Health Sciences Center, Section of Pulmonary/Critical Care: Kyle I. Happel, MD (principal investigator), Richard S. Tejedor, MD (co-principal investigator), Marie C. Sandi, RN, MN, FNP-BC (principal clinic coordinator), Connie Romaine, RN, FNP, Callan J. Burzynski (coordinators); former members: Arleen Antoine, Jonathan Cruse. National Jewish Health, Denver: Rohit Katial, MD (principal investigator), Flavia Hoyte, MD, Dan Searing, MD (co-principal investigators), Trisha Larson (principal clinic coordinator), Nina Phillips (recruiter and data entry operator); former member: Holly Currier, RN. Nemours Children’s Clinic-University of Florida Consortium, Jacksonville: John Lima, PharmD (principal investigator), Kathryn Blake, PharmD, Jason Lang, MD, Edward Mougey, PhD (co-principal investigators), Nancy Archer, RN, BSN (principal clinic coordinator), Deanna Seymour, RN, BSN, Mary Warde, RN, BSN (coordinators). Hofstra-NSLIJ School of Medicine, Beth Thalheim Asthma Center, New Hyde Park, NY: Rubin Cohen, MD (principal investigator), Maria Santiago, MD (co-principal investigator), Ramona Ramdeo, MSN, FNP-C, RN, RT (principal clinic coordinator), Maureen Dreyfus (data entry operator). Vermont Lung Center at the University of Vermont, Colchester: Charles Irvin, PhD (principal investigator), Anne E. Dixon, MD, David A. Kaminsky, MD, Thomas Lahiri, MD(co-principal investigators), Stephanie M. Burns (principal clinic coordinator). The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: John Mastronarde, MD(principal investigator), Jonathan Parsons, MD (co-investigator), Janice Drake, CCRC (principal clinic coordinator), Joseph Santiago, BS; former members: David Cosmar, BA, Rachael A. Compton (coordinators). Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College: Allen J. Dozor, MD (principal investigator), Sankaran Krishnan, MD, MPH, Joseph Boyer, MD (co-principal investigators); Agnes Banquet, MD, Elizabeth de la Riva-Velasco, MD, Diana Lowenthal, MD, Suzette Gjonaj, MD, Y. Cathy Kim, MD, Nadav Traeger, MD, John Welter, MD, Marilyn Scharbach, MD, Subhadra Siegel, MD (study physicians); Ingrid Gherson, MPH (lead research coordinator), Lisa Monchil, RRT, CCRC (coordinator); Tara M. Formisano, BS, Jessica Williams (data entry operators). St Louis Asthma Clinical Research Center, Washington University, St Louis: Mario Castro, MD, MPH (principal investigator), Leonard Bacharier, MD, Kaharu Sumino, MD (co-principal investigators); Jaime J. Tarsi, RN, MPH (principal clinic coordinator), Brenda Patterson, MSN, RN, FNP(coordinator); Terri Montgomery (data entry operator); former members: Raymond G. Slavin, MD (co-principal investigator), Deborah Keaney Chassaing, RN (coordinator). University of Arizona, Tucson: Lynn B. Gerald, PhD, MSPH (principal investigator), James L. Goodwin, PhD, Mark A. Brown, MD, Kenneth S. Knox, MD (co-principal investigators), Tara F. Carr, MD, Cristine E. Berry, MD, MHS, Fernando D. Martinez, MD, Wayne J. Morgan, MD, Cori L. Daines, MD, Michael O. Daines, MD, Roni Grad, MD, Dima Ezmigna, MBBS(study physicians), Monica M. Vasquez, MPH, MEd (principal clinic coordinator), Jesus A. Wences, BS, Silvia S. Lopez, RN, Janette C. Priefert (coordinators); former members: Monica T. Varela, LPN, Rosemary Weese, RN (coordinators), Katherine Chee, Andrea Paco, BS (data entry operator). University of Miami, Miami-University of South Florida, Tampa: Adam Wanner, MD (principal investigator, Miami), Richard Lockey, MD, (principal investigator, Tampa), Andreas Schmid, MD, Michael Campos, MD (co-principal investigators, Miami), Monroe King, DO (co-principal investigator, Tampa), Eliana S. Mendes, MD (principal clinic coordinator, Miami), Patricia D. Rebolledo, Johana Arana, Lilian Cadet, Rebecca McCrery, Sarah M. Croker, BA (coordinators); former member: Shirley McCullough, BS (coordinator). University of Missouri, Kansas City School of Medicine, Kansas City: Gary Salzman, MD (principal investigator), Asem Abdeljalil, MD, Dennis Pyszczynski, MD, Abid Bhat, MD (co-principal investigators), Patti Haney, RN, BSN, CCRC (principal clinic coordinator). University of California, San Diego: Stephen Wasserman, MD (principal investigator), Joe Ramsdell, MD, Xavier Soler, MD PhD, (co-principal investigators), Katie Kinninger, RCP (principal clinic coordinator), Paul Ferguson, MS, Amber Martineau, Samang Ung (coordinators); former member: Tonya Greene (clinic coordinator). University of Virginia, Charlottesville: W. Gerald Teague, MD (principal investigator), Kristin W. Wavell, BS (principal clinic coordinator); former members: Donna Wolf, PhD, Denise Thompson-Batt, RRT, CRP (coordinators). Chairman’s Office, University of Alabama, Birmingham (formerly at Respiratory Hospital, Winnipeg, Manitoba, Canada): William C. Bailey, MD, Nicholas Anthonisen, MD, PhD (research group chair). Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: Robert Wise, MD (center director), Janet Holbrook, PhD, MPH (deputy director), Razan Yasin, MHS (principal coordinator), Debra Amend-Libercci, Anna Adler, MS, Anne Shanklin Casper, MA, Marie Daniel, BA, Adante Hart, Andrea Lears, BS, Gwen Leatherman, BSN, MS, RN, Deborah Nowakowski, Nancy Prusakowski, MS, Sobharani Rayapudi, MD, ScM, Alexis Rea, Joy Saams, RN, David Shade, JD, Elizabeth Sugar, PhD, April Thurman, Christine Wei, MS; former members: Alicia Newcomer (principal coordinator), Christian Bime, MD MSc, Ellen Brown, MS, Charlene Levine, BS, Suzanna Roettger, MA, Nienchun Wu, Weijiang Shen, Lucy Wang, Johnson Ukken. Data and Safety Monitoring Board: Stephen C. Lazarus, MD (chair), William J. Calhoun, MD, MichelleCloutier, MD, Peter Kahrilas, MD, Bennie McWilliams, MD, Andre Rogatko, PhD, Christine Sorkness, PharmD, Scott Sicherer, MD; National Heart, Lung, and Blood Institute Office: Gail Weinmann, MD, Michelle Freemer, MD, Robert Smith, PhD, Virginia Taggart, MPH, Lisa Webber, BSN, RN, Gang Zheng, PhD. Project Office, American Lung Association, NewYork: Elizabeth Lancet, MPH (project officer), Norman H. Edelman, MD (scientific consultant), Susan Rappaport, MPH.
Footnotes
Author Contributions: Drs Smith and Holbrook had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Smith, Kalhan, Wise, Sugar, Irvin, Dozor, Holbrook.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Smith, Sugar, Lima, Dozor.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Sugar, Holbrook.
Obtained funding: Smith, Wise, Lima, Holbrook.
Administrative, technical, or material support: Smith, Wise, Sugar, Lima, Irvin, Holbrook.
Study supervision: Smith, Wise, Dozor, Holbrook.
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01052116
+ Supplemental content at jama.com
Group Information: The American Lung Association Asthma Clinical Research Centers are listed at the end of this article.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Smith reports receipt of personal fees for Data and Safety Monitoring Board membership from Merck and co-chairing an educational program from Scientific Therapeutics Information. Dr Kalhan reports receipt of personal fees from Forest Laboratories, Boerhinger Ingelheim, Merck, and Quantia Communications and grants to his institution from Boerhinger Ingelheim, GlaxoSmithKline, PneumRx, and Spiration. Dr Wise reports receipt of personal fees for consultancy from Boerhinger Ingelheim, GlaxoSmithKline, Forest Laboratories, Novartis, Sunovion, AstraZeneca, Janssen, Genentech, Bristol-Myers Squibb, Pulmonx, Merck, Spiration, and Teva and grants/grants pending to his institution from GlaxoSmithKline/American Lung Association, AstraZeneca, BIPI, Forest Laboratories, Pearl, and Sunovion. No other disclosures were reported.
