Abstract
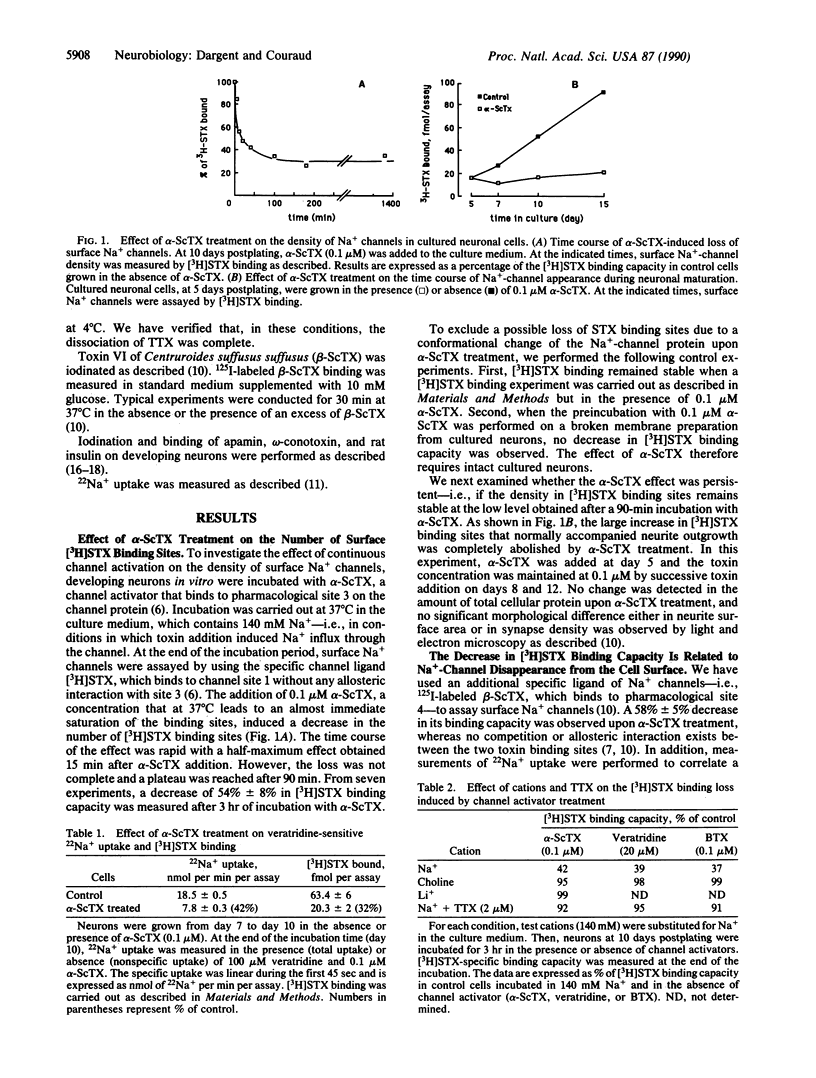
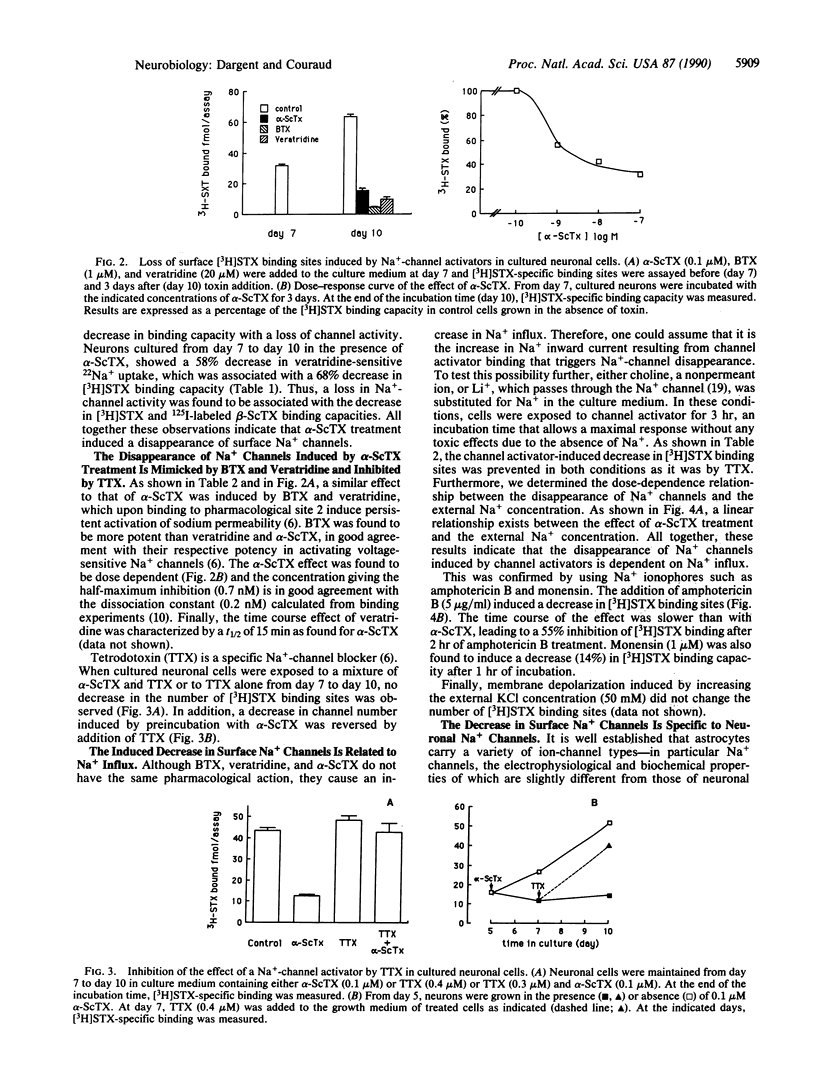
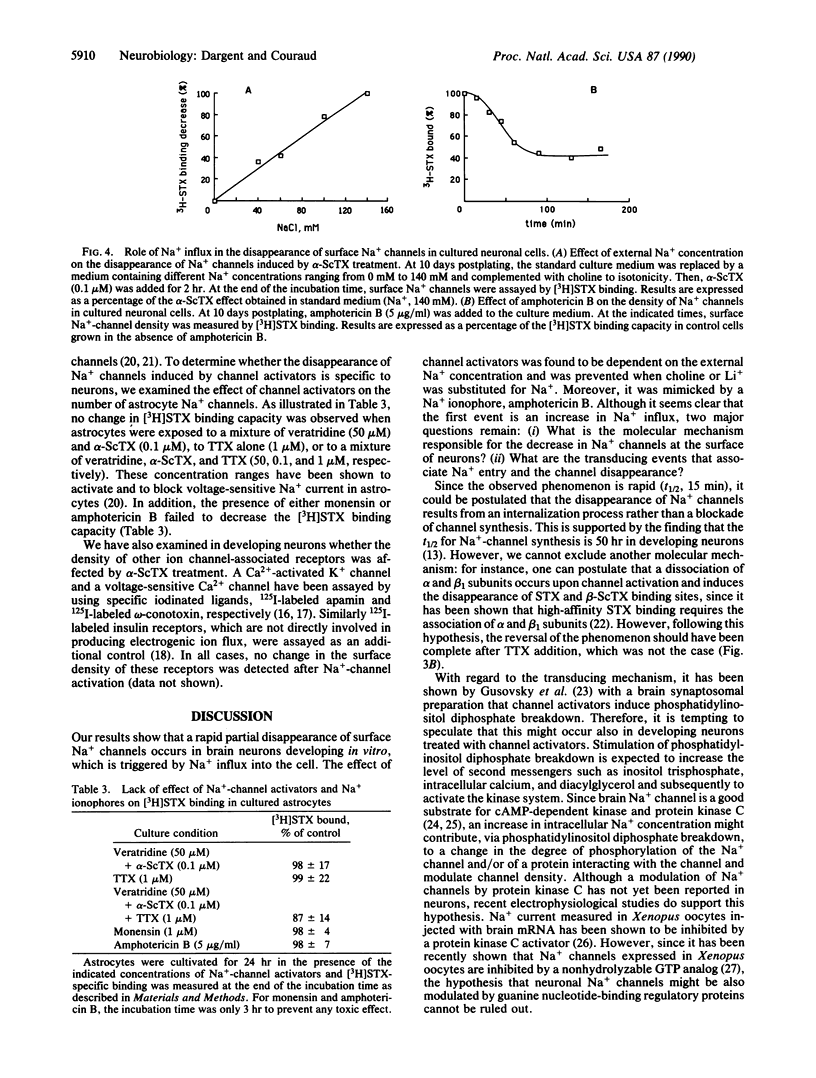
To address the issue of whether regulatory feedback exists between the electrical activity of a neuron and ion-channel density, we investigated the effect of Na(+)-channel activators (scorpion alpha toxin, batrachotoxin, and veratridine) on the density of Na+ channels in fetal rat brain neurons in vitro. A partial but rapid (t1/2, 15 min) disappearance of surface Na+ channels was observed as measured by a decrease in the specific binding of [3H]saxitoxin and 125I-labeled scorpion beta toxin and a decrease in specific 22Na+ uptake. Moreover, the increase in the number of Na+ channels that normally occurs during neuronal maturation in vitro was inhibited by chronic channel activator treatment. The induced disappearance of Na+ channels was abolished by tetrodotoxin, was found to be dependent on the external Na+ concentration, and was prevented when either choline (a nonpermeant ion) or Li+ (a permeant ion) was substituted for Na+. Amphotericin B, a Na+ ionophore, and monensin were able to mimick the effect of Na(+)-channel activators, while a KCl depolarization failed to do this. This feedback regulation seems to be a neuronal property since Na(+)-channel density in cultured astrocytes was not affected by channel activator treatment or by amphotericin B. The present evidence suggests that an increase in intracellular Na+ concentration, whether elicited by Na(+)-channel activators or mediated by a Na+ ionophore, can induce a decrease in surface Na+ channels and therefore is involved in down-regulation of Na(+)-channel density in fetal rat brain neurons in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Prives J. Negative modulation of sodium channels in cultured chick muscle cells by the channel activator batrachotoxin. J Biol Chem. 1985 Apr 25;260(8):4740–4744. [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Beckh S., Noda M., Lübbert H., Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989 Dec 1;8(12):3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwald-Netter Y., Martin-Moutot N., Koulakoff A., Couraud F. Na+-channel-associated scorpion toxin receptor sites as probes for neuronal evolution in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1245–1249. doi: 10.1073/pnas.78.