Abstract
Introduction
Individuals with idiopathic subglottic stenosis (SGS) are at risk for voice disorders prior to and following surgical management. This study examined the nature and severity of voice disorders in patients with SGS before and after a revised cricotracheal resection procedure designed to minimize adverse effects on voice function.
Method
Eleven women with idiopathic SGS provided presurgical and postsurgical audio recordings. Voice Handicap Index (VHI) scores were also collected. Cepstral, signal-to-noise, periodicity, and fundamental frequency analyses were undertaken for connected speech and sustained vowel samples. Listeners made auditory-perceptual ratings of overall quality and monotonicity.
Results
Paired samples statistical analyses revealed that mean fundamental frequency decreased from 215 Hz (SD=40 Hz) to 201 Hz (SD=65 Hz) following surgery. In general, VHI scores decreased after surgery. Voice disorder severity based on the cepstral spectral index of dysphonia™ for sustained vowels decreased (improved) from 41 (SD=41) to 25 (SD=21) points; no change was observed for connected speech. Semitone standard deviation (2.2 semitones) did not change from pre- to post-treatment. Auditory-perceptual ratings demonstrated similar results.
Conclusions
These preliminary results indicate that this revised cricotracheal resection procedure is promising in minimizing adverse voice effects while offering a longer-term surgical outcome for SGS. Further research is needed to determine causal factors for pretreatment voice disorders, as well as to optimize treatments in this population.
Keywords: subglottic stenosis (SGS), cricotracheal resection (CTR), voice disorders, semitone standard deviation (STSD)
INTRODUCTION
Idiopathic subglottic stenosis (SGS) causes dyspnea that can have significant adverse effects on quality of life and activities of daily living. Often patients with SGS endure a prolonged course of treatment involving endoscopic or open airway reconstruction. Some have undergone numerous endoscopic procedures in efforts to maintain airway patency and avoid a tracheostomy.1–3 Open airway procedures such as cricotracheal resection (CTR) have a high success rate in treating SGS, albeit with higher risks. While early tracheal reconstruction surgeries have been reported since the 1950s,4 surgical procedures have evolved over the following decades.
Patients with SGS often present with voice disorders.5,6 It has been suggested that airway turbulence caused by subglottic narrowing influences vocal fold vibration.7 In addition to airway patency, the potential effects on voice production are also considered in SGS management. Several studies have recently documented the effects of endoscopic procedures on the voice, reporting that they usually improve or avoid worsening voice function.2,5,7–10 CTR involves removal of the entire region of the stenosis and anastomosis in the upper and lower portions of the airway.11 More recently, CTR has emerged as a potentially preferable method for treating SGS.12–14 Although more invasive than endoscopic procedures such as microlaryngoscopy, carbon dioxide laser, and balloon dilation, CTR can produce lasting airway patency and potentially avoid the need for subsequent surgical procedures or tracheostomy.12
One of the concerns with CTR has been the possible worsening of voice problems in patients with SGS, including reduced fundamental frequency (F0) range, lower mean habitual F0, reduced overall intensity, breathiness, hoarseness, inability to project the voice, and vocal strain.5,8,10,15,16 Therefore, a voice-sparing CTR procedure could potentially offer long-term airway management while maintaining or improving voice function. The purpose of this investigation was to examine the effects of a revised CTR procedure that minimizes cricothyroid disturbance, thus improving voice outcomes.
MATERIALS AND METHODS
Participants
This study included 11 women (ages 33 to 73 years; M = 50 years; SD = 12 years) with idiopathic SGS who received CTR at The University of Utah Voice Disorders Center between 2008 and 2014. Inclusion criteria were the presence of SGS requiring surgical management, being over 18 years of age, and the availability of both presurgical and postsurgical audio recordings. Participants were excluded only if quality audio recordings were not available. All participants had a previous history of microlaryngoscopy with dilation procedures performed by the senior author (M.E.S.). At the time of CTR, all presented with either Grade I or Grade II SGS. A revised CTR procedure aimed to minimize adverse voice effects was performed on all participants by the senior author (M.E.S.). The CTR procedure involved several modifications from published descriptions:13,14 1) instead of transecting the cricothyroid muscles, the pars rectus bellies were peeled off the anterior cricoid ring but left attached to the thyroid cartilage, 2) when the anterior arch of the cricoid was resected, the lateral portions of the arch remained, resulting in a resection of 25–40% of the anterior cricoid, 3) if the stenosis did not involve the cricothyroid membrane, then this was not resected and the tracheal flap was then sewn to the cricothyroid membrane and not to the thyroid cartilage, and 4) after the tracheal flap was positioned and the cricothyroid anastomosis completed, the cricothyroid muscle flaps were replaced into their anatomic position by tacking them onto tracheal rings over the anastomosis to reinforce the repair. These modifications to the CTR procedure are illustrated in Figures 1 and 2. All study-related procedures were approved by the Institutional Review Boards at The University of Utah (IRB#00045048) and Brigham Young University (IRB#E14524).
Figure 1.
