Abstract
Central sleep apnea (CSA) and continuous positive airway pressure (CPAP) emergent CSA are common in patients for whom opioids have been prescribed for chronic pain management. It is not known if opioids are the potential cause of CSA. We report the case of a patient who underwent multiple full nights of polysomnography testing while on opioids, off opioids, and with various positive airway pressure devices. While on opioids, the patient had severe CSA that persisted during both CPAP and bilevel titration but was eliminated with adaptive servoventilation therapy. Some time later, opioid use was discontinued by the patient. Repeat polysomnography showed resolution of the sleep-disordered breathing. Later—while the patient was still off opioids—she had gained weight and become symptomatic; polysomnography showed obstructive sleep apnea without CSA. This time, therapy with CPAP showed elimination of sleep apnea without emergent CSA. These data collectively indicate that opioids were the cause of CSA as well as emergent CSA.
Citation:
Javaheri S, Patel S. Opioids cause central and complex sleep apnea in humans and reversal with discontinuation: a plea for detoxification. J Clin Sleep Med. 2017;13(6):829–833.
Keywords: adaptive servoventilation, central sleep apnea
INTRODUCTION
Opioids use disorder is a widespread health care crisis.1 Many young individuals on opioids are found deceased in bed. Opioids (at times with other drugs) are found in the bloodstream at autopsy, but the actual cause of death remains unknown. Respiratory depression2 or torsade de pointes3 could be the cause of death in these individuals.
Fatal opioids-induced ventilatory depression occurs during sleep, in particular with disordered breathing events. These events include central and obstructive apneas, and hypoventilation.2,4,5
We report the case of a patient who underwent multiple sleep studies on and off opioids and with and without continuous positive airway pressure (CPAP) therapy. These studies strongly indicate that opioids were the cause of her central sleep apnea (CSA), and also emergent CSA with commencement of CPAP and bilevel positive airway pressure (PAP) therapy. CSA and PAP-emergent CSA were reversed when opioids were withdrawn. A preliminary report has been previously presented in abstract form.6
REPORT OF CASE
In May 2006, a 44-year-old woman was referred for evaluation of sleep apnea. She complained of snoring, frequent nocturnal awakenings, and excessive daytime sleepiness. She had undergone decompression for Chiari type 1 malformation with syringomyelia 6 years prior to referral. Since then, she suffered headaches and was treated with oxycodone 40 mg three times daily and hydrocodone 5 mg, two to four times daily. In addition, the patient was taking duloxetine 20 mg daily for depression. Her body mass index was 33.5 kg/m2; physical examination was otherwise normal. The patient's Epworth Sleepiness Scale score was 17 of 24.
Full-night attended polysomnography was performed using standard techniques and definitions detailed previously.7 Here we only emphasize apnea distinction that is critical to the case report. Airflow was qualitatively monitored using an oronasal thermistor (Model 1459, Sleepmate Technologies, Midlothian, Virginia, United States). While the patient was wearing a PAP device, airflow was monitored by the pneumotach of the device. An apnea was defined as cessation of airflow for ≥ 10 seconds. An obstructive apnea was defined as the absence of airflow in the presence of rib cage and abdominal excursions. A central apnea was defined as the absence of airflow with absence of rib cage, abdominal, and sum of rib and abdominal excursions (flat signals from these probes).7
The patient had severe sleep apnea with an apnea-hypopnea index (AHI) of 64 events/h. The central apnea index (CAI) was 30 events/h with mild desaturation (Table 1, Figure 1). Titration with CPAP (up to a pressure of 8 cm H2O) was performed, and the patient's AHI decreased to 42 events/h due to reduction in obstructive events, but her CAI increased to 35 events/h. She complained of excess pressure and underwent a full-night bilevel titration. CAI increased further to 52 events/h (Table 1, Figure 2). With adaptive servoventilation titration, AHI decreased to 17 events/h with virtual elimination of apneas but persistent residual hypopneas, a common polysomnographic observation.7
Table 1.
Polysomnographic data on and off opioids, and off and on continuous positive air pressure therapy.
