Abstract
Introduction
Subjective cognitive decline (SCD) and biomarker-based “at-risk” concepts such as “preclinical” Alzheimer's disease (AD) have been developed to predict AD dementia before objective cognitive impairment is detectable. We longitudinally evaluated cognitive outcome when using these classifications.
Methods
Memory clinic patients (n = 235) were classified as SCD (n = 122): subtle cognitive decline (n = 36) and mild cognitive impairment (n = 77) and subsequently subclassified into SCDplus and National Institute on Aging–Alzheimer's Association (NIA-AA) stages 0 to 3. Mean (standard deviation) follow-up time was 48 (35) months. Proportion declining cognitively and prognostic accuracy for cognitive decline was calculated for all classifications.
Results
Among SCDplus patients, 43% to 48% declined cognitively. Among NIA-AA stage 1 to 3 patients, 50% to 100% declined cognitively. The highest positive likelihood ratios (+LRs) for subsequent cognitive decline (+LR 6.3), dementia (+LR 3.4), and AD dementia (+LR 6.5) were found for NIA-AA stage 2.
Discussion
In a memory clinic setting, NIA-AA stage 2 seems to be the most successful classification in predicting objective cognitive decline, dementia, and AD dementia.
Keywords: Memory clinic, Alzheimer's disease, Prediction, Neuropsychology, Dementia, Mild cognitive impairment, Clinical progression, Diagnosis, Classification
1. Introduction
The pathophysiological processes underlying Alzheimer's disease (AD) are assumed to develop many years before clinical evidence of a manifest dementia state [1]. Current research focuses on identifying characteristics of the early stages of AD, and several concepts have been developed to that end. In 2011, the US National Institute on Aging–Alzheimer's Association (NIA-AA) group presented recommendations to identify “the preclinical stage of AD,” referring to a stage characterized by the presence of biomarker signs of AD but absence of verified cognitive impairment [2], [3]. The NIA-AA stages were subdivided into stages reflecting a suggested temporal sequence of the pathway to AD: stage 0 = both amyloid and neurodegeneration markers negative; stage 1 = evidence of amyloidosis (e.g., lowered cerebrospinal fluid (CSF) amyloid β (Aβ)42 concentrations, neurodegeneration markers negative); stage 2 = evidence of amyloidosis and neurodegeneration (e.g., increased CSF tau concentrations); and stage 3 = biomarker pattern as in stage 2 but also with “subtle cognitive decline.” This model is not uniformly accepted, in part, because of the findings that place neurodegeneration temporally before amyloidosis [4], [5], [6].
Subjective cognitive decline (SCD)—self-perceived decline despite objectively unimpaired cognitive function—represents a possible presymptomatic stage of mild cognitive impairment (MCI) and AD [7]. SCD may be present, but is not required, in the NIA-AA “preclinical AD” stages. However, the target populations of these concepts are indeed overlapping. SCD has been reported 15 years before MCI [8] and mounting evidence implicates SCD as a risk factor for dementia [9], [10], [11], [12] and AD-biomarker abnormalities [13], [14].
Studies of the predictive value of subjective cognitive and/or memory symptoms have been limited because of the lack of conceptual and methodological consensus. Recently, the SCD Initiative suggested a conceptual framework for investigating SCD, including research criteria and features, which should be reported in SCD studies [15] (see Supplementary Material for these features specified for the present study). Additional features were listed under the term “SCDplus,” to further increase the likelihood of identifying preclinical AD: (1) subjective decline in memory rather than other cognitive domains; (2) onset of SCD within the last 5 years; (3) age at onset of SCD ≥60 years; (4) concerns (worries) associated with SCD; (5) feeling of worse performance than others of the same age group; (6) confirmation of cognitive decline by an informant; and if available (7) the presence of the apolipoprotein E (APOE ɛ4) genotype; and (8) biomarker evidence for AD (defines preclinical AD). Both the SCD and SCDplus criteria are described as research criteria that “require continuous refinement and validation to eventually serve as a standardized indicator for biomarker-based preclinical AD detection” [15]. Molinuevo et al. [16] recently presented recommendations on how to apply the SCD criteria.