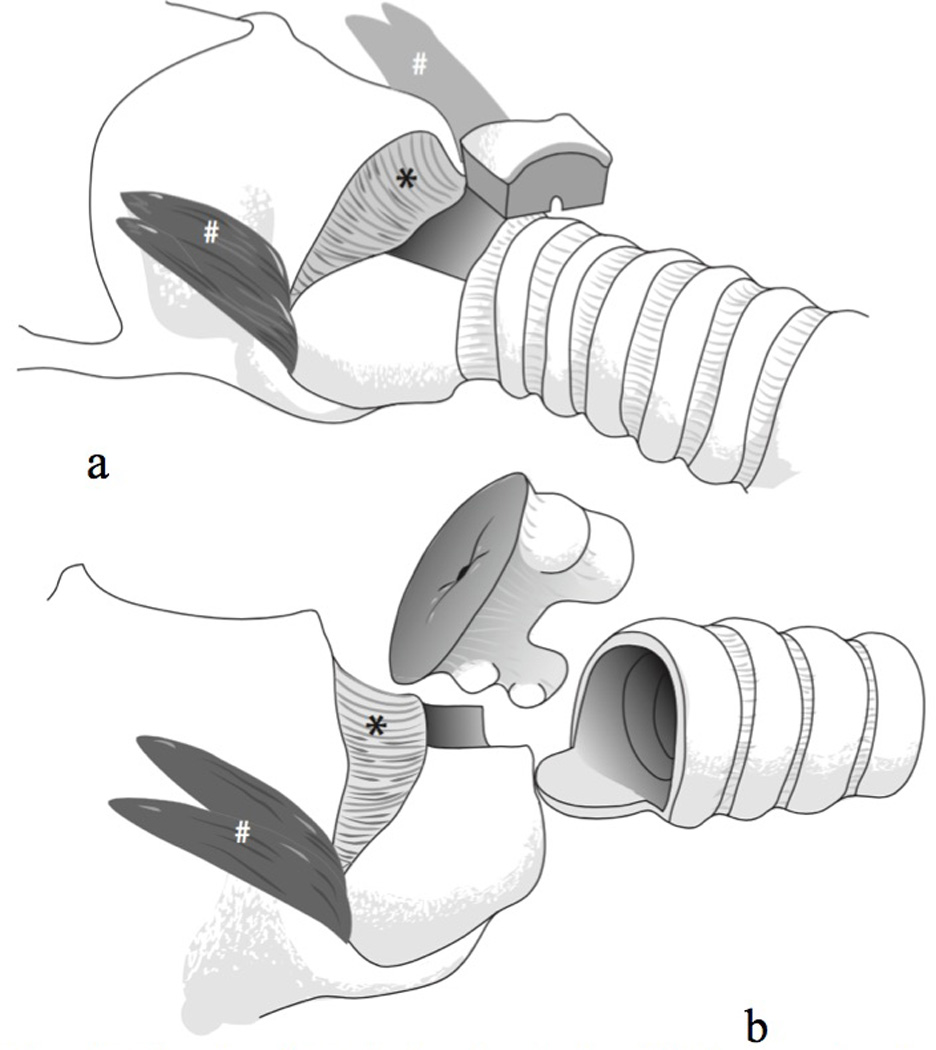
Two views of cricotracheal resection (CTR), showing modifications to spare the voice.
Figure 2.
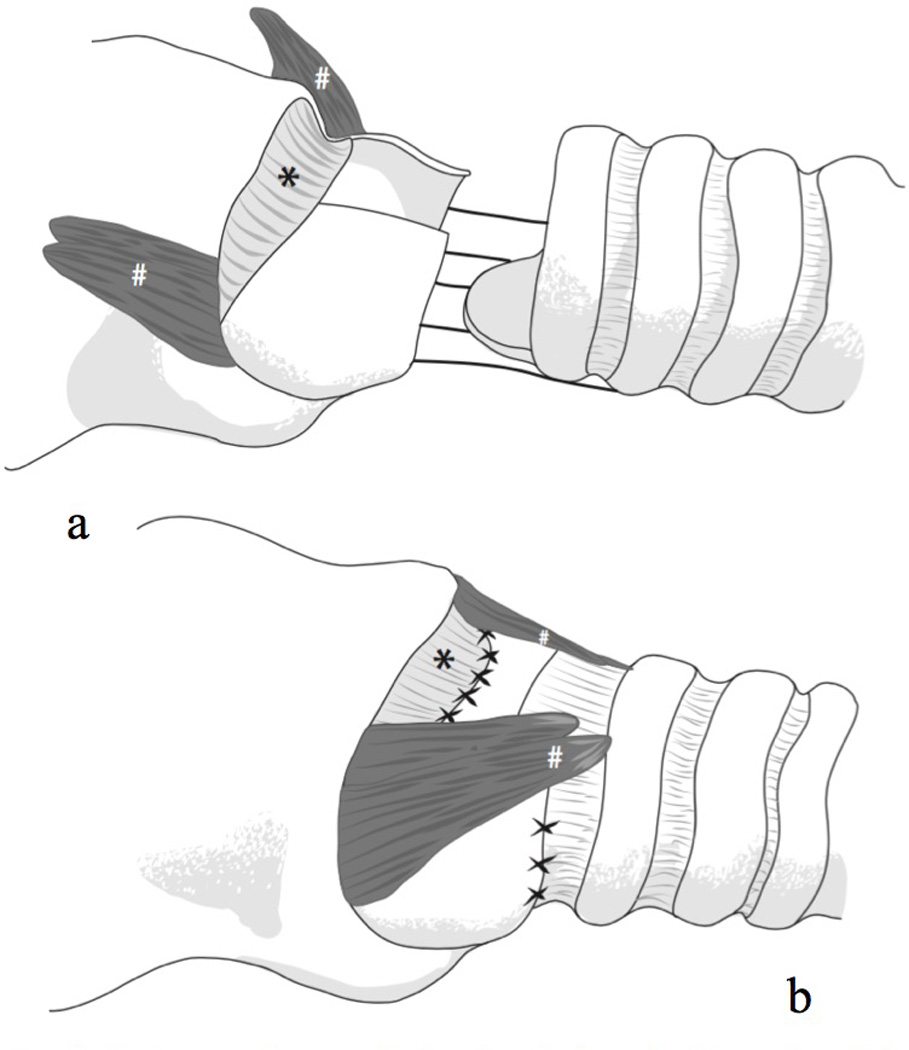
Two views of reconstruction after cricotracheal resection (CTR).
Speech Samples
Presurgical samples were collected on average 67 days (SD = 120 days) prior to CTR, and postsurgical samples were collected on average 158 days (SD = 119 days) following CTR. For purposes of the present investigation, speech stimuli included the Rainbow Passage17 and sustained /ɑ/ vowels. For purposes of acoustic and auditory-perceptual evaluation, the second and third sentences of the Rainbow Passage and the central three seconds of the sustained vowel were extracted using Audacity (v. 2.0.6; audacity.sourceforge.net, La Jolla, California) and resaved at 44.1 kHz (16 bit). Subsequent acoustic analyses were performed using this sampling rate, with the exception of the cepstral spectral index of dysphonia (CSID™), periodicity, and F0 analyses, which were automatically downsampled to 25 kHz within the Analysis of Dysphonia in Speech and Voice (ADSV™) and Multidimensional Voice Program (MDVP™) software packages (version 5109; KayPentax; Montvale, New Jersey, USA). Additionally, for purposes of auditory-perceptual analyses, speech samples were normalized for amplitude and saved as stereo files so that listeners could hear samples binaurally via headphones.
