Abstract
Background
Oesophageal diverticula are rare outpouchings of the oesophagus which may be classified anatomically as pharyngeal (Zenker’s), mid-oesophageal and epiphrenic. While surgery is indicated for symptomatic patients, no consensus exists regarding the optimum technique for non-Zenker’s oesophageal diverticula. The aim of this study was to determine the outcome of surgery in patients with non-Zenker’s oesophageal diverticula.
Methods
PubMed, MEDLINE and the Cochrane Library (January 1990 to January 2016) were searched for studies which reported outcomes of surgery in patients with non-Zenker’s oesophageal diverticula. Primary outcome measure was the rate of staple line leakage.
Results
Twenty-five observational studies involving 511 patients (259 male, median age 62 years) with mid-oesophageal (n = 53) and epiphrenic oesophageal (n = 458) diverticula who had undergone surgery [thoracotomy (n = 252), laparoscopy (n = 204), thoracoscopy (n = 42), laparotomy (n = 5), combined laparoscopy and thoracoscopy (n = 8)] were analysed. Myotomy was performed in 437 patients (85.5%), and anti-reflux procedures were performed in 342 patients (69.5%). Overall pooled staple line leak rates were reported in 13.3% [95% c.i. (11.0–15.7), p < 0.001] and were less common after myotomy (12.4%) compared with no myotomy (26.1%, p = 0.002).
Conclusions
No consensus exists regarding the surgical treatment of non-Zenker’s oesophageal diverticula, but staple line leakage is common and is reduced significantly by myotomy.
Keywords: Oesophageal diverticula, Myotomy
Introduction
Oesophageal diverticula (OD) are rare outpouchings of the oesophagus with a prevalence of up to 3% based on radiologic and endoscopic studies.1 , 2 OD may be classified anatomically as pharyngeal (Zenker’s) which is the most common type (70%), middle and distal oesophageal (epiphrenic).3 The aetiology of non-Zenker’s OD can be divided into traction and pulsion. Traction diverticula are true diverticula (include all layers of the oesophagus) which are due to chronic mediastinal diseases.4 Pulsion diverticula are false diverticula (an outpouching of the mucosa or submucosa) caused by increased intraluminal pressure secondary to a motility disorder or mechanical obstruction.5 , 6
While surgery is indicated for symptomatic patients, no consensus exists regarding the optimum technique for non-Zenker’s OD (transabdominal versus transthoracic, open versus minimally invasive, diverticulectomy versus diverticulopexy, routine versus selective myotomy and the need for an anti-reflux procedure). This is because alterations in oesophageal motility are not simply detected despite great improvements in the understanding of the pathophysiology of oesophageal functional diseases.5 , 7 Although various disorders such as achalasia, hypertensive lower oesophageal sphincter and diffuse oesophageal spasm have been found to be associated with non-Zenker’s OD,8 histologic abnormalities of the oesophageal myenteric plexus were reported in 80% of patients in the absence of a specific motility disorder.9
Surgery is an effective treatment for non-Zenker’s OD but is associated with significant morbidity of up to 75% including staple line leak rates of up to 33% and mortality of up to 11%.8 , 10 – 13 These outcomes have not changed despite advancements in minimally invasive surgery and stapling devices.14 , 15 In the absence of randomised controlled trials, we conducted a systematic review and meta-analysis of observational studies to determine the optimal surgical approach in patients with non-Zenker’s OD.
Materials and Methods
Search Strategy
A systematic review of published work was conducted according to the Meta-Analysis of Observational Studies and Epidemiology (MOOSE)16 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)17 guidelines. A systematic search of PubMed, MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews and Cochrane Controlled Trials Register, Cochrane Library, was performed by DC on March 1, 2016. A sensitive search strategy that combined the exploded thesaurus term for oesophageal diverticula or free text terms in the title or abstract for “oesophageal, epiphrenic diverticula” was developed. The searches were limited to human studies published in the English language from 1990 onwards. Further articles were identified by hand-searching reference lists of all articles retrieved to identify potentially relevant studies. Searches were cross-referenced on PubMed using the related articles function.
Inclusion Criteria
Studies reporting surgical outcomes in patients with non-Zenker’s OD were included. When there were multiple articles by the same authors analysing data from the same or similar patient group, the most recent publication was included if the study periods overlapped.
Exclusion Criteria
Studies of patients with pharyngeal (Zenker’s) diverticulum were excluded. Studies with less than five patients, review articles, case reports, nationwide databases based on coding, experimental studies and unpublished data from conference abstracts were excluded.
Data Extraction
Data were extracted independently by the authors using a standard protocol. Any discrepancies were dealt with by discussion, and consensus was reached. The following information was extracted from each study: first author, year of publication, study design, country of origin, total number of patients, age, median follow-up, site and size of diverticula, presence of motility disorder, details of surgery, staple line leak, morbidity, mortality, reoperation, recurrence rates and presence of reflux symptoms at follow-up. Authors were not contacted for incomplete data. The primary outcome measure was the rate of staple line leakage. This was defined as a clinically relevant leakage over the diverticulectomy staple line which was confirmed radiologically. Secondary outcome measures include successful treatment (defined as symptom improvement or resolution at follow-up), morbidity, mortality, reoperation and recurrence rates and the presence of reflux symptoms at follow-up.
