Abstract
Study Objectives:
To evaluate changes in rates of family physician (FP) management of insomnia in Australia from 2000–2015.
Methods:
The Bettering the Evaluation And Care of Health (BEACH) program is a nationally representative cross-sectional survey of 1,000 newly randomly sampled family physicians' activity in Australia per year, who each record details of 100 consecutive patient encounters. This provided records of approximately 100,000 encounters each year. We identified all encounters with patients older than 15 years where insomnia or difficulty sleeping was managed and assessed trends in these encounters from 2000–2015.
Results:
There was no change in the management rate of insomnia from 2000–2007 (1.54 per 100 encounters [95% confidence interval [CI]: 1.49–1.58]). This rate was lower from 2008–2015 (1.31 per 100 encounters [95% CI: 1.27–1.35]). There was no change in FP management: pharmacotherapy was used in approximately 90% of encounters; nonpharmacological advice was given at approximately 20%; and onward referral at approximately 1% of encounters. Prescription of temazepam changed from 54.6 [95% CI: 51.4–57.9] per 100 insomnia problems in 2000–2001 to 43.6 [95% CI: 40.1–47.0] in 2014–2015, whereas zolpidem increased steadily from introduction in 2000 to 14.6 [95% CI: 12.2–17.1] per 100 insomnia problems in 2006–2007, and then decreased to 7.3 [95% CI: 5.4–9.2] by 2014–2015.
Conclusions:
Insomnia management frequency decreased after 2007 in conjunction with ecologically associated Australian media reporting of adverse effects linked to zolpidem use. Australian FPs remain reliant on pharmacotherapy for the management of insomnia.
Citation:
Miller CB, Valenti L, Harrison CM, Bartlett DJ, Glozier N, Cross NE, Grunstein RR, Britt HC, Marshall NS. Time trends in the family physician management of insomnia: the Australian experience (2000–2015). J Clin Sleep Med. 2017;13(6):785–790.
Keywords: epidemiology, family physician, insomnia, pharmacotherapy
INTRODUCTION
Insomnia is a common and costly complaint that affects approximately 6% to 10% of the population.1–3 Annual financial costs of insomnia to society are estimated to be up to $107 billion in the United States,4 $6.6 billion (CAD) in the Canadian province of Quebec,5 and $1.5 billion (AUD) in Australia.6 Insomnia is common in family physician (FP) settings and is predominantly treated by pharmacotherapy in the United States and elsewhere.7–10 Current evidence-based recommendations published by the American Academy of Sleep Medicine and American College of Physicians recommend cognitive behavioral therapy (CBT) as the first-line and most efficacious therapy.2,11 Despite this, no health system has enough skilled CBT practitioners to treat even a small proportion of insomnia cases.12,13 Patients are dissatisfied with current approaches and prefer nonpharmacological strategies but continue to report that hypnotics are an attractive quick-fix solution.14
In the United Kingdom, three waves of the National Mental Health Survey reported an almost doubling in the prevalence of participant reported insomnia (3.1% to 5.8%) and a doubling in the prevalence of pharmacotherapy for insomnia between 1993 and 2007 (0.4% to 0.8% of the population).7 An increase in the prevalence of occasional insomnia symptoms has also been reported in Finland.15 However, these participant-reported rates may be underreported or overreported when compared to clinician record.16 These data made us wonder whether such an increase in insomnia may be detectible in other countries, such as Australia.
BRIEF SUMMARY
Current Knowledge/Study Rationale: To describe how insomnia has been managed by family physicians in Australia and how this has changed through time.
Study Impact: Insomnia management rates per 100 family physician-patient encounters decreased after 2007–2008, when there was a high level of Australian media reporting of adverse effects of zolpidem. Through 2000–2015 there was a steady rate of approximately 90% of insomnia problems that were managed with pharmacotherapy. However, the pattern of pharmacotherapeutic agents being prescribed through time changed. Temazepam was the most commonly prescribed medication but zolpidem prescriptions notably fell since a peak in 2006–2007, and slow-release melatonin gradually rose in popularity since release in 2009.
