Abstract
OBJECTIVES
To investigate the effect of changing the bladder filling rate during cystometry in younger (2–3 months) and older (13–14 months) C57BL/6J male mice.
METHODS
Cystometry was performed on mice under anesthesia. Voiding cycles were established in each mouse at a pump delivery rate of 17 μl/min. After 30 min, the rate was increased sequentially to 25, 33, 41 and 49 μl/min. Each rate was maintained for 30 min. The following cystometric parameters were quantified: peak pressure amplitude, intercontractile interval (ICI), compliance, micturition pressure threshold and voiding efficiency.
RESULTS
Bladder weights were significantly greater in older mice (42 mg vs. 27 mg, P < 0.01), but functional capacities were not different. The pressure amplitudes did not change as filling rate increased, nor did they differ between the 4-month and 13-month-old males. ICIs were not significantly different between young and mature mice. However, both groups exhibited a non-linear reduction in ICI with increasing filling rate, best described by a power curve (R2 > 0.93). Compliance was higher in the older mice at low filling rates (17 and 25 μl/min) but this difference diminished at higher rates. Compliance decreased with increasing flow rate in a non-linear manner, again with greater effects at low filling rates. Micturition pressure thresholds increased with increasing flow rate in a linear manner and older mice began voiding at higher pressures than younger. Both young and old mice exhibited voiding efficiencies of ~70%.
CONCLUSIONS
The rate of volume delivery has complex effects on the timing of voiding and compliance. These findings argue for greater standardization of cystometry protocols and further investigation into afferent signaling to higher centers at different filling rates.
Keywords: mouse, cystometrogram, urodynamics, filling rate, volume delivery, compliance
INTRODUCTION
Bladder cystometry is the measurement of intrabladder pressure recorded during continuous liquid infusion and reports on all phases of the micturition process. It is a widely used methodology for the study of integrated physiological factors of bladder function, as well a way to study the consequences of injury, genetic manipulation and obstruction. Cystometry on small animal models has been used to gather fundamental insights into lower urinary tract (LUT) function and the pathophysiology of its clinical conditions [1,2]. Although cystometry is useful, it is a delicate and complex procedure with a number of critical steps. Interpretation of cystometric data is complicated by the fact that it is often performed in different ways by different laboratories. Methodological variables include varying concentrations of urethane for anesthesia [3], length of rest period following surgery, infusion for different times prior to beginning measurement, tubing diameter, and methods of insertion/suturing of the catheter. Depending on the skill of the operator, bladder/nerve damage, particularly for mice, is an ever-present variable. To further complicate the interpretation of cystometric data, different labs emphasize different parameters in the analyses of the pressure:volume curves. These variations in methodology and analysis create problems when comparing results gathered from different laboratories [4].
To begin to understand the importance of specific experimental parameters in mouse bladder cystometry, we have investigated the effect of changing the infusion-rate into the bladder. This was motivated in part by the surprising observation that cystometrograms (CMGs) performed by two different groups on age-matched females of three different inbred strains of mice had similar intercontractile intervals (ICIs) despite a two-fold difference in infusion rates [4]. This difference could have been due to a number of environmental as well as operational variables, but we wished to more rigorously investigate the dependence of ICI on filling rate. Accordingly, we altered the bladder-filling rate in groups of younger (2–3 months) and older (13–14 months) C57BL/6J male mice and recorded pressure:volume curves at incremental filling rates of 17, 25, 33, 41 and 49 μl/min, for at least 30 min. The median life span for males of this strain is 29.6 months (http://phenome.jax.org/). Therefore, the older mice we tested can be considered equivalent to middle-aged by human standards. As such they may be thought of as a mature cohort rather than truly aged. For humans this represents a point in life at which bladder problems start to become more common both in men and women.
MATERIALS AND METHODS
The work described in this article has been carried out in accordance with the EU Directive 2010/63/EU for animal experiments and the Uniform Requirements for manuscripts submitted to biomedical journals.