Statistical Analysis
The meta-analysis was performed in line with the recommendations from the Cochrane Collaboration and PRISMA guidelines using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Meta-analysis was used to pool study estimates of the outcome measures as detailed above. The pooled estimated outcomes were calculated using generic inverse variance random-effects meta-analysis using data from studies which reported at least one event in the outcome under investigation with standardised mean differences and 95% confidence intervals (c.i.) quoted. Patients who did not undergo diverticulectomy were excluded from calculations of staple line leak rates.
Subgroup analyses were conducted according to the surgical approach. Heterogeneity among study estimates was quantified using the I 2 value and associated test for heterogeneity which was reported for each analysis. Where heterogeneity was apparent, the DerSimonian and Laird random-effects method was used to pool estimates with inverse variance weights. The fixed-effects method of Mantel-Haenszel was applied otherwise.
Study Quality
The quality of non-randomised studies was assessed using the Newcastle-Ottawa Scale which examines patient selection methods, comparability of study groups and assessment of outcome. A score of at least 6 stars from a maximum of 9 was considered to indicate higher quality.
Results
Characteristics of Included Studies
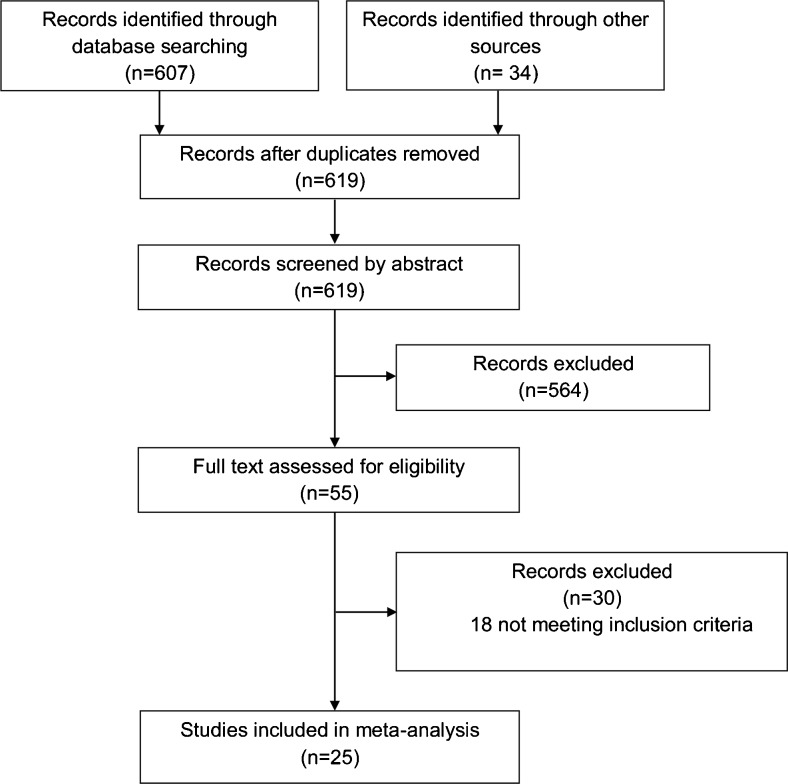
The search identified 641 studies of which 25 were suitable for inclusion (Fig. 1). All studies analysed were observational cohorts, of which one had a prospective design11 (Table 1).
Fig. 1.
Identification process for eligible studies
Table 1.
Characteristics of included studies
| Author | Year | Country | Total | Age (years) | Approach | Myotomy | Anti-reflux | F/Ua (months) | NOb |
|---|---|---|---|---|---|---|---|---|---|
| Allaix et al.35 | 2015 | USA | 13 | 65 | Laparoscopy | 13 | 13 | 24 | 5 |
| Altorki et al.18 | 1993 | USA | 17 | 65 | Open | 17 | 17 | 84 | 5 |
| Bagheri et al.23 | 2014 | Iran | 17 | 39 | Open | 12 | 0 | 12 | 5 |
| Benacci et al.8 | 1993 | USA | 33 | 65 | Open | 23 | 6 | 83 | 6 |
| Bowman et al.11 | 2015 | USA | 44 | 70 | Laparoscopy | 44 | 44 | 39 | 6 |
| Castrucci et al.21 | 1998 | Italy | 27 | 55 | Open | 22 | 17 | 47 | 4 |
| DJourno et al.20 | 2009 | Canada | 23 | 58 | Open | 23 | 22 | 61 | 5 |
| Fekete and Vonns10 | 1992 | France | 27 | 63 | Open | 15 | 14 | 6 | 5 |
| Fumagalli et al.33 | 2012 | Italy | 30 | 62 | Laparoscopy | 30 | 30 | 52 | 5 |
| Gonzalez-Calatayud et al.12 | 2014 | Spain | 6 | 64 | Laparoscopyc | 6 | 6 | 62 | 5 |
| Hauge et al.13 | 2014 | Norway | 11 | 60 | Both | 3 | 3 | 27 | 6 |
| Hudspeth et al.24 | 1993 | USA | 9 | 62 | Open | 6 | 0 | 36 | 5 |
| Jordan and Kinner25 | 1999 | USA | 19 | 59 | Open | 13 | 4 | – | 4 |
| Klaus et al.6 | 2003 | USA | 11 | 68 | Laparoscopyc | 10 | 10 | 26 | 5 |
| Macke et al.26 | 2015 | USA | 57 | 71 | Laparoscopyc | 47 | 24 | 21 | 6 |
| Matthews et al.27 | 2003 | USA | 5 | 64 | Laparoscopyc | 5 | 4 | 16 | 6 |
| Melman et al.32 | 2009 | USA | 13 | 67 | Laparoscopy | 13 | 12 | 14 | 6 |
| Nehra et al.7 | 2002 | USA | 18 | 66 | Open | 17 | 17 | 24 | 5 |
| Rossetti et al.30 | 2013 | Italy | 21 | 59 | Laparoscopy | 21 | 21 | 78 | 6 |
| Soares et al.29 | 2011 | USA | 23 | 57 | Both | 21 | 23 | 34 | 6 |
| Streitz et al.22 | 1992 | USA | 16 | 62 | Open | 13 | 0 | 84 | 5 |
| Tedesco et al.34 | 2005 | USA | 7 | 73 | Laparoscopy | 7 | 7 | 60 | 4 |