Between 2000–2015, there are likely to have been substantive changes in the mix of pharmacotherapy as new hypnotics were released in Australia and Z-class hypnotics came under considerable scrutiny because of concerns about safety. In particular, zolpidem (marketed as Stilnox in Australia17 and Am-bien in the United States)18,19 was first released in Australia in late 2000.20 Zolpidem-related side effects became the subject of intense media scrutiny in 2007–2008.21,22 Later in 2008, the Australian Therapeutic Goods Administration imposed a black box warning in the product information for zolpidem.23,24 Only the United States and Australia seem to have been subject to sustained media interest in these side effects.21,25
In Australia, from April 1998 to March 2016, FP activity was monitored by the Bettering the Evaluation And Care of Health (BEACH) program,26 an annual nationally representative cross-sectional survey of FP clinical activity. Earlier cross-sectional analyses of these data (2006–2008)27 found that pharmacotherapy was prescribed by FPs at a rate of 95.2 medications per 100 insomnia problems. Since that report was published, significant changes in insomnia management are likely to have occurred due to the zolpidem-related stimulated reporting event21,22,25,28 and the introduction of important new hypnotics such as slow-release melatonin (marketed as Circadin in Europe since 2007 and Australia since 2009).29,30 In addition, there have been anecdotal reports of off-label prescriptions of low doses (50 mg) of the antipsychotic quetiapine (tradename: Seroquel) for insomnia.31–35 The objectives of this study are to describe changes in the management rates of insomnia in Australia and to characterize any changes in management actions by FPs over the period 2000 to 2015. Specifically, we aim to:
describe insomnia management rates by FPs and describe rates in specific age and sex groups;
identify and describe any changes in pharmacotherapeutic interventions, nonpharmacotherapeutic interventions, or onward referral rates by FPs for insomnia.
METHODS
These are secondary analyses of data from the BEACH program. One year weighted datasets from April 2000–March 2001 to March 2014–April 2015 are used.36,37 The method is described fully elsewhere.26 In summary, BEACH was an ongoing cross-sectional survey of FP clinical activity in Australia, enrolling about 1,000 newly randomly sampled FPs each year. Each physician recorded details of 100 consecutive encounters with consenting patients, on structured paper encounter forms. FP recording periods were evenly spread across the calendar year. Data include patient characteristics, and in free text, the problems managed (up to four per encounter) at the highest diagnostic level possible on the evidence currently available. FPs nominated the status of each problem (tick box) as either a “new” problem: defined as the first encounter of a problem, including the first encounter of a recurrence of a previously resolved problem, but excluding the encounter of a problem first assessed by another provider; or an “old” problem: a previously assessed problem that requires ongoing care, including followup for a problem or an initial encounter of a problem previously assessed by another provider.36 For each problem managed, FPs recorded the treatments provided at the encounter. Each recorded clinical action (including medications, clinical treatments, tests ordered, and referrals made) is linked by the FP to the specific problem being managed.
The free text problems managed and nonpharmacological treatments are secondarily classified by trained coders, according to the International Classification of Primary Care, Second Edition (ICPC-2)38 and coded more specifically using ICPC-2 PLUS,39 an Australian FP clinical interface terminology. Insomnia problems were defined as ICPC-2 PLUS codes: P06001 (Unable (to); sleep), and P06003 (Insomnia). We restricted our analyses to encounters with patients 15 years or older. The BEACH program and analyses of the BEACH data have ethical approval and oversight from the Human Research Ethics Committee of the University of Sydney (Ref: 2012/130). The ethics committee of the Australian Institute of Health and Welfare also approved this study in the 2000–2011 data year.36
Statistical Methods
The BEACH program is the only national Australian dataset that contains representative data on the content of FP encounters. This program has a cluster sample study design in which the primary sampling unit is the FP, and the unit of analysis is the FP-patient encounter. Results are reported as rates per 100 or 100,000 encounters, rates per 100 insomnia problems managed, and patient age- and sex-specific insomnia management rates, with robust 95% confidence intervals (CIs) that adjust for the cluster design effect. Rates were calculated using the “survey means” procedures in SAS (v9.1.3 Inc, Cary, North Carolina, United States) with adjustment for the cluster study design.
