Abstract
Background
Despite clinical trial evidence and clinical experience supporting the efficacy of transcatheter aortic valve replacement (TAVR), data demonstrating the benefit of TAVR specifically in the very elderly patients are limited, as they often represent only a small proportion of the clinical trial populations.
Objectives
To compare the outcomes of nonagenarians to younger patients undergoing TAVR in current clinical practice.
Methods
National U.S. data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry were analyzed. Outcomes at 30-days and 1-year were compared between patients ≥90 vs <90 years of age using cumulative incidence curves. Quality of life was assessed with the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12).
Results
Between November 2011 and September 2014, 24,025 patients underwent TAVR in 329 participating hospitals, of which 3,773 (15.7%) were age ≥90. The 30-day and 1-year mortality was significantly higher among nonagenarians (≥90 vs. <90: 30-day: 8.8% vs. 5.9%, p<0.001; 1-year: 24.8% vs. 22.0%, p<0.001, absolute risk 2.8%, relative risk 12.7%). However, nonagenarians had a higher mean STS PROM score(10.9% vs. 8.1%; p<0.001) and therefore had similar ratios of observed to expected rates of 30-day death (≥90 vs. <90: 0.81, 95% CI 0.70–0.92 vs. 0.72, 95% CI 0.67–0.78). There were no differences in the rates of stroke, aortic valve reintervention or myocardial infarction at 30-days or 1-year. Nonagenarians had lower (worse) median KCCQ-12 scores at 30-days; however, there was no significant difference at 1-year.
Conclusions
In current U.S. clinical practice, approximately 16% of patients undergoing TAVR are ≥90 years of age. Although 30-day and 1-year mortality was statistically higher compared with younger patients undergoing TAVR, the absolute and relative differences were clinically modest. TAVR also improves quality of life to the same degree in nonagenarians as in younger patients. These data support safety and efficacy of TAVR in select very elderly patients.
Keywords: Transcatheter aortic valve replacement, Transcatheter aortic valve implantation, TAVI, Centenarians, Quality of life, elderly
Introduction
It is estimated that the number of people age 90 or greater (nonagenarians) in the United States will quadruple by the year 2050 to reach 8.7 million.(1) As such, clinicians are being confronted with an increasing number of nonagenarians with severe aortic stenosis (AS), which significantly reduces quality of life and survival. Due to the morbidity and mortality of surgical aortic valve replacement (SAVR) in patients at advanced age, surgery is often denied to very elderly patients.(2,3) Over the last 10 years, transcatheter aortic valve replacement (TAVR) has emerged as a viable treatment option for patients with severe AS who are inoperable or at high-surgical risk, prolonging survival and improving quality of life in the majority of patients.(4,5) However, the effect of TAVR in nonagenarians is largely unknown, as they represented only a small fraction of patients enrolled in the pivotal clinical trials. A few small, single-center series have reported outcomes of TAVR in the very elderly and showed acceptable results.(6–9) Due to this lack of outcomes data for TAVR in the very elderly, decision-making for TAVR in nonagenarians is complicated. As such, the aim of this study was to compare the procedural, 30-day, and 1-year outcomes of nonagenarians with patients under the age of 90 undergoing TAVR in current clinical practice using comprehensive data from the STS/ACC Transcatheter Valve Therapy (TVT) Registry.
Methods
The STS/ACC TVT Registry
The TVT Registry collects clinical information including patient demographics, comorbidities, functional status, quality of life, and procedural details in addition to postoperative, 30-day, and 1-year outcomes using standardized definitions on virtually all patients undergoing TAVR with a commercially approved device in the U.S..(10,11) The Registry has been approved by the Chesapeake Central Institutional Review Board (IRB) and the Duke University School of Medicine IRB. Both IRB committees granted a waiver of informed consent and authorization for this study.
Study Cohort
Nonagenarians were defined as patients aged 90 or above at the time of the procedure, and thus a small number of centenarians were included in this study (n=24). TVT Registry clinical records for procedures performed from November 2011 through September 2014 were linked to Medicare administrative claims using direct patient identifiers (name and social security number) by CMS. Per the CMS National Coverage Determination for reimbursement, all patients were required to have site documentation of echocardiographic defined severe AS and an assessment by 2 cardiothoracic surgeons who independently deemed the patients as at high or prohibitive surgical risk of mortality from SAVR. Of the 24,025 index TVT procedure records, 8,502 were not linked to Medicare, either because of patient nonparticipation in the Medicare Parts A and B fee-for-service program at the time of the index procedure or an inability to link the index admission to a Medicare inpatient claim. For quality of life, the study cohort was limited to procedures performed on or before July 17, 2014 for 30-day assessment and procedures on or before August 1, 2013 for 1-year assessment to allow for appropriate follow-up.
Study Endpoints
Primary outcomes studied included death, stroke, rehospitalization due to heart failure, aortic valve reintervention, myocardial infarction (MI) and quality of life (QOL) at 30 days and 1 year. QOL was assessed with the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12), a 12-item condensed psychometrically valid version of the full KCCQ.(12) A disease-specific health status survey originally developed to describe and monitor health status in patients with heart failure, the KCCQ has also been validated in patients with aortic stenosis.(13) For this study, we focused on the overall summary score of the KCCQ-12 (KCCQ-os), which ranges from 0 to 100 with higher scores indicating less symptom burden, less physical and social limitations, and better quality of life.