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudier J. A., Berwald-Netter Y., Dellmann H. D., Boudier J. L., Couraud F., Koulakoff A., Cau P. Ultrastructural visualization of Na+-channel associated [125I]alpha-scorpion toxin binding sites on fetal mouse nerve cells in culture. Brain Res. 1985 May;352(1):137–142. doi: 10.1016/0165-3806(85)90097-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M., Sokolovsky M., Dascal N. Modulation of the voltage-dependent sodium channel by agents affecting G-proteins: a study in Xenopus oocytes injected with brain RNA. Brain Res. 1989 Sep 4;496(1-2):197–203. doi: 10.1016/0006-8993(89)91066-4. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Phosphorylation of the alpha subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984 Sep;4(3):291–297. doi: 10.1007/BF00733592. [DOI] [PubMed] [Google Scholar]
- Couraud F., Jover E., Dubois J. M., Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Couraud F., Martin-Moutot N., Koulakoff A., Berwald-Netter Y. Neurotoxin-sensitive sodium channels in neurons developing in vivo and in vitro. J Neurosci. 1986 Jan;6(1):192–198. doi: 10.1523/JNEUROSCI.06-01-00192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein N. G., Catterall W. A., Moon R. T. Identification of a 33-kilodalton cytoskeletal protein with high affinity for the sodium channel. Biochemistry. 1988 Mar 22;27(6):1818–1822. doi: 10.1021/bi00406a003. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Merrick D., Auld V., Dunn R., Goldin A. L., Davidson N., Catterall W. A. Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusovsky F., Hollingsworth E. B., Daly J. W. Regulation of phosphatidylinositol turnover in brain synaptoneurosomes: stimulatory effects of agents that enhance influx of sodium ions. Proc Natl Acad Sci U S A. 1986 May;83(9):3003–3007. doi: 10.1073/pnas.83.9.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Heidenreich K. A., de Vellis G., Gilmore P. R. Functional properties of the subtype of insulin receptor found on neurons. J Neurochem. 1988 Sep;51(3):878–887. doi: 10.1111/j.1471-4159.1988.