Statistical significance of year-to-year differences is judged by nonoverlapping 95% CIs. In order to test whether FP management of insomnia decreased in conjunction with the stimulated reporting event, we pooled all encounters for the years 2000–2007 and all encounters for the years 2008–2015 and used a z test with alpha set at P < .05. There was a 1-year exclusion period because the event occurred during 2007–2008.21
RESULTS
Patient Sample
Between April 2000 and March 2015, 14,716 FPs participated in BEACH, recording details of 1,471,600 encounters. Of these, 1,285,787 (87%) were with adults 15 years or older, and 775,955 (60%) were with female patients.
Changes in the Management of Insomnia Encounters
Figure 1 shows the management rate of insomnia cases per 100,000 encounters per BEACH year (2000–2015). After removing the BEACH year 2007–2008 because it was the year of the stimulated reporting event, the annual management rate of insomnia was higher before the stimulated reporting event (2000–2007; 1,535 per 100,000 [95% CI: 1,490–1,580]) than after (2008–2015; 1,309 [95% CI: 1,270–1,350], z = 6.46, P < .0001). Sex-specific management rates for 2000–2015 are plotted in Figure S1 in the supplemental material. Females accounted for approximately 62% of patients at encounters where insomnia was managed throughout the study period. Figure S2 in the supplemental material shows the management rate of insomnia in older individuals (age 75 years or older) decreased significantly from 3,348 [95% CI: 2,894–3,802] in 2000–2001 to 2,229 [95% CI: 1,901–2,557] per 100,000 encounters in 2014–2015. For all other age groups there was no significant change in the management rate over time.
Figure 1. Management rate of insomnia cases per 100,000 encounters per year.
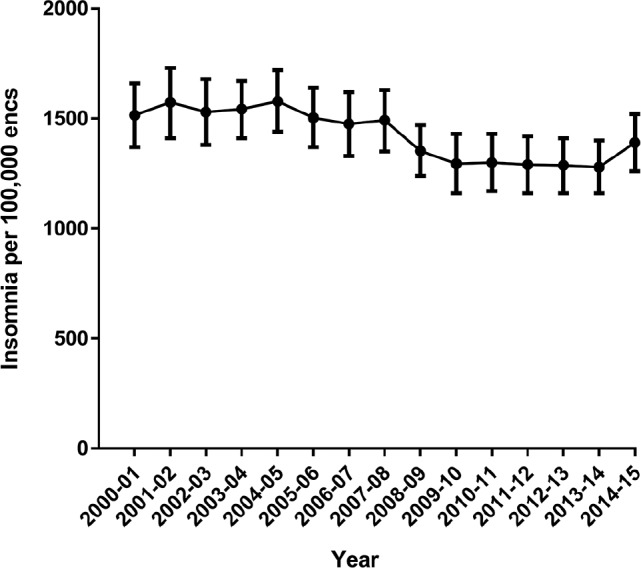
Error bars indicate 95% confidence intervals.
Changes in Management Approaches
Figure 2 charts the proportion of insomnia management occasions at which at least one of each type of management action occurred, by year. Throughout the study period, medications were by far the most common (approximately 90%) management choice, followed by counseling/advice (eg, sleep hygiene, medication use, relaxation techniques: approximately 20%) and very rarely (approximately 1%) referral. Figure S3 in the supplemental material profiles nondrug clinical treatments for insomnia problems through time. Provision of advice/education about sleep increased over the study period from 2.9 [95% CI: 1.4–4.5] in 2000–2001 to 8.8 [95% CI: 6.6–11.0] per 100 insomnia problems in 2014–2015. Figure S4 in the supplemental material plots FP referrals to psychologists, sleep clinics, counselors, and psychiatrists.
Figure 2. Proportion (%) of insomnia problems that resulted in at least one of each type of management action per year.