VHI
In order to compare changes in acoustic measures with patient-reported outcomes, participants were asked to complete the VHI18 prior to and after undergoing surgery. Six of the 11 participants did not complete pretreatment VHIs, but all completed posttreatment VHIs. Data by participants are included in Table 1.
Table 1.
Voice Handicap Index (VHI) Scores for Presurgical versus Postsurgical Dates
| Subject | Pre VHI of 120 | # Days before CTR | Post VHI of 120 | # Days after CTR |
|---|---|---|---|---|
| 1 | -- | -- | 3 | 148 |
| 2 | 42 | 41 | 38 | 183 |
| 3 | 14 | 6 | 12 | 407 |
| 4 | -- | -- | 4 | 99 |
| 5 | -- | -- | 7 | 176 |
| 6 | -- | -- | 27 | 148 |
| 7 | 31 | 27 | 20 | 85 |
| 8 | 48 | 1 | 35 | 401 |
| 9 | 59 | 7 | 9 | 90 |
| 10 | 10 | 57 | 33 | 0 |
| 11 | -- | -- | 7 | 120 |
Acoustic Analysis Procedures
F0 and voice signal periodicity measures were made using the MDVP program. Cepstral measures were computed using the ADSV program. Additional spectral analysis and F0 variability measures were extracted with a custom Matlab application provided by the seventh author (E.H.). All acoustic analyses are described in detail below.
F0 analysis
To quantify the relationship between SGS on F0, as well as possible changes following surgical management, two primary F0 analyses were undertaken. First, mean F0 during sustained vowel samples was calculated. This measure was selected primarily due to previous research indicating that F0 decreases after CTR.16 This could be due to possible negative effects on cricothyroid muscle activity, or also possibly that SGS itself might be related to presurgical changes in F0 due to the potential influence of the subglottis on vocal fold oscillation. Similarly, F0 variation might be reduced following surgical management of SGS due to changes in cricothyroid function. Therefore, F0 semitone standard deviation (STSD) during connected speech was also calculated. Periodicity measures, including percent jitter and percent shimmer from sustained vowel phonation were also computed.
Pitch strength analysis
In this context, pitch strength is a calculated estimation of the listener tonal auditory-perceptual correlates of a sound.19 In other words, pitch strength estimates how a listener may judge the pitch saliency of a sound. Sound spectra that are characterized by more energy surrounding the F0 and harmonics are associated with greater pitch strength. Thus as pitch strength increases corresponding changes in spectral slope are also observed. Pitch strength has also been shown to correlate with auditory-perceptual judgments of tonality of sound samples.20 Additionally, pitch strength may relate to vibratory types associated with voice production.21 Because patients with SGS can sound breathy, it is reasonable that pitch strength might improve as breathiness decreases.
Cepstral analysis
Recent literature indicates that cepstral characteristics of the voice sample are significantly associated with perceptual ratings of voice disorder severity.22,23 Because individuals with SGS might have voice disorders both prior to and following surgical management, the present study attempted to quantify acoustic voice disorder severity in presurgical and postsurgical voice samples. Fourier transformation of a Fourier transform generates the cepstral spectrum, with the cepstral peak prominence (CPP) highly correlated with perceptual estimates of voice disorder severity.22,23 Cepstral peak prominence, along with the cepstral slope and central tendency parameters—similar to moments of the long-term average spectrum—are used to calculate the CSID. To that end, CPP and variation measures were calculated to generate the CSID for connected speech and sustained vowel samples.
Auditory-Perceptual Analysis Procedures
Because individuals might experience voice changes that have unique auditory-perceptual features, auditory-perceptual analysis of presurgical and postsurgical speech samples was undertaken. Auditory-perceptual analyses were performed by 13 graduate students in the Department of Communication Disorders at Brigham Young University, all of whom had taken the graduate voice disorders course from the first author (K.T.). Although the target number of listeners for this study was 10, auditory-perceptual ratings were oversampled in case some listeners proved unreliable in their ratings and thus would necessarily be excluded from statistical analysis. All listeners passed a standard hearing screening at 20 dB HL at 500, 1000, 2000, and 4000 Hz.
Listener tasks included ratings of overall voice severity, monotonicity, and overall F0 increase, decrease, or lack of change. Prior to initiation of the listening task, listeners were oriented to a range of voice disorder severity (i.e., from normal to severe) for the Rainbow Passage and sustained vowels, and listeners practiced the rating tasks. Once the listening experiment was initiated, 10% of samples were randomly repeated to estimate intrajudge reliability. Each of the three listening tasks was performed separately, such that listeners rated voice severity for all connected speech and sustained vowel samples prior to proceeding to monotonicity and F0 change ratings. Both voice severity and monotonicity ratings were performed using an approximately 10 cm visual analog scale (VAS), with an interactive computer program permitting a cursor to be placed at any point on the scale, the extreme left indicating normal voice or no monotonicity, and the extreme right indicating extreme voice disorder severity or monotonicity. The third listening task consisted of a three-item response indicating the auditory-perceptual judgment of overall F0 increase, decrease, or no change for connected speech and sustained vowel samples. All ratings were accomplished via a custom Matlab computer program developed by the second author (C.D.). All listening tasks were accomplished in a single-walled sound booth while listeners wore Sennheiser (HD 558) headphones. Listeners were permitted to adjust sample presentation to a comfortable loudness level, and were able to repeat sample presentations as many times as desired prior to moving to the next sample or sample pair.
Results
Acoustic and auditory-perceptual data met the assumptions for parametric statistical analysis based on Levene’s Test for Equality of Variances and examination of central tendency for sample groups. Therefore, two-tailed paired-samples t tests were employed to examine differences from pretreatment to posttreatment based on the dependent variables, using a Bonferroni correction for multiple comparisons and an alpha level of .05.
