Abstract
Introduction
The objective of this study was to evaluate the real-world safety and effectiveness of adalimumab with methotrexate (MTX) in disease-modifying antirheumatic drug (DMARD)- and biologic-naïve Japanese patients with rheumatoid arthritis (RA) at risk of progressive structural joint damage.
Methods
This multicenter, prospective, observational, postmarketing surveillance study was conducted between February 2013 and April 2015 at 84 centers in Japan. Patients with RA at risk of progressive structural joint damage were enrolled and initiated treatment with adalimumab and MTX. Adverse events were recorded up to week 28. Effectiveness/disease activity was assessed using the Disease Activity Score based on a 28-joint count with erythrocyte sedimentation rate and C-reactive protein (DAS28-4ESR and DAS28-4CRP), Clinical Disease Activity Index, and Simplified Disease Activity Index at 0, 4, 12, and 24 weeks. DAS28-4CRP response was evaluated in the low-dose (<8 mg/week) and high-dose (≥8 mg to ≤16 mg/week) MTX groups at week 24.
Results
One hundred fifty-seven of 163 patients comprised the safety cohort: mean (SD) age, 56.5 (13.9) years; females, 65.6%; rheumatoid factor positive, 73.2%; anti-cyclic citrullinated peptide antibody positive, 66.9%; bone erosions, 51.6%; mean disease duration, 9.5 months. The majority of patients (≥80%) had moderate or high disease activity at baseline, and ≥50% with available data achieved remission or low disease activity at week 24 (DAS28-4CRP <3.2). Five serious adverse drug reactions occurred in four patients, including pyelonephritis, Pneumocystis jiroveci pneumonia, interstitial lung disease, pleurisy, and pericarditis; the outcomes were either recovered or recovering. Significant improvements/reductions in disease activity over 24 weeks were noted in all effectiveness measures (P < 0.0001). Most of the population achieved DAS28-4CRP remission (<2.6) at week 24 regardless of the MTX dose.
Conclusion
Adalimumab in combination with MTX could be a beneficial treatment option for DMARD- and biologic-naïve Japanese patients with RA at risk of progressive structural joint damage.
Funding: AbbVie GK and Eisai.
Trial Registration: ClinicalTrials.gov identifier, NCT01783730.
Electronic supplementary material
The online version of this article (doi:10.1007/s40744-017-0059-1) contains supplementary material, which is available to authorized users.
Keywords: Adalimumab, Effectiveness, Methotrexate, Postmarketing surveillance, Rheumatoid arthritis, Safety
Introduction
Adalimumab, a fully human anti-tumor necrosis factor (TNF)-alpha monoclonal antibody, was approved for the treatment of rheumatoid arthritis (RA) in April 2008 in Japan with the data of the CHANGE study and an overseas clinical trial [1, 2]. The key findings that led to its initial approval were significantly improved American College of Rheumatology (ACR) outcomes achieved with adalimumab compared with placebo in patients with RA previously failing treatment with at least one disease-modifying antirheumatic drug (DMARD). Therefore, the initial approval for adalimumab was limited to use in patients who had inadequately responded to DMARDs (established patients), and a request was made to conduct a postmarketing observational study (PMOS) involving established and DMARD and biologic treatment-naïve patients with RA to obtain sufficient safety and effectiveness data on adalimumab to optimize its use in RA in clinical practice.
A double-blind, placebo-controlled study was also conducted to evaluate the preventive effect on progressive joint destruction in Japanese patients with RA (HOPEFUL-1 study) [3]. Based on the results of the HOPEFUL-1 study, the Japanese regulatory authority [Pharmaceuticals and Medical Devices Agency (PMDA)] approved an additional indication for adalimumab for the prevention of structural joint damage in patients with RA. This approval offered DMARD-naïve patients at risk of early structural joint damage a chance to receive adalimumab as a first-line treatment for RA. We designed the present PMOS to examine the real-world safety profile and effectiveness of adalimumab plus methotrexate (MTX) for the treatment of patients with RA.
