Abstract
Study Objectives:
In approximately 56% to 75% of patients with obstructive sleep apnea (OSA), the frequency and duration of apneas are influenced by body position. This is referred to as position-dependent OSA or POSA. Patients with POSA can be treated with a small device attached to either the neck or chest. These devices—a new generation of devices for positional therapy (PT)—provide a subtle vibrating stimulus that prevents patients adopting the supine position. The objectives of this study were to determine whether PT is effective in improving sleep study variables and sleepiness, and to assess compliance.
Methods:
A systematic review and meta-analysis.
Results:
Three prospective cohort studies and four randomized controlled trials were included in this review. Combined data for studies reporting on the effect of PT show that there was a mean difference of 11.3 events/h (54% reduction) in apnea-hypopnea index and 33.6% (84% reduction) in percentage total sleeping time in the supine position. The standardized mean difference for both parameters demonstrated a large magnitude of effect (> 0.8 in both cases).
Conclusions:
There is strong evidence that the new generation of devices for PT are effective in reducing the apnea-hypopnea index during short-term follow-up. These devices are simple-to-use for patients and clinicians and are reversible. Under study conditions with short-term follow-up, compliance is high; however, long-term compliance cannot be assessed because of lack of reliable data. Additional long-term, high-quality studies are needed to confirm the role of PT as a single or as a combination treatment modality for OSA patients and to assess long-term compliance.
Citation:
Ravesloot MJ, White D, Heinzer R, Oksenberg A, Pépin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13(6):813–824.
Keywords: compliance, meta-analysis, obstructive sleep apnea, positional, positional therapy, systematic review
INTRODUCTION
In approximately 56% to 75% of patients with obstructive sleep apnea (OSA), the frequency and duration of apneas are influenced by body position.1–5 Various definitions of position-dependent obstructive sleep apnea (POSA) have been applied in literature, but the most common classification system and definition marks a distinction between two groups of patients: positional patients (PP)6 and nonpositional patients (NPP).3,4,7 In PP, desaturations, cyclic variations in heart rate, loud snoring, and apneas and hypopneas appear almost exclusively in the supine position.8 Cartwright first described the arbitrary cutoff point of a difference of 50% or more in apnea index between supine and nonsupine positions.3
In the medical literature many have applied modified versions of Cartwright's criteria. In 1998, Marklund et al. defined supine-dependent sleep apnea as a supine apnea-hypopnea index (AHI) ≥ 10 events/h, together with a lateral AHI < 10 event/h.7 In studies by Permut et al. and Mador et al., POSA was defined as AHI < 5 events/h while in the nonsupine position as well as decreased AHI in the nonsupine positions compared to the supine position.9,10 In the study by Bignold et al., when patients met the following criteria, they were deemed position-dependent: overall AHI ≥ 15 events/h, supine AHI ≥ twice the nonsupine AHI, ≥ 20 minutes of sleep in supine and nonsupine postures, and nonsupine AHI < 15.11 The Amsterdam Positional OSA Criteria (APOC) aim to accurately identify candidates who will benefit from a clinically signifi-cant improvement in OSA with positional therapy (PT). The APOC focused on the percentage of total sleep time (TST) in both the worst sleeping position (WSP) and best sleeping position and the AHI in best sleeping position.12
The prevalence of POSA is thought to be higher in Asian populations.13,14 Most but not all PP still snore when sleeping in the lateral posture, but their sleep is interrupted with only a few apneas or hypopneas or not at all, and they consequently achieve a better sleep quality.15 By avoiding the supine position, their sleep is no longer fragmented. PP who have undergone treatment enjoy a more restful sleep and are more alert during daytime hours.16
The prevalence of POSA decreases as the severity of sleep apnea increases. The majority of PP (70% to 80%) have mild or moderate OSA.2,4,5,10 In Asian patients with mild OSA, up to 87% can be classified as PP.13 Those in the PP group compared to those in the NPP group have a lower body mass index (BMI) and are younger.2,4,5,17 One study found that of the patients on the waiting list for surgical weight loss, only 34% could be classified as PP.18 Those in the NPP group can become positional after weight loss, and the opposite is also true.18,19
Studies using a variety of diagnostic modalities in awake patients have suggested that PP compared to NPP have a more backward positioning of the lower jaw, lower facial height, longer posterior airway space (PAS) measurements, and a smaller volume of lateral pharyngeal wall tissue, resulting in a greater lateral diameter and elliptoid shape of the upper airway.20–24 Also, PP tend to have a smaller neck circumference.10 The current hypothesis is that even though the anterior-posterior diameter in both PP and NPP is reduced as a result of the effect of gravity in the supine position, because of the greater lateral diameter in PP, there is sufficient preservation of airway space and avoidance of complete upper airway collapse.14 Furthermore, it has been suggested that a decrease in functional residual lung capacity when moving from lateral to supine position may be an important triggering factor in the occurrence of OSA in PP.25
PT is aimed at preventing patients from sleeping in the WSP.2 In most cases, sleeping supine is the WSP for breathing function. Various techniques have been described, but the majority of studies on PT use the so-called tennis ball technique (TBT): a bulky mass strapped to the patient's back.1 Even though TBT is simple and cheap, as well as effective in reducing the AHI, results are unsatisfactory. It appears that discomfort is the main responsible factor for poor compliance and the subsequent disappointing long-term results.1 Compliance rates reported in the literature range from 40% to 70% in the short term to only 10% in the long term.1,16,26,27
Recent developments have seen the introduction of a new generation of devices for PT, a small device attached to either the neck or chest, that prevents the patient from adopting the supine position through a subtle vibrating stimulus.1,11,28,29 Encouraging data have been published suggesting that this simple therapy has good compliance and successfully prevents PP from adopting the supine position without negatively influencing sleep efficiency. Of additional value, the new generation of devices for PT, unlike TBT, provide objective compliance data. This is not only valuable for research purposes but also from a patient perspective. It has been suggested that new devices for PT show promise as a stand-alone treatment or as an additional measure to increase the success rate of other established treatment methods,35 but where is the evidence?
Objectives
The objectives of this study were to determine whether the new generation of devices for PT are effective in reducing the AHI by preventing PP from adopting the supine position in comparison to inactive PT (meaning a device is fastened to the patient, but it is not switched on so there is no vibrating feedback when the patient adopts the supine position) and to assess compliance with PT. A secondary objective was to evaluate whether the new devices for PT are able to improve the following metrics: arousal index, sleep efficiency, Epworth Sleepiness Scale (ESS) score, and awakenings.