Spontaneous void spot assays
Void spot assay (VSA) was performed for 4 h as described in the previous studies [5,6]. Briefly, individual mice were placed on Blick Cosmos Blotting paper (Dick Blick Art Materials, Galesburg, IL) cut to the dimensions of a typical mouse cage and placed in bottom. Mice were provided with standard dry mouse chow but no water. At the end of 4 h, mice were removed, and the filter paper was retrieved, dried, and imaged under ultraviolet light in a UVP Chromato-Vue C75 UV viewing cabinet. Filters were then photographed and the images analyzed and quantitated using UrineQuant software developed by us in collaboration with the Imaging and Data Analysis Core at Harvard Medical School.
Mouse bladder cystometry
Four young (2–3 months old) and four older C57BL/6J male mice (13–14 months old) had cystometry performed under urethane anesthesia (intraperitoneal injection of 1.2–1.4 g/kg). Each mouse had an initial dose at 1.2 g/kg and if it became necessary for maintenance of anesthetic plane, additional aliquots were injected [6]. PE50 tubing was implanted through the dome of the bladder with the use of an inserted 25-gauge × 1.5 inch needle, then secured with purse string surgical suture. The catheter was connected to a pressure transducer and syringe pump, coupled to a computerized recording system (LabChart). Following surgery, the mice were given 30 min of recovery time before experiments commenced and bladders were infused with sterile saline at room temperature (22°C). Filling rates of 17, 25, 33, 41 and 49 μl/min were used, in that order, on each animal for at least 30 min and the cystometric parameters; pressure amplitude at voiding (peak pressure to basal), ICI, compliance, micturition pressure threshold and voiding efficiency were quantitated. Compliance was measured by finding the inverse of the pressure slope during bladder filling and prior to the voiding event. Bladder voiding efficiency was assessed at the end of the experiment by emptying the bladder, running a single-fill CMG with voiding at 49 μl/min and then measuring the residual volume. Efficiency was calculated by using equation ((inflow volume − residual volume)/inflow volume)) × 100, where inflow volume = flow rate × time of single fill. Following cystometry, bladders were excised, blotted dry on Kimwipes and weighed.
Statistics
Data are expressed as means ± standard error of the mean (SE). Statistical analysis was performed in Excel using two-tailed t tests. Statistical significance was identified at P < 0.05.
RESULTS
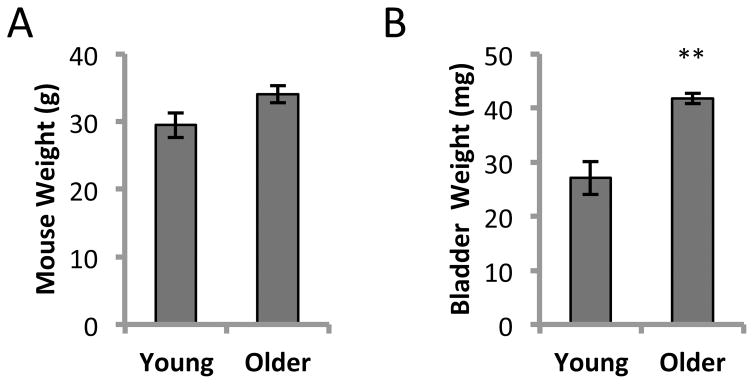
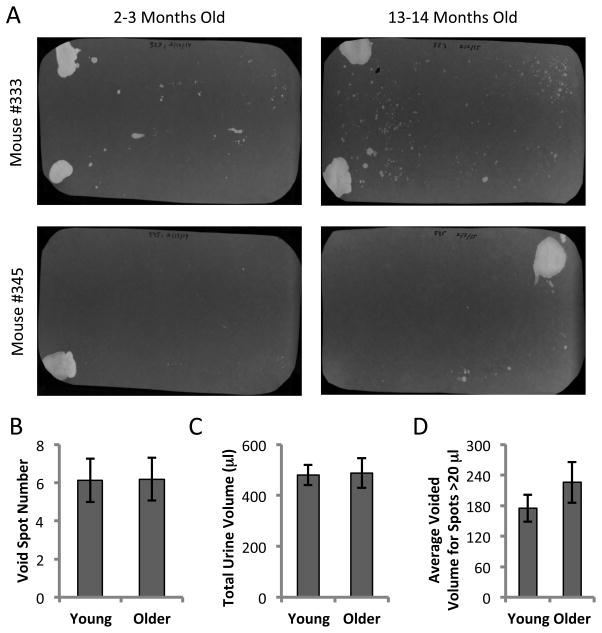
At 13–14 months of age, the bladders were significantly larger than at 2–3 months (P < 0.01) despite the mice being similar in weight (Fig. 1A and 1B). VSA was performed in triplicate (3 separate days) on the same five mice at 2–3 months of age and again when they were 13–14 months old. Examples of UV illuminated filters are shown for two mice when they were young and after they had aged (Fig. 2A). Following image analysis and based on area:volume calibrations, we eliminated any small spots that corresponded to a volume of less than 5 μl. Artifactual spotting of this kind (perhaps due to fur contamination) can be seen particularly for mouse #333 (Fig. 2A, top). Quantitation of urine spot numbers (Fig. 2B), and total urine volumes (Fig. 2C) indicated that there were no significant differences between younger and older mice. Furthermore analyzing the average size of all spots larger than 20 μl (considered true voids) showed the average voided volume to be ~200 μl and was not significantly different at the older age. This suggests that the increase in bladder weight was not associated with any change to function or capacity, as the mice remained continent exhibiting typical controlled voids near the corners of the cage [6].