In-hospital outcomes were collected as part of the TVT Registry. Valve Academic Research Consortium (VARC) definitions were used for major bleeding.(10,11) All site-reported stroke and valve reintervention events were adjudicated by a board-certified cardiologist using VARC definitions. This process involved review of specific site queries and de-identified source records as needed. Following hospital discharge, death was identified using the Medicare Denominator file. Medicare in-hospital administrative claims files were used for detection of re-hospitalization events through October 2014 using the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: for stroke; 433.x1, 434.x1, 997.02, 436, 437.1, 437.9, 430, 431, and 432.x; for heart failure, 398.x, 402.x1, 404.x1, 404.x3, and 428.x; for aortic valve reintervention, 35.11, 35.21, 35.22, 35.01, 35.05, 35.06, and 35.09; and for MI, 410.x0, 410.x1. For rehospitalization, follow-up was censored at the end of fee-for-service coverage, loss of Part A or B coverage, or end of the follow-up period (October 31, 2014), whichever occurred first.
Statistical Analysis
Baseline characteristics and in-hospital outcomes of the study population were presented according to the age. Categorical variables were summarized as percentages and continuous variables as medians with interquartile ranges (IQRs). The baseline characteristics and in-hospital outcomes of patients ≥ 90 and < 90 years of age were then compared using the Pearson chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Cumulative incidences of death and nonfatal outcomes at 30 days and 1 year post TAVR were estimated for patients ≥ 90 and < 90 years of age. For nonfatal outcomes - including stroke, heart failure readmission, aortic valve reintervention and post-procedural myocardial infarction - death was considered a competing risk, and therefore the cumulative incidence indicated the probability of a nonfatal outcome occurring given that death may impede its occurrence.(14) The 30-day observed to expected mortality ratios were calculated based on the baseline STS Predicted Risk of Operative Mortality (PROM) score, with 95% confidence intervals (CIs) obtained using a normal approximation to the binomial distribution.(15)
The unadjusted and adjusted effects of age on 30-day and 1-year mortality were assessed using Cox proportional hazards models. Nonfatal outcomes were assessed using Fine and Gray proportional subdistribution hazards models in the presence of competing risk of death.(14,16) The multivariable models included the covariates in the recently developed TVT model for in-hospital mortality.(17) The risk of adverse outcomes for patients ≥ 90 vs. < 90 years of age was reported using hazard ratios (HRs) with 95% CIs.
Complete case analysis was performed for 30-day and 1-year KCCQ-os scores. KCCQ-os scores were summarized as medians with IQRs and compared between patients ≥ 90 and < 90 years of age using the Wilcoxon rank sum test. The effect of age on follow-up KCCQ-os scores was assessed using linear regression models that included a binary indicator variable for age group. Models were constructed as unadjusted (including age only) and adjusted, including age, baseline STS PROM score, and baseline KCCQ-os score. As the STS PROM score is calculated including age as well as its interactions with several risk factors, this complicates the multivariable adjustment. As such, the baseline STS PROM score was re-calculated assuming all patients were 85 years of age, the median age of the study population. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc.), and a p-value < 0.05 was considered statistically significant.
Results
Patient Cohort
From November 2011 through September 2014, 24,025 patients, of which 3,773 (15.7%) were nonagenarians, underwent TAVR at 329 participating hospitals. The median age was 92 years in nonagenarians and 82 years in the younger cohort. Compared to patients under age 90, nonagenarians were more likely to be female and less likely to have high-risk features including prior non-aortic valve cardiac surgery procedure, diabetes, prior stroke, and prior myocardial infarction (MI), but, overall, had higher estimated surgical mortality (STS PROM scores, ≥90 vs. <90: 9.2% vs. 6.3%; p<0.001). Although nonagenarians had slower five-meter walk tests (8.7 vs. 8.0; p <0.001), they had better self-reported QOL (KCCQ-os: 41.7 vs. 37.5; p <0.001) prior to TAVR (Table 1).
Table 1.