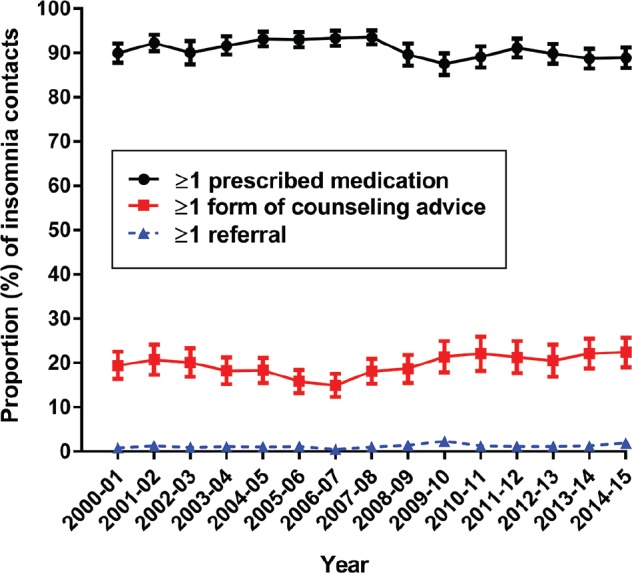
Error bars indicate 95% confidence intervals and may not be visible at times due to the scale of the graph. Counseling advice includes sleep hygiene, medication use, and relaxation techniques. Totals add to more than 100% per year because problems can be managed by more than one action per encounter.
Pharmacotherapy Changes 2000–2015
Figure 3A shows the change in prescribed medication rate per 100 insomnia problems for the top five most prescribed medications ranked from 2014–2015. Figure 3B shows the changes in the next five most prescribed medications ranked from 2014–2015. In 2014–2015, these 10 medications (Figure 3A and 3B) account for 90% of the pharmaceutical agents used for insomnia management. The prescription rate for quetiapine (Figure 3B) was so low in most years that confidence limits could not be reliably calculated.
Figure 3. Prescriptions per 100 insomnia problems managed.

(A) top 1 to 5 most commonly prescribed medications ranked from 2014– 2015 data and (B) top 6 to 10 most commonly prescribed medications ranked from 2014–2015 data. Error bars indicate 95% confidence intervals and are not calculated when the numerator is 3 or fewer because the event rate is not statistically distinguishable from zero.
Post Hoc Analysis: Differences Between New and Old Problem Management Before and After 2007–2008
After seeing the results presented in Figures 1 and 3 we developed a post hoc hypothesis that management rate and/or the prescription patterns for initial “new” (newly emergent or re-emergent) and previously managed (ie, “old”) insomnia may have been affected by the stimulated reporting event in different ways. Figure 4 shows that the management rate for “new” insomnia did not change. The apparent decrease in the management rate of “old” insomnia problems between 2006 to 2008 was not statistically significant using the nonoverlap-ping 95% CI rule. However, because of our previous observations about the stimulated reporting event year,22,25 we then decided to apply the same z test to data pooled before and after the stimulated reporting event in 2007–2008. Pooling all “old” insomnia managements for BEACH years 2000–2007 and for BEACH years 2008–2015 yielded a statistically signifi-cant decrease after the stimulated event (2000–2007; 1,329 per 100,000 [95% CI: 1,280–1,381] versus 2008–2015; 1,117 [95% CI: 1,073–1,164], z = 6.08, P < .0001). Management rates of “new” problems did not change significantly (2000–2007; 206 per 100,000 [95% CI: 188–223] versus 2008–2015; 192 [95% CI: 175–209], z = 0.38, P = .352: see Figure 4). Figure 5 plots the changes in pharmacotherapy for both “new” (Figure 5A) and “old” (Figure 5B) management encounters of insomnia for our key medications: temazepam (traditional market leader), zolpidem, and melatonin.
Figure 4. Management rate of “old” and “new” insomnia cases per 100,000 encounters per year.