Acoustic Analyses
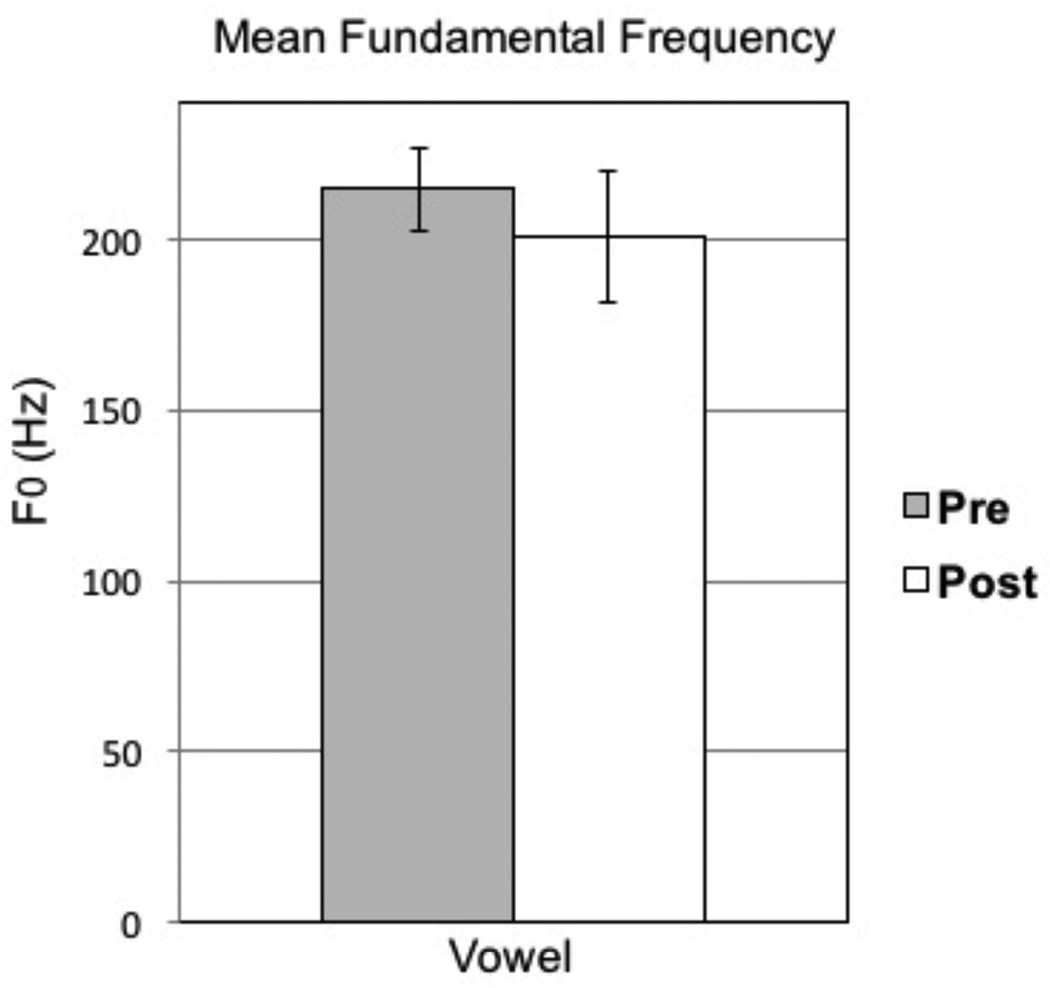
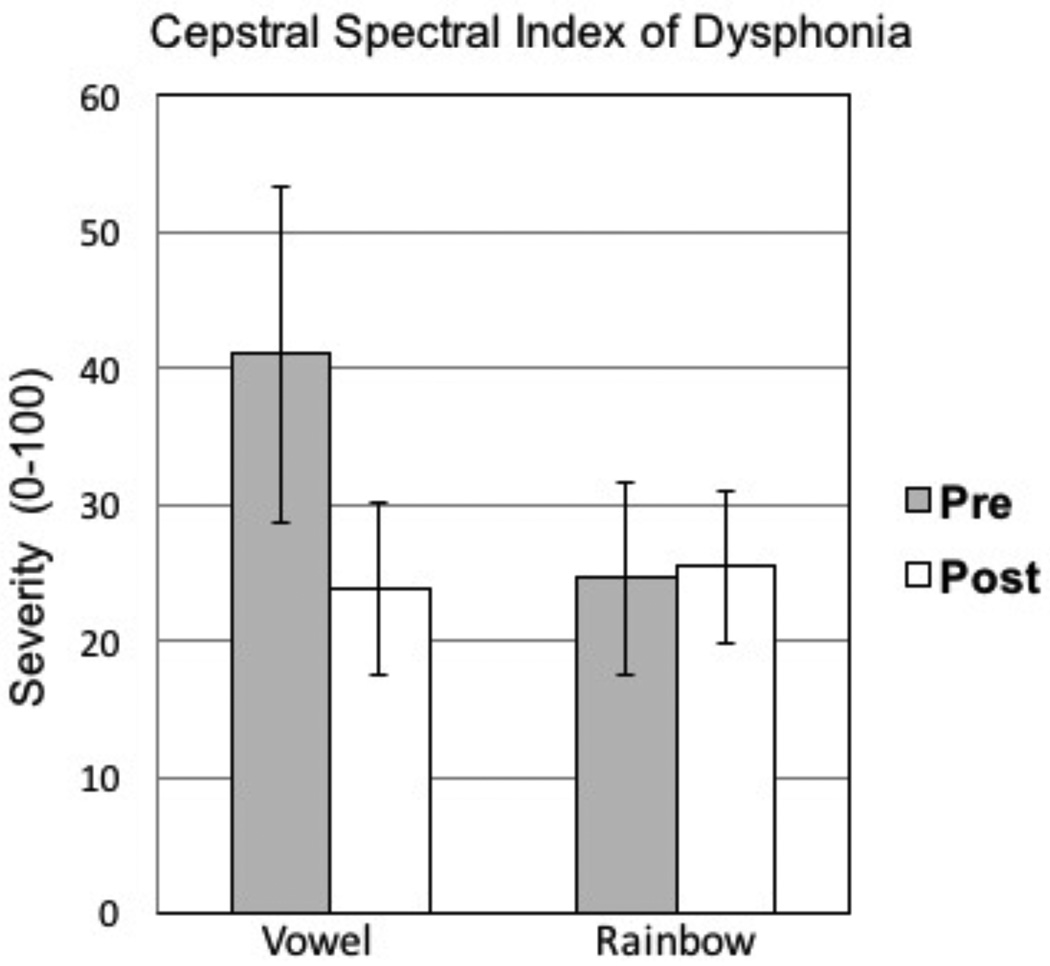
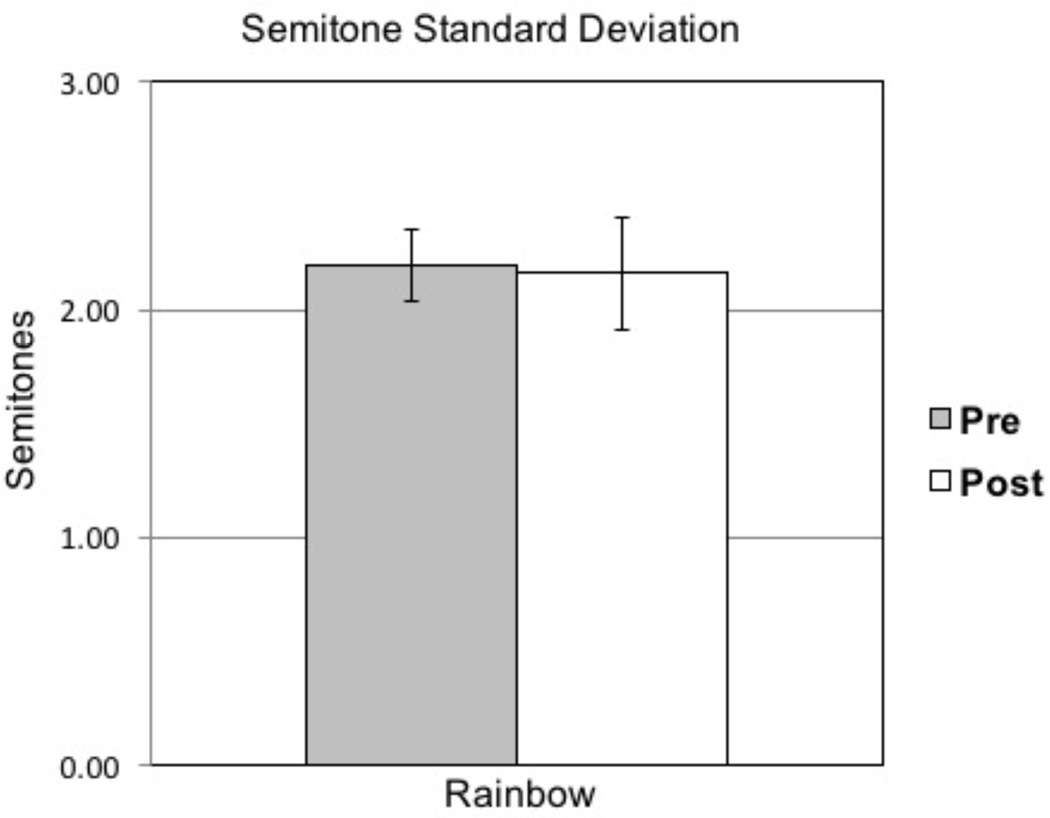
The results from paired-samples t tests are presented in Table 2. No significant differences were observed between presurgical and postsurgical recordings (Bonferroni correction .05/9 = .005). To compare these data set with previous SGS treatment studies, select variables are illustrated. Mean F0 during sustained phonation is presented in Figure 3. CSID and STSD are presented in Figures 4 and 5, respectively.
Table 2.
Acoustic Measures of Voice Disorder Severity for Presurgical versus Postsurgical Samples
| Acoustic Variable | Mean (SD) | Min | Max | Normative Mean (SD) | t score | p value |
|---|---|---|---|---|---|---|
| Mean Fundamental Frequency (Vowel) | ||||||
| Pre-surgical group | 214.9(39.8) | 153.3 | 278.3 | 244.0 (27.5) | .927 | .376 |
| Post-surgical group | 201.0(64.8) | 84.4 | 311.8 | |||
| Jitter, % (Vowel) | ||||||
| Pre-surgical group | 2.2(1.6) | .6 | 5.6 | 0.6(.4) | −.649 | .531 |
| Post-surgical group | 2.6(3.2) | .4 | 10.0 | |||
| Shimmer, % (Vowel) | ||||||