A recent review concluded, after having summarized the current knowledge of CSF AD biomarkers in SCD, that there is emerging evidence that biomarkers differentiate between declining and stable SCD patients [17], although studies in clinical settings are scarce as are studies combining biomarkers and subjective or subtle cognitive decline [3], [18]. Both the NIA-AA stages and SCD/SCDplus are recent concepts that need concurrent evaluation in longitudinal clinical samples to assess the clinical utility in predicting cognitive decline and AD-type dementia (ADD).
In this study, we examined SCD, SCDplus, SCDplusbio (i.e., SCDplus + APOE ɛ4 and biomarkers), NIA-AA stages 0 to 3 of preclinical AD, and MCI in patients seeking care at a memory clinic, with respect to
-
(1)
the proportion of cognitively stable and declining patients over time (descriptive report),
-
(2)
the ability of the classifications to predict cognitive decline, dementia, and ADD specifically, and
-
(3)
the individual contribution of each feature included in the classifications to predict cognitive decline and dementia.
2. Methods
2.1. Participants
All patients (n = 235) in the present study were included in the ongoing clinical prospective single-center Gothenburg MCI study [19] between 1999 and 2013. In the present study, inclusion criteria were age 50 to 79 years; self-reported cognitive decline with a duration of ≥6 months, assessed by specialist clinicians through interviews; available data at baseline with respect to CSF biomarkers, SCDplus features, and neuropsychological tests used to discriminate between SCD and MCI. Systemic and other somatic diseases possibly causing cognitive impairment, for example, subdural hemorrhage, brain tumor, hypothyroid state, encephalitis, unstable heart disease, and psychiatric disorders such as major affective disorder (not minor depressive disorder), schizophrenia, substance abuse, and confusion, were cause for exclusion. Of the 250 patients meeting inclusion criteria, 235 (94%) were followed up and were thus included in the analyses. There were no significant differences in age, Mini–Mental State Examination (MMSE) [20], or years of education between patients who were excluded (n = 152) because of missing data (missing data at baseline, n = 137; missing data at follow-up, n = 15) and the included patients (n = 235) (excluded patients' mean [SD = standard deviation] age, 63 (9); mean (SD) years of education, 13 (4); and mean (SD) MMSE, 29 (1). Included patients' mean (SD) age, 64 (8); mean (SD) years of education, 12 (4); and mean (SD) MMSE, 29 (2)). Baseline characteristics are presented in Table 1. The most common reason for missing data was missing informant report. Some patients lived alone or were reluctant to ask their spouses or children to fill out a symptom questionnaire. All patients lived in the extended Gothenburg region in Sweden and sought care at the Sahlgrenska memory clinic. Patients were referred via primary health care, and a minor part was self-referrals.
Table 1.
Baseline characteristics
| Baseline data | Total sample | No detectable cognitive impairment |
Detectable cognitive impairment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total group (=SCD) | SCDplus | SCDplus-bio | NIA-AA preclinical AD stage 0 | NIA-AA preclinical AD stage 1 | NIA-AA preclinical AD stage 2 | Total group | NIA-AA preclinical AD stage 3 | MCI | Unclassified | ||
| N | 235 | 122 | 98 | 69 | 46 | 10 | 21 | 113 | 6 | 77 | 30 |
| Age, mean (SD) | 64 (8) | 62 (7)∗ | 63 (7) | 64 (7) | 59 (6) | 64 (9) | 67 (7) | 65 (8)∗ | 65 (7) | 66 (8) | 65 (7) |
| Years of education, M (SD) | 12 (4) | 13 (4)∗ | 13 (3) | 13 (4) | 13 (3) | 14 (5) | 12 (4) | 11 (3)∗ | 9 (1) | 11 (3) | 12 (4) |
| Male/female, % | 44/56 | 50/50, ns | 55/45 | 51/49 | 59/41 | 30/70 | 48/52 | 37/63, ns | 67/33 | 33/68 | 43/57 |
| MMSE, mean (SD) | 29 (2) | 29 (1), ns | 29 (1) | 29 (1) | 29 (2) | 29 (1) | 29 (1) | 28 (2), ns | 28 (1) | 28 (2) | 29 (1) |
| Months followed, M (SD) | 48 (35) | 58 (39)∗ | 56 (37) | 59 (40) | 55 (36) | 49 (39) | 54 (34) | 38 (28)∗ | 32 (18) | 38 (29) | 40 (28) |
| Aβ42, M (SD) | 611 (214) | 637 (211)† | 643 (217) | 626 (237) | 684 (140) | 402 (80) | 372 (78) | 584 (214)† | 396 (77) | 573 (226) | 647 (176) |