Additional Contributions: We acknowledge Archer Daniels Midland for providing the soy isoflavone supplement and the matching placebo and for the periodic chemical analyses to ensure isoflavone stability and potency. We also acknowledge the staff at the individual study sites and the data coordinating center for their expertise and dedication and the study participants, without whom this study could not have been done.
Contributor Information
Lewis J. Smith, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Ravi Kalhan, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Robert A. Wise, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland.
Elizabeth A. Sugar, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland.
John J. Lima, Nemours Children’s Clinic, Jacksonville, Florida.
Charles G. Irvin, Department of Medicine, University of Vermont, Burlington.
Allen J. Dozor, Department of Pediatrics, New York Medical College, Valhalla.
Janet T. Holbrook, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 2.Raviv S, Smith LJ. Diet and asthma. Curr Opin Pulm Med. 2010;16(1):71–76. doi: 10.1097/MCP.0b013e3283323b73. [DOI] [PubMed] [Google Scholar]
- 3.Smith LJ, Holbrook JT, Wise R, et al. American Lung Association Asthma Clinical Research Centers Dietary intake of soy genistein is associated with lung function in patients with asthma. J Asthma. 2004;41(8):833–843. doi: 10.1081/jas-200038447. [DOI] [PubMed] [Google Scholar]
- 4.Bime C, Wei CY, Holbrook J, Smith LJ, Wise RA. Association of dietary soy genistein intake with lung function and asthma control: a post-hoc analysis of patients enrolled in a prospective multicentre clinical trial. Prim Care Respir J. 2012;21(4):398–404. doi: 10.4104/pcrj.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalhan R, Smith LJ, Nlend MC, Nair A, Hixon JL, Sporn PH. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin Exp Allergy. 2008;38(1):103–112. doi: 10.1111/j.1365-2222.2007.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogarty A, Lewis SA, Scrivener SL, et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003;33(10):1355–1359. doi: 10.1046/j.1365-2222.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty A, Lewis SA, Scrivener SL, et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respir Med. 2006;100(1):174–179. doi: 10.1016/j.rmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59(8):652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Cullen KW, Watson K, Zakeri I. Relative reliability and validity of the Block Kids Questionnaire among youth aged 10 to 17 years. J Am Diet Assoc. 2008;108(5):862–866. doi: 10.1016/j.jada.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 15.Bime C, Wei CY, Holbrook JT, Sockrider MM, Revicki DA, Wise RA. Asthma Symptom Utility Index: reliability, validity, responsiveness, and the minimal important difference in adult asthmatic patients. J Allergy Clin Immunol. 2012;130(5):1078–1084. doi: 10.1016/j.jaci.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992;45(5):461–472. doi: 10.1016/0895-4356(92)90095-5. [DOI] [PubMed] [Google Scholar]
- 17.Asmussen L, Olson LM, Grant EN, Fagan J, Weiss KB. Reliability and validity of the Children’s Health Survey for Asthma. Pediatrics. 1999;104(6):e71. doi: 10.1542/peds.104.6.e71. [DOI] [PubMed] [Google Scholar]
- 18.Mastronarde JG, Anthonisen NR, Castro M, et al. American Lung Association Asthma Clinical Research Centers Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360(15):1487–1499. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishi N, Mano N, Asakawa N. Direct injection method for quantitation of endogenous leukotriene E4 in human urine by liquid chromatography/electrospray ionization tandem mass spectrometry with a column-switching technique. Anal Sci. 2001;17(6):709–713. doi: 10.2116/analsci.17.709. [DOI] [PubMed] [Google Scholar]
- 20.Stumpf K, Pietinen P, Puska P, Adlercreutz H. Changes in serum enterolactone, genistein, and daidzein in a dietary intervention study in Finland. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1369–1372. [PubMed] [Google Scholar]