| Pre-surgical group | 6.6(4.2) | 2.4 | 15.7 | 2.0(.79) | −.161 | .875 |
| Post-surgical group | 6.8(7.0) | 1.4 | 22.1 | |||
| Cepstral Peak Prominence (Vowel) | ||||||
| Pre-surgical group | 8.2 (2.1) | 3.3 | 10.6 | -- | −.427 | .678 |
| Post-surgical group | 8.6 (3.7) | .2 | 13.0 | |||
| Cepstral Peak Prominence (Rainbow) | ||||||
| Pre-surgical group | 5.8(1.5) | 2.0 | 7.2 | -- | .829 | .426 |
| Post-surgical group | 5.6(1.4) | 2.9 | 7.2 | |||
| Cepstral Spectral Index of Dysphonia (Vowel) | ||||||
| Pre-surgical group | 41.0(40.8) | 11.3 | 153.9 | -- | 1.115 | .291 |
| Post-surgical group | 24.6(21.0) | −5.8 | 60.6 | |||
| Cepstral Spectral Index of Dysphonia (Rainbow) | ||||||
| Pre-surgical group | 23.9 (23.7) | 1.2 | 76.4 | -- | −.482 | .640 |
| Post-surgical group | 25.5 (18.6) | −.04 | 58.0 | |||
| Pitch Strength (Vowel) | ||||||
| Pre-surgical group | 38.1 (10.8) | 19.9 | 50.1 | -- | .062 | .952 |
| Post-surgical group | 38.0 (16.9) | 0.0 | 54.7 | |||
| Semitone Standard Deviation (Rainbow) | ||||||
| Pre-surgical group | 2.2 (.5) | 1.4 | 3.0 | -- | .125 | .903 |
| Post-surgical group | 2.2 (.8) | 1.2 | 4.2 |
Figure 3.