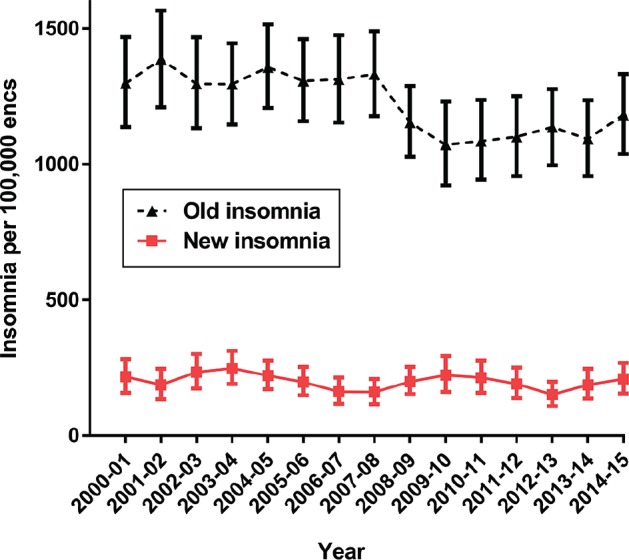
Error bars indicate 95% confidence intervals.
Figure 5. Temazepam, melatonin and zolpidem prescriptions per 100 insomnia problems managed.
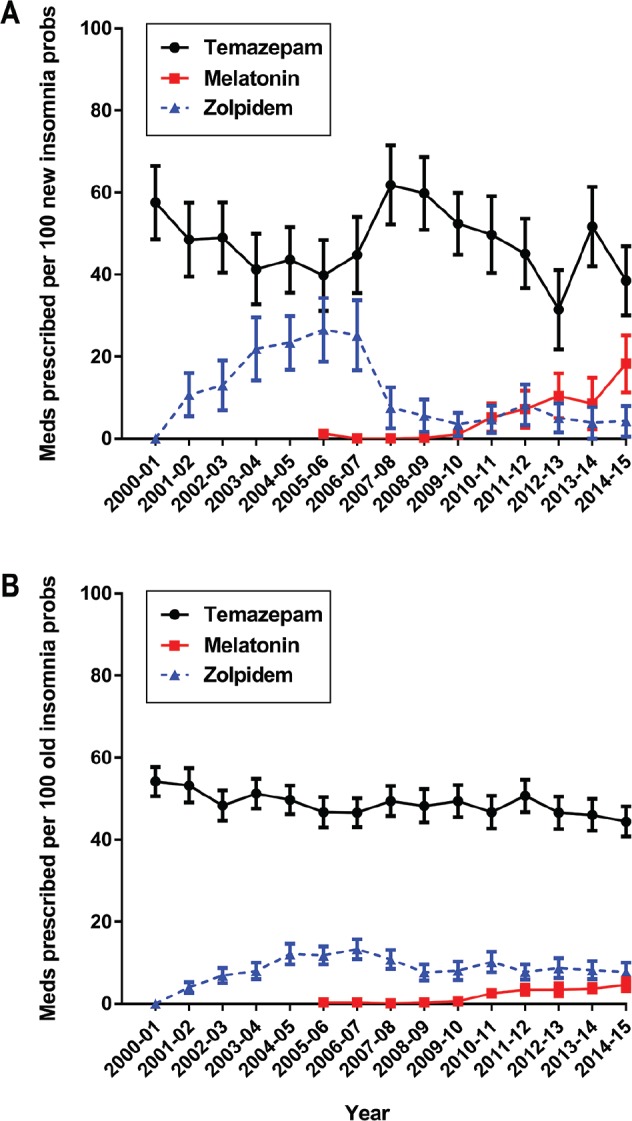
(A) “new” problem management and (B) “old” problem management. Error bars indicate 95% confidence intervals and are not calculated when the numerator is 3 or fewer because the event rate is not statistically distinguishable from zero.
DISCUSSION
The insomnia management rate in Australian family practice remained stable between 2000 and 2007 and then decreased in 2007–2008 in conjunction with an ecologically associated stimulated reporting event surrounding zolpidem.22,25 Between 2009 and 2015, insomnia management remained stable at a lower rate. Over the study period there was no change in the proportion of female patients (approximately 62%) but there was a change in the age distribution of patients managed for insomnia. The only age group to significantly decline was people aged 75 years and older. This may be due to increasing concerns about the safety profiles of benzodiazepines and zolpidem coupled with a lack of access to nonpharmacological alternatives.12,40,41 Despite major treatment guidelines for insomnia recommending CBT as a first-line therapy, there were no changes in management actions, possibly due to a lack of health system capacity.13,42 However, it is encouraging that advice and education about sleep steadily increased over the course of the study period, be it from a very low base.