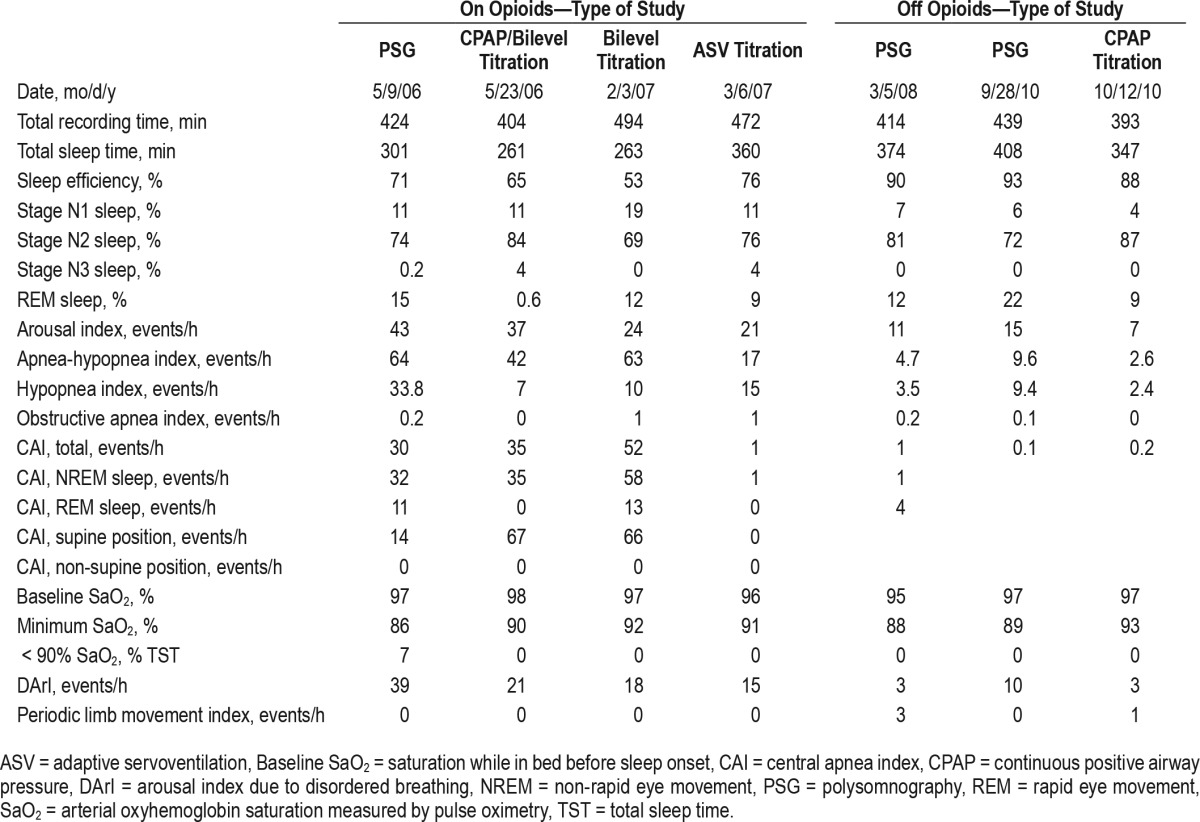
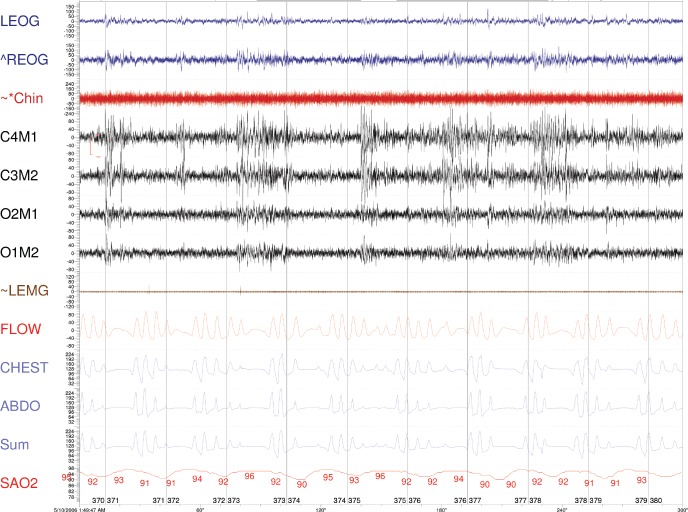
Figure 1. Five-minute epoch from May 2006.