| van der Peet et al.28 | 2001 | Netherlands | 5 | 58 | Laparoscopyc | 2 | 0 | – | 4 |
| Varghese et al.19 | 2007 | USA | 35 | 71 | Open | 33 | 34 | 45 | 6 |
| Zaninotto et al.31 | 2012 | Italy | 24 | 61 | Laparoscopy | 21 | 24 | 96 | 5 |
aMedian follow-up
bNewcastle-Ottawa score
cStudies which also utilised thoracoscopy
Patient Demographics and Diagnosis
Analysis was carried out on 511 patients [259 male, median age (range) 62 years (16–96) with mid-oesophageal (n = 53) and epiphrenic (n = 458) OD]. The median size (range) of diverticulum was 5 (1–16) cm. Preoperative manometry was performed in 408 patients (79.8%), and oesophageal motility disorders were identified in 363 patients (71%).
Indications for Surgery
Only one study advocated surgery in asymptomatic patients with non-Zenker’s OD.18 Dysphagia and regurgitation were reported in 416 (81.4%) and 365 (71.4%) patients, respectively. Respiratory symptoms of cough and aspiration were reported in 129 (25.2%) patients.
Surgical Approach
Eleven studies reported outcomes of open surgical approach7 , 8 , 10 , 18 – 25 in 257 patients (51.6%) [left thoracotomy (n = 186), right thoracotomy (n = 66) and laparotomy (n = 5)]. Seven studies utilised the thoracoscopic approach6 , 12 , 13 , 26 – 29 in 42 patients. Seven studies utilised laparoscopy alone11 , 30 – 35 in 204 patients. Three studies utilised a combined laparoscopic and thoracoscopic approach12 , 26 , 28 in eight patients. Nine patients (3.7%) required conversion to open procedure [thoracoscopy to thoracotomy (n = 6), laparoscopy to thoracotomy (n = 1), laparoscopy to laparotomy (n = 1)].
Management of Diverticulum
Thirteen studies reported outcomes of routine diverticulectomy.11 , 12 , 22 , 24 , 26 – 34 Diverticulectomy and diverticulopexy were performed in 456 (89.2%) and 17 (3.3%) patients, respectively. The diverticulum was left in situ in 38 patients (7.4%) who underwent myotomy with or without an anti-reflux procedure.
Myotomy
Myotomy was performed in 437 (23 mid and 414 distal OD) patients (85.5%). Selective and routine approaches to myotomy were adopted in 156 – 8 , 10 , 13 , 19 , 21 – 26 , 28 , 29 , 31 and 1011 , 12 , 18 , 20 , 27 , 30 , 32 – 35 studies, respectively. Myotomy was performed on the contralateral and ipsilateral sides to the diverticulectomy in 11 studies7 , 10 , 18 – 21 , 23 , 24 , 27 , 32 , 33 (n = 237) and 1 study,30 respectively (n = 21) and on either side in 1 study26 (n = 47) and anteriorly in 12 studies6 , 8 , 11 – 13 , 22 , 25 , 28 , 29 , 31 , 34 , 35 (n = 132).
Fundoplication
Fundoplication was performed in 355 patients (69.5%) [Dor (n = 148), Belsey Mark IV (n = 100), Toupet (n = 63), Nissen (n = 44)]. Four studies did not report the use of fundoplication.22 – 24 , 28
Outcomes
Staple Line Leak
Individual study outcomes are shown in Table 2. One study did not report long-term outcomes following surgery.28 Staple line leaks were diagnosed either at contrast study or endoscopy in all papers. Staple line leaks occurred in 51 patients, 8 of whom had died. Sixteen patients were treated conservatively with antibiotics and parenteral nutrition, 17 required percutaneous drainage, 15 returned to theatre and 3 patients were stented successfully. Twenty-three studies6 – 8 , 10 – 13 , 18 , 19 , 21 – 26 , 28 – 36 reported at least one staple line leak and were included in the overall pooled estimated leak rate of 13.3% [95% c.i. (11.0–15.7), p < 0.001] (Fig. 2). Pooled staple line leak rates according to surgical approach are shown in Table 3.
Table 2.