Means and standard error bars for pretreatment versus posttreatment mean fundamental frequency (F0).
Figure 4.
Means and standard error bars for pretreatment versus posttreatment voice disorder severity based on the cepstral-spectral index of dysphonia (CSID).
Figure 5.
Means and standard error bars for pretreatment versus posttreatment semitone standard deviation (STSD).
Auditory-Perceptual Analyses
Eleven of the 13 original graduate student raters met inclusion criteria based on a Pearson r correlation of .89 for repeated ratings of 10% of the samples. For those 11 raters, the single measures Intraclass Correlation Coefficient was .701 and the average measures Intraclass Correlation Coefficient was .963, F (26, 260) = 26.808, p < .001, indicating acceptable agreement among raters. Therefore, ratings from those 11 individuals were averaged and included in the auditory-perceptual analysis.
Data from pretreatment and posttreatment comparisons were analyzed using paired samples t tests (Bonferroni correction .05/3 = .016). No significant differences were observed. Specifically, mean connected speech voice severity ratings were 16.8 (SD = 11.9) of 100 points for pretreatment samples and 15.5 (SD = 15.5) of 100 points for posttreatment, t(10) = .302, p = .769. Mean connected speech voice monotonicity ratings were 19.3 (SD = 8.7) of 100 points for pretreatment samples and 23.3 (SD = 16.0) of 100 points for posttreatment, t(10) = −.888, p = .395. Mean sustained vowel voice severity ratings were 36.5 (SD = 23.2) of 100 points for pretreatment samples and 33.5 (SD = 29.4) of 100 points for posttreatment, t(10) = .510, p = .621.
Additionally, auditory-perceptual judgments of pitch change after CTR were undertaken. Ordinal data were collected including ratings of no change, pitch increase, or pitch decrease. When the raters’ judgments were averaged, 53.7% of presented sample pairs were judged to decrease in pitch from pretreatment to posttreatment; 36.4% of sample pairs were judged to stay the same and 9.9% were judged to increase in pitch from pretreatment to posttreatment. Figure 6 illustrates vocal fold elongation during modal and high pitch in a 48-year-old participant, demonstrating notable ability to modulate pitch following CTR surgery.
Figure 6.
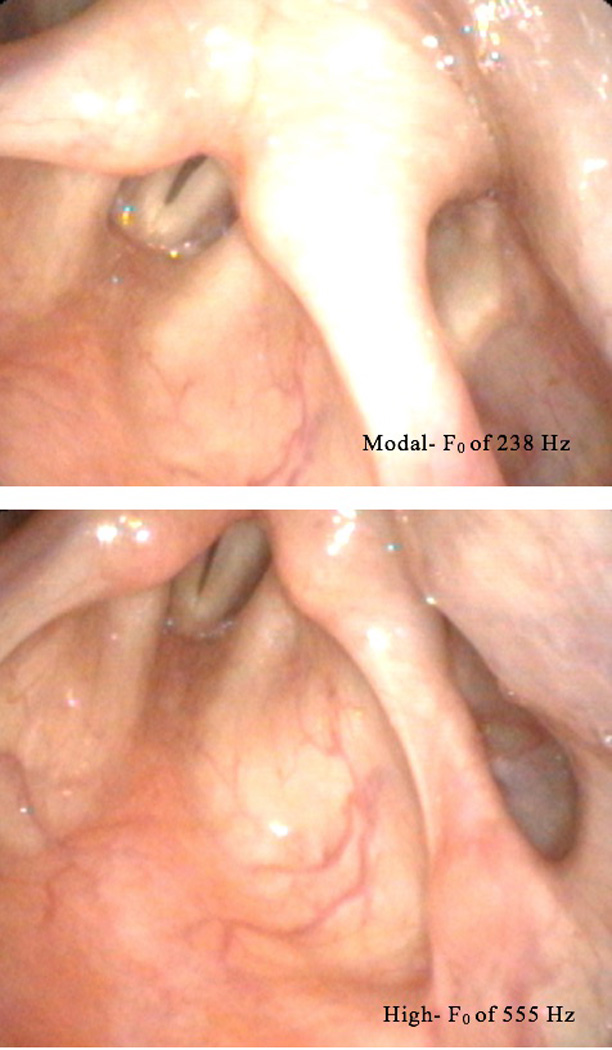
Modal fundamental frequency (F0) of 238 Hz and High F0 of 555 Hz.
DISCUSSION
The purpose of the present investigation was twofold: to quantify the acoustic and auditory-perceptual features of SGS and to examine possible changes in these features following CTR. Participants included a subset of adult female patients with idiopathic SGS from a single laryngology practice who received a CTR surgical procedure. All participants had received a prior microlaryngoscopy and balloon dilation procedures with subsequent recurrence to Grade I or II subglottic narrowing. To this end, recordings were retrieved from a clinical audio archive from patients with pretreatment and posttreatment samples. In general, analysis of the results indicated that these pretreatment SGS patients demonstrated mild or mild-moderate voice disorder severity based on acoustic, auditory-perceptual ratings and VHI scores. Neither acoustic nor auditory-perceptual voice disorder severity measures significantly changed following the CTR procedure. VHI scores indicated similar results. Auditory-perceptual ratings of pitch change indicated that the majority of participants decreased in pitch from pretreatment to posttreatment, but not significantly so. These findings indicate that the revised CTR procedure might be a viable alternative to less permanent subglottic dilation procedures, with minimal adverse effects on voice quality.