Figure 1. Mouse and bladder weights from 2–3 months old and 13–14 months old C57BL/6J males.
A. Mouse weights were not significantly different. The average weight of young males (n = 4) was 29.5 ± 1.8 g compared to 34.1 ± 1.3 g for older males (n = 4). B. Bladder wet weights. Older mice had significantly heavier bladders (41.7 ± 0.9 mg vs. 27.0 ± 3.0 mg; mean ± SEM; **P < 0.01).
Figure 2. Void spot assays on five mice at 2–3 months and 13–14 months of age.
A. Representative filters from 2 individual mice at the younger and older ages. Mouse #333 exhibits greater small spot dispersion which may be related to marking behavior or fur contamination. B–D. Data are from 5 mice tested three times each at each age and averaged. All spots with an area corresponding to < 5 μl were eliminated from the analysis. B. Total number of spots > 5 μl; C. Total urine volumes; D. Average volume of voids larger than 20 μl. Data are mean ± SEM. Paired t-test showed no difference in any parameter with age.
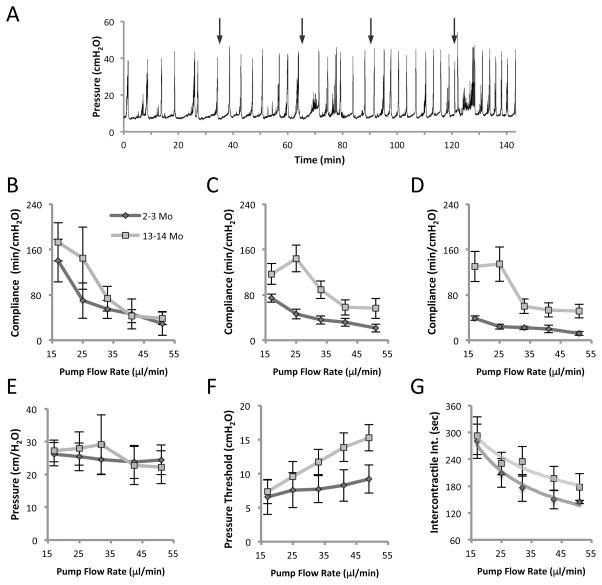
We performed anesthetized cystometry on eight mice and examined the effect of slowly increasing the pump speed, and therefore the bladder filling rate, on several parameters. Filling rates of 17, 25, 33, 41 and 49 μl/min were used. An example of the raw data for a 4-month-old mouse can be seen in Figure 3A with the times at which filling rate was increased indicated by arrows. It can be seen that with higher fill rates the voiding interval diminishes. An approximately 3-fold increase in pump speed from 17 μl/min to 49 μl/min, resulted in several measurable changes to cystometric parameters. Of these parameters however, peak voiding pressures did not change at any filling rate and were not different between younger and older mice (Fig. 3E). Therefore, older age did not influence the maximal voiding pressures and increasing the flow-rate did not cause compensatory changes in expulsive pressure.
Figure 3. Cystometric parameters of 2–3 and 13–14 months old C57BL/6J males at incremental filling rates of 17, 25, 33, 41 and 49 μl/min.