Methods
Study Design
This multicenter prospective PMOS was conducted between February 2013 and April 2015 at 84 centers in Japan. The primary objective was to examine the safety profile of adalimumab in daily clinical practice of patients with RA showing rapid progression of structural damage of the joints who have no prior history of treatment with DMARDs or biologic agents. The safety profile of adalimumab was compared between DMARD-naïve patients and established patients (n = 7740) included and analyzed in the completed all-case PMOS [4]. The secondary objective was to establish the real-world effectiveness of adalimumab plus MTX on disease activity as assessed using Disease Activity Score based on the 28-joint count (DAS28), Clinical Disease Activity Index (CDAI), and Simplified Disease Activity Index (SDAI) in DMARD- and biologic-naïve patients with RA showing rapid progression of structural joint damage. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Subjects
The sample size was based on the adverse event (AE) profile of a previous PMOS in Japanese patients with RA treated with adalimumab [4]. The study reported that the most frequent serious AEs (SAEs) were “infections and infestations,” which occurred at an incidence rate of 2.4% in patients with RA showing inadequate response to existing therapies. In a subcohort of 4129 patients receiving concomitant adalimumab and MTX, the incidence of the SAE “infections and infestations” was 2.15% (90/4129). This incidence rate was used for setting the 150-patient sample size for this study, considering that the number of patients needed for the detection of at least one patient with a serious “infection and infestation” at a statistical power of 95% was calculated to be 138. Numeric data were presented as the number of non-missing values, means, standard deviations (SDs), minimum, median, and maximum. Japanese RA patients meeting all of the following requirements were enrolled in this study: High disease activity, poor prognostic factors [e.g., rheumatoid factor-positive, anti-cyclic citrullinated peptide (CCP) antibody-positive, and radiographic evidence of bone erosion], indication for the combination of adalimumab and MTX, and no prior history of treatment with DMARDs and biologic agents. Patients with known contraindications to adalimumab were excluded from the analysis [5].
Treatment
Eligible patients initiated treatment with combination adalimumab and MTX (≤16 mg/week) and were followed for 24 weeks or until early discontinuation of adalimumab. Adalimumab was prescribed by the physician in accordance with the approved label for use in patients with RA. MTX administration was started either on the same day of adalimumab administration or the next day of adalimumab administration.
Safety
AEs were recorded up to week 28 and were coded according to the Medical Dictionary for Regulatory Activities (version 18.1) system organ class and preferred term. All AEs, including abnormal laboratory findings, regardless of their causal relationships to adalimumab treatment, were documented during the study period or until discontinuation of adalimumab. Each AE was described in terms of the date of its onset, seriousness, causal relationship to adalimumab, progress, treatment provided, outcome, and laboratory findings.
Measurements
Patients underwent baseline assessments, including evaluation of prior treatments and concomitant drug or nondrug treatments. Efficacy was measured by changes in DAS28 with erythrocyte sedimentation rate (ESR; DAS28-4ESR) and C-reactive protein (CRP; DAS28-4CRP), CDAI (morning stiffness, tender and swollen joints, and global disease activity; remission ≤2.8), and SDAI (CDAI assessment along with CRP; remission ≤3.3) [6], at 0, 4, 12, and 24 weeks or at discontinuation of adalimumab before 24 weeks. Thereafter, patients were grouped into those receiving low-dose (<8 mg/week) or high-dose (≥8 mg to ≤16 mg/week) MTX at baseline and at week 24. Subsequently, DAS28-4CRP response was evaluated in the low-dose and high-dose MTX groups at week 24. The DAS28-4CRP response based on disease duration (<3, ≥3 to <6, ≥6 to <12, ≥12 to <24, and ≥24 months) was also assessed at week 24. All data from the study were recorded in a case report form.
Statistical Analyses
Categorical data were described by number and percentage of patients in each category. Change in the proportion of patients with a DAS28-4CRP from ≥3.2 at baseline to <3.2 at week 24 was evaluated using the McNemar test. Change in DAS28 over 24 weeks was assessed using a paired t test. Multivariate logistic regression analysis was used to identify demographic and baseline variables that could significantly affect the risk of AEs related to adalimumab plus MTX or the response to combination therapy. The odds ratio (OR) and its two-sided Wald 95% confidence interval (CI) were estimated for each covariate. The goodness of fit of the regression model to data was assessed using the Hosmer-Lemeshow goodness-of-fit test. To help select potential covariates to be included in the model, a univariate logistic regression analysis was performed using the safety analysis set and its multivariate logistic regression subset, and Spearman’s rank correlation was used to assess the subset between each covariate and the risk of adverse drug reactions (ADRs). One of two well-correlated (Spearman’s rank correlation coefficient [r] > 0.8) variables was excluded from the model. Inferential statistics were performed at a nominal significance level of 0.05 (two-sided).