Baseline characteristics of the study population
| Variable | Nonagenarians (Age ≥ 90) (N=3773) | Others (Age < 90) (N=20252) | p-value |
|---|---|---|---|
| Age* | 92.0 (90.0–93.0) | 82.0 (76.0–86.0) | <0.001 |
| Femaley | 1953 (51.76) | 9976 (49.26) | 0.005 |
| STS PROMxx score* | 9.22 (6.73–13.25) | 6.34 (4.20–9.77) | <0.001 |
| NYHA yy Class I & IIy | 670 (17.76) | 3459 (17.08) | 0.347 |
| NYHA yy Class III & IVy | 3055 (80.97) | 16479 (81.37) | |
| LVEF{* | 58.00 (48.00–65.00) | 56.00 (45.00–63.00) | <0.001 |
| Prior CABGx, y | 693 (18.37) | 6873 (33.94) | <0.001 |
| Prior Other Cardiac Surgeryy | 150 (3.98) | 1477 (7.29) | <0.001 |
| Prior Aortic Valve Procedurey | 646 (17.12) | 3239 (15.99) | 0.086 |
| Permanent Pacemaker/ICDy | 817 (21.65) | 3209 (15.85) | <0.001 |
| Atrial fibrillationy | 1619 (42.91) | 8175 (40.37) | 0.004 |
| Prior Strokey | 352 (9.33) | 2587 (12.77) | <0.001 |
| Transient Ischemic Attacky | 394 (10.44) | 1719 (8.49) | <0.001 |
| Peripheral Arterial Diseasey | 970 (25.71) | 6606 (32.62) | <0.001 |
| Hypertensiony | 3266 (86.56) | 18077 (89.26) | <0.001 |
| Diabetes Mellitusy | 730 (19.35) | 8179 (40.39) | <0.001 |
| Insulin Dependent Diabetesy | 149 (20.41) | 3192 (39.03) | <0.001 |
| Moderate-Severe Chronic Lung Diseasey | 581 (15.40) | 6096 (30.10) | <0.001 |
| Prior MI#,y | 786 (20.83) | 5258 (25.96) | <0.001 |
| Five Meter Walk Test - Time (sec)* | 8.67 (6.67–11.33) | 8.00 (6.00–10.67) | <0.001 |
| KCCQ-12jj Overall Score* | 41.67 (23.96–59.38) | 37.50 (21.88–56.77) | <0.001 |
| BMIy (kg/m2)* | 24.67 (22.20–27.56) | 27.25 (23.88–31.77) | <0.001 |
| Currently on Dialysisy | 39 (1.03) | 945 (4.67) | <0.001 |
| Creatinine (mg/dL)* | 1.10 (0.90–1.40) | 1.10 (0.90–1.50) | 0.002 |
| Triple Vessel Diseasey | 786 (20.83) | 5709 (28.19) | <0.001 |
| Left Main Stenosis >= 50%y | 325 (8.61) | 2241 (11.07) | <0.001 |
| Moderate-Severe Aortic Insufficiencyy | 663 (17.57) | 4171 (20.60) | <0.001 |
Median with interquartile range
Number of patients and percentage
BMI = Body mass index
CABG = Coronary artery bypass grafting
KCCQ-12 = 12-item Kansas City Cardiomyopathy Questionnaire
LVEF = Left ventricular ejection fraction
MI = Myocardial infarction
NYHA = New York Heart Association
STS PROM = STS Predicted Risk of Operative Mortality
In-hospital outcomes
Compared with patients under age 90, nonagenarians were more likely to experience in-hospital stroke, major vascular assess site complications, and major bleeding events (Table 2). They also had longer ICU-stays and increased rates of blood transfusions. In-hospital death was higher among nonagenarians (6.5% vs. 4.5%; p <0.001), and they were also more likely to be discharged to extended care/TCU/rehabilitation or nursing home.
Table 2.
In-hospital outcomes of nonagenarians and patients under age 90
| Variable | Nonagenarians (Age ≥ 90) (N=3773) | Others (Age < 90) (N=20252) | p-value |
|---|---|---|---|
| Myocardial Infarction | 25 (0.67) | 104 (0.52) | 0.248 |
| Stroke | 102 (2.72) | 426 (2.11) | 0.021 |
| Atrial Fibrillation | 258 (6.87) | 1320 (6.54) | 0.458 |
| Major Vascular Access Site Complication | 38 (1.01) | 133 (0.66) | 0.019 |
| Minor Vascular Access Site Complication | 82 (2.18) | 353 (1.75) | 0.068 |
| New Requirement for Dialysis | 60 (1.60) | 373 (1.85) | 0.289 |
| Aortic Valve Reintervention | 10 (0.27) | 73 (0.36) | 0.361 |
| VARCx Major Bleeding Event | 299 (8.11) | 1354 (6.81) | 0.004 |
| In-Hospital Mortality | 244 (6.47) | 915 (4.52) | <0.001 |
| Discharge Location Home | 1848 (52.38) | 12793 (66.19) | <0.001 |
| Extended Care/TCU/Rehab | 1319 (37.39) | 5317 (27.51) | |
| Nursing Home | 296 (8.39) | 922 (4.77) | |
| Other | 62 (1.76) | 279 (1.44) | |
| RBCz/Whole Blood Transfusion | 1690 (44.79) | 7753 (38.28) | <0.001 |
| Length of Stay (days)* | 6.00 (4.00–10.00) | 6.00 (4.00–9.00) | <0.001 |
| Number of Hours in ICUy* | 46.00 (24.00–78.00) | 44.00 (24.00–74.30) | <0.001 |
Median with interquartile range
ICU = intensive care unit
RBC = Red blood cells
VARC = Valve Academic Research Consortium
Post-discharge outcomes
No differences in stroke rate, aortic valve reintervention, or MI were evident after 30-days or 1-year (Table 3, Figure 1 and Figure 2). There was a higher rate of heart failure readmission after 30 days for nonagenarians (5.3% vs 4.3%; p = 0.014; Figure 3) but not after 1 year (14.9% vs 14.5%; p = 0.377). The 30-day and 1-year mortality rates were significantly higher among nonagenarians (≥90 vs. <90: 30-day: 8.8% vs. 5.9%, p<0.001; 1-year: 24.8% vs. 22.0%, p<0.001, absolute risk 2.8%, relative risk 12.7%; Figure 4). Among the small number of patients’ ≥100 years of age (n=15), 30-day mortality was 0% and 1-year mortality was 6.7%.