METHODS
Search
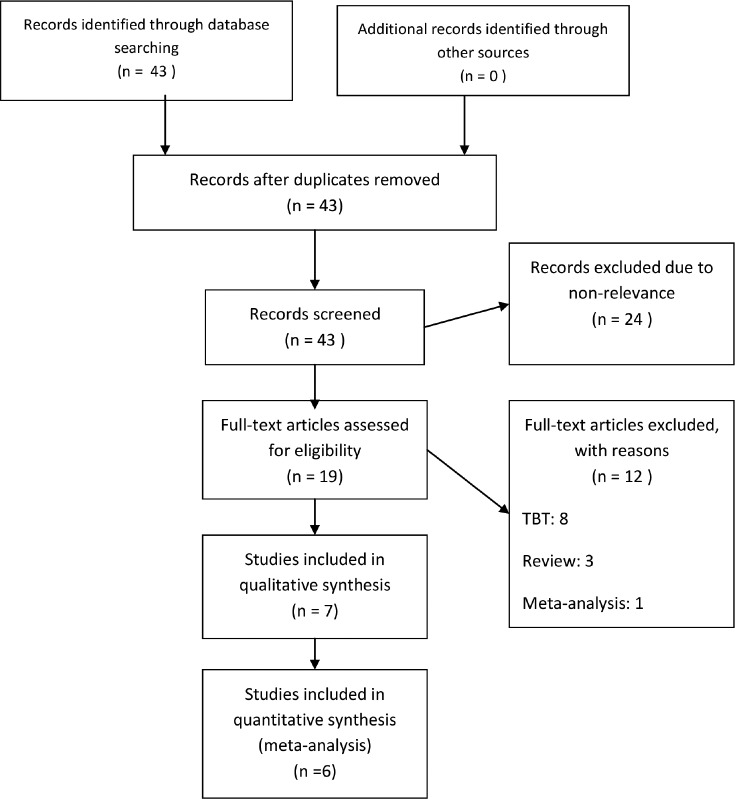
A search strategy was used with a combination of Medical Subject Headings (MeSH) and keywords (Appendix 1) in the MEDLINE and EMBASE databases. The reference lists of both review articles and primary studies were checked for additional references. Two investigators independently assessed the titles and abstracts. The number of records identified, included, and excluded were recorded in a PRISMA flow diagram.31 To identify ongoing studies and to check for unpublished completed studies, the ClinicalTrials.gov database was searched.
Inclusion and Exclusion Criteria
Studies evaluating the effect of a device either strapped to the neck or chest providing vibrotactile feedback if the supine position is adopted were included. Only studies analyzing adults with POSA diagnosed by polysomnography (PSG) or polygraphy (PG) were included.
Study Outcomes
The primary outcome measures of this review were AHI and compliance. Secondary outcome measures included percentage supine sleep time, arousal index, awakenings, and sleep efficiency.
Risk of Bias in Individual Studies
Risk of bias in individual studies was evaluated using the Cochrane Collaboration “Risk of bias” tool in the RevMan 5.3 software (Review Manager [RevMan] software, The Cochrane Collaboration, Copenhagen).
Statistical Analysis: Meta-Analysis
The mean AHI and percentage TST in the supine position were collected at baseline and with active treatment. These data were pooled from each study as to describe the effect of PT. Authors of the trials were contacted to attain mean and standard deviation data if not reported in the published article. The data were pooled using the inverse variance method for fixed-effects meta-analysis and rendered a mean difference (MD) and its associated 95% confidence interval. The magnitude of the effect was interpreted through the value of the standardized mean difference (SMD), defined as: small = 0.2, medium = 0.5 and large = 0.8.32
Interstudy heterogeneity was assessed using the χ2 and I2 tests and evaluated according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.33 P < .1 and I 2 > 50% were considered indicative of statistical heterogeneity, in which case the random-effects model was adopted. Statistical analyses were performed using RevMan 5.3 software.
Funnel plot graphical expressions were chosen to search and identify for bias. Visual inspection of the funnel plots was performed.
RESULTS
The results of the search strategy are depicted in Figure 1. The search was completed on October 13, 2015. Seven studies met the inclusion criteria for this review; three prospective cohort studies and four randomized controlled trials (RCT). A summary of the included studies can be found in Table 1 and Table 2. Table 3 and Table 4 show an overview of the objective outcome measures reported in the manuscripts. Five studies evaluated the efficacy of a chest-worn device, and two studies evaluated the efficacy of a neck-worn device.
Figure 1. Results of literature search.
PRISMA 2009 flow-diagram. PRISMA = preferred reporting items for systematic reviews and meta-analyses, TBT = tennis ball technique.
Table 1.
Summary of included randomized controlled trial studies.
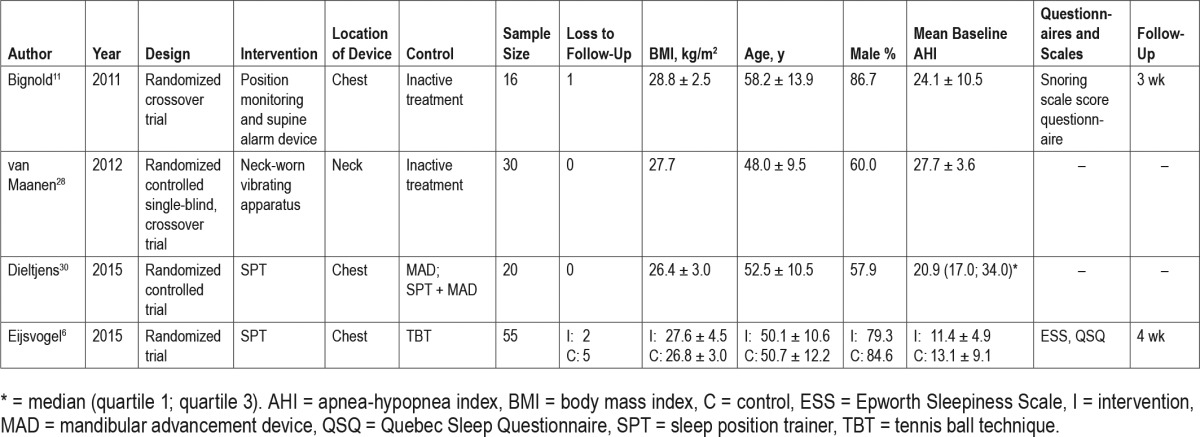
Table 2.
Summary of included single-group cohort studies.
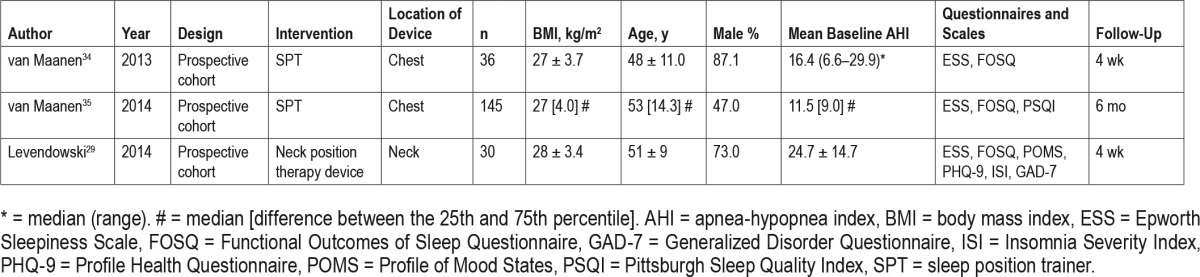
Table 3.
Overview of the results of the included randomized controlled trial studies.
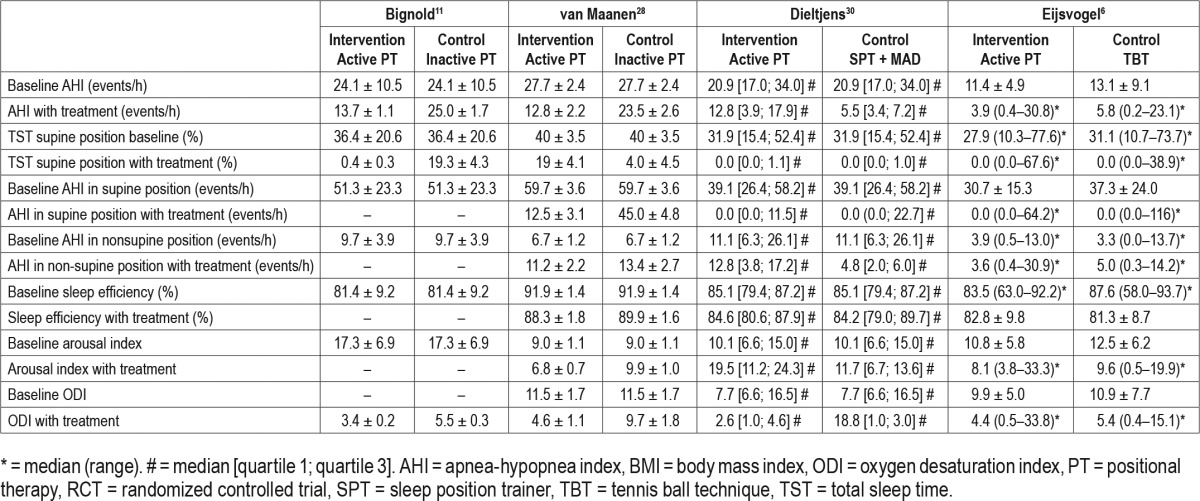
Table 4.