Results
Patient Demographics and Baseline Characteristics
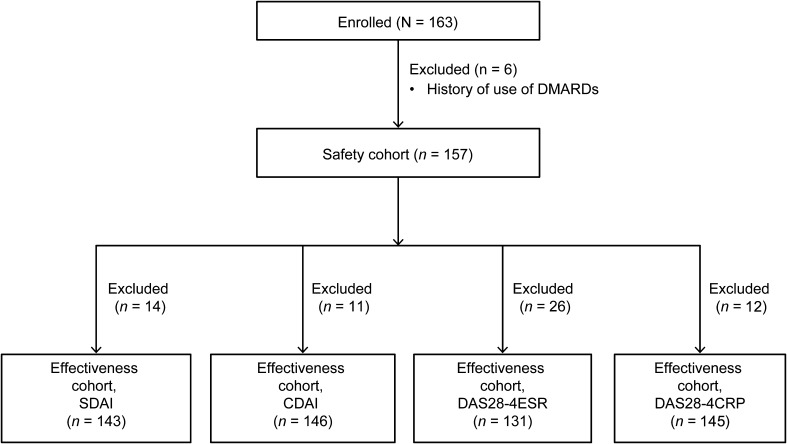
A total of 163 patients were enrolled, of whom 157 were included in the safety cohort. Patients with available DAS28 data were included in the effectiveness cohorts (Fig. 1). The mean (SD) age of the safety cohort was 56.5 (13.9) years, and most patients were women (65.6%); 73.2% and 66.9% of patients were rheumatoid factor-positive and anti-CCP antibody–positive, respectively. The mean (SD) duration of RA was 9.5 (34.4) months, and 40.8% of patients had a DAS28-4CRP score of >5.1. Bone erosions were present in approximately 81 patients, while other reasons for arthrosis damage were present in 45 patients. Thirty-seven patients had a history of treatment with adrenal corticosteroids, whereas 51 patients had received concomitant treatment with adrenal corticosteroids. Fourteen patients received concomitant DMARDs other than MTX (Table 1).
Fig. 1.
Patient flow chart. CDAI Clinical Disease Activity Index, DAS28-4ESR/-4CRP Disease Activity Score based on 28-joint count and using erythrocyte sedimentation rate/C-reactive protein, DMARD disease-modifying antirheumatic drug, SDAI Simplified Disease Activity Index
Table 1.
Patient demographics and baseline characteristics
| Demographics and baseline characteristics | Safety cohort |
|---|---|
| No. of patients | 157 |
| Male/female, % | 34.4/65.6 |
| Age, mean (SD), years | 56.5 (13.9) |
| Weight, mean (SD), kg | 57.77 (13.02) |
| Duration of RA, mean (SD), months | 9.5 (34.4) |
| Duration of smoking (ex-smokers), mean (SD), years | 20.1 (14.5) |
| Duration of smoking (current smokers), mean (SD), years | 35.7 (8.9) |
| HBs antigen test, mean (SD) | |
| Positive | 0 |
| Negative | 153 (99.4) |
| Unspecified | 1 (0.6) |
| Not conducted | 3 (1.9) |
| HBs antibody test, mean (SD) | |
| Positive | 14 (10.1) |
| Negative | 124 (89.9) |
| Not conducted | 19 (12.1) |
| HBc antibody test, mean (SD) | |
| Positive | 15 (10.6) |
| Negative | 126 (89.4) |
| Not conducted | 16 (10.2) |
| β-d-glucan in the blood, mean (SD) | |
| Positive | 2 (1.4) |
| Negative | 137 (98.6) |
| Not conducted | 18 (11.5) |
| Peripheral white blood cell count, mean (SD), /mm3 | |
| ≥4000 | 152 (97.4) |
| <4000 | 4 (2.6) |
| Not conducted | 1 (0.6) |
| Peripheral blood lymphocyte count, mean (SD), /mm3 | |
| ≥1000 | 141 (91.6) |
| <1000 | 13 (8.4) |
| Not conducted | 3 (1.9) |
| Baseline DAS28-4CRP score, mean (SD) | |
| ≤5.1 | 78 (49.7) |
| >5.1 | 64 (40.8) |
| Rheumatoid factor, positive, IU/ml | 115 (73.2) |
| Anti-CCP antibody, positive, U/ml | 105 (66.9) |
| Bone erosions, present, mean (SD) | 81 (51.6) |
| Other reasons for faster arthrosis damage, present, mean (SD) | 45 (28.7) |
| Comorbidities, yes, n (%) | 80 (51.0) |
| Cardiovascular | 28 (35.0) |
| Respiratory | 15 (18.8) |
| Hematologic | 5 (6.3) |
| Hepatic | 15 (18.8) |
| Renal | 1 (1.3) |
| Diabetes | 11 (13.8) |
| Malignancy | 0 |
| Others | 52 (65.0) |
| Prior morbidities, yes, n (%) | 47 (29.9) |
| History of allergies, yes, n (%) | 16 (10.2) |
| Steinbrocker’s stage, % | |
| I | 51.0 |
| II | 36.3 |
| III | 9.6 |
| IV | 3.2 |
| Steinbrocker’s class, % | |
| 1 | 35 |
| 2 | 45.9 |
| 3 | 17.2 |
| 4 | 1.9 |
| MTX initial dose, n (%) | |
| <8 mg/week | 81 (51.6) |
| ≥8 mg/week | 76 (48.4) |
| MTX dose classification, n (%) | |
| High dose at baseline, high dose at week 24 | 63 (40.1) |
| High dose at baseline, low dose at week 24 | 2 (1.3) |
| Low dose at baseline, high dose at week 24 | 43 (27.4) |
| Low dose at baseline, low dose at week 24 | 29 (18.5) |
| Other than the above mentioned | 20 (12.7) |
| Prior use of corticosteroids, yes, n (%) | 37 (23.6) |
| Concomitant use of corticosteroids, yes, n (%) | 51 (32.5) |
| Concomitant use of DMARDs other than MTX, yes, n (%) | 14 (8.9) |
CCP cyclic citrullinated peptide, DAS28-4CRP Disease Activity Score based on 28-joint count and using C-reactive protein, DMARD disease-modifying antirheumatic drug; HBc hepatitis B core, HBs, hepatitis B surface, MTX methotrexate, RA rheumatoid arthritis, SD standard deviation
Change in MTX Dosage at Week 24
Of the patients enrolled, 142 were receiving MTX at baseline. A greater proportion of patients on low-dose MTX (≤8 mg/week MTX; 44/74) escalated to high-dose MTX (>8 mg/week) at week 24, while 30 patients continued to receive low-dose MTX. A greater majority of patients who entered the study on high-dose MTX (66/68) continued to receive high-dose MTX; 2 patients switched to low-dose MTX. In the safety cohort (n = 157), MTX was discontinued at week 24 in 19 patients due to insufficient effect (n = 3 [15.8%]), AEs (n = 5 [26.3%]), and other reasons (n = 11 [57.9%]).
Safety
To examine the safety profile of adalimumab in combination with MTX in clinical practice in DMARD- and biologic-naïve patients at risk of early structural joint damage, we analyzed AE data obtained from the 157 patients included in the safety analysis set. A total of 37 ADRs occurred in 29 patients (18.47%; Table 2). The most common ADRs were “infections and infestations” (n = 7; 4.46%) and “general disorders and administration site conditions” (n = 4; 2.55%).