Please note that with central apnea, airflow, as measured by thermocouple, from the rib cage, abdominal, and the sum excursions are all flat. ABDO = abdomen respiratory inductance plethysmography, FLOW = thermocouple, CHEST = rib cage respiratory inductance plethysmography, Chin = chin electromyogram, ECG = central electroencephalogram, LEOG = left electro-oculogram, LEMG = leg electromyogram, REOG = right electro-oculogram, SaO2 = arterial oxyhemoglobin saturation, Sum = sum of the CHEST and ABDO. The amplitude of the signals are not proportional.
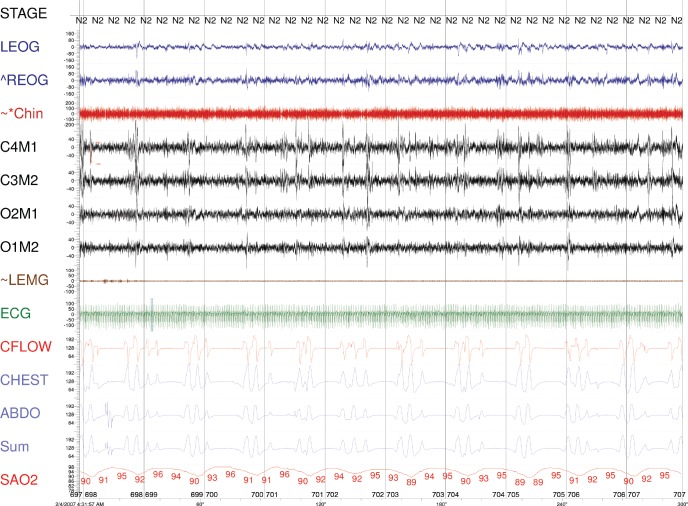
Figure 2. Five-minute epoch from February 2007 on CPAP of 8 cm H2O.
Please note that with central apnea, airflow monitored by the pneumotach, from the rib cage, abdominal, and the sum excursions are all flat. ABDO = abdomen respiratory inductance plethysmography, CFLOW = pneumotach, CHEST = rib cage respiratory inductance plethysmography, Chin = chin electromyogram, ECG = central electroencephalogram, LEOG = left electro-oculogram, LEMG = leg electromyogram, REOG = right electro-oculogram, SaO2 = arterial oxyhemoglobin saturation, Sum = sum of the CHEST and ABDO. The amplitude of the signals are not proportional.
The patient remained intolerant to therapy and complained of claustrophobia. She failed to make any additional appointments. However, she was followed by her physcian who ordered polysomnography in March 2008, 4 months after the patient completed narcotic detoxification. The patient was still taking duloxetine. She was asymptomatic when the polysomnography was performed, and it showed an AHI of 4.7 events/h and CAI of 1 event/h (Table 1).
Two years later, the patient complained of the return of excessive daytime sleepiness and nonrestorative sleep, presumably due to significant weight gain. She remained off opioids but continued taking duloxetine. Polysomnography showed mild obstructive sleep apnea with AHI of 10 events/h and CAI of zero events/h. Because of the patient's excessive daytime sleepiness, CPAP titration was performed. This led to improved AHI without the emergence of CSA (Table 1).
DISCUSSION
This patient underwent seven full nights of polysomnography testing in our center over several years. All polysomnographies were reviewed by an author (SJ). Initially, she had severe sleep apnea with many central apneas consistent with opioid-associated sleep-disordered breathing. Central apneas persisted with CPAP and bilevel therapy, but were eliminated with adaptive servoventilation therapy consistent with previous observations.7
The remaining 3 polysomnographies were performed after the patient ceased taking opioids. The first polysomnogram, obtained 4 months after discontinuation of opioids, showed virtual elimination of disordered breathing. The second polysomno-gram, obtained when the patient had gained weight and became symptomatic with excessive daytime sleepiness, showed mild obstructive sleep apnea without CSA. Importantly, in contrast to the previous observation, CPAP titration (during the third polysomnographic study off opioids) showed improvement in AHI and an absence of emergent CSA (Table 1).
This evolution of sleep-disordered breathing over several years is consistent with the notion that—in this patient—opioid use was the cause of CSA and persistent CSA with CPAP and bilevel therapy. The reduction of the AHI and CAI following detoxification further supports this notion.