Overview of the results of the included single-group cohort studies.
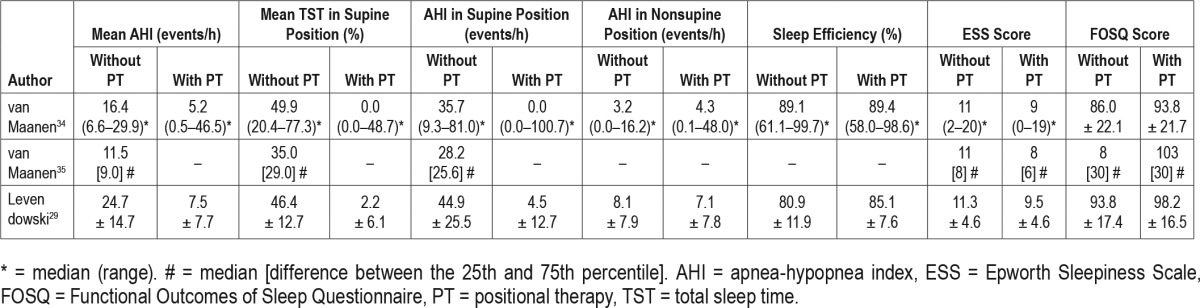
The chest-worn devices studied were both small, lightweight, battery-powered devices (see Figure 2 and Figure 3). The “position monitoring and supine alarm device” and sleep position trainer (SPT) weigh 50 g and 25 g, respectively, and have the following dimensions: 80 × 40 × 20 mm and 72 × 35 × 10 mm, respectively. Both devices are fastened to the chest with a strap, so that the device is located on the sternum. The SPT is placed in a pocket of the strap and closed with a Velcro tab. Both devices provide a vibrating stimulus if the supine position is identified, this is measured using internal position-sensitive tilt switches11 or a three-dimensional digital accelerometer.6,30,34,35 The device described by Bignold et al.11 gives off a vibration if the supine position is detected for 5 s consecutively. The SPT starts the stimulus if the supine position is detected and no turning movement is subsequently detected. The stimulus increases gradually until a nonsupine position is detected. If no reaction is monitored, the vibrations are paused and reinitiated after 2 minutes. Both devices have the capacity to store and register data, such as sleeping posture, that can be uploaded through custom software via a USB connection and personal computer.6,11,30,34,35
Figure 2. Position monitoring and supine alarm device.

Reprinted from Journal of Clinical Sleep Medicine, Vol 7(4), Bignold JJ et al. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device, pages 376-383, Copyright 2011, with permission of the American Academy of Sleep Medicine.11
Figure 3. Sleep position trainer.
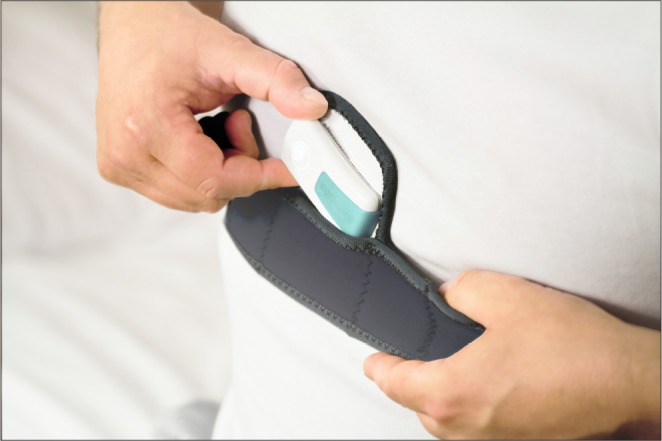
Copyright 2017, reprinted with permission of NightBalance B.V.
Concerning the neck-worn devices, the device (see Figure 4) described by van Maanen et al.28 consisted of a small vibrating apparatus (30 × 30 × 10 mm, powered by three small batteries). The position sensor provides a vibrating stimulus with a delay of 10 seconds after the supine position is detected, causing the device to vibrate with gradual incremental strength until a different sleeping position is detected, in which case the vibrations ceased immediately. The small device was worn secured to the skin of the neck with hypoallergenic adhesive tape and connected to the polysomnography system.28
Figure 4. Neck-worn positional therapy device.
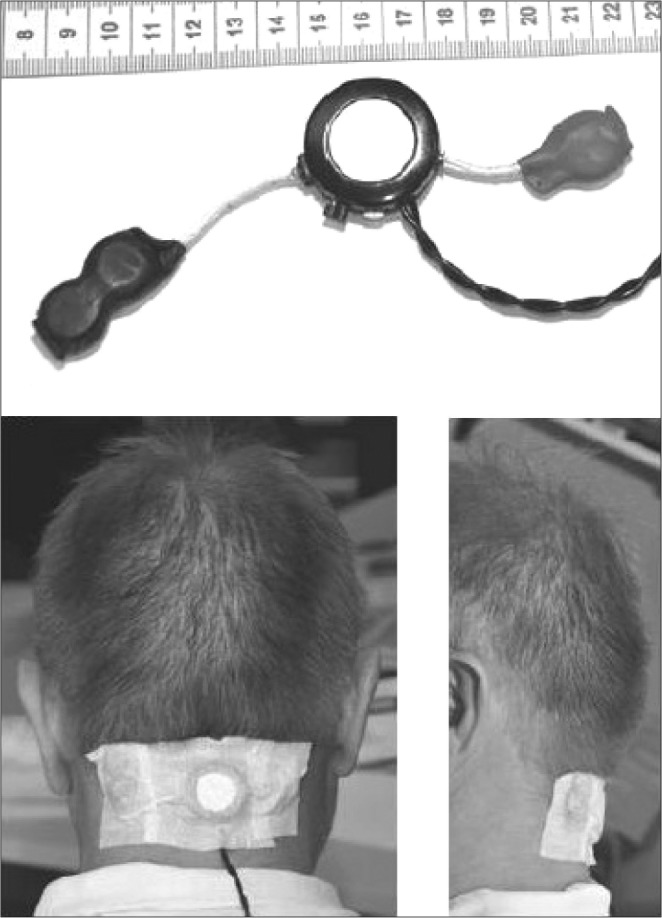
The middle part (ring structure) of the apparatus shown consists of a small vibrating motor (such as the one used in cell phones) and a position sensor. Three small round batteries (two positioned on the left, one on the right) are connected via the white cables. The braided black cables connect the device to the polysomnograph system. Reprinted from Journal of Sleep Research, Vol 21(3), van Maanen JP et al. Evaluation of a new simple treatment for positional sleep apnoea patients, pages 322-329, Copyright 2012, with permission of John Wiley & Sons, Inc.28
The neck-worn device as described by Levendowski et al.29 is a small, battery-powered device, weighing 44 g (55 × 38 × 16 mm) affixed to the back of the neck with an adjustable nonlatex silicone rubber strap secured by a magnetic clasp (see Figure 5). Using a three-dimensional digital accelerometer to detect sleeping position, a vibrating stimulus is provided by two 1G haptic motors if the supine position is adopted. Positional feedback is modulated by setting the number of motors to be excited (one or both) and varying the duration of the motor(s) excitation. Feedback is initiated at a very low frequency/duration, and gradually increased until the user is no longer in the supine position. At any given intensity level, the feedback is repeated six times with an interfeedback interval of 2 seconds. This device is also capable of storing data on supine sleeping time, frequency and duration of feedback, snoring, and sleep/wake.29
Figure 5. Neck position training device.