Table 2.
Adverse drug reaction profile based on MTX dose
| MTX dosage groups at baseline | Total | Total | High dose (≥8 mg/week) | Low dose (<8 mg/week) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTX dosage groups at week 24 | n = 157 | High dose n = 66 | Low dose n = 2 | High dose n = 43 | Low dose n = 30 | ||||||
| Non-serious | Serious | Non-serious | Serious | Non-serious | Serious | Non-serious | Serious | Non-serious | Serious | ||
| No. of ADR onset cases | 25 | 4 | 29 | 7 | 0 | 2 | 0 | 9 | 1 | 2 | 1 |
| No. of ADRs | 32 | 5 | 37 | 9 | 0 | 2 | 0 | 13 | 2 | 3 | 1 |
| Incidence of ADRs, % | 16.3 | 2.6 | 18.5 | 11.1 | 0 | 100 | 0 | 20.9 | 2.3 | 6.9 | 3.4 |
| Infectious and parasitic diseases | 7 | 2 | 9 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 |
| Gastroenteritis | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nasopharyngitis | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pyelonephritis | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Herpes zoster | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pulpitis dental | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonia | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonia bacterial | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumocystis jiroveci pneumonia | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Immune system disorders | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychiatric disorder | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dysphoria | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cardiac disorder | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pericarditis | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Interstitial lung disease | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurisy | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Alopecia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Rash | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorder | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Arthralgia | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gastrointestinal disorder | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | 4 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Injection site reaction | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chills | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pyrexia | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Injection site erythema | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Peripheral edema | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Event that cannot be evaluated | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Laboratory tests | 10 | 0 | 10 | 4 | 0 | 2 | 0 | 3 | 0 | 1 | 0 |
| Increase in alanine aminotransferase | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Increase in aspartate aminotransferase | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Decrease in white blood cell count | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Abnormal liver function test | 3 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Elevation of liver enzyme | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abnormal liver enzymes | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Increase in β-d-glucan in the blood | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Decrease in blood platelet count | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
ADR adverse drug reaction, MTX methotrexate
Multivariate logistic regression analysis of the incidence of ADRs was performed to identify demographic or baseline variables influencing the safety of adalimumab plus MTX (data not shown). All potential covariates included in the regression model had an OR for which the two-sided Wald 95% CI was wide and crossed 1. Therefore, there was no clear indication for the direction of their association with the risk of ADRs during combination therapy. There were five SAEs in four patients (2.55%) >60 years of age. The SAEs included pyelonephritis, Pneumocystis jiroveci pneumonia, interstitial lung disease, and pleurisy. The outcomes of these patients from these SAEs were either recovered or recovering. Pericarditis was the only unexpected event according to the current Japanese label. The patient was a 62-year-old female, without a history or comorbidities of cardiovascular disease, who had initiated treatment with adalimumab in June 2013. The patient was diagnosed with acute pericarditis 4 months after the start of treatment; adalimumab was discontinued 5 months after administration. Pericarditis resolved by 8 months after administration; however, miliary tuberculosis was then diagnosed in this patient and she died. According to the treating physician, the diagnosis of miliary tuberculosis was not considered related to treatment.