- 21.Wang GJ, Lapcík O, Hampl R, et al. Time-resolved fluoroimmunoassay of plasma daidzein and genistein. Steroids. 2000;65(6):339–348. doi: 10.1016/s0039-128x(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 22.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3):S65–S87. doi: 10.1016/j.jaci.2011.12.986. suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dweik RA, Boggs PB, Erzurum SC, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical Applications An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med. 2006;354(16):1667–1669. doi: 10.1056/NEJMp068070. [DOI] [PubMed] [Google Scholar]
- 26.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49(2):171–174. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grievink L, Smit HA, Ocké MC, van’t Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998;53(3):166–171. doi: 10.1136/thx.53.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115(6):1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 29.McKeever TM, Britton J. Diet and asthma. Am J Respir Crit Care Med. 2004;170(7):725–729. doi: 10.1164/rccm.200405-611PP. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Mannino DM, Redd SC. Serum antioxidant concentrations among US adults with self-reported asthma. J Asthma. 2004;41(2):179–187. doi: 10.1081/jas-120026075. [DOI] [PubMed] [Google Scholar]
- 31.Ram FS, Rowe BH, Kaur B. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2004;3:CD000993. doi: 10.1002/14651858.CD000993.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Garcia V, Arts IC, Sterne JA, Thompson RL, Shaheen SO. Dietary intake of flavonoids and asthma in adults. Eur Respir J. 2005;26(3):449–452. doi: 10.1183/09031936.05.00142104. [DOI] [PubMed] [Google Scholar]
- 33.Messina M. Modern applications for an ancient bean: soybeans and the prevention and treatment of chronic disease. J Nutr. 1995;125(3):567S–569S. doi: 10.1093/jn/125.3_Suppl.567S. suppl. [DOI] [PubMed] [Google Scholar]
- 34.Schabath MB, Hernandez LM, Wu X, Pillow PC, Spitz MR. Dietary phytoestrogens and lung cancer risk. JAMA. 2005;294(12):1493–1504. doi: 10.1001/jama.294.12.1493. [DOI] [PubMed] [Google Scholar]
- 35.Polkowski K, Mazurek AP. Biological properties of genistein: a review of in vitro and in vivo data. Acta Pol Pharm. 2000;57(2):135–155. [PubMed] [Google Scholar]
- 36.Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003;167(2):185–192. doi: 10.1164/rccm.200205-420OC. [DOI] [PubMed] [Google Scholar]
- 37.Janssen LJ, Lu-Chao H, Netherton S. Responsiveness of canine bronchial vasculature to excitatory stimuli and to cooling. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L930–L937. doi: 10.1152/ajplung.2001.280.5.L930. [DOI] [PubMed] [Google Scholar]
- 38.Regal JF, Fraser DG, Weeks CE, Greenberg NA. Dietary phytoestrogens have anti-inflammatory activity in a guinea pig model of asthma. Proc Soc Exp Biol Med. 2000;223(4):372–378. doi: 10.1046/j.1525-1373.2000.22353.x. [DOI] [PubMed] [Google Scholar]
- 39.Rüfer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54(8):2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- 40.Miyake Y, Sasaki S, Ohya Y, et al. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the Osaka Maternal and Child Health Study. J Allergy Clin Immunol. 2005;115(6):1176–1183. doi: 10.1016/j.jaci.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Wakai K, Egami I, Kato K, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33(2):139–145. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- 42.International Study of Asthma and Allergies in Childhood Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351(9111):1225–1232. [PubMed] [Google Scholar]
- 43.Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer Agents Med Chem. 2012;12(10):1264–1280. doi: 10.2174/187152012803833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev. 2011;69(8):432–448. doi: 10.1111/j.1753-4887.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- 45.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence f habitual diet. Proc Society Exp Biol Med. 1998;217(3):335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 46.National Heart, Lung, and Blood Institute. Asthma Action Plan. http://www.nhlbi.nih.gov/health/resources/lung/asthma-action-plan.htm. Accessed Accessed May 5, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