There are two case reports8,9 of reversal of CSA with discontinuation of opioids; however, in one report9 oximetry was used after withdrawal of opioids, showing elimination of de-saturation. The notion that opioids can cause CSA in humans is consistent with animal studies10,11 that show opioids suppress breath rhythm generation via interaction on the μ receptors on the pre-Bötzinger complex in the brainstem, the presumed site of the respiratory rhythm generation.
CSA persisted in this patient during titration with CPAP and bilevel PAP. This finding is consistent with our previous report of persistent CSA in patients on opioids after weeks of adherence to CPAP.7 We previously reported that in patients with OSA in whom treatment-emergent CSA developed, CSA was eliminated with continued use of CPAP in almost all patients.12 One exception among these patients was the group of patients on opioids. Meanwhile, in a large group of patients on opioids with obstructive sleep apnea and without CSA, treatment-emergent CSA developed in almost all patients, with a prevalence much higher than treatment-emergent CSA in OSA patients who do not use opioids.12 We therefore suggest that the pathobiological mechanisms of this treatment-emergent and persistent CSA in opioid users is different than the garden variety transitory complex sleep apnea.7,12 In opioid users, CPAP is effective in eliminating obstructive events but remains ineffective for CSA, because of the continued suppression of rhythm generation by opioids. We acknowledge, however, that in some opioid users with OSA, CPAP-emergent CSA does not develop.
Our patient also suffered from Chiari malformation for which she had undergone craniotomy. This malformation is a known cause of CSA; however, it is very unlikely that this malformation was the reason for CSA in our patient, because after detoxification CSA was eliminated and did not reemerge with CPAP therapy.
We also note that after detoxification, when the patient was asymptomatic, AHI decreased to 4.7 events/h. Later when she was symptomatic, AHI was 9 events/h (Table 1). We have no accurate records of the patient's weight during this period when she was followed by her physician.
In animal studies from two different laboratories,10,11,13 opioids have been shown to decrease hypoglossal nerve activity at two different sites within the brainstem. It is therefore conceivable that detoxification contributed to attenuation of obstructive sleep-disordered breathing.
We conclude that this case report strongly suggests that opioids can be a cause of severe CSA and CPAP-persistent CSA in humans. Many individuals on opioids are found dead in bed, without any apparent cause. It is suspected that a terminal apnea could be a potential cause of death. It is our hope that by limiting the use of opioids, sleep-disordered breathing can be eliminated and death may be prevented in these young individuals.
DISCLOSURE STATEMENT
Work for this study was performed at Sleepcare Diagnostics. Both authors have seen and approved the manuscript. There was no financial support provided for this study, and the authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Randerath WJ. Opioid-induced central sleep apnea: mechanisms and therapies. Sleep Med Clin. 2014;9(1):49–56. [Google Scholar]
- 3.Kao D, Bartelson BB, Khatri V, et al. Trends in reporting methadone-associated cardiac arrhythmia, 1997-2011. Ann Intern Med. 2013;158:735–740. doi: 10.7326/0003-4819-158-10-201305210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farney RJ, McDonald AM, Boyle KM, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J. 2013;42(2):394–403. doi: 10.1183/09031936.00120012. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Cao M, Yue HJ, Chawla P. Obstructive sleep apnea and chronic opioid use. Lung. 2010;188(6):459–468. doi: 10.1007/s00408-010-9254-3. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Patel S. Opioids cause central sleep apnea in humans. Am J Respir Crit Care Med. 2016;193:A4222. [Google Scholar]
- 7.Javaheri S, Harris N, Howard J, Chung E. Adaptive servo-ventilation for treatment of opioids-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637–643. doi: 10.5664/jcsm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MJ, Livingston M, Scharf SM. Reversal of central sleep apnea following discontinuation of opioids. J Clin Sleep Med. 2012;8(5):579–580. doi: 10.5664/jcsm.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramar K. Reversal of sleep-disordered breathing with opioid withdrawal. Pain Pract. 2009;9(5):394–398. doi: 10.1111/j.1533-2500.2009.00295.x. [DOI] [PubMed] [Google Scholar]
- 10.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7(3):232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31(4):1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javaheri S, Smith J, Chung J. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 13.Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587(Pt 11):2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]