The incidence of ADRs was also analyzed by MTX dosage at baseline and week 24. Twenty patients were excluded from the safety data set because of missing data at these time points. The patients treated with ≥8 mg/week MTX at baseline experienced no serious ADRs. Two of the patients treated with <8 mg/week MTX at baseline experienced serious ADRs; one patient treated with <8 mg/week MTX at baseline and ≥8 mg/week MTX at week 24 experienced pericarditis and pleurisy and one patient treated with <8 mg/week MTX at both baseline and week 24 experienced pyelonephritis. There was no numerical difference in the incidence of ADRs between MTX dosage at baseline and week 24.
DAS28-4CRP Response at 24 Weeks Based on Disease Duration
Nearly all of the patients in the DAS28-4CRP effectiveness cohort (134/140 [95.7%]) had disease duration <2 years; of these patients, approximately ≥80% had moderate or high disease activity (DAS28-4CRP ≥3.2) at baseline. After 24 weeks of treatment, approximately ≥50% patients achieved remission or low disease activity (DAS28-4CRP <3.2). In comparison, only two of six patients (33.3%) with RA ≥2 years duration achieved remission or low disease activity (DAS28-4CRP <3.2) at week 24 (Table 3).
Table 3.
DAS28-4CRP response at 24 weeks based on disease duration (n = 140)
| Duration of RA (months) | n | DAS28-4CRP at baseline | DAS28-4CRP at week 24 | McNemar’s testb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <3.2 | ≥3.2 | <3.2 | ≥3.2 | ||||||||
| n | %a | n | %a | n | %a | n | %a | n | P value | ||
| <3 | 55 | 6 | 10.9 | 46 | 83.6 | 32 | 58.2 | 1 | 1.8 | 33 | <0.0001 |
| ≥3 to <6 | 53 | 1 | 1.9 | 46 | 86.8 | 28 | 52.8 | 5 | 9.4 | 29 | <0.0001 |
| ≥6 to <12 | 20 | 3 | 15.0 | 16 | 80.0 | 11 | 55.0 | 0 | 0.0 | – | – |
| ≥12 to <24 | 6 | 0 | 0.0 | 6 | 100.0 | 3 | 50.0 | 2 | 33.3 | – | – |
| ≥24 | 6 | 0 | 0.0 | 6 | 100.0 | 2 | 33.3 | 0 | 0.0 | – | – |
DAS28-4CRP Disease Activity Score based on 28-joint count and using C-reactive protein, RA rheumatoid arthritis
aThe denominator of the ratio is the number of target cases of each RA disease activity classification
bMcNemar’s test evaluated the change in proportion of patients with DAS28-4CRP ≥3.2 at baseline to <3.2 at week 24; analysis could not be performed because of missing data in patients with a disease duration ≥6 months
A significant proportion of patients with disease duration <6 months (<3 months and from ≥3 to <6 months) had a decrease in DAS28-4CRP from ≥3.2 to <3.2 at week 24 (McNemar’s test; P < 0.0001) compared with baseline. Because of the small number of patients, the DAS28-4CRP response at 24 weeks could not be evaluated statistically in patients with disease duration ≥6 months (Table 3).
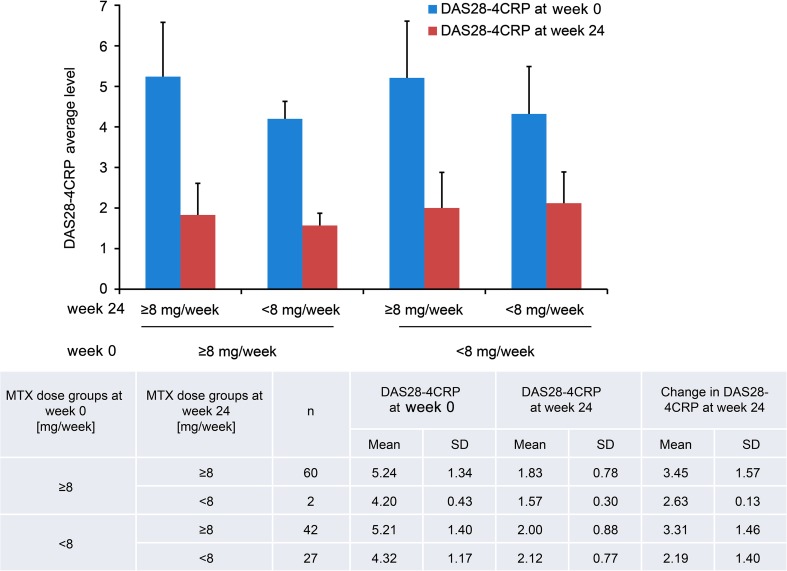
DAS28-4CRP Response at 24 Weeks Based on MTX Dose
DAS28-4CRP remission (<2.6) was achieved in patients in both the low-dose and high-dose MTX groups at week 24 (Fig. 2).