A. Pressure tracing of a 4 month old mouse. Arrows indicate the time points at which the flow rate was increased. It can be seen that voiding intervals diminish with fill rate. This effect is most pronounced at the slowest filling velocity. B–D. Effect of bladder filling rate on compliance during early third (B), middle third (C) and final third (D) of bladder filling phase. E. Pressure amplitudes at voiding (peak pressure to basal) in younger and older mice. F. Micturition pressure threshold at different flow rates in younger and older mice. G. Intercontractile interval (time between voids) at different filling rates. Both age groups showed a non-linear reduction in ICI with increasing filling rate best described by a power curve (R2 > 0.93). Data is mean ± SEM, N = 4 mice/group. For each flow rate a minimum of 4 voiding cycles was analyzed and a mean calculated.
Bladder wall compliance was segmentally analyzed at early, middle and late thirds of the filling phase (prior to initiation of voiding contractions) (Fig. 3B–3D). Several features of this data set are noteworthy. Firstly, compliance diminishes as flow rate increases. This suggests that mechanosensory feedback, likely mediated neurogenically, causes gradual compensatory stiffening of the detrusor smooth muscle and bladder wall as the bladder is more rapidly stretched. In general, particularly at the lower filling rates, older mice had greater compliance.
Secondly, upon inspection of Figure 3B there is no significant difference in the earliest stage of filling between younger and older mice. The bladders appear equally compliant. However, clear differences open up during the later stages of filling, with young mice showing lower compliance in the middle third (Fig. 3C) and even lower during the final third of filling just before micturition (Fig. 3D). These data would suggest that older bladders accommodate more easily even as filling stretch forces increase and the velocity of stretch increases.
Analysis of the pressure threshold at which the bladder begins contraction to initiate voiding, revealed an approximately linear increase in both younger and older mice as the flow rate increased (Fig. 3F). Interestingly therefore, as compliance is reduced, the pressure threshold to begin voiding increases. Older mice appeared to have reduced sensitivity on some level as they required higher pressure thresholds before voiding.
The intercontractile voiding interval gradually diminished in both age groups as the rate of saline infusion increased (Fig. 3G). This reduction in ICI was not unexpected, based on the simple hypothesis that at faster fill-rates, the bladder wall would experience arrival at the micturition pressure threshold earlier. However, the increased frequency of voiding was not a linear function of the flow-rate and in fact was much better fit by a power relationship [y = 0.0032x−0.43, R2 = 0.99 (2–3 months) and y = 0.0035x−0.31, R2 = 0.93 (13–14 months)]. Reflex voiding was more sensitive to small changes at the slowest fill rates, with the largest reduction in ICI occurring between 17 and 25 μl/min. Further increases in filling rate of saline (from 33, 41 to 49 μl/min) had progressively smaller effects on the ICI, as the voiding reflexes became less sensitive to the rate of wall stretch.
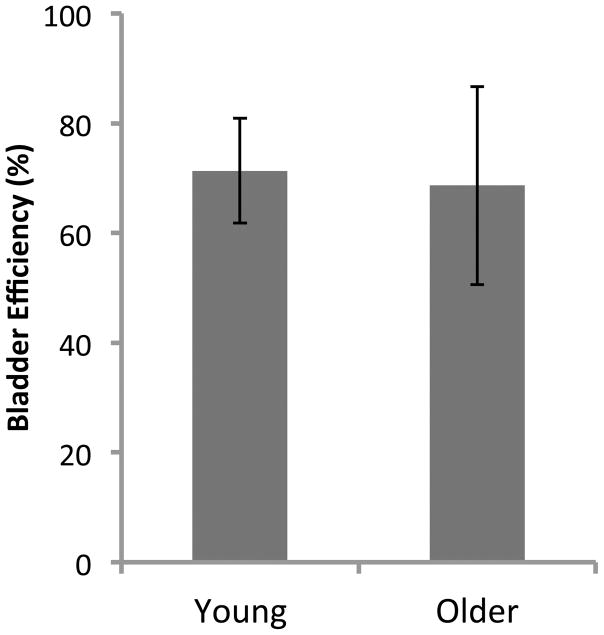
Upon finishing each CMG we performed a final, single void cystometric cycle to assess residual volume and therefore gain an estimate of bladder efficiency [7]. In this maneuver, the pump is stopped, the bladder emptied completely by drainage, the tubing reconnected and pump started, a single voiding cycle initiated, at the completion of which, the residual fluid is collected and measured. The age difference had little influence on voiding efficiency, which was about 70% at both 4 months and 13 months of age (Fig. 4).