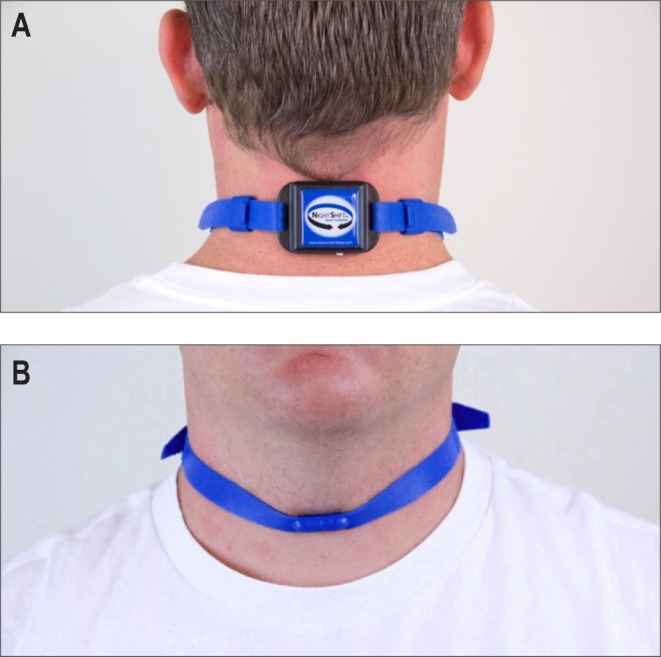
Reprinted from Journal of Clinical Sleep Medicine, Vol 10(8), Levendowski DJ et al. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea, pages 863-871, Copyright 2014, with permission of the American Academy of Sleep Medicine.29
Randomized Controlled Trials
A summary of the included RCT and their results can be found in Table 1 and Table 3, respectively.
Two articles report randomized crossover trials comparing active versus inactive PT. Bignold et al.11 evaluated the efficacy of a chest-worn device (see Figure 2) in 15 patients fulfilling the following criteria: overall AHI ≥ 15 events/h, supine AHI twice or greater than nonsupine AHI, ≥ 20 min of sleep in supine and nonsupine positions, and a nonsupine AHI < 20 events/h. Subjects were assigned to receive the active PT or inactive PT in a random order for a week followed by a 1-week washout period before commencing the alternative treatment. The device consists of a position monitoring and supine alarm device fastened to the chest. The mean baseline AHI (24.1 events/h) was reduced approximately 45% with active treatment.11
van Maanen et al.28 performed a study evaluating the efficacy of a neck-worn device (see Figure 4), including 30 PP according to Cartwright's criteria.3 Randomly in one of the two test recordings the device was active, in the other it was inactive. Patients were blinded to the chosen activity state of the device. In the treatment group, there was a statistically signifi-cant mean decrease in AHI from 27.7 events/h to 12.8 events/h (54%) and in 23% there was an overall AHI < 5 events/h. The device did not have a significant effect on sleep efficiency.28
Eijsvogel et al.6 observed, without taking compliance into consideration, that SPT (see Figure 3) and TBT were equally effective in reducing respiratory indices in 55 patients in an RCT. Both therapies reduced time spent in the supine sleep position to a median of 0%. An AHI < 5 events/h was achieved in 43% and 68% of the patients treated with TBT and SPT, respectively (P = .087). Compliance, defined as ≥ 4 h/night and ≥ 5 nights/wk, was 75.9% for the SPT and 42.3% for TBT users (P = .01). There were more dropouts in the TBT group (5 versus 2). Reasons for dropout were shoulder/back pain, inability to adopt a supine position, or vibrating noise. A mean disease alleviation of 48.6% and 70.5% for TBT and SPT, respectively, was observed (P = .005). Objective sleep quality parameters and awakenings had greater improvement in the SPT group, as well as the Quebec Sleep Questionnaire (QSQ) scores.6
Dieltjens et al.30 evaluated the additional effect of SPT in patients with residual POSA during mandibular advancement (MAD) therapy in an RCT performed in 20 patients. One arm received SPT alone prior to SPT with MAD, while the second group received SPT with MAD, followed by SPT alone. The SPT reduced the time spent in supine sleeping position compared to baseline and MAD therapy. Both MAD and SPT were individually effective in reducing the overall AHI significantly when compared to baseline from a median 20.9 events/h at baseline to 11.0 events/h (47%) and 12.8 events/h (39%) with MAD or SPT respectively. When treated with a combination of SPT with MAD, the AHI was further reduced to 5.5 events/h (76%). This was statistically significantly lower when compared with baseline, MAD alone, and SPT alone. It was concluded that the combination of SPT and MAD leads to a higher therapeutic efficacy in patients with residual POSA during MAD therapy in comparison with either therapy alone.30
Risk of Bias
A summary of the risk of bias can be found in Figure 6. All of the studies were randomized, but the method used to generate the allocation sequence was not described, nor the method used to conceal the allocation sequence. In two studies it was unrealistic to blind patients to their allocated treatment modality; SPT versus SPT and MAD and SPT versus TBT.6,30 Bignold et al. indicate that it was difficult to blind patients to active versus inactive treatment allocation.11 In all studies the individuals assigned to score the polysomnographic details were blinded for the treatment assignment. Loss to follow-up and reasons for dropout were described. An intention-to-treat analysis was performed in four studies6,11,28,29; one was a per protocol analysis35 and in the remaining two no specifics were mentioned.30,34 In one of these two there was no loss to follow-up,30 and in the other five patients withdrew.34
Figure 6. Risk of bias summary for the included studies.