Table 3.
Post-discharge outcomes in nonagenarians and patients under age 90 and unadjusted hazard ratio
| Nonagenarians (Age ≥ 90) | Others (Age < 90) | Unadjusted hazard ratio | |||||
|---|---|---|---|---|---|---|---|
| Outcome | No. of Events | Rate, % (95% CI) | No. of Events | Rate, % (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| 30-day | |||||||
| Mortality | 232 | 8.8 (7.8, 10.0) | 755 | 5.9 (5.5, 6.3) | <0.001 | 1.53 (1.32, 1.78) | <0.001 |
| Stroke | 77 | 2.9 (2.4, 3.7) | 305 | 2.4 (2.1, 2.6) | 0.087 | 1.24 (0.97, 1.60) | 0.087 |
| Heart Failure | 140 | 5.3 (4.5, 6.3) | 548 | 4.3 (3.9, 4.6) | 0.014 | 1.26 (1.05, 1.52) | 0.014 |
| Aortic Valve Reintervention | 10 | 0.4 (0.2, 0.7) | 51 | 0.4 (0.3, 0.5) | 0.912 | 0.96 (0.49, 1.90) | 0.913 |
| Myocardial infarction | 22 | 0.8 (0.6, 1.3) | 98 | 0.8 (0.6, 0.9) | 0.683 | 1.10 (0.69, 1.75) | 0.683 |
| 1-year | |||||||
| Mortality | 570 | 24.8 (23.1, 26.7) | 2324 | 22.0 (21.2, 22.9) | <0.001 | 1.21 (1.11, 1.33) | <0.001 |
| Stroke | 108 | 4.4 (3.7, 5.4) | 456 | 3.9 (3.6, 4.3) | 0.183 | 1.16 (0.94, 1.42) | 0.178 |
| Heart Failure | 345 | 14.9 (13.5, 16.5) | 1588 | 14.5 (13.8, 15.2) | 0.377 | 1.06 (0.94, 1.19) | 0.354 |
| Aortic Valve Reintervention | 14 | 0.6 (0.3, 0.9) | 75 | 0.6 (0.5, 0.8) | 0.738 | 0.91 (0.51, 1.61) | 0.741 |
| Myocardial infarction | 46 | 2.0 (1.5, 2.7) | 230 | 2.3 (2.0, 2.6) | 0.783 | 0.96 (0.70, 1.32) | 0.804 |
Figure 1. Adjusted age effects on post-discharge outcomes.
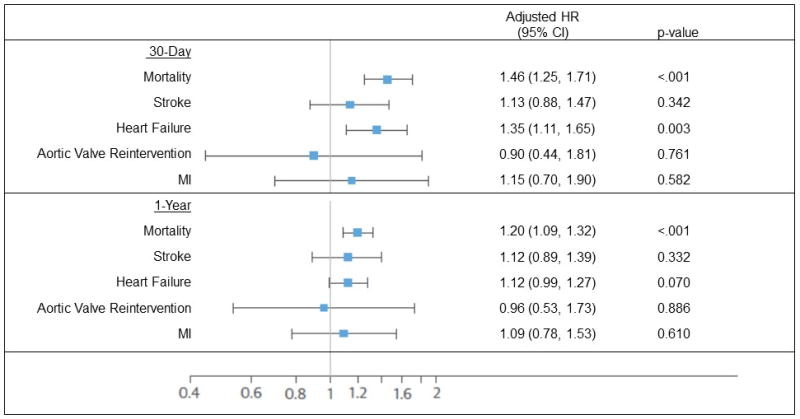
Adjusted age effects on post-discharge outcomes after 30 days and 1-year. (HR = hazard ratio, MI = myocardial infarction)
Figure 2. Stroke.
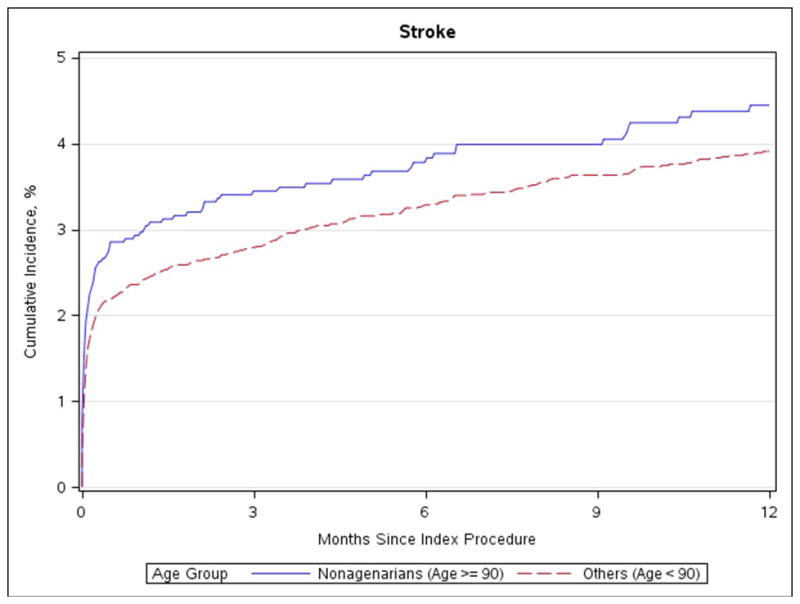
Cumulative incidence of stroke in nonagenarians and patients under age 90
Figure 3. Heart failure readmission.