The pharmacotherapy mix changed after zolpidem was approved in 199920 and then marketed in Australia in late 2000.17 Zolpidem prescriptions rose until 2006–2007, prior to the stimulated reporting event21 and then sharply decreased. Changes in pharmacotherapy were particularly notable in new cases of insomnia. The other notable change was the slow but steady increase in prescriptions for slow-release melatonin (Circadin) since its release in 2009.30 Prescription rates for all other medications examined remained statistically unchanged except for the overall decline in temazepam. Contrary to reports of substantial prescribing of the antipsychotic quetiapine (Seroquel) for insomnia,31–35 we found barely discernible rates of use.
Limitations
The BEACH study used year-by-year nationally representative sampling of FPs that provided data to describe specific insomnia management rates by age and sex, and the drug management of insomnia over the 15 years reported in this study. Because data were collected by the FP-completed form we do not know if patients actually did obtain the drug from a pharmacist or whether or not they used it. Patients who actively seek non-pharmacological therapy may not present to FPs and may have accessed other forms of treatment for insomnia including CBT through a non-FP referred psychologist or online platform. In addition, because we chose to focus on management of insomnia in adults we cannot make conclusions about the care of insomnia in younger patients (younger than 15 years).
CONCLUSIONS
The management rate for insomnia in Australian family practice was stable between 2000–2015, except for a decrease in 2007–2008 in conjunction with adverse media interest regarding zolpidem. It has become progressively less common for older Australians (age 75 years or older) to be treated for insomnia. Australian FPs remain reliant on pharmacotherapy for the management of insomnia but the agents prescribed over the 15 years studied has changed, especially for new insomnia cases.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Grunstein has received funding to attend an advisory board meeting for Merck. This was not related to the current study.
Financial Support: Research supported by Australian NHMRC grants to RRG 571421 & 1060992.
Current major contributors to the BEACH Study (CH and HB):
AstraZeneca Pty Ltd (Australia) (1998–2016)
Australian Government Department of Health (1998–2004, 2007–2016)
Novartis Pharmaceuticals Australia Pty Ltd (2009–2016)
bioCSL (Australia) Pty Ltd (2010–2016)
Sanofi-Aventis Australia Pty Ltd (2006–2012, 2015–2016)
Some financial support for the BEACH program was also provided by:
Australian Government Department of Veterans' Affairs (2004–2016)
In past years covered in this study contributors to the BEACH project have been:
AbbVie Pty Ltd (2014–2015)
Merck, Sharpe and Dohme (Australia) Pty Ltd (2002–2013)
Pfizer Australia (2004–2013)
National Prescribing Service (2005–2009, 2012–2013)
GlaxoSmithKline Australia Pty Ltd (2010–2012)
Bayer Australia Ltd (2010–2011)
Janssen-Cilag Pty Ltd (2000–2010)
Abbott Australasia Pty Ltd (2006–2010)
Wyeth Australia Pty Ltd (2008–2010, then merged with Pfizer)
Roche Products Pty Ltd (1998–2006)
Aventis Pharma Pty Ltd (1998–2002)
Ethics: The BEACH program has Ethics approval and oversight from the Human Research Ethics Committee of the University of Sydney (Ref: 2012/130).
ACKNOWLEDGMENTS
Author Contributions: Study design: HCB, LV, CMH; Data collection: HCB, LV, CMH; Data analysis: LV; Interpretation of results: CBM, LV, NSM, NG; Preparation of the manuscript: CBM, LV, CMH, HCB, NSM, NG, NC, RRG.
ABBREVIATIONS
- BEACH
Bettering the Evaluation And Care of Health
- CBT
cognitive behavioral therapy
- CI
confidence interval
- FP
family physician
- ICPC
International Classification of Primary Care
REFERENCES
- 1.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.Kraus SS, Rabin LA. Sleep America: managing the crisis of adult chronic insomnia and associated conditions. J Affect Disord. 2012;138(3):192–212. doi: 10.1016/j.jad.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10:427–438. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Deloitte Access Economics. Re-awakening Australia: the economic cost of sleep disorders in Australia, 2010. Sleep Health Foundation website. [Accessed June 2016]. http://www.sleephealthfoundation.org.au/pdfs/news/Reawakening%20Australia.pdf. Published October 2011.