| T-tau, M (SD) | 397 (249) | 336 (192)∗ | 354 (189) | 421 (184) | 197 (61) | 177 (80) | 604 (198) | 461 (286)∗ | 799 (313) | 490 (296) | 325 (162) |
| p-tau, M (SD) | 59 (30) | 55 (25)† | 55 (23) | 63 (24) | 37 (6) | 35 (9) | 86 (34) | 65 (34)† | 128 (42) | 66 (32) | 50 (22) |
| Informant-reported memory decline, % | 89 | 87, ns | 96 | 96 | 88 | 44 | 91 | 91, ns | 67 | 96 | 87 |
| CIMP-QUEST memory scale, mean (SD) | 4 (3) | 3 (3)† | 4 (2) | 4 (3) | 4 (3) | 2 (3) | 4 (3) | 4 (3)† | 4 (4) | 4 (3) | 4 (3) |
| Depressive symptomatology, % | 10 | 12, ns | 9 | 8 | 14 | 44 | 10 | 8, ns | 33 | 5 | 12 |
Abbreviations: Aβ42, amyloid β 42; AD, Alzheimer's disease; CIMP-QUEST, Cognitive Impairment Questionnaire; M, mean; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; NIA-AA, National Institute on Aging–Alzheimer's Association; ns, nonsignificant difference; p-tau, phosphorylated tau; SCD, subjective cognitive decline; SD, standard deviation; T-tau, total-tau.
NOTE. Continuous variables are presented as the means and SD. Unclassified patients = patients with subtle cognitive decline but at least one negative biomarker (=not fulfilling criteria for preclin-3). Baseline data for the SCD subgroups (SCDplus; SCDplusbio; NIA-AA stages 0 to 2) and the NIA-AA stage 3 group were not compared as the samples were partly overlapping.
Statistically significant difference between groups with detectable versus nondetectable cognitive impairment, P < .01.
Statistically significant difference between groups with detectable versus nondetectable cognitive impairment, P < .05.
Healthy controls, n = 101; mean (SD) age, 65 (6); mean (SD) years of education, 12 (3); and mean (SD) MMSE, 29 (1), were recruited through information meetings (in, e.g., senior organizations), and some were relatives of patients. Inclusion and exclusion criteria were identical for control subjects and patients, except control subjects had neither subjectively reported nor objective cognitive impairment, assessed by a clinician. Healthy control subjects were only included in the present study to generate cutoff values for neuropsychological test scores.
2.2. Assessments
All participants were examined at baseline and completed at least one biannual follow-up. Baseline assessments were carried out during 4 to 5 patient visits. At baseline and follow-up, the Global Deterioration Scale (GDS) staging of cognitive impairment [21] was performed by a specialist physician or a registered nurse using a previously described algorithm based on cognitive screening instruments [19]. The stages included stage 1 (no subjective or objective cognitive decline); GDS stages 2 to 3 (some subjective and/or objective cognitive decline, but no dementia); and GDS stage ≥4 (objective cognitive decline at the level of probable dementia or beyond, in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). GDS was used to exclude patients with no subjective or objective cognitive decline (GDS 1) or probable dementia (GDS stage ≥4) at baseline and as follow-up dementia screening.
Informants, predominantly spouses or adult children of patients, completed the Cognitive Impairment Questionnaire, an instrument designed to identify dementia-related symptoms by informant report [22]. Licensed psychologists administered a neuropsychological battery during two visits, each lasting 1.5 to 2 hours. Depressive symptoms were assessed using the Montgomery-Asberg Depression Rating Scale [23] or the 20-item Geriatric Depression Scale [24], [25], using ≥10 and ≥6 as cutoff scores, respectively, for depressive symptomatology.
CSF and whole blood was drawn from all participants, using procedures and subsequent analyses as previously described [19]. Briefly, we used enzyme-linked immunosorbent assays to measure Aβ, total tau (t-tau), and phosphorylated tau (p-tau). APOE genotyping was performed using solid-phase mini-sequencing as previously described [26]. We used CSF AD marker cutoffs previously used for prediction of AD [27]: Aβ42 ≤ 482 ng/L; t-tau ≥320 ng/L; and p-tau ≥52 ng/L.
Written informed consent was obtained from all participants, and the local ethics committee approved the study.