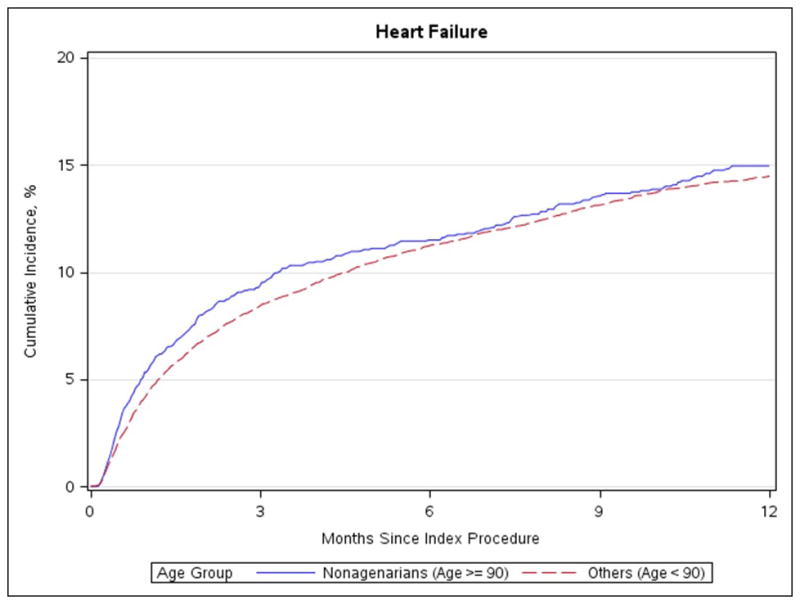
Cumulative incidence of heart failure readmission in nonagenarians and patients under age 90
Figure 4. Mortality.
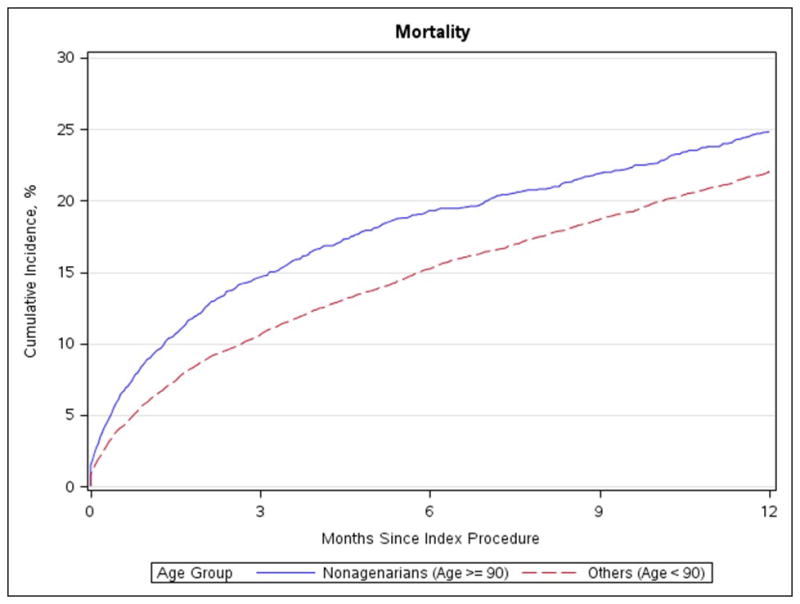
Cumulative incidence of mortality in nonagenarians and patients under age 90
After adjusting for multiple demographic and clinical factors (see appendix), nonagenarians continued to show an increased risk of mortality at 30-days (adjusted HR=1.46, 95% CI 1.25–1.71 p < 0.001), heart failure at 30-days (adjusted HR=1.35, 95% CI 1.11–1.65, p=0.003), and mortality at 1-year (adjusted HR=1.20, 95% CI 1.09–1.32, p<0.001). However, as nonagenarians had a higher STS PROM than the younger cohort, the observed to expected mortality ratios were 0.81 (95% CI: 0.70–0.92) and 0.72 (95% CI: 0.67–0.78) respectively.
Quality of Life
Nonagenarians had a lower median 30-day KCCQ-os score compared with younger patients (70.8 vs. 72.9, p = 0.006) but similar KCCQ-os scores at 1-year after TAVR (79.2 vs. 81.3, p = 0.539; Table 4). After adjusting for STS PROM risk score and baseline KCCQ, nonagenarians had, on average, 3.57-point lower KCCQ-os scores at 30-days but no significant difference at 1 year.
Table 4.