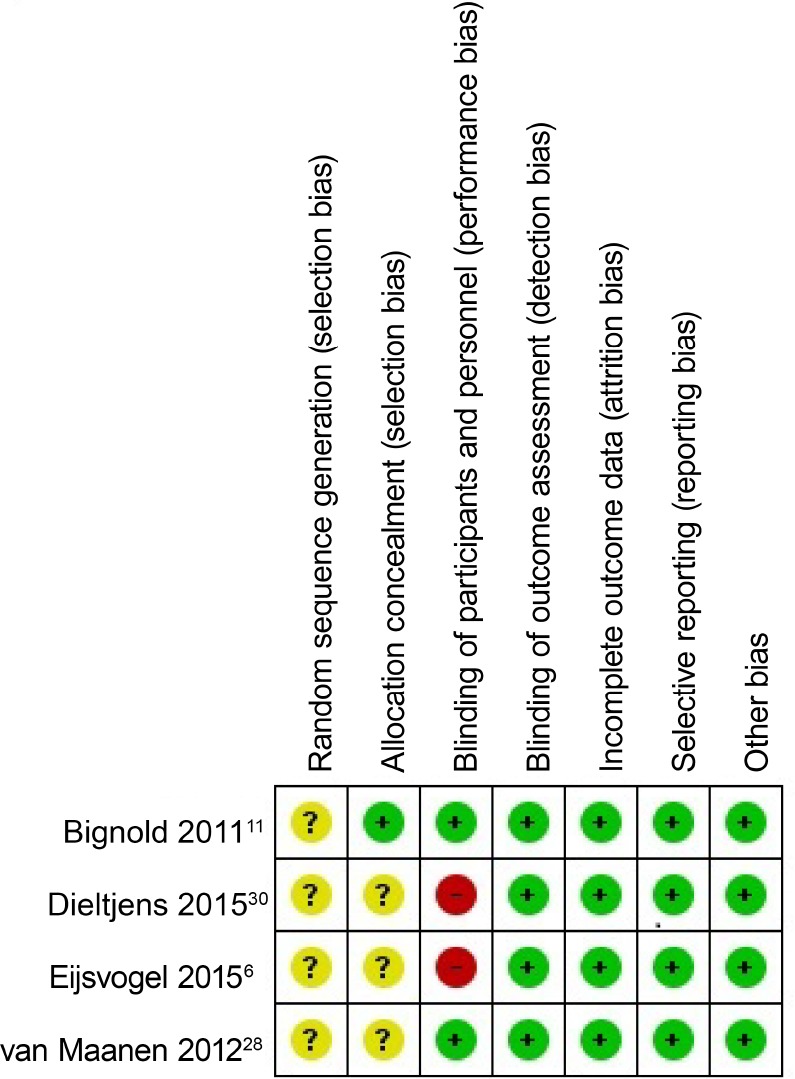
Green circle with plus sign = low risk of bias, yellow circle with question mark = unclear risk of bias, red circle with minus sign = high risk of bias.
Single-Group Cohort Studies
A summary of the included single-group studies and the results can be found in Table 2 and Table 4, respectively. In studies by van Maanen et al.34 and Levendowski et al.29 patients were instructed to wear either a chest-worn or neck-worn device for 30 days, respectively (see Figure 3 and Figure 5). In both studies upon completion, patients were requested to complete a variety of questionnaires including the ESS and underwent a follow-up PSG with active treatment.
van Maanen et al.34 included 36 PP according to Cartwright's criteria.3 The AHI decreased from a median 16.4 events/h to 5.2 events/h (68.3%) (P < .001).
Thirty patients with an AHI ≥ 5 events/h, an overall AHI ≥ 1.5 times the nonsupine AHI, and ESS ≥ 5 were included by Levendowski et al. The AHI decreased from a mean 24.7 events/h to 7.5 events/h (69.6%) (P < .001). Objective compliance data showed that the median 1-month compliance rate was 96% (71% to 100%), defined as the number of nights the device was worn 4 hours or longer divided by the days during the intention-to-treat period.29
van Maanen et al. evaluated the long term-effectiveness of and compliance with SPT. One hundred forty-five patients were included with mild to moderate OSA. Patients received a battery of questionnaires at baseline and after 1, 3, and 6 months. There was a significant decrease in ESS, increase in Functional Outcomes of Sleep Questionnaire (FOSQ) and decrease in Pittsburgh Sleep Quality Index (PSQI) compared with baseline. In addition, patients were asked to register their SPT in the online database in order to retrieve the percentage supine sleep time and objective compliance data. The median percentage of supine sleep time decreased significantly from 21% to 2%, 2% and 3%, after 1, 3, and 6 months respectively. After 6 mo, on average, the patients not lost to follow-up (n = 53 of 145), used their SPT 6.7 h/night and were 100% compliant (> 4 h/night, 7 nights/wk).34
Ongoing Studies
Three ongoing studies (as of October 13, 2015) were found on ClinicalTrials.gov. One study hopes to evaluate the effectiveness of PT in comparison with no treatment in an RCT, focusing on both subjective and objective parameters.36 A second study intends to compare PT with MAD therapy in patients with POSA in an RCT.37 A third proposes to judge the cost-effectiveness of SPT by comparing SPT with continuous positive airway pressure (CPAP), MAD, and the combination of MAD and SPT in patients with moderate POSA.38
Meta-Analysis
Apnea-hypopnea index with and without PT
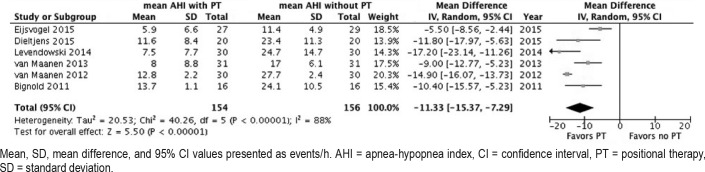
Data addressing the comparison of AHI with and without PT were available from six studies. Pooling of the results demonstrated that the AHI was significantly reduced when PT was applied in comparison to baseline from a mean 21.8 ± 7.2 to 9.9 ± 10.6 (53.6%). Random-effects analysis demonstrated an AHI MD of −11.33 events/h (95% CI −15.37, −7.29), Z-value = 5.50 (P < .00001), see Figure 7. The AHI SMD was −1.94 (95% CI −2.90, −0.97) (large effect).
Figure 7. Forest plot comparing mean apnea-hypopnea index with and without treatment.
Mean, SD, mean difference, and 95% CI values presented as events/h. AHI = apnea-hypopnea index, CI = confidence interval, PT = positional therapy, SD = standard deviation.
Percentage total sleep time in supine position with and without PT
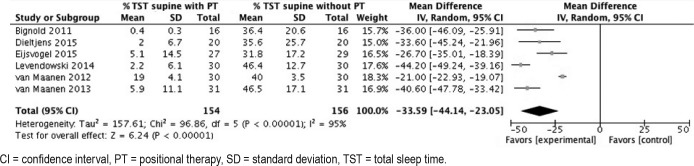
Data addressing the comparison of % TST in supine position with and without PT were available from six studies. Pooling of the results showed that the % TST in the supine position was significantly reduced when PT was applied in comparison with baseline from a mean 40.1% ± 17.2 to 6.5% ± 10.8 (83.8% reduction). Random-effects meta-analysis demonstrated a MD of −33.59, 95% CI −44.14, −23.05, Z-value = 6.24, P < .00001; Figure 8). The SMD was −3.02 (−4.09, −1.94) (large effect).
Figure 8. Forest plot comparing percentage of total sleep time in the supine position with and without treatment.
CI = confidence interval, PT = positional therapy, SD = standard deviation, TST = total sleep time.
Sensitivity analysis
Visual inspection of the funnel plot including all six studies showed no obvious asymmetry, nevertheless there were two outliers, most likely due to the small number, small study population, and methodological diversity (such as different inclusion criteria for example) of the studies. There was a normal distribution of the six effects.
On visual inspection of the funnel plot including the four studies evaluating trunk-worn devices, there was no asymmetry nor outliers, but the distribution of the effects was non-normal. A funnel plot of the effects of the non-RCT studies, showed no asymmetry, but there were two outliers and a non-normal distribution of the effects.
Subgroup analysis was not feasible due to the small number of studies evaluating neck-worn devices.