In the present investigation, no significant worsening was observed following the revised CTR procedure specifically modified to preserve cricothyroid function. Although mean F0 of sustained vowels did decrease slightly posttreatment, it remained within normal limits and not significantly different from pre-operative values. In our previous study of the effects on voice from traditional CTR technique, average sustained vowel F0 decreased from 221 Hz to 169 Hz, and average speaking F0 dropped from 190 Hz to 159 Hz.16 The results of the current study are similar to voice outcomes we found after CTR with the cricoid arch was not excised.16 Although not all participants completed a VHI both prior to and after surgery, the results indicate VHI scores generally decreased after surgery. Additionally, the finding that STSD did not change from pretreatment to posttreatment is particularly interesting given that traditional CTR techniques transected the cricothyroid musculature, resulting in a more monotone voice. Additionally, the results indicate that, although F0 decreased slightly posttreatment, this change was not significant and did not influence the patient’s ability to produce prosodic changes during connected speech sampling (i.e., during oral reading of the Rainbow Passage) based both on auditory-perceptual and acoustic analyses. These findings indicate that posttreatment changes in voice quality are likely to exclude prosodic limitations, thus resulting in a more favorable surgical outcome. Future studies, such as randomized experimental trials, are warranted to determine which surgical management procedures produce the most successful outcomes for both voice function and sustained airway management.
Studies related to presurgical voice function in patients with SGS indicate that voice disorders are relatively common in this population. Yet only a few studies have attempted to quantify the nature and severity of these voice disorders, including both perceptual and objective measures, prior to surgical management. The majority have included patient self-report as the primary outcome variable for examining voice function at baseline. Recently, Hseu et al.2 observed mild to mild-to-moderate voice severity in a subset of patients with SGS prior to surgery based on self-report ratings from the VHI. Smith et al.16 also observed voice disorders in the mild-to-moderate range based on selected acoustic measures prior to surgical management of idiopathic SGS in a similar group of patients to those in the present study. Most recently, Hoffman, Brand, and Dailey9 performed a study of 10 patients with SGS who received balloon dilation as an alternative to the potentially more detrimental effects of open laryngeal surgeries such as CTR. The authors documented presurgical voice disorder severity in the very mild range based on the VHI and the severe range based on the auditory-perceptual GRBAS scale.23 The authors concluded that additional studies were needed to document voice disorders in this population, including treatment response.
The findings from the present study are consistent with those documenting a mild to moderate voice disorder in patients with SGS whose stenosis was severe enough to warrant surgical intervention. Some variability in findings among studies might be explained by differences in the measurement tool used, sampling techniques, patient instructions, and methods for analysis. For example, it is possible that patients tend to underestimate their voice disorder severity based on dyspnea symptoms that might eclipse or interact with voice concerns. This could explain why examiner-based and objective measurement tools seem to indicate greater voice disorder severity in this population. Regardless of these possible reasons for variability across studies, the results from the present investigation indicate that patients with SGS present with voice disorder severity ranging from mild to moderate based on auditory-perceptual, aerodynamic, acoustic analyses and patient-reported measures. Presumably, the greater the severity of the stenosis, the more severe the voice disorder; however, this is yet to be proven because many patients seek out and receive surgical intervention in the form of balloon dilation prior to open laryngeal procedures.9
Balloon dilation procedures have become the more commonly used interventions in managing SGS. This shift in clinical practice seems to relate to both the negative impact of more invasive surgical procedures such as SGS on voice function, as well as the less invasive nature of microlaryngoscopy dilation procedures. Hoffman et al.9 reported improved VHI and Dysphonia Severity Index24 scores, reduced acoustic voice severity, and improved glottal function postdilation. In other recent work, the effects of balloon dilation were compared in patients with laryngotracheal versus SGS. The authors documented that voice disorders were significantly more severe in laryngotracheal or multilevel stenoses, whereas SGS patients presented with more mild voice problems. Additionally, patient-reported voice-related quality of life (V-RQOL)25 improved postdilation in patients with SGS versus other stenoses of the airway. Similar findings were observed by Hatcher, Dao, and Simpson.26 Unfortunately, dilation procedures are not long-lasting; many patients have to return within 1–2 years for repeated procedures. In some cases, multiple dilations result in a lengthier period of airway patency, but this result varies. Therefore, the advantages of a less invasive dilation procedure resulting in improved airway patency and voice function are often offset by the transiency of the improvement and likelihood of recurrence.
Several caveats should be considered when examining the results from the present investigation. First, the sample size in this study was somewhat low, thus minimizing statistical power and the ability to detect subtle voice changes following surgery. Although idiopathic SGS is a relatively rare condition, larger sample sizes are needed to more thoroughly evaluate differences in voice and airway outcomes. Ultimately, multi-center, blinded, randomized clinical trials will be required to evaluate which surgical procedures provide the optimal outcome for this patient population. Second, it should be noted that the recordings in the present study were obtained in a clinical setting; thus, additional potentially useful tasks such as pitch glides and F0 during extemporaneous speech were not analyzed because they were not obtained consistently in this cohort. Despite this limitation, STSD did not change from pretreatment to posttreatment, indicating that cricothyroid function for the speaking voice was likely preserved following the revised CTR procedure. Additionally, a few patient samples contained vocal fry posttreatment, influencing interpretation of the results related to possible F0 change after surgery. Although there are several possible reasons why patients might use vocal fry following surgery but not before (e.g., changes in respiratory patterns for speech), this should be considered when examining postsurgical F0 in this study population.
CONCLUSION
In summary, this study examined the effects of a revised CTR procedure on voice production in treatment of idiopathic SGS in women. The results indicated that this procedure was effective in preserving voice function while producing the desired airway patency outcomes. Future studies should examine this CTR procedure as compared to other treatments to determine the best methods for preserving voice function while managing the airway. Additionally, research involving the causative factors for voice disorders in this population is important to inform novel treatment development. The differences in cost-benefit ratio between the less invasive endoscopic procedures and revised CTR should be examined with respect to invasiveness of the procedure and the permanence of the surgical result.
Supplementary Material
This clinical video illustrates changes in glissando. This video is of a 48-year old female who underwent cricotracheal resection (CTR) demonstrating modal, high pitch, and glissando phonation.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders, National Institutes of Health, R01 DC009616, and the David O. McKay School of Education and Office of Research and Creative Activities, Brigham Young University. We wish to thank Katie Johnson for her assistance with audio sample retrieval during this study. We would also like to thank Robert Pierce for his design of the surgical illustrations.
Footnotes
The authors have no financial relationships or conflicts of interest to disclose related to this study and manuscript.