30-day and 1-year KCCQ-12 overall summary scores in nonagenarians and others under age 90
| Nonagenarians (Age ≥ 90) | Others (Age < 90) | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | N | Mean | Median (IQR) | N | Mean | Median (IQR) | p-value |
| 30 Day KCCQ-os* Score | 1571 | 66.25 | 70.83 (51.04, 86.46) | 8686 | 68.11 | 72.92 (52.60, 87.50) | 0.006 |
| 1 Year KCCQ-os* Score | 623 | 74.77 | 79.17 (61.46, 93.75) | 3089 | 75.20 | 81.25 (62.50, 93.75) | 0.539 |
KCCQ-os = overall summary score of the 12-item Kansas City Cardiomyopathy Questionnaire
Discussion
This is the largest study comparing short- and mid-term outcomes of nonagenarians with younger patients undergoing TAVR in U.S. clinical practice. Patient demographics of nonagenarians in this study highlight that nonagenarians who are currently undergoing TAVR represent a highly selected group. Factors that make a patient higher risk for poor outcomes after TAVR, such as reduced ejection fraction, prior cardiac surgery, and prior stroke, are less commonly present in nonagenarians compared to the younger population, thus making very advanced age the primary factor making the nonagenarian high risk for surgery. While these patients are at increased risk for morbidity and mortality after TAVR simply based on their age, our findings show that many nonagenarians have good outcomes after TAVR - with prolonged survival and improved QOL - making TAVR reasonable to consider in select nonagenarians with severe AS.
Most existing studies report single-center experiences with small patient cohorts and thus may only provide limited decision-making information on the outcomes of nonagenarians undergoing TAVR.(6–9,18) In the largest previous study on TAVR in nonagenarians (346 nonagenarians out of 2,254 enrolled patients; reported from the French National Transcatheter Aortic Valve Implantation Registry (FRANCE-2)), Yamamoto and colleagues demonstrated a 30-day mortality of 11.2% in nonagenarians without any significant difference compared with patients age 80 to 84 or 85 to 89.(19) We found a lower 30-day mortality rate in nonagenarians (8.8%), which may reflect differences in patient selection and improvements in devices and techniques. Although the mortality rate for nonagenarians in our study remained higher than observed in younger patients (5.9%), this rate may be acceptable, particularly given the QOL benefits observed among surviving nonagenarians. This is further illustrated by the similar observed to expected ratios. Of note, the observed nonagenarian mortality rate is not only lower than that previously reported on nonagenarians but is also comparable to published mortality rates for octogenarians.(19,20)
In regard to long-term survival, the FRANCE-2 data showed a trend towards decreased survival in older patients, but the study did not meet statistical significance due to the limited number of patients at risk by 1-year.(19) The current study confirms a higher mortality rate in nonagenarians at 1-year, but due to the high mortality rates in both groups, it is doubtful that the measured difference of 2.8% is clinically significant or simply reflects the shorter underlying life expectancy of nonagenarians. Some clarity on this issue can be achieved by comparing our 1-year outcomes to the life expectancy of an age-matched general population. The younger patient cohort showed a 1-year mortality of 22%, which is significantly higher compared to the age-matched general population (6.1% in males and 4.4% in females).(21) In nonagenarians, however, the observed difference between 1-year mortality in TAVR patients (24.8%) and the same age general population (male 20.5% and female 16.5%) was much smaller (Figure 5). Thus we confirm the promising results from other reports about TAVR in select nonagenarians and additionally show that the difference between outcomes of select nonagenarians and younger patients might not be of major clinical relevance.
Figure 5. Mortality compared to age-matched general population.
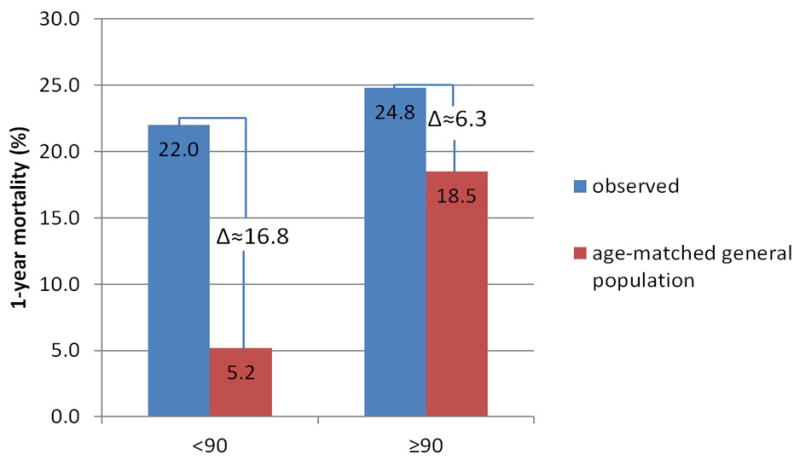
1-year mortality observed versus age-matched general population
We further describe the outcomes of a very small cohort of patients ≥100 years of age undergoing TAVR. Overall, 24 centenarians were treated in the TVT registry, thus, this is the largest report on TAVR in this age group. We are aware that our patient cohort (n=15 with CMS linkage) is too small to give a reliable statement, but nevertheless, the procedural outcomes are encouraging. There was no mortality at 30-days likely indicative of very careful patient selection. The very low 1-year mortality (6.7%) is excellent, especially considering the high 1-year death probability in a 100 year old patient (36% in males and 31% in females). Of note, Bridges and colleagues, reviewing the STS national database, reported that only 5 centenarians underwent cardiac surgery in the US between 1997 and 2000.(22)
Increased age alone may heighten the risk of particular post-procedural complications. As vascular complications were one of the most common complications, assessing age-related differences is relatively straightforward. The increasing prevalence of vascular complications in very old patients has been demonstrated by several studies, but, because of varying definitions, rates of vascular complications are difficult to compare between studies. Havakuk and colleagues reported that the rate of minor vascular injuries is increased in patients 85 and older (7.5% vs. 16%, p = 0.02), without any difference in major vascular complications (4.3% vs. 2.5%, p = 0.41).(23) On the other hand, our data, similar to those of Yamamoto et al, shows higher rates of major vascular complications in the very elderly but no significant difference in minor vascular injury rates.(9)
Additional in-hospital complications that differed between nonagenarians and younger patients were major bleeding events, need for blood transfusion, and stroke, all of which were higher in the nonagenarian group. The in-hospital stroke rate was higher for nonagenarians in the present study, but we did not detect any significant effect of age on incidence of stroke following TAVR at 30-days nor at 1-year.(9,19,23) The observed 30-day stroke rate was similar to prior reports on elderly patients undergoing TAVR.(6,9,20,23,24). The increased incidences of these in-hospital complications are comprehensively reflected in the longer ICU stays and higher likelihood of discharge to extended care or rehabilitation facilities experienced by nonagenarians.