DISCUSSION
All studies published on the new generation of devices for PT have shown that PT is efficacious in reducing the AHI in patients with OSA. It is a simple, well-tolerated (at least in the short term), and reversible treatment. In PP, it must be emphasized that severity of disease is directly related to the sleeping time spent or not spent in the supine position. Therefore, the effectiveness of PT is dependent on the residual % TST in supine position, the AHI in nonsupine position, and compliance with therapy. We found that the mean % TST in supine position was reduced by 83.8% and that the mean % TST in supine position with PT was 6.5%. The mean AHI was reduced to a mean AHI of 9.9 events/h, a 53.6% reduction. Comparing these results with other treatment modalities for OSA (CPAP, surgery, etc.) is difficult. PP are generally younger and have a lower BMI, hindering such comparisons. Previous studies comparing the efficacy of TBT, or modifications of it, with the new generation of devices for PT indicate similar results.1,9,16,39–46 However, under study conditions with short-term follow-up, compliance is higher with the newer generation PT devices. Therefore, greater therapeutic effectiveness seems likely.
When interpreting results of treatment approaches, it is of importance to bear in mind that the effectiveness of conservative treatment reducing respiratory indices depends both on its effect on airway obstruction and compliance.47–51 In the case of PT these are residual % TST in supine position, AHI in non-supine position, and compliance.
The importance of compliance is illustrated in the study by Eijsvogel et al.6 Although therapeutic efficacy was comparable between PT and TBT, compliance with PT was superior. Taking compliance into account, a mean disease alleviation of 48.6% and 70.5% was achieved for TBT and the new generation PT respectively.6
Both CPAP and—to a lesser extent—MAD therapy are hampered by compliance issues. With the advent of objective monitoring, in two prospective small-scale studies, a median use of MAD therapy for 6.4 h/night was reported after 3 months and a mean use of 6.1 h/night after 1 year.52,53 Approximately 29% to 83% of patients using CPAP are noncompliant.54 Eight percent to 15% of patients refuse CPAP treatment after a single night's use and 20% to 40% will discontinue CPAP after 3 months.55
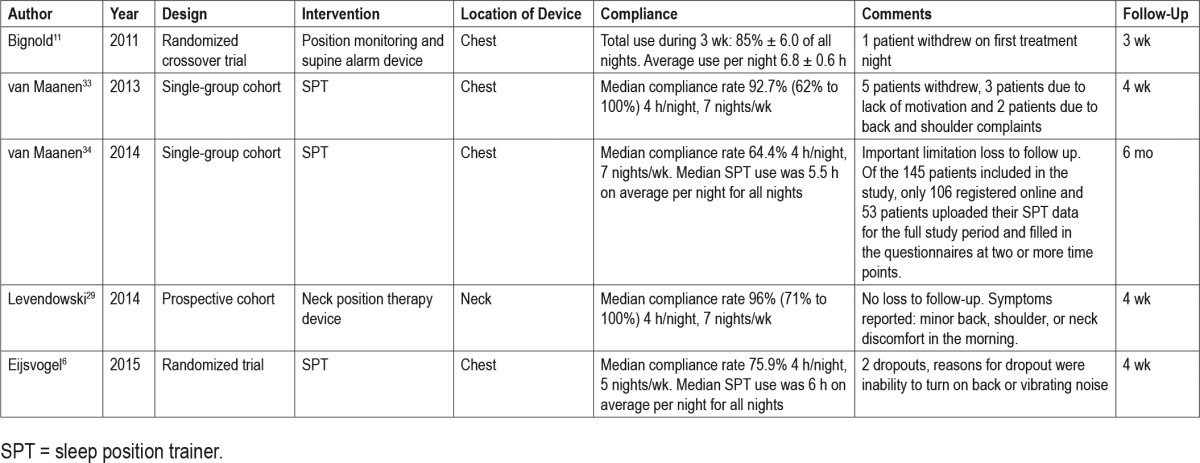
Under study conditions, short-term (1 month) follow-up median compliance of new-generation PT devices is high, varying from 76% to 96%, when compliance is defined as 4 h/night, 7 nights/wk.6,29,34 van Maanen et al. also reported on long-term follow-up. Unfortunately these results are hampered by a 50% loss to follow-up, making interpretation of results difficult35 (see Table 5). Average new-generation PT device use per night was 6.8 h/night over a 3-week period and a median of 6.5 h/night and 5.5 h/night over a 1-month and 6-month period, respectively. A median 92.7% to 96% of patients were compliant over a 1-month period, with compliance defined as ≥ 4 h/ night, 7 nights/wk. Lack of reliable data on long-term compliance has serious implications when applying PT in clinical practice and needs specific attention in future research.
Table 5.
Compliance data for new-generation positional therapy devices.
Two types of new-generation PT devices were analyzed in the various articles, either neck-worn or chest-worn. It would be interesting to perform a subanalysis comparing the efficacy (reduction in AHI) of neck-worn versus chest-worn devices and their effect on sleep quality and comfort. One could hypothesize that a chest-worn device may be more comfortable than a neck-worn device, but might be more likely to shift during sleep.
One study demonstrated the importance of head position, separate from trunk position, as an important parameter to measure in PP.56 The authors conclude that in one-fourth of all patients with OSA, the head position is an important factor, in addition to the position of the trunk. In these patients, the AHI calculated while the head is in a supine position is higher than the AHI determined over the time when the trunk is in the supine position. Furthermore, 6.5% of patients were not position-dependent based on the position sensor on the trunk and were classified as only head-supine–dependent. This finding may have consequences for the methods used for future diagnosis and treatment of OSA.
In the past, Cartwright showed that the “combined effect of PT and a tongue retaining device was better than one of the treatment modalities alone.”57 The role of combination therapy is again advocated by Dieltjens et al.30 This is particularly interesting when one considers that the majority of PP experience mild or moderate OSA and that patients with mild or moderate OSA can be suitable candidates for MAD therapy. The prevalence of residual POSA in patients under MAD therapy is 33.9%58 according to Cartwright's criteria.3 Further studies are needed to evaluate long-term benefit and compliance of combined therapy.
Limitations of the Study
The quality of the included studies was high, concerning risk of bias. Unfortunately in the majority of studies it was not feasible to blind the patients to their allocated treatment modality. We estimate that this will not negatively influence polysomnographic data because of its objective nature, but risk of bias concerning subjective data such as the ESS is present in some studies.
Pooling of results was only feasible for PT itself, because of substantial clinical diversity between studies, particularly with respect to the control intervention type. Comparison across studies was complicated by the application of different definitions of POSA and different inclusion criteria. Hours of use of PT were not readily available for all studies; therefore, we were unable to keep compliance into consideration. In three studies median summary data were reported, due to non-normal distribution of the data. By using the mean for pooling of results, we may be underestimating the effect.
Clinical Implications of New Devices for PT
Suitable candidates for PT are those who will benefit from a clinically significant improvement of their OSA with PT, bearing in mind that patients could benefit from a combination of therapies.59
Description of patients who will or will not benefit from this mode of therapy have been explored.60 In one paper three categories were defined12:
A PP has a nonsupine AHI < 5 events/h. By avoiding the supine position, the patient can be cured.
An NPP will not benefit from PT because his or her breathing abnormalities during sleep are not influenced by sleeping position.
- A multifactorial patient can benefit from PT, but not be cured. The multifactorial patient's OSA severity is influenced in part by sleep position.
- Patients with a nonsupine AHI in a lower OSA severity category than the overall AHI. If treated with PT, the patient can theoretically decrease in overall AHI and OSA severity category. As a consequence patients can undergo less aggressive primary treatment (less invasive surgery, for example).
- In patients who do not tolerate CPAP or oral appliances, PT can be considered salvage therapy. For example, as the AHI decreases, so does the CPAP pressure needed, potentially improving adherence.