Fig. 2.
DAS28-4CRP response at 24 weeks based on MTX dose (n = 131). DAS28-4CRP Disease Activity Score based on 28-joint count and using C-reactive protein, MTX methotrexate, SD standard deviation
Efficacy Based on Change in Disease Activity
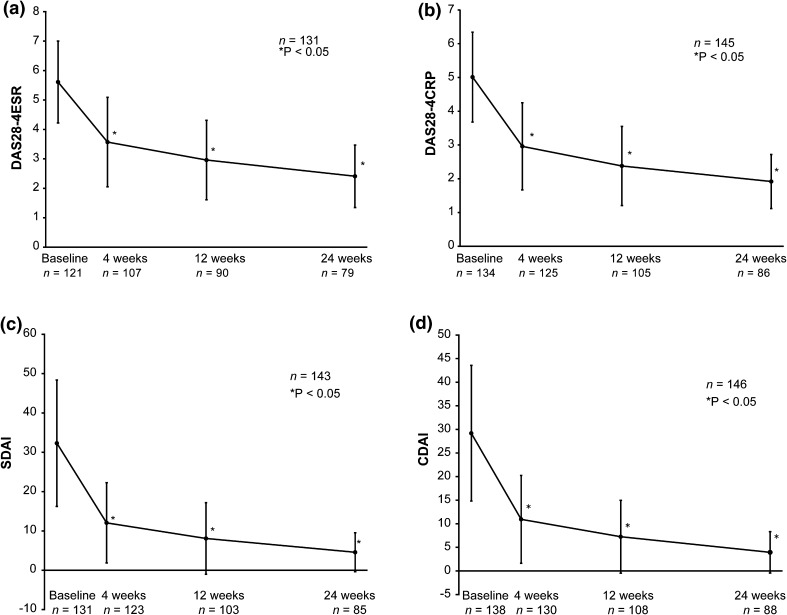
There was a significant improvement/reduction in disease activity over 24 weeks assessed by change in DAS28-4ESR, DAS28-4CRP, SDAI, and CDAI scores from 5.61, 5.01, 32.30, and 29.18 at baseline to 2.41, 1.92, 4.58, and 3.96 at week 24, respectively (P < 0.05). A steep decline in disease activity was seen as early as week 4, and this improvement in disease activity was sustained for 24 weeks (Fig. 3). Overall, 37 patients (23.6%) from the safety cohort discontinued adalimumab before week 24 because of insufficient effect (n = 12), AEs (n = 7), lost to follow-up (n = 6), remission (n = 4), financial reasons (n = 3), patient preference (n = 2), change in hospital (n = 1), and other reasons (n = 2). Multivariate regression analysis showed that baseline patient characteristics such as cardiac disorder (OR, 4.1; 95% CI, 0.9–18.5), blood disorder (OR, 2.9; 95% CI, 0.1–83.5), history of drug allergy (OR, 1.6; 95% CI, 0.1–25.2), peripheral white blood cell count ≥4000/mm3 (OR, 6.5; 95% CI, 0.2–193.3), and concomitant use of adrenal corticosteroids (OR, 1.8; 95% CI, 0.7–4.8) are likely to negatively impact remission (Table S1). Similarly, univariate regression analysis showed that baseline patient characteristics such as age ≥65 years (OR, 1.6; 95% CI, 0.8–3.5), weight ≥50 to <60 kg (OR, 1.8; 95% CI, 0.6–5.2), peripheral blood lymphocyte count ≥1000/mm3 (OR, 1.6; 95% CI, 0.5–5.4), and concomitant use of adrenal corticosteroids (OR, 1.6; 95% CI, 0.7–3.4) are likely to negatively impact remission (Table S2).
Fig. 3.
Change in DAS as assessed using a DAS28-4ESR, b DAS28-4CRP, c SDAI, and d CDAI scores. CDAI Clinical Disease Activity Index, DAS28-4ESR/-4CRP Disease Activity Score based on 28-joint count and using erythrocyte sedimentation rate/C-reactive protein, SDAI Simplified Disease Activity Index. *Paired t test
Discussion
This study provides the first prospective real-world evidence from routine clinical practice of combination therapy initiated in DMARD and biologic treatment-naïve patients with RA at risk of progressive joint damage. Results indicate that treatment with adalimumab in combination with MTX is safe and effective.