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Jover E., Massacrier A., Cau P., Martin M. F., Couraud F. The correlation between Na+ channel subunits and scorpion toxin-binding sites. A study in rat brain synaptosomes and in brain neurons developing in vitro. J Biol Chem. 1988 Jan 25;263(3):1542–1548. [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marqueze B., Martin-Moutot N., Levêque C., Couraud F. Characterization of the omega-conotoxin-binding molecule in rat brain synaptosomes and cultured neurons. Mol Pharmacol. 1988 Aug;34(2):87–90. [PubMed] [Google Scholar]
- Messner D. J., Catterall W. A. The sodium channel from rat brain. Role of the beta 1 and beta 2 subunits in saxitoxin binding. J Biol Chem. 1986 Jan 5;261(1):211–215. [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Ascher P., Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987 Jan;7(1):101–109. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord J., Catterall W. A. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989 May;2(5):1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Poli M. A., Mende T. J., Baden D. G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol Pharmacol. 1986 Aug;30(2):129–135. [PubMed] [Google Scholar]
- Rossie S., Gordon D., Catterall W. A. Identification of an intracellular domain of the sodium channel having multiple cAMP-dependent phosphorylation sites. J Biol Chem. 1987 Dec 25;262(36):17530–17535. [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986 Aug 1;46(3):437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- Seagar M. J., Granier C., Couraud F. Interactions of the neurotoxin apamin with a Ca2+-activated K+ channel in primary neuronal cultures. J Biol Chem. 1984 Feb 10;259(3):1491–1495. [PubMed] [Google Scholar]
- Sherman S. J., Catterall W. A. Electrical activity and cytosolic calcium regulate levels of tetrodotoxin-sensitive sodium channels in cultured rat muscle cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):262–266. doi: 10.1073/pnas.81.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y., Elmer L., Davis J., Bennett V., Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988 May 12;333(6169):177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]