Although our in-hospital results might suggest worse short-term outcomes for nonagenarians, 30-day and, more importantly, 1-year outcomes are more suitable to determine the appropriateness of TAVR in the nonagenarian age group. However, it is important to note that survival is not the sole factor defining good outcomes in TAVR, especially in the elderly population where survival with reasonable functional capacity and QOL is what matters most. Improvement in QOL should also be included to evaluate whether a procedure should be reasonably offered to nonagenarians.
In a review of the PARTNER trial, Thourani and colleagues have previously demonstrated that QOL improves and stabilizes 6 months after TAVR in nonagenarians.(25) Our data confirm these findings. We found that there was a significant increase in KCCQ scores by 30-days, but scores were significantly lower in nonagenarians compared to younger patients. However, there were no differences in QOL between age groups by 1 year after TAVR. These findings suggest that nonagenarians likely recover more slowly after TAVR and thus need more time until the beneficial effect of the procedure is measurable. However, if given time to recover, older patients are able to achieve similar QOL levels as younger patients. This information may be important for patients to know prior to undergoing TAVR, for post-procedure planning and setting realistic expectations of recovery times.
Limitations
This analysis should be interpreted in light of several important potential limitations. The TVT registry only captures information on patients receiving commercially approved devices. As several newer TAVR devices are currently under investigation in the US, our data thus do not represent an all-comers population nor the most recent iterations of transcatheter devices. Furthermore, a large number of patients (35%; 8502/24025) could not be included in long-term outcome analysis due to inability to link with CMS. Compared with patients without CMS linkage, patients with CMS linkage were more likely to be females, to have prior TIA, higher LVEF, STS PROM, and KCCQ-12 score. They were less likely to have prior aortic valve procedure, diabetes, NYHA class III/IV, and to be currently on dialysis. Furthermore, there was a high rate of missingness of KCCQ data (50% at 30-day and 62% at 1-year follow-up). Compared with patients with non-missing KCCQ-os scores, patients with missing KCCQ scores at follow-up were more likely to be males, have slower five meter walk test, lower LVEF, higher STS PROM, lower baseline KCCQ score, have insulin dependent diabetes, and to be on dialysis. It is unclear how inclusion of these patients with missing follow-up data would have altered our findings. However, our study still represents the largest study of nonagenarians who have undergone TAVR and provides much needed data to describe the outcomes of these high-risk patients.
Conclusion
Given their limited life expectancy, it has been unclear whether to perform TAVR in the very elderly and existing data on outcomes of these patients have been limited. The primary concerns are that nonagenarians might not survive the procedure as frequently, recover from the procedure as quickly and nor experience an improved functional outcome and quality of life. We report in current U.S. clinical practice that approximately 16% of patients undergoing TAVR are nonagenarians or older. Although 30-day and 1-year mortality rates were higher in this age group compared with younger TAVR patients, the absolute and relative differences were clinically modest. Furthermore, while nonagenarians generally take longer to recover their physical function and QOL than younger patients, TAVR improved long-term QOL to a similar degree in both age groups. As such, the reported data support both the safety and the efficacy of TAVR in select elderly patients, suggesting that TAVR should not be denied solely based on patient age.
COMPETENCY IN MEDICAL KNOWLEDGE
Although 30-day and 1-year mortality rates were higher in nonagenarians compared with younger patients undergoing TAVR, the absolute and relative differences were clinically modest.
COMPETENCY IN PATIENT CARE
Selected nonagenarians should be considered for TAVR to improve symptoms and quality of life.
TRANSLATIONAL OUTLOOK
More information from clinical studies is needed to clarify which patients, regardless of their age, benefit from TAVR. This would improve patient selection, especially if the Indication for TAVR is expanded to patients at lower risk.