Outcomes of individual studies
| Author | Total | Diverticulectomy | Leak | Morbidity | Reoperation | Mortality | Recurrence |
|---|---|---|---|---|---|---|---|
| Allaix et al.35 | 13 | 6 (46.2) | 1 (15.7) | 1 (7.7) | 0 | 0 | 0 |
| Altorki et al.18 | 17 | 14 (82.4) | 1 (7.1) | 1 (5.9) | 0 | 1 (5.9) | 0 |
| Bagheri et al.23 | 17 | 13 (76.5) | 1 (7.7) | 3 (17.6) | 0 | 0 | 0 |
| Benacci et al.8 | 33 | 32 (97.0) | 6 (18.8) | 11 (33.3) | 2 (6.1) | 3 (9.1) | 0 |
| Bowman et al.11 | 44 | 44 (100.0) | 8 (18.2) | 33 (75.0) | 0 | 0 | 0 |
| Castrucci et al.21 | 27 | 17 (63.0) | 2 (11.8) | 3 (11.1) | 2 (7.4) | 2 (7.4) | 0 |
| DJourno et al.20 | 23 | 13 (56.5) | 0 | 2 (8.7) | 0 | 0 | 0 |
| Fekete and Vonns10 | 27 | 23 (85.2) | 2 (8.7) | 5 (18.5) | 1 (3.7) | 3 (11.1) | 2 (7.4) |
| Fumagalli et al.33 | 30 | 30 (100.0) | 1 (3.3) | 2 (6.7) | 1 (3.3) | 0 | 0 |
| Gonzalez-Calatayud et al.12 | 6 | 6 (100.0) | 2 (33.3) | 2 (33.3) | 0 | 0 | 0 |
| Hauge et al.13 | 11 | 9 (81.2) | 3 (33.3) | 3 (27.3) | 2 (18.2) | 0 | 0 |
| Hudspeth et al.24 | 9 | 9 (100.0) | 1 (11.1) | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Jordan and Kinner25 | 19 | 16 (84.2) | 1 (6.3) | 1 (5.3) | 0 | 0 | 0 |
| Klaus et al.6 | 11 | 6 (54.5) | 1 (16.7) | 2 (18.2) | 1 (9.1) | 0 | 0 |
| Macke et al.26 | 57 | 57 (100.0) | 4 (7.0) | 18 (31.6) | 4 (7.0) | 1 (1.8) | 0 |
| Matthews et al.27 | 5 | 5 (100.0) | 0 | 0 | 0 | 0 | 0 |
| Melman et al.32 | 13 | 13 (100.0) | 1 (7.7) | 2 (15.4) | 1 (7.7) | 0 | 0 |
| Nehra et al.7 | 18 | 14 (77.8) | 1 (7.1) | 3 (16.7) | 2 (11.1) | 1 (9.1) | 0 |
| Rossetti et al.30 | 21 | 21 (100.0) | 5 (23.8) | 6 (28.6) | 0 | 1 (4.8) | 0 |
| Soares et al.29 | 23 | 23 (100.0) | 1 (4.3) | 5 (21.7) | 1 (4.3) | 1 (4.3) | 0 |
| Streitz et al.22 | 16 | 16 (100.0) | 1 (6.3) | 6 (37.5) | 0 | 0 | 0 |
| Tedesco et al.34 | 7 | 7 (100.0) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 0 | 0 |
| van der Peet et al.28 | 5 | 5 (100.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 0 | 1 (20.0) |
| Varghese et al.19 | 35 | 33 (94.3) | 2 (6.1) | 5 (14.3) | 1 (2.9) | 1 (2.9) | 0 |
| Zaninotto et al.31 | 24 | 24 (100.0) | 4 (16.7) | 6 (25.0) | 0 | 0 | 0 |
Percentages in parentheses
Fig. 2.
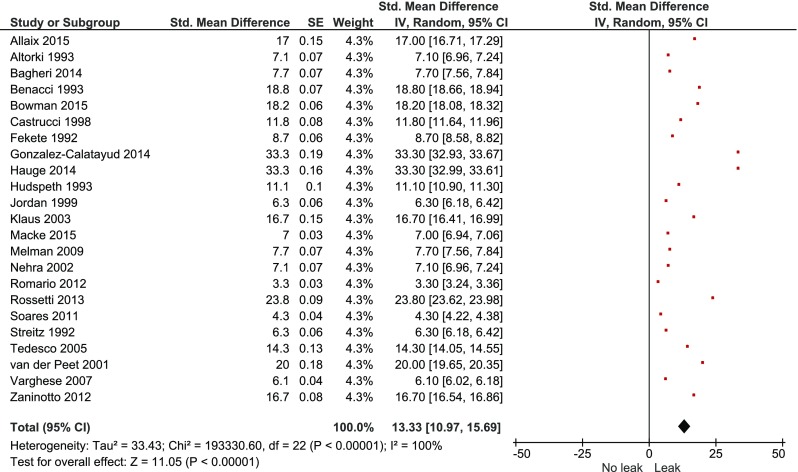
Overall pooled staple line leak rate
Table 3.
Pooled staple line leak rates according to surgical approach
| Surgical approach | Pooled staple line leak rates, (95% c.i.) | p value |
|---|---|---|
| Open | 11.3 (8.4–14.2) | 0.347 |
| Minimally invasive | 15.2 (11.4–19.0) | |
| Myotomy | 12.4 (9.2–15.6) | 0.002 |
| No myotomy | 26.1 (18.3–33.9) | |
| Anti-reflux | 14.7 (10.8–18.5) | 0.45 |
| No anti-reflux | 13.3 (9.9–16.7) |
Treatment Success
The overall pooled estimated treatment success rate was 88.5% [95% c.i. (84.8–92.2), p < 0.001] (Fig. 3). The treatment success rates according to surgical approach are shown in Table 4.