REFERENCES
- 1.Damrose EJ. On the development of idiopathic subglottic stenosis. Med Hypotheses. 2008;71:122–125. doi: 10.1016/j.mehy.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124:736–741. doi: 10.1002/lary.24410. [DOI] [PubMed] [Google Scholar]
- 3.Valdez TA, Shapshay SM. Idiopathic subglottic stenosis revisited. Ann Oto Rhinol Laryn. 2002;111:690–695. doi: 10.1177/000348940211100806. [DOI] [PubMed] [Google Scholar]
- 4.Conley JJ. Reconstruction of the subglottic air passage. Ann Oto Rhinol Laryn. 1953;62:477–495. doi: 10.1177/000348945306200219. [DOI] [PubMed] [Google Scholar]
- 5.Ettema SL, Tolejano CJ, Thielke RJ, Toohill RJ, Merati AL. Perceptual voice analysis of patients with subglottic stenosis. Otolaryng Head Neck. 2006;135:730–735. doi: 10.1016/j.otohns.2006.06.1249. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope. 2014;124:498–503. doi: 10.1002/lary.24287. [DOI] [PubMed] [Google Scholar]
- 7.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Oto Rhinol Laryn. 2015;124:413–418. doi: 10.1177/0003489414564249. [DOI] [PubMed] [Google Scholar]
- 8.Bryans L, Palmer AD, Schindler JS, Andersen PE, Cohen JI. Subjective and objective parameters of the adult female voice after cricotracheal resection and dilation. Ann Oto Rhinol Laryn. 2013;122:707–716. doi: 10.1177/000348941312201108. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman MR, Brand WT, Dailey SH. Effects of Balloon Dilation for Idiopathic Laryngotracheal Stenosis on Voice Production. Ann Oto Rhinol Laryn. 2015;125:12–19. doi: 10.1177/0003489415595425. [DOI] [PubMed] [Google Scholar]
- 10.Houlton JJ, de Alarcon A, Johnson K, et al. Voice outcomes following adult cricotracheal resection. Laryngoscope. 2011;121:1910–1914. doi: 10.1002/lary.21915. [DOI] [PubMed] [Google Scholar]
- 11.Hartley BE, Cotton RT. Pediatric airway stenosis: laryngotracheal reconstruction or cricotracheal resection. Clin Otolaryngol Allied Sci. 2000;25:342–349. doi: 10.1046/j.1365-2273.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandu K, Monnier P. Cricotracheal resection. Otolaryng Clin N Am. 2008;41:981–998. x. doi: 10.1016/j.otc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Monnier P, Lang F, Savary M. Cricotracheal resection for pediatric subglottic stenosis. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S283–S286. doi: 10.1016/s0165-5876(99)00175-5. [DOI] [PubMed] [Google Scholar]
- 14.White DR, Rutter MJ. Cricotracheal resection and reanastomosis. Oper Techn Otolaryng. 2009;20:236–240. [Google Scholar]
- 15.Grillo HC, Mathisen DJ, Ashiku SK, Wright CD, Wain JC. Successful treatment of idiopathic laryngotracheal stenosis by resection and primary anastomosis. Ann Oto Rhinol Laryn. 2003;112:798–800. doi: 10.1177/000348940311200909. [DOI] [PubMed] [Google Scholar]
- 16.Smith ME, Roy N, Stoddard K, Barton M. How does cricotracheal resection affect the female voice? Ann Oto Rhinol Laryn. 2008;117:85–89. doi: 10.1177/000348940811700202. [DOI] [PubMed] [Google Scholar]
- 17.Fairbanks G. Voice and articulation drillbook. New York, NY: Harper; 1960. [Google Scholar]
- 18.Jacobson BH, Johnson A, Grywalski C, Silbergliet A, Jacobson G, Benninger MS. The Voice Handicap Index (VHI) Am J Speech Lang Pathol. 1997;6:66–70. [Google Scholar]
- 19.Camacho A. On the use of auditory models' elements to enhance a sawtooth waveform inspired pitch estimator on telephone-quality signals; In Information Science, Signal Processing and their Applications (ISSPA), 2012 11th International Conference on Information Science, Signal Processing, and their Applications; 2012. pp. 1107–1112. [Google Scholar]
- 20.Shrivastav R, Eddins DA, Anand S. Pitch strength of normal and dysphonic voices. J Acoust Soc Am. 2012;131:2261–2269. doi: 10.1121/1.3681937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopf L, Shrivastav R, Eddins D, Skowronski M, Hunter EJ. American Speech-Language-Hearing Association (ASHA) Convention. Orlando, FL: 2014. A comparison of voice signal typing and pitch strength. [Google Scholar]
- 22.Awan SN, Roy N, Dromey C. Estimating dysphonia severity in continuous speech: application of a multi-parameter spectral/cepstral model. Clin Linguist Phonet. 2009;23:825–841. doi: 10.3109/02699200903242988. [DOI] [PubMed] [Google Scholar]
- 23.Peterson EA, Roy N, Awan SN, Merrill RM, Banks R, Tanner K. Toward validation of the cepstral spectral index of dysphonia (CSID) as an objective treatment outcomes measure. J Voice. 2013;27:401–410. doi: 10.1016/j.jvoice.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Wuyts FL, De Bodt MS, Molenberghs G, et al. The dysphonia severity index: an objective measure of vocal quality based on a multiparameter approach. J Speech Lang Hear Res. 2000;43:796–809. doi: 10.1044/jslhr.4303.796. [DOI] [PubMed] [Google Scholar]
- 25.Kupfer RA, Hogikyan EM, Hogikyan ND. Establishment of a normative database for the Voice-Related Quality of Life (V-RQOL) measure. J Voice. 2014;28:449–451. doi: 10.1016/j.jvoice.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Hatcher JL, Dao AM, Simpson CB. Voice outcomes after endoscopic treatment of laryngotracheal stenosis. Ann Otol Rhinol Laryngol. 2015;124:235–239. doi: 10.1177/0003489414551980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This clinical video illustrates changes in glissando. This video is of a 48-year old female who underwent cricotracheal resection (CTR) demonstrating modal, high pitch, and glissando phonation.