Figure 4. Efficiency of voiding.
After continuous flow cystometry, the pump was stopped, the bladder was emptied and then a single fill CMG was performed. Upon initiation of a void the pump was stopped again. Following completion of the void, the remaining residual volume in the bladder was measured. Efficiency was calculated by ((inflow volume [flow rate × time of single fill] − residual volume)/inflow volume) × 100. Data is mean ± SEM; n = 4 for both groups.
DISCUSSION
This is a limited study which is likely to be underpowered for the detection of subtle changes in group urodynamics with modest aging. However the experimental approach in which each individual animal acts as its own control was sufficient to detect consistent differences in pressure:volume curves over multiple voids at each flow rate. The data revealed several interesting and important phenomena.
Heavier bladders did not equate to functionally larger bladders. VSA showed there were similar total volumes of urine voided by both the younger and older groups and similar per-void volumes. This conclusion is further supported by the similar ICI’s at each flow rate (Fig. 3G) and the similar bladder efficiencies (Fig. 4). A steady increase in bladder weight with age has been noted by others [8,9]. Extensive remodeling particularly in old age is associated with disorganization of the urothelium, increased collagen density and thickness within the lamina propria and collagen infiltration into the muscle bundles [10]. Despite the fact that bladders became significantly heavier with age, the expulsive pressure was maintained — at all filling rates (Fig. 3E). This is consistent with the findings of Smith et al. [8] who observed that even very aged mice (up to 26 months) showed no loss of detrusor strength. However, sensitivity to filling was reduced, consistent with altered neural processing [8]. Our pressure threshold data (Fig. 3F) would suggest some loss of sensitivity to filling also. Further, we can add to their observations by noting that the rate of filling does not affect contractile pressures.
The subtle differences in ICI curves, with the older mice showing slightly longer intervals at the higher fill rates, perhaps also suggests the beginning of a loss of filling sensitivity [8]. We would need to test mice that were older still, to see if this trend became more pronounced.
From a simple mechanical perspective, one might assume that during continuous filling, intravesical pressure would reach a micturition threshold, causing activation of mechanosensitive bladder afferents to initiate voiding, regardless of the amount of time it took to reach that pressure. In such a scenario, the rate of filling — within reasonable limits — would not matter and the ICI would change in a linear manner with flow rate. Our data clearly show that this is incorrect and that the ICI was most sensitive at the slower filling rates and less so as the velocity of filling increased. This may indicate that normal mechanical feedback to the central nervous system becomes overwhelmed or desensitized at high rates.
Bladder compliance diminished with increased filling rates. Since compliance is a measure of the ease (or lack of resistance) to filling, the drop we note equates to greater wall resistance. Compliance is due to contributions from passive (extracellular matrix) and active (detrusor muscle) elements. Since passive elements will not change in these acute experiments, we conclude that sensory signaling to bladder afferents causes a compensatory increase in wall tone at supraphysiological filling rates. Changes in smooth muscle tone have been shown to reflect mechanosensory feedback [11]. Indeed this was shown in rats [12]. At a physiological filling rate, there was a decrease in pressure at which afferent firing rate peaked. As the filling rate increased, the afferent firing rate continued to decrease and the activation pressure threshold for the afferent units increased.
Smith et al. have shown that compliance is centrally modulated, i.e. controlled at the level of the brain. In elegant experiments, they showed that abolition of central control, either by isoflurane anesthesia or by death decreased compliance during cystometry, in identical fashion [13]. For isoflurane, the effect was reversible upon removal of the anesthetic. They also showed that bladder compliance in mice, increases with age [14]. Our data are in agreement with their findings. Our older mice showed approximately 80% greater compliance at a filling rate of 25 μl/min. However, this differential disappeared at higher rates. Overall, their findings led them to conclude that greater compliance with aging is likely to result from decreased detrusor muscle activity (micromotional or autonomous activity) and that this occurs due to diminished mechanosignaling to the brain during filling. The slightly longer ICIs in our mature mice are consistent with their hypothesis.