Fig. 3.
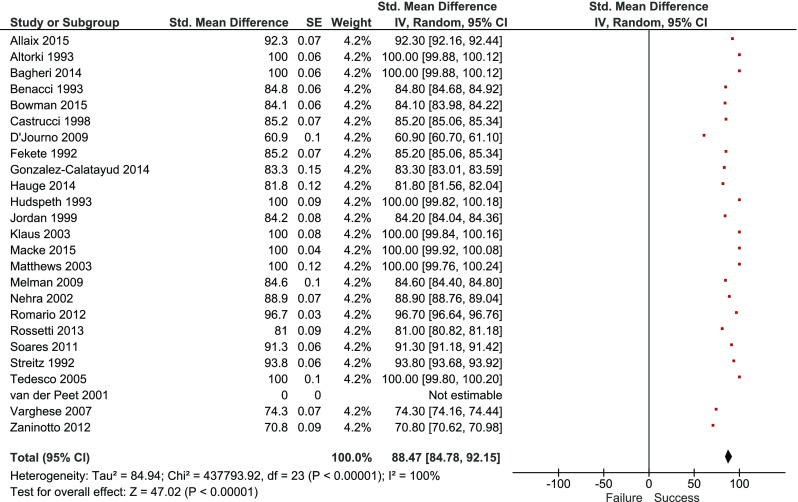
Overall pooled treatment success rate
Table 4.
Pooled treatment success rates according to surgical approach
| Surgical approach | Pooled treatment success rates (95% c.i.) | p value |
|---|---|---|
| Open | 87.4 (81.8–93.0) | 0.56 |
| Minimally invasive | 89.6 (84.6–94.5) | |
| Diverticulectomy | 85.0 (80.9–89.1) | 0.02 |
| No diverticulectomy | 65.4 (55.6–75.2) |
Morbidity
Morbidity was reported in 111 patients (staple line leak = 51, wound infection = 3, cardiovascular = 17, respiratory = 27, urinary tract infection = 3, bleeding = 3 and “other” = 7). Twenty-four studies6 – 8 , 10 – 13 , 18 – 26 , 28 – 36 reported at least one complication and were included in the overall pooled estimated morbidity rate of 21.1% [95% c.i. (14.4–27.7), p < 0.001] (Fig. 4). Morbidity of open vs. minimally invasive approaches was 17.3% [95% c.i. (12.1–22.5)] and 25.7% [95% c.i. (12.1–39.3), p = 0.145], respectively.
Fig. 4.
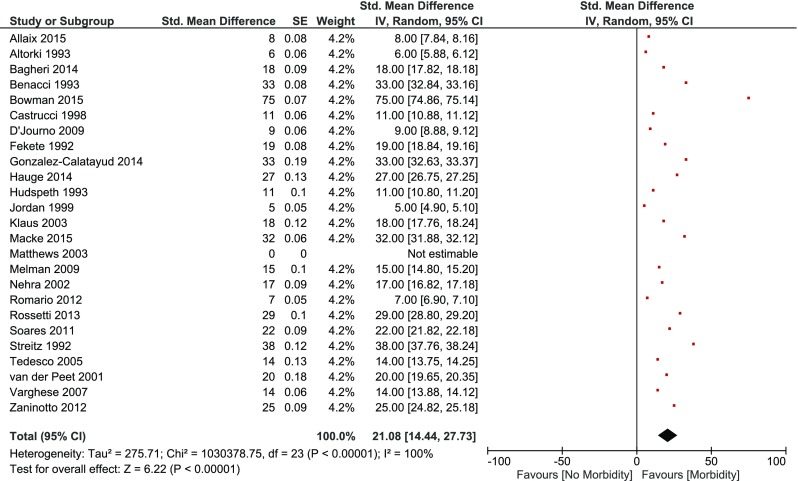
Overall pooled morbidity rate
Reoperation
Twenty patients required reoperations for staple line leak (n = 15), bleeding (n = 3), port site hernia (n = 1), acute paraoesophageal hernia (n = 1) and splenic injury requiring splenectomy (n = 1). Thirteen6 – 8 , 10 , 13 , 19 , 24 , 26 , 28 , 29 , 32 – 34 studies reported at least one reoperation and were included in the overall pooled estimated reoperation rate of 9.4% [95% c.i. (7.7–11.1), p < 0.001].
In-Hospital Mortality
Fourteen patients died in hospital following surgery due to staple line leak (n = 8), pneumonia (n = 2), myocardial infarction (n = 3) and port site hernia (n = 1). These were reported in nine studies7 , 8 , 10 , 18 , 19 , 21 , 26 , 29 , 30 which were included in the overall pooled estimated in-hospital mortality rate of 5.9% [95% c.i. (4.0–7.8), p < 0.001].
Recurrence and Reflux
At a median follow-up of 46 months, three patients developed recurrence of the ED which required reoperation, one of whom did not have a myotomy at the index procedure. Postoperative reflux was assessed with routine 24-h pH monitoring in five studies.20 , 21 , 29 – 31 Two studies utilised quantitative assessment of reflux with a modified Likert score,11 , 34 and one study used the GERD-HRQOL questionnaire.26 The rest of the studies assessed postoperative reflux symptoms by simple questioning.