- 7.Calem M, Bisla J, Begum A, et al. Increased prevalence of insomnia and changes in hypnotics use in England over 15 years: analysis of the 1993, 2000, and 2007 National Psychiatric Morbidity Surveys. Sleep. 2012;35(3):377–384. doi: 10.5665/sleep.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asnis GM, Thomas M, Henderson MA. Pharmacotherapy treatment options for insomnia: a primer for clinicians. Int J Mol Sci. 2015;17(1):E50. doi: 10.3390/ijms17010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riemann D, Spiegelhalder K, Espie C, et al. Chronic insomnia: clinical and research challenges-an agenda. Pharmacopsychiatry. 2011;44(1):1–14. doi: 10.1055/s-0030-1267978. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 11.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 12.Kathol RG, Arnedt JT. Cognitive behavioral therapy for chronic insomnia: confronting the challenges to implementation. Ann Intern Med. 2016;165(2):149–150. doi: 10.7326/M16-0359. [DOI] [PubMed] [Google Scholar]
- 13.Parthasarathy S, Carskadon MA, Jean-Louis G, et al. Implementation of sleep and circadian science: recommendations from the Sleep Research Society and National Institutes of Health workshop. Sleep. 2016;39(12):2061–2075. doi: 10.5665/sleep.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung JMY, Bartlett DJ, Armour CL, Glozier N, Saini B. Insomnia patients' help-seeking experiences. Behav Sleep Med. 2014;12(2):106–122. doi: 10.1080/15402002.2013.764529. [DOI] [PubMed] [Google Scholar]
- 15.Kronholm E, Partonen T, Harma M, et al. Prevalence of insomnia-related symptoms continues to increase in the Finnish working-age population. J Sleep Res. 2016;25(4):454–457. doi: 10.1111/jsr.12398. [DOI] [PubMed] [Google Scholar]
- 16.Knox SA, Harrison CM, Britt HC, Henderson JV. Estimating prevalence of common chronic morbidities in Australia. Med J Aust. 2008;18(2):66–70. doi: 10.5694/j.1326-5377.2008.tb01918.x. 9. [DOI] [PubMed] [Google Scholar]
- 17.Australian Adverse Drug Reactions Bulletin, Vol 26, No 1. Australian Government Department of Health: Therapeutic Goods Administration website. [Accessed July 2016]. https://www.tga.gov.au/publication-issue/australian-adverse-drug-reactions-bulletin-vol-26-no-1. Published February 2007.
- 18.Drug Approval Package: Approval Letter(s) & Printed Labeling (PDF) United States Food and Drug Administration website. [Accessed May 2016]. http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019908_S000_AmbienTOC.cfm. Published December 16, 1992. Updated July 13, 2005.
- 19.Ambien Product Information Sheet. United States Food and Drug Administration website. [Accessed March 2017]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019908s037lbl.pdf. Published October 2014.
- 20.Product Information Stilnox Tablets. Australian Government Department of Health: Therapeutic Goods Administration website. [Accessed July 2016]. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05721-3&d=2016072516114622483. Published September 10, 1999.
- 21.Marshall N, Glozier N, Grunstein RR. Thirty-fold spike in adverse event reporting associated with zolpidem use in Australia was most likely caused by the media. Sleep. 2009;32:A393. Abstract Suppl. [Google Scholar]
- 22.Ben-Hamou M, Marshall NS, Grunstein RR, Saini B, Fois RA. Spontaneous adverse event reports associated with zolpidem in Australia 2001-2008. J Sleep Res. 2011;20(4):559–568. doi: 10.1111/j.1365-2869.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 23.Zolpidem (‘Stilnox’) Australian Government Department of Health: Therapeutic Goods Administration website. [Accessed July 2016]. https://www.tga.gov.au/alert/zolpidem-stilnox. Published February 21, 2008.