Recommendations for Future Perspectives
Current data available are promising, but more research is needed.61 Although we are stating the obvious, there is a demand for high-quality evidence from more and larger RCTs evaluating the efficacy and cost-effectiveness of new-generation PT devices. There should be a focus on the effect of PT on quality-of-sleep metrics, such as sleep fragmentation, sleep continuity, and sleep stage changes. Also needed are long-term follow-up studies, assessing compliance and the effect of PT on daytime sleepiness, other daytime symptoms, health-related quality of life, and cardiovascular, metabolic and cognitive parameters. Furthermore, it is essential to evaluate PT failures and the possibility of adaptation to the vibratory stimuli with long-term use.
Evaluating the efficacy of PT is hindered by the fact that there are no universally used POSA criteria. Use of a universal classification can facilitate collection of data across multiple centers and comparison of results across studies. The most common classification and definition used to date is Cart-wright's classification.3 Various classifications, especially modified versions of Cartwright's criteria, have been applied in the literature, aiming to improve clinical relevance.7,9–12
The potential extensive application of PT also needs to be explored. Other populations that may benefit from PT are pregnant woman with POSA,62 patients with positional central sleep apnea,63 and position-dependent habitual snorers.64
Some hypothesize that by using new-generation PT, patients are trained to avoid the supine sleep position, resulting in self-avoidance of the supine position during sleep. Research on this topic is needed.65
Studies have shown that the WSP is the supine position in nearly three-fourths of patients.2 Research is needed to better understand patients with an alternate WSP. New-generation PT devices have the potential to also treat these patients.
Although there are some interesting data on the anatomical basis for the phenotype distinction between patients with positional and nonpositional OSA, more research on this topic is required.20
Various predictors of POSA have become apparent: age, AHI, BMI and ethnicity, for example. A few studies have explored associations between craniofacial characteristics and POSA. Studies in larger populations and different ethnic groups are needed. This is not only important to better understand the pathophysiology of POSA but also to identify pheno-types associated with the development and severity of POSA.
CONCLUSIONS
All studies published on new-generation PT devices have shown that PT is effective in reducing the AHI during short-term follow-up. Furthermore, they are simple-to-use for both patients and clinicians, and they are reversible. Under study conditions with short-term follow-up, compliance is high; however, long-term compliance cannot be assessed because of lack of reliable data. PT has potential as a treatment modality for patients with OSA, in particular those with mild or moderate OSA. Additional high-quality, long-term studies are needed to confirm the promising role of PT.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. White is a medical advisor for NightBalance, a consultant for Philips Respironics, and chief medical officer of Apnicure Inc. Drs. Pépin and Heinzer are medical advisors for NightBalance. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank H. Marti-Soler, PhD from the Institute of Social and Preventive Medicine, University of Lausanne, Switzerland for her contributions and assistance with the sensitivity analysis from.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APOC
Amsterdam Positional OSA Classification
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- GAD-7
Generalized Disorder Questionnaire
- ISI
Insomnia Severity Index
- MAD
mandibular advancement device
- MeSH
medical subject heading
- NPP
patient with non-positional OSA
- OSA
obstructive sleep apnea
- PAS
posterior airway space
- PHQ-9
Profile Health Questionnaire
- POMS
Profile of Mood States
- POSA
position-dependent obstructive sleep apnea
- PP
patient with POSA
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- PT
positional therapy
- QSQ
Quebec Sleep Questionnaire
- RCT
randomized controlled trial
- RoB
risk of bias
- SD
standard deviation
- SPT
sleep position trainer
- TBT
tennis ball technique
- WSP
worst sleeping position
APPENDIX
Appendix 1: Search Syntax
(“Sleep Apnea, Obstructive”[Mesh] OR OSAS[ti/ab] OR OSAHS[ti/ab] OR OSA[ti/ab] OR POSA[ti/ab] OR (sleep[ti/ab] AND (apnea[ti/ab] OR apneas[ti/ab] OR apnei*[ti/ab] OR apneu*[TI/AB] OR apnoea[ti/ab] OR apnoeas[ti/ab]))) AND ((position[ti/ab] OR positional[ti/ab] OR posture[ti/ab] OR supine[ti/ab])) AND (PT[ti/ab] OR therapy[ti/ab] OR device[ti/ab] OR trainer[ti/ab] OR treatment[ti/ab]))
REFERENCES
- 1.Ravesloot MJ, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17(1):39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravesloot MJ, Frank MH, van Maanen JP, Verhagen EA, de Lange J, de Vries N. Positional OSA part 2: retrospective cohort analysis with a new classification system (APOC) Sleep Breath. 2016;20(2):881–888. doi: 10.1007/s11325-015-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 4.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 5.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263(10):946–950. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 6.Eijsvogel MM, Ubbink R, Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11(2):139–147. doi: 10.5664/jcsm.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114(6):1630–1635. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 8.Oksenberg A, Gadoth N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res. 2014;23(2):204–210. doi: 10.1111/jsr.12097. [DOI] [PubMed] [Google Scholar]
- 9.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 10.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128(4):2130–2137. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 11.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7(4):376–383. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank MH, Ravesloot MJ, van Maanen JP, Verhagen E, de Lange J, de Vries N. Positional OSA part 1: towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath. 2015;19(2):473–480. doi: 10.1007/s11325-014-1022-9. [DOI] [PubMed] [Google Scholar]
- 13.Mo JH, Lee CH, Rhee CS, Yoon IY, Kim JW. Positional dependency in Asian patients with obstructive sleep apnea and its implication for hypertension. Arch Otolaryngol Head Neck Surg. 2011;137(8):786–790. doi: 10.1001/archoto.2011.122. [DOI] [PubMed] [Google Scholar]
- 14.Teerapraipruk B, Chirakalwasan N, Simon R, et al. Clinical and polysomnographic data of positional sleep apnea and its predictors. Sleep Breath. 2012;16(4):1167–1172. doi: 10.1007/s11325-011-0627-5. [DOI] [PubMed] [Google Scholar]
- 15.Oksenberg A, Gadoth N. Continuous and loud snoring only in the supine posture. J Clin Sleep Med. 2015;11(12):1463–1464. doi: 10.5664/jcsm.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope. 2006;116(11):1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 17.Itasaka Y, Miyazaki S, Ishikawa K, Togawa K. The influence of sleep position and obesity on sleep apnea. Psychiatry Clin Neurosci. 2000;54(3):340–341. doi: 10.1046/j.1440-1819.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 18.Morong S, Benoist LB, Ravesloot MJ, Laman DM, de Vries N. The effect of weight loss on OSA severity and position dependence in the bariatric population. Sleep Breath. 2014;18(4):851–856. doi: 10.1007/s11325-014-0955-3. [DOI] [PubMed] [Google Scholar]
- 19.Oksenberg A, Dynia A, Nasser K, Gadoth N. Obstructive sleep apnoea in adults: body postures and weight changes interactions. J Sleep Res. 2012;21(4):402–409. doi: 10.1111/j.1365-2869.2011.00988.x. [DOI] [PubMed] [Google Scholar]
- 20.Saigusa H, Suzuki M, Higurashi N, Kodera K. Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology. 2009;110(4):885–890. doi: 10.1097/ALN.0b013e31819b5d57. [DOI] [PubMed] [Google Scholar]
- 21.Chang ET, Shiao GM. Craniofacial abnormalities in Chinese patients with obstructive and positional sleep apnea. Sleep Med. 2008;9(4):403–410. doi: 10.1016/j.sleep.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Walsh JH, Leigh MS, Paduch A, et al. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep. 2008;31(11):1543–1549. doi: 10.1093/sleep/31.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soga T, Nakata S, Yasuma F, et al. Upper airway morphology in patients with obstructive sleep apnea syndrome: effects of lateral positioning. Auris Nasus Larynx. 2009;36(3):305–309. doi: 10.1016/j.anl.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Pevernagie DA, Stanson AW, Sheedy PF, 2nd, Daniels BK, Shepard JW., Jr Effects of body position on the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;152(1):179–185. doi: 10.1164/ajrccm.152.1.7599821. [DOI] [PubMed] [Google Scholar]
- 25.Joosten SA, Sands SA, Edwards BA, et al. Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology. 2015;20(5):819–827. doi: 10.1111/resp.12549. [DOI] [PubMed] [Google Scholar]
- 26.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5(5):428–430. [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzer RC, Pellaton C, Rey V, et al. Positional therapy for obstructive sleep apnea: an objective measurement of patients' usage and efficacy at home. Sleep Med. 2012;13(4):425–428. doi: 10.1016/j.sleep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 28.van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21(3):322–329. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 29.Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863–871. doi: 10.5664/jcsm.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19(2):637–644. doi: 10.1007/s11325-014-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum; 1988. [Google Scholar]
- 33.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2008 Version 5.0.0. The Cochrane Collaboration. [Google Scholar]
- 34.van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17(2):771–779. doi: 10.1007/s11325-012-0764-5. [DOI] [PubMed] [Google Scholar]
- 35.van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014;37(7):1209–1215. doi: 10.5665/sleep.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tønnesen P Glostrup University Hospital Copenhagen. Night Balance for Positional Obstructive Sleep Apnea Syndrome (POSAS) [Accessed October 13, 2015]. ClinicalTrials.gov Identifier: NCT02114424. ClinicalTrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02114424. Published April 10, 2014.