Of the 157 patients evaluated for safety, 29 patients (18.47%) experienced an ADR. The most common ADRs in this study were “infections and infestations” (n = 9; 5.73%) and “general disorders and administration site conditions” (n = 4; 2.55%). There was one case of pericarditis; however, this case resolved and was not considered to be related to treatment. In the postmarketing surveillance of 7740 established patients with RA, the incidence of ADRs was 24%; the most common ADRs were “skin and subcutaneous disorders” (7.2%), followed by “infections and infestations” (7.0%) [4]. Thus, these results indicate that no particular ADR was observed in the present study in contrast to the previous case study [4]. There was no apparent relationship between the incidence of ADRs and the MTX dose. Four SAEs were reported in this study: pyelonephritis, Pneumocystis jiroveci pneumonia, interstitial lung disease, and pleurisy occurred in one patient each, and pericarditis of unknown severity occurred in one patient. Although the outcomes of all SAEs were either recovered or recovering (including the case of pericarditis), the causal relationship between adalimumab and pericarditis cannot be ruled out, as the event developed after adalimumab administration. Overall, the ADR profile and frequency in DMARD- and biologic-naïve RA patients at risk of progressive joint damage were similar to those previously reported in patients with established RA, suggesting that the early intensive therapy with adalimumab did not adversely affect the existing safety profile.
There were 37 patients who discontinued the combination treatment with adalimumab and MTX before week 24, of which 12 patients discontinued because of insufficient effect. This suggests that there were TNF-alpha non-responders even among patients who had no prior history of exposure to DMARDs and biologics. Therefore, physicians should select appropriate treatment options besides TNF-alpha inhibitors on the basis of careful observation and accurate diagnosis.
Of note, approximately ≥50% of patients achieved remission or low disease activity (DAS28-4CRP response) at week 24. This patient population included 95.5% of patients with a disease duration <2 years and ≥80% of patients with moderate or high disease activity at baseline. These results were consistent with previous reports of clinical trials with the combination therapy [3, 7]. In both Japanese and international clinical trials, poor prognostic factors (rheumatoid factor-positive, anti-CCP antibody–positive, or bone erosion) were features observed in patients with a disease duration of <2 years. ACR guidelines recommend the use of anti-TNF inhibitors with or without MTX in patients with disease duration of <6 months with high disease activity and features of poor prognosis [8]. Eventually, the intensive use of biologics for the patients in this study was deemed to be reasonable.
Japanese studies have evaluated the clinical effectiveness of adalimumab in combination with higher doses of MTX (>8 mg/week) using both small [9, 10] and large sample sizes [4, 11]. In the present study, we observed DAS28-4CRP remission (<2.6) in most patients at week 24 regardless of the MTX dose. The real-world observational nature of the study indicates that a dose of MTX based on the patient’s background, condition, and response to the therapies, as assessed by the treating physician, would be beneficial for patients. The present results are consistent with a post hoc analysis of the MELODY study, which suggested that 8 mg/week of MTX should be an adequate dose for biologic-naïve patients [11], and the HOPEFUL-1 study, which indicated that adalimumab with MTX is safe and efficacious in suppressing disease progression (with radiographic assessments) and improving clinical outcomes in Japanese patients with disease duration of <2 years and high disease activity [3]. A similar response was seen in Western patients with RA, in whom increasing doses of MTX in combination with adalimumab showed a statistically significant trend toward improved clinical outcomes; however, in patients with disease duration of <1 year, the efficacy of MTX at 10- and 20-mg/week was equivalent [12]. Both univariate and multivariate regression analysis in the present study also indicated that the dose of MTX is not likely to affect remission. However, baseline patient characteristics, such as cardiac disorder, blood disorder, history of drug allergy, peripheral white blood cell count ≥4000/mm3, concomitant use of adrenal corticosteroids, age ≥65 years, weight ≥50 to <60 kg, and peripheral blood lymphocyte count ≥1000/mm3, were likely to have a negative impact on remission.
We observed a steep decline in disease activity as early as week 4, and this improvement was sustained for 24 weeks. All disease activity scores (DAS28-4ESR, DAS28-4CRP, SDAI, and CDAI) showed significant improvement approaching remission at week 24. This improvement in disease activity was similar to that reported in the HOPEFUL-1 study [7], indicating that the combination of adalimumab and MTX was equally effective in reducing disease activity in the real-world scenario. The results suggest that in patients with disease duration of <2 years with high disease activity and anticipated progressive joint damage, the MTX dose used in combination with adalimumab was appropriately assessed in routine clinical practice.