Our focus in these experiments was to add to our knowledge regarding an important experimental variable in cystometry. A literature search revealed that instillation rates for CMGs in mice has varied from 13 μl/min [4] up to 100 μl/min [15,16]. The non-linearity of fill rate with voiding interval illustrates the complexity of effects that volume delivery rate has on the timing of voiding and the difficulty of data comparisons when methodology is different. However, this is not to say that faster flow rates are inappropriate. Clearly this is a decision which may be justified for a number of reasons relevant to the strain under investigation or the particular physiology of different mouse models.
Since sensitivity to filling appears to diminish with higher fill rates, we recommend that investigators should err on the side of slower fill rates and not exceed 25 μl/min in mice for routine cystometry in C57BL/6J mice. These findings argue for greater standardization of cystometry protocols between labs and further fundamental investigations into afferent signaling to the higher centers at different filling rates.
Acknowledgments
Support for this work was provided by NIDDK grants RO1 DK83299 and P20 DK097818.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Author contributions
Kim AK and Hill WG conceived and supervised the study; Kim AK and Hill WG designed experiments; Kim AK performed experiments; Hill WG provided new tools and reagents; Kim AK and Hill WG analyzed data; Kim AK and Hill WG wrote the manuscript; Kim AK and Hill WG made manuscript revisions.
References
- 1.Andersson KE, Soler R, Füllhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30:636–646. doi: 10.1002/nau.21108. [DOI] [PubMed] [Google Scholar]
- 2.Dolber PC, Jin H, Nassar R, Coffman TM, Gurley SB, et al. The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice. Neurourol Urodyn. 2015;34:72–78. doi: 10.1002/nau.22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010;29:1344–1349. doi: 10.1002/nau.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, et al. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol. 2015;308:1369–1378. doi: 10.1152/ajprenal.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, et al. Aging Research Using Mouse Models. Curr Protoc Mouse Biol. 2015;5:95–133. doi: 10.1002/9780470942390.mo140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, et al. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol. 2014;306:1296–1307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshiyama M, Roppolo JR, Takeda M, de Groat WC. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol. 2013;304:390–396. doi: 10.1152/ajprenal.00574.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2011;302:577–586. doi: 10.1152/ajpregu.00508.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jost SP. Postnatal growth of the mouse bladder. J Anat. 1985;143:39–43. [PMC free article] [PubMed] [Google Scholar]
- 10.Schueth A, Spronck B, van Zandvoort MAMJ, van Koeveringe GA. Age-related changes in murine bladder structure and sensory innervation: a multiphoton microscopy quantitative analysis. Age (Dordr) 2016;38:17. doi: 10.1007/s11357-016-9878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southern JB, Frazier JR, Miner AS, Speich JE, Klausner AP, et al. Elevated steady-state bladder preload activates myosin phosphorylation: detrusor smooth muscle is a preload tension sensor. Am J Physiol Renal Physiol. 2012;303:1517–1526. doi: 10.1152/ajprenal.00278.2012. [DOI] [PubMed] [Google Scholar]
- 12.De Wachter S, De Laet K, Wyndaele J. Does the cystometric filling rate affect the afferent bladder response pattern? A study on single fibre pelvic nerve afferents in the rat urinary bladder. Neurourol Urodyn. 2006;25:162–167. doi: 10.1002/nau.20157. [DOI] [PubMed] [Google Scholar]
- 13.Smith PP, Deangelis AM, Kuchel GA. Evidence of central modulation of bladder compliance during filling phase. Neurourol Urodyn. 2011;31:30–35. doi: 10.1002/nau.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PP, DeAngelis A, Simon R. Evidence of increased centrally enhanced bladder compliance with ageing in a mouse model. BJU Int. 2014;115:322–329. doi: 10.1111/bju.12669. [DOI] [PubMed] [Google Scholar]
- 15.Jusuf AA, Kojima S, Matsuo M, Tokuhisa T, Hatano M. Vesicourethral sphincter dysfunction in ncx deficient mice with an increased neuronal cell number in vesical ganglia. J Urol. 2001;165:993–998. [PubMed] [Google Scholar]
- 16.Sutherland RS, Kogan BA, Piechota HJ, Bredt DS. Vesicourethral function in mice with genetic disruption of neuronal nitric oxide synthase. J Urol. 1997;157:1109–1116. [PubMed] [Google Scholar]