Twelve studies6 – 8 , 10 , 13 , 18 , 20 – 22 , 29 , 32 , 35 reported reflux symptoms at follow-up and were included in the pooled estimated incidence of reflux symptoms which was similarly irrespective of whether an anti-reflux procedure was performed [19.0 (95% c.i. 7.1–30.9%)] or not [21.0 (95% c.i. 13.1–28.9%), p = 0.243].
Sensitivity Analysis and Heterogeneity
Sensitivity analysis of higher quality studies with at least 10 patients revealed a similar pooled staple line leak rate of 14.9% [95% c.i. (10.2–19.6), p < 0.001]. Heterogeneity was significant in all analyses.
Discussion
Main Findings
The main findings from this meta-analysis of 25 studies of over 500 patients with non-Zenker’s OD were that diverticulectomy resulted in better symptom resolution, and staple line leak rates can be reduced significantly by routine myotomy. Both open and minimally invasive approaches resulted in similar outcomes, and the addition of anti-reflux procedures did not significantly improve postoperative reflux symptoms.
Strengths
The strengths of this study are the large sample size analysed. Due to the rarity of non-Zenker’s OD, the controversies surrounding the surgical treatment of these patients will not be answered by randomised trials. This is the only comprehensive meta-analysis of the outcomes of surgery in over 500 patients with non-Zenker’s OD which has identified the optimum treatment. The largest case series to date only included 57 patients over a 15-year period.26 A nationwide population database of 1056 patients with non-Zenker’s OD reported a leak rate of 3.1%37 which is at odds with the findings of our study. Hospital coding was used in this database which may have underestimated the complication rates. These types of studies were therefore not included in our meta-analysis.
Limitations
This study has limitations. Meta-analysis of retrospective cohort studies is regrettably sensitive to confounding and selection bias. However, there are no randomised trials comparing the various surgical approaches. A variety of procedures were used in the studies included in the meta-analysis resulting in significant heterogeneity. The outcomes (staple line leakage and success rates) were not explicitly defined in all papers and not stratified according to the site of the diverticula. The assessment of symptoms at follow-up also varied significantly between studies. We therefore broadly defined success rates as symptom improvement or resolution at follow-up which was reported in all studies. Subgroup analysis was limited as not all studies reported separate outcomes according to the presence of motility disorders or individual surgical approach. Nevertheless, a sensitivity analysis of higher quality studies revealed similar results to the overall analysis, thereby strengthening the conclusions.
Surgical Approach
Despite the increased use of minimally invasive approaches since 2000, the open approach is still widely adopted. Over half of patients in this cohort underwent open surgery usually via a left thoracotomy. Although the treatment success rates were similar between the two approaches, there was a non-significant trend towards higher staple line leak and overall morbidity rates in patients who underwent minimally invasive surgery. Short-term outcomes, for example length of hospital stay, appear to be shorter in individual series reporting the minimally invasive approach,6 , 29 , 34 but this could not be analysed as only less than half of the studies included in this meta-analysis reported length of hospital stay. The choice of approach depends not only on the location of the OD, need for myotomy and anti-reflux procedure but, more importantly, on local expertise. Minimally invasive approaches should only be performed by surgeons experienced in both open and minimally invasive oesophageal surgeries.26
Management of Diverticulum
The majority of patients in this study underwent excision of the OD. Castrucci et al. did not perform a diverticulectomy in the presence of wide-necked diverticula without food retention in the pouch, pulmonary aspiration or mucosal lesions.21 D’Journo et al. advocated suspension of wide-necked diverticula when there was no dependent portion of the diverticular sac and myotomy alone in the presence of multiple small diverticula.20 Small diverticula are usually less symptomatic6 , 21 and should arguably be treated non-surgically32 unless the predominant symptom is dysphagia secondary to achalasia. Diverticulectomy resulted in improvement or resolution in symptoms in 85% of patients compared with 65% who underwent diverticulopexy or myotomy alone. Excision of the OD should therefore be performed in the presence of symptoms directly related to the OD such as food regurgitation.
Myotomy
Another contentious issue is the need for myotomy. The pathogenesis of non-Zenker’s OD is not fully understood. The diagnosis of oesophageal motility disorders is challenging and the current method of investigation is not tolerated by all patients. Some studies have identified motility disorders in almost all patients with non-Zenker’s OD7 , 11 , 30 , 35 whereas others have identified motor disorders in less than 20%.6 , 13 These differences between series may be explained by a variation in criteria used to reach a diagnosis38 or the intermittent dysfunction that is not detected by oesophageal motility studies.7 Oesophageal motor disorders were identified in just over 70% of patients, and myotomy was performed in 85% of patients in this meta-analysis. Just as Belsey5 pointed out over half a century ago, the underlying cause leading to the blow out must be addressed if successful surgery is expected. We have shown that myotomy significantly reduces the staple line leak rate from 26 to 12.4%.