Although direct comparisons cannot be made, the decline in disease activity is consistent with results from adalimumab and MTX combination therapy used in randomized controlled trials conducted in Japan [3, 7] and in the West in the treatment with DMARDs, MTX, and/or biologic-naïve patient populations with early RA [12–16]. Although not evident from this study, adalimumab and MTX combination therapy could contribute to inhibit bone erosion [17]. Therefore, early adalimumab treatment with MTX is expected to improve clinical function in patients with high disease activity and can be considered effective in this patient population.
Study limitations included the open-label, observational study design, which reflects real-world practice. There was no restriction on the treatment or the use of concomitant medications. There were limitations for obtaining the data, leading to missing data at some specified time points. As this was a mandatory postmarketing study requested by the PMDA, we did not include the radiological examination, influence of quality of life and cost benefit evaluation. In addition, the collection of effectiveness data for this study might have been limited by the perceived clinical need for collecting data for the care of the patient in routine clinical practice.
Conclusions
This PMOS is the first to examine the real-world safety and effectiveness of adalimumab and MTX in DMARD- and biologic-naïve Japanese patients with RA at risk of progressive structural joint damage. The results from the study raised no new concerns about the safety of adalimumab with MTX therapy and confirmed the previous findings that combination therapy significantly decreased disease activity assessed using various measures and helped achieve clinical remission. This study suggests that adalimumab in combination with MTX could be a beneficial treatment option for this patient population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Sponsorship for this study and article processing charges were funded by AbbVie GK and Eisai. We would like to thank the Statistical Analysis Department, CDM Division of CMIC Co., Ltd., for data analysis and consultation. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Editorial support, in the form of medical writing, assembling tables and creating high-resolution images based on the authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Annirudha Chillar, MD, PhD, of Cactus Communications, and was funded by AbbVie GK and Eisai. Finally, we would like to thank all of the patients and investigators.
The design, study conduct, and financial support for the postmarketing surveillance were provided by AbbVie and Eisai. AbbVie participated in the interpretation of data, review, and approval of this manuscript.
Disclosures
Yukiko Ito is an employee of AbbVie GK. Kaori Hozumi is an employee of AbbVie GK. Yukiko Okada is an employee of AbbVie GK. Sarina Kurimoto is an employee of AbbVie GK.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/E6F7F0605DB820A1.
References
- 1.van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyasaka N, The CHANGE Study Investigators Clinical investigation in highly disease- affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18:252–262. doi: 10.3109/s10165-008-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi T, Yamanaka H, Ishiguro N, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2014;73:536–543. doi: 10.1136/annrheumdis-2012-202433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of 7740 patients. Mod Rheumatol. 2014;24:390–398. doi: 10.3109/14397595.2013.843760. [DOI] [PubMed] [Google Scholar]
- 5.Humira® Prescribing Information. http://www.rxabbvie.com/pdf/humira.pdf. Accessed Sept 2016.
- 6.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka H, Ishiguro N, Takeuchi T, et al. Recovery of clinical but not radiographic outcomes by the delayed addition of adalimumab to methotrexate-treated Japanese patients with early rheumatoid arthritis: 52-week results of the HOPEFUL-1 trial. Rheumatology (Oxford) 2014;53:904–913. doi: 10.1093/rheumatology/ket465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh K, Ito S, Unno M, et al. The rate of decrease in the disease activity of rheumatoid arthritis during treatment with adalimumab depends on the dose of methotrexate. Intern Med. 2015;54:1035–1041. doi: 10.2169/internalmedicine.54.4085. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima Y, Miyahara H, Kondo M, et al. Impact of methotrexate dose on efficacy of adalimumab in Japanese patients with rheumatoid arthritis: results from registered data analyses. Mod Rheumatol. 2017;27:15–21. doi: 10.3109/14397595.2016.1170958. [DOI] [PubMed] [Google Scholar]
- 11.Koike T, Harigai M, Ishiguro N, et al. Effect of methotrexate plus adalimumab on the achievement of rheumatoid arthritis therapeutic goals: post hoc analysis of Japanese patients (MELODY study) Rheumatol Ther. 2016;3:129–141. doi: 10.1007/s40744-015-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmester GR, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis. 2015;74:1037–1044. doi: 10.1136/annrheumdis-2013-204769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 14.Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detert J, Bastian H, Listing J, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. 2013;72:844–850. doi: 10.1136/annrheumdis-2012-201612. [DOI] [PubMed] [Google Scholar]
- 16.Keystone EC, Breedveld FC, van der Heijde D, et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol. 2014;41:5–14. doi: 10.3899/jrheum.130543. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Yamanaka H, Ishiguro N, et al. Adalimumab discontinuation in patients with early rheumatoid arthritis who were initially treated with methotrexate alone or in combination with adalimumab: 1 year outcomes of the HOPEFUL-2 study. RMD Open. 2016;2:e000189. doi: 10.1136/rmdopen-2015-000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.