Abbreviations
- AS
Aortic stenosis
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- IRB
Institutional Review Board
- MI
myocardial infarction
- QOL
quality of life
- KCCQ-12
12-item Kansas City Cardiomyopathy Questionnaire
- KCCQ-os
overall summary score of the KCCQ-12
- VARC
Valve Academic Research Consortium
- IQR
interquartile range
- STS
Society of Thoracic Surgeons
- STS PROM
STS Predicted Risk of Operative Mortality
- CI
confidence interval
Footnotes
Sponsor Statement
STS/ACC TVT RegistryTM is an initiative of The Society of Thoracic Surgeons and the American College of Cardiology.
Disclaimer
This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR.
References
- 1.Vincent GKVV. The Next Four Decades: The Older Population in the United States 2010 to 2050. USC Bureau; Washington, DC: 2010. [Google Scholar]
- 2.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? European heart journal. 2005;26:2714–20. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 3.Assmann A, Minol JP, Mehdiani A, Akhyari P, Boeken U, Lichtenberg A. Cardiac surgery in nonagenarians: not only feasible, but also reasonable? Interact Cardiovasc Thorac Surg. 2013;17:340–3. doi: 10.1093/icvts/ivt125. discussion 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42:S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 6.Noble S, Frangos E, Samaras N, et al. Transcatheter aortic valve implantation in nonagenarians: effective and safe. European journal of internal medicine. 2013;24:750–5. doi: 10.1016/j.ejim.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Mack MC, Szerlip M, Herbert MA, et al. Outcomes of Treatment of Nonagenarians With Severe Aortic Stenosis. The Annals of thoracic surgery. 2015;100:74–80. doi: 10.1016/j.athoracsur.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Akin I, Kische S, Paranskaya L, et al. Morbidity and mortality of nonagenarians undergoing CoreValve implantation. BMC cardiovascular disorders. 2012;12:80. doi: 10.1186/1471-2261-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Meguro K, Mouillet G, et al. Comparison of effectiveness and safety of transcatheter aortic valve implantation in patients aged >/=90 years versus <90 years. The American journal of cardiology. 2012;110:1156–63. doi: 10.1016/j.amjcard.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. European heart journal. 2011;32:205–17. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. European heart journal. 2012;33:2403–18. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circulation Cardiovascular quality and outcomes. 2015 doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circulation Heart failure. 2013;6:61–7. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 15.DeLong ER, Peterson ED, DeLong DM, Muhlbaier LH, Hackett S, Mark DB. Comparing risk-adjustment methods for provider profiling. Statistics in medicine. 1997;16:2645–64. doi: 10.1002/(sici)1097-0258(19971215)16:23<2645::aid-sim696>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Jason PF, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 17.O’Brien SM, Cohen DJ, Rumsfeld JS, et al. Variation in Hospital Risk-Adjusted Mortality Rates Following Transcatheter Aortic Valve Replacement in the United States: A Report from the Society of Thoracic Surgeons and American College of Cardiology National Transcatheter Valve Therapies Registry. 2015 doi: 10.1161/CIRCOUTCOMES.116.002756. Submitted to JACC. [DOI] [PubMed] [Google Scholar]
- 18.Murashita T, Greason KL, Suri RM, et al. Aortic valve replacement for severe aortic valve stenosis in the nonagenarian patient. The Annals of thoracic surgery. 2014;98:1593–7. doi: 10.1016/j.athoracsur.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Mouillet G, Meguro K, et al. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry. The Annals of thoracic surgery. 2014;97:29–36. doi: 10.1016/j.athoracsur.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 20.Buellesfeld L, Gerckens U, Erbel R, et al. Age-stratified baseline and outcome characteristics of patients undergoing transcatheter aortic valve implantation: results from the German multicenter registry. The Journal of invasive cardiology. 2012;24:531–6. [PubMed] [Google Scholar]
- 21.Administration SS. Period life table for the Social Security area population. 2011. [Google Scholar]
- 22.Bridges CR, Edwards FH, Peterson ED, Coombs LP, Ferguson TB. Cardiac surgery in nonagenarians and centenarians. Journal of the American College of Surgeons. 2003;197:347–56. doi: 10.1016/S1072-7515(03)00384-3. discussion 356–7. [DOI] [PubMed] [Google Scholar]
- 23.Havakuk O, Finkelstein A, Steinvil A, et al. Comparison of outcomes in patients </=85 versus >85 years of age undergoing transcatheter aortic-valve implantation. The American journal of cardiology. 2014;113:138–41. doi: 10.1016/j.amjcard.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Verouhis D, Yamasaki K, Ivert T, Ruck A, Settergren M. Transcatheter aortic valve implantation is feasible and safe in nonagenarians. Journal of the American Geriatrics Society. 2014;62:189–90. doi: 10.1111/jgs.12620. [DOI] [PubMed] [Google Scholar]
- 25.Thourani VH, Jensen HA, Babaliaros V, et al. Outcomes in Nonagenarians Undergoing Transcatheter Aortic Valve Replacement in the PARTNER-I Trial. The Annals of thoracic surgery. 2015 doi: 10.1016/j.athoracsur.2015.05.021. [DOI] [PubMed] [Google Scholar]