Anti-reflux
The need for an anti-reflux procedure and the type of fundoplication are widely debated topics. Over two thirds of patients in this study underwent an anti-reflux procedure, the majority of whom had a partial fundoplication. The staple line leak rates and postoperative reflux rates were similar regardless of whether a fundoplication was performed or not. However, these results should be interpreted with caution as the reporting of symptomatic reflux outcomes varied between studies. Moreover, it was not possible to identify the optimum type of fundoplication in this meta-analysis as the outcomes of the various procedures were not reported separately. The choice of fundoplication is therefore dependent on the patients’ symptoms and surgeon preference.
Conclusion
In conclusion, this comprehensive meta-analysis of over 500 patients with non-Zenker’s OD has shown that the optimum surgical treatment is diverticulectomy along with routine myotomy with or without an anti-reflux procedure. Both open and minimally invasive approaches are equally effective.
Acknowledgments
Author Contributions
David S.Y. Chan: Conception and design of the work, acquisition, analysis, interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Antonio Foliaki: Acquisition, analysis, interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Wyn G. Lewis: Acquisition, analysis, interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Geoffrey W.B. Clark: Acquisition, analysis, interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guy R.J.C. Blackshaw: Acquisition, analysis, interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Presented at the Association of Upper GI Surgeons (AUGIS), September 2016, Leeds, UK.
References
- 1.Thomas ML, Anthony AA, Fosh BG, Finch JG, Maddern GJ. Oesophageal diverticula. Br J Surg. 2001;88:629–642. doi: 10.1046/j.1365-2168.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoghooghi D, Coakley FV, Breiman RS, Qayyum A, Yeh BM. Frequency and etiology of midesophageal diverticula at barium esophagography. Clinical Imaging. 2006;30:245–247. doi: 10.1016/j.clinimag.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Tobin RW. Esophageal rings, webs, and diverticula. Journal of Clinical Gastroenterology. 1998;27:285–295. doi: 10.1097/00004836-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 4.do Nascimento FA, Lemme EM, Costa MM. Esophageal diverticula: pathogenesis, clinical aspects, and natural history. Dysphagia. 2006;21:198–205. doi: 10.1007/s00455-006-9028-5. [DOI] [PubMed] [Google Scholar]
- 5.Belsey R. Functional disease of the esophagus. J Thorac Cardiovasc Surg. 1966;52:164–188. [PubMed] [Google Scholar]
- 6.Klaus A, Hinder RA, Swain J, Achem SR. Management of epiphrenic diverticula. J Gastrointest Surg. 2003;7:906–911. doi: 10.1007/s11605-003-0038-4. [DOI] [PubMed] [Google Scholar]
- 7.Nehra D, Lord RV, DeMeester TR, Theisen J, Peters JH, Crookes PF, Bremner CG. Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg. 2002;235:346–354. doi: 10.1097/00000658-200203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benacci JC, Deschamps C, Trastek VF, Allen MS, Daly RC, Pairolero PC. Epiphrenic diverticulum: results of surgical treatment. Ann Thorac Surg. 1993;55:1109–1113. doi: 10.1016/0003-4975(93)90016-B. [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Goldblum JR, Yearsley MM, Shay SS, Reznik SI, Murthy SC, Mason DP, Blackstone EH. Myenteric plexus abnormalities associated with epiphrenic diverticula. Eur J Cardiothorac Surg. 2009;35:22–27. doi: 10.1016/j.ejcts.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Fekete F, Vonns C. Surgical management of esophageal thoracic diverticula. Hepatogastroenterology. 1992;39:97–99. [PubMed] [Google Scholar]
- 11.Bowman TA, Sadowitz BD, Ross SB, Boland A, Luberice K, Rosemurgy AS. Heller myotomy with esophageal diverticulectomy: an operation in need of improvement. Surg Endosc. 2016;30:3279–3288. doi: 10.1007/s00464-015-4655-2. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Calatayud M, Targarona EM, Balague C, Rodriguez-Luppi C, Martin AB, Trias M. Minimally invasive therapy for epiphrenic diverticula: Systematic review of literature and report of six cases. J Minim Access Surg. 2014;10:169–174. doi: 10.4103/0972-9941.141498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauge T, Johnson E, Sandstad O, Johannessen HO, Trondsen E. Surgical treatment of epiphrenic oesophageal diverticulum. Tidsskr Nor Laegeforen. 2014;134:1047–1050. doi: 10.4045/tidsskr.13.1336. [DOI] [PubMed] [Google Scholar]
- 14.Fernando HC, Luketich JD, Samphire J, Alvelo-Rivera M, Christie NA, Buenaventura PO, Landreneau RJ. Minimally invasive operation for esophageal diverticula. Ann Thorac Surg. 2005;80:2076–2080. doi: 10.1016/j.athoracsur.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Del Genio A, Rossetti G, Maffetton V, Renzi A, Brusciano L, Limongelli P, Cuttitta D, Russo G, Del Genio G. Laparoscopic approach in the treatment of epiphrenic diverticula: long-term results. Surg Endosc. 2004;18:741–745. doi: 10.1007/s00464-003-9044-6. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altorki NK, Sunagawa M, Skinner DB. Thoracic esophageal diverticula. Why is operation necessary? J Thorac Cardiovasc Surg. 1993;105:260–264. [PubMed] [Google Scholar]