- 24.Zolpidem and sleep-related behaviours. NPS Medicinewise website. [Accessed September 2016]. http://www.nps.org.au/publications/health-professional/nps-radar/2008/august-2008/brief-item-zolpidem. Published August 1, 2008.
- 25.Wong CK, Marshall NS, Grunstein RR, et al. Spontaneous adverse event reports associated with zolpidem in the United States 2003-2012. J Clin Sleep Med. 2017;13(2):223–234. doi: 10.5664/jcsm.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britt H, Miller G. BEACH program update. Aust Fam Physician. 2015;44(6):411–414. [PubMed] [Google Scholar]
- 27.Charles J, Harrison C, Britt H. Insomnia. Aust Fam Physician. 2009;38(5):283. [PubMed] [Google Scholar]
- 28.Cheung JMY, Atternäs K, Melchior M, Marshall NS, Fois RA, Saini B. Primary health care practitioner perspectives on the management of insomnia: a pilot study. Aust J Prim Health. 2014;20(1):103–112. doi: 10.1071/PY12021. [DOI] [PubMed] [Google Scholar]
- 29.Circadin : EPAR - Summary for the public. European Medicines Agency website. [Accessed May 2016]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000695/human_med_000701.jsp&mid=WC0b01ac058001d124. Published May 2010. Updated August 13, 2015.
- 30.AusPAR: Melatonin. Australian Government Department of Health: Therapeutic Goods Administration website. [Accessed May 2016]. https://www.tga.gov.au/auspar/auspar-melatonin-0. Published November 28, 2009.
- 31.Anderson SL, Vande Griend JP. Quetiapine for insomnia: a review of the literature. Am J Health Syst Pharm. 2014;71(5):394–402. doi: 10.2146/ajhp130221. [DOI] [PubMed] [Google Scholar]
- 32.DUSC Review on the Utilisation of Antipsychotics - August 2013. The Pharmaceutical Benefits Scheme website. [Accessed May 2016]. http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2013-08/antipsychotics. Published August 2013.
- 33.Thompson W, Quay TA, Rojas-Fernandez C, Farrell B, Bjerre LM. Atypical antipsychotics for insomnia: a systematic review. Sleep Med. 2016;22:13–17. doi: 10.1016/j.sleep.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Lee TC, Desforges P, Murray J, Saleh RR, McDonald EG. Off-label use of quetiapine in medical inpatients and postdischarge. JAMA Intern Med. 2016;176(9):1390–1391. doi: 10.1001/jamainternmed.2016.3309. [DOI] [PubMed] [Google Scholar]
- 35.Hermes ED, Sernyak M, Rosenheck R. Use of second-generation antipsychotic agents for sleep and sedation: a provider survey. Sleep. 2013;36(4):597–600. doi: 10.5665/sleep.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britt H, Miller GC, Henderson J, et al. General practice activity in Australia 2014-15. The University of Sydney eScholarship Repository website. [Accessed May 2016]. https://ses.library.usyd.edu.au/handle/2123/11882. Published 2015.
- 37.Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2000-01 to 2009-10: 10 year data tables. Australian Institute of Health and Welfare website. [Accessed May 2016]. http://www.aihw.gov.au/publication-detail/?id=6442472440. Published December 8, 2010.
- 38.WONCA International Classification Committee. International Classification of Primary Care: ICPC-2. 2nd ed. Oxford, England: Oxford University Press; 1998. [Google Scholar]
- 39.ICPC-2 PLUS: the BEACH coding system. The University of Sydney website. [Accessed May 2016]. http://www.fmrc.org.au/icpc2plus/. Updated July 25, 2012.
- 40.Levy HB. Non-benzodiazepine hypnotics and older adults: what are we learning about zolpidem? Expert Rev Clin Pharmacol. 2014;7(1):5–8. doi: 10.1586/17512433.2014.864949. [DOI] [PubMed] [Google Scholar]
- 41.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kathol RG, Arnedt JT. Cognitive behavioral therapy for chronic insomnia: confronting the challenges to implementation. Ann Intern Med. 2016;165(2):149–150. doi: 10.7326/M16-0359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