- 37.de Ruiter MHT. Academisch Medisch Centrum - Universiteit van Amsterdam, St. Lucas Andreas Ziekenhuis Hospital. RCT: Oral Appliance Therapy and Sleep Position Trainer in Patients With Position Dependant Obstructive Sleep Apnea (POSA) [Accessed October 13, 2015]. ClinicalTrials.gov Identifier: NCT02045576. ClinicalTrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02045576. Published November 26, 2013.
- 38.Benoist LBL. Onze Lieve Vrouwe Gasthuis, University Hospital Antwerp. Economic Evaluation of Treatment Modalities for Position Dependent Obstructive Sleep Apnea. [Accessed October 13, 2015]. ClinicalTrials.gov Identifier: NCT02553902. ClinicalTrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02553902. Published September 14, 2015.
- 39.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 40.Maurer JT, Stuck BA, Hein G, Verse T, Hormann K. Treatment of obstructive sleep apnea with a new vest preventing the supine position. Dtsch Med Wochenschr. 2003;128(3):71–75. doi: 10.1055/s-2003-36658. [DOI] [PubMed] [Google Scholar]
- 41.Braver HM, Block AJ. Effect of nasal spray, positional therapy, and the combination thereof in the asymptomatic snorer. Sleep. 1994;17(6):516–521. doi: 10.1093/sleep/17.6.516. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel S, Smith E, Leiacker R, Fischer Y. Efficacy and longterm compliance of the vest preventing the supine position in patients with obstructive sleep apnea. Laryngorhinootologie. 2007;86(8):579–583. doi: 10.1055/s-2007-966179. [DOI] [PubMed] [Google Scholar]
- 43.Loord H, Hultcrantz E. Positioner-a method for preventing sleep apnea. Acta Otolaryngol. 2007;127(8):861–868. doi: 10.1080/00016480601089390. [DOI] [PubMed] [Google Scholar]
- 44.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus nCPAP in the management of position‐dependent obstructive sleep apnoea syndrome. Respirology. 2008;13(5):708–715. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 45.Choi JH, Park YH, Hong JH, et al. Efficacy study of a vest‐type device for positional therapy in position dependent snorers. Sleep Biol Rhythms. 2009;7(3):181–187. [Google Scholar]
- 46.Kavey NB, Blitzer A, Gidro-Frank S, Korstanje K. Sleeping position and sleep apnea syndrome. Am J Otolaryngol. 1985;6(5):373–377. doi: 10.1016/s0196-0709(85)80015-6. [DOI] [PubMed] [Google Scholar]
- 47.Ravesloot MJ, de Vries N, Stuck BA. Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea. Laryngoscope. 2014;124(1):344–345. doi: 10.1002/lary.24302. [DOI] [PubMed] [Google Scholar]
- 48.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34(1):105–110. doi: 10.1093/sleep/34.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16(5):921–927. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland K, Phillips CL, Cistulli PA. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. Journal of Dental Sleep Medicine. 2015;2(4):175–181. [Google Scholar]
- 51.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieltjens M, Braem MJ, Vroegop AV, et al. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502. doi: 10.1378/chest.13-0613. [DOI] [PubMed] [Google Scholar]
- 53.Dieltjens M, Verbruggen AE, Braem MJ, et al. Determinants of objective compliance during oral appliance therapy in patients with sleep-related disordered breathing: a prospective clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(10):894–900. doi: 10.1001/jamaoto.2015.1756. [DOI] [PubMed] [Google Scholar]
- 54.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014;(1):CD007736. doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 56.van Kesteren ER, van Maanen JP, Hilgevoord AA, Laman DM, de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. Sleep. 2011;34(8):1075–1081. doi: 10.5665/SLEEP.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14(6):546–552. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- 58.Dieltjens M, Braem MJ, Van de Heyning PH, Wouters K, Vanderveken OM. Prevalence and clinical significance of supine-dependent obstructive sleep apnea in patients using oral appliance therapy. J Clin Sleep Med. 2014;10(9):959–964. doi: 10.5664/jcsm.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gadoth N, Oksenberg A. Positional therapy in obstructive sleep apnea: for whom and for whom not. In: de Vries N, Ravesloot M, van Maanen JP, editors. Positional Therapy in Obstructive Sleep Apnea. Switzerland: Springer International Publishing; 2015. pp. 383–394. [Google Scholar]
- 60.Oksenberg A, Silverberg DS. The effect of body posture on sleep-related breathing disorders: facts and therapeutic implications. Sleep Med Rev. 1998;2(3):139–162. doi: 10.1016/s1087-0792(98)90018-1. [DOI] [PubMed] [Google Scholar]
- 61.Oksenberg AS. Positional therapy for sleep apnea: a promising behavioral therapeutic option still waiting for qualified studies. Sleep Med Rev. 2014;18(1):3–5. doi: 10.1016/j.smrv.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Morong S, Hermsen B, de Vries N. Sleep-disordered breathing in pregnancy: a review of the physiology and potential role for positional therapy. Sleep Breath. 2014;18(1):31–37. doi: 10.1007/s11325-013-0849-9. [DOI] [PubMed] [Google Scholar]
- 63.Jannsen H, Verbraecken JA, Pervernagie D, Overeem S. Positional central sleep apnea. In: de Vries N, Ravesloot M, van Maanen JP, editors. Positional Therapy in Obstructive Sleep Apnea. Switzerland: Springer International Publishing; 2015. pp. 209–219. [Google Scholar]
- 64.Benoist LB, Morong S, van Maanen JP, Hilgevoord AA, de Vries N. Evaluation of position dependency in non-apneic snorers. Eur Arch Otorhinolaryngol. 2014;271(1):189–194. doi: 10.1007/s00405-013-2570-5. [DOI] [PubMed] [Google Scholar]
- 65.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1985;8(2):87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]