- 19.Varghese TK, Jr, Marshall B, Chang AC, Pickens A, Lau CL, Orringer MB. Surgical treatment of epiphrenic diverticula: a 30-year experience. Ann Thorac Surg. 2007;84:1801–1809. doi: 10.1016/j.athoracsur.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 20.D’Journo XB, Ferraro P, Martin J, Chen LQ, Duranceau A. Lower oesophageal sphincter dysfunction is part of the functional abnormality in epiphrenic diverticulum. Br J Surg. 2009;96:892–900. doi: 10.1002/bjs.6652. [DOI] [PubMed] [Google Scholar]
- 21.Castrucci G, Porziella V, Granone PL, Picciocchi A. Tailored surgery for esophageal body diverticula. Eur J Cardiothorac Surg. 1998;14:380–387. doi: 10.1016/S1010-7940(98)00201-2. [DOI] [PubMed] [Google Scholar]
- 22.Streitz JM, Jr, Glick ME, Ellis FH., Jr Selective use of myotomy for treatment of epiphrenic diverticula. Manometric and clinical analysis. Archives of Surgery. 1992;127:585–587. doi: 10.1001/archsurg.1992.01420050109014. [DOI] [PubMed] [Google Scholar]
- 23.Bagheri R, Maddah G, Mashhadi MR, Haghi SZ, Tavassoli A, Ghamari MJ, Sheibani S. Esophageal diverticula: Analysis of 25 cases. Asian Cardiovasc Thorac Ann. 2013;22:583–587. doi: 10.1177/0218492313515251. [DOI] [PubMed] [Google Scholar]
- 24.Hudspeth DA, Thorne MT, Conroy R, Pennell TC. Management of epiphrenic esophageal diverticula. A fifteen-year experience. Am Surg. 1993;59:40–42. [PubMed] [Google Scholar]
- 25.Jordan PH, Jr, Kinner BM. New look at epiphrenic diverticula. World J Surg. 1999;23:147–152. doi: 10.1007/PL00013158. [DOI] [PubMed] [Google Scholar]
- 26.Macke RA, Luketich JD, Pennathur A, Bianco V, Awais O, Gooding WE, Christie NA, Schuchert MJ, Nason KS, Levy RM. Thoracic Esophageal Diverticula: A 15-Year Experience of Minimally Invasive Surgical Management. Ann Thorac Surg. 2015;100:1795–1802. doi: 10.1016/j.athoracsur.2015.04.122. [DOI] [PubMed] [Google Scholar]
- 27.Matthews BD, Nelms CD, Lohr CE, Harold KL, Kercher KW, Heniford BT. Minimally invasive management of epiphrenic esophageal diverticula. Am Surg. 2003;69:465–470. [PubMed] [Google Scholar]
- 28.van der Peet DL, Klinkenberg-Knol EC, Berends FJ, Cuesta MA. Epiphrenic diverticula: minimal invasive approach and repair in five patients. Dis Esophagus. 2001;14:60–62. doi: 10.1111/j.1442-2050.2001.00151.x. [DOI] [PubMed] [Google Scholar]
- 29.Soares RV, Montenovo M, Pellegrini CA, Oelschlager BK. Laparoscopy as the initial approach for epiphrenic diverticula. Surg Endosc. 2011;25:3740–3746. doi: 10.1007/s00464-011-1779-x. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti G, Fei L, del Genio G, Maffettone V, Brusciano L, Tolone S, Cimmino M, Moccia F, Terrone A, Romano G, Guerriero L, del Genio A. Epiphrenic diverticula mini-invasive surgery: a challenge for expert surgeons--personal experience and review of the literature. Scand J Surg. 2013;102:129–135. doi: 10.1177/1457496913482242. [DOI] [PubMed] [Google Scholar]
- 31.Zaninotto G, Parise P, Salvador R, Costantini M, Zanatta L, Rella A, Ancona E. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:218–222. doi: 10.1053/j.semtcvs.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Melman L, Quinlan J, Robertson B, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Matthews BD. Esophageal manometric characteristics and outcomes for laparoscopic esophageal diverticulectomy, myotomy, and partial fundoplication for epiphrenic diverticula. Surg Endosc. 2009;23:1337–1341. doi: 10.1007/s00464-008-0165-9. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli Romario U, Ceolin M, Porta M, Rosati R. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:213–217. doi: 10.1053/j.semtcvs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Tedesco P, Fisichella PM, Way LW, Patti MG. Cause and treatment of epiphrenic diverticula. Am J Surg. 2005;190:891–894. doi: 10.1016/j.amjsurg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Allaix ME, Borraez Segura BA, Herbella FA, Fisichella PM, Patti MG. Is resection of an esophageal epiphrenic diverticulum always necessary in the setting of achalasia? World J Surg. 2015;39:203–207. doi: 10.1007/s00268-014-2770-1. [DOI] [PubMed] [Google Scholar]
- 36.Sonbare DJ. Pulsion Diverticulum of the Oesophagus: More than just an Out Pouch. Indian J Surg. 2015;77:44–48. doi: 10.1007/s12262-013-0955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onwugbufor MT, Obirieze AC, Ortega G, Allen D, Cornwell EE, 3rd, Fullum TM. Surgical management of esophageal diverticulum: a review of the Nationwide Inpatient Sample database. J Surg Res. 2013;184:120–125. doi: 10.1016/j.jss.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 38.Richter JE. Oesophageal motility disorders. Lancet. 2001;358:823–828. doi: 10.1016/S0140-6736(01)05973-6. [DOI] [PubMed] [Google Scholar]