2.3. Classifications—step 1: SCD, subtle cognitive decline, and MCI
SCD was not measured with a specific instrument, but was based on patients' active help-seeking and self-reported cognitive decline during ≥6 months, assessed in baseline clinical interviews. The presence of cognitive symptoms and duration ≥6 months were noted by the clinician in tick-boxes in the fixed study protocol. Informant corroboration of symptoms was not a part of the SCD criteria, but it was a part of the SCDplus criteria (specified in Section 2.4 and Fig. 1). To distinguish SCD from subtle cognitive decline/MCI while ruling out the presence of objectively measurable cognitive signs associated with early AD, we used four tests from our test battery that previously predicted conversion to AD [28]. The neuropsychological tests used were as follows: the silhouettes subtest from the visual objects and space perception (VOSP; visual object perception) battery [29]; the Rey auditory verbal learning test (RAVLT; word list memory test), immediate recall [30]; the Boston naming test (BNT; confrontation naming) [31]; and the Rey complex figure test (RCFT), delayed recall (visuospatial memory) [32]. Although there are recently suggested operationalizations of subtle cognitive decline [33], there are no definitive criteria. We categorized patients as having subtle cognitive decline if they scored less than the cutoff value (1.5 SD less than the healthy control mean) on one neuropsychological test. Patients with two or more scores less than the cutoff value were categorized as MCI. Patients categorized as either subtle cognitive decline or MCI were considered as having “detectable cognitive impairment.” The remaining patients had no scores less than the cutoff value and were categorized as SCD. Stratified cutoff scores (1.5 SD less than control means): VOSP silhouettes 16.8/20; BNT 49.3/60 (<12 years of education) or 50.5 (≥12 years of education); RAVLT A6 4.6/15 (<12 years of education) or 4.4 (≥12 years of education); and RCFT delayed recall: 7.1/36. There were no significant differences between older (≥65 years) and younger (<65 years) control subjects. Consequently, we did not stratify the cutoff scores by age.
Fig. 1.
Operationalization of SCD and NIA-AA stage subclassifications.
2.4. Classifications—step 2: SCD subgroups and NIA-AA stages
Patients were subsequently categorized following the algorithms presented in Fig. 1 (see also Supplementary Table 1). Important to note, although NIA-AA stages 0 to 2 require absence of objective cognitive impairment when studied in a dementia context, SCD in itself is not a prerequisite of the NIA-AA stages [15]. However, the characteristics of the current sample (all patients being active help-seekers with SCD or more advanced cognitive impairment) imply that all patients eligible to be categorized into NIA-AA stages 0 to 2 were in fact patients with SCD, which would be the case in most memory clinic settings. NIA-AA stages were by definition independent samples, but the SCDplus groups were partly overlapping with each other and with the NIA-AA stages (clarified in Supplementary Fig. 1). We separated SCDplus from SCDplusbio to explore different variants of the SCDplus features—SCDplusbio including invasive and SCDplus including noninvasive methods. In our operationalization of SCDplus, biomarkers and APOE status are therefore not accounted for, and SCDplusbio is a subcategory of SCDplus. In a subanalysis, NIA-AA stages 2 and 3 were pooled because of small group sizes.
2.5. Follow-up assessment
Patients were followed up for 12 to 142 months, mean (SD) 48 (35) months. The varying follow-up intervals reflect the broad time range of inclusion (14 years) of consecutive patients at the memory clinic. The mean follow-up time did not differ significantly between subclassifications of SCD or between stable and declining patients (data not shown). The mean follow-up time was significantly shorter for patients with subtle cognitive decline or MCI, which was expected as these patients on average may be temporally closer to advanced cognitive impairment, limiting possibilities of further follow-up.
Neuropsychological testing and diagnostic assessment of clinical dementia were performed at all follow-up rounds. The cognitive decline outcome was defined as decline in neuropsychological test results (using delta values) or to clinical dementia (using GDS and criteria for dementia), at follow-up. For patients with GDS stage ≥4 at follow-up, we determined the presence of manifest dementia syndrome and specific dementia diagnosis using standardized criteria [34], [35], [36], [37] (specified in Table 2 legend). Neuropsychological decline was assessed for all patients. Patients not meeting criteria for decline were considered stable.
Table 2.
Cognitive outcome for different classifications
| Follow-up data | Total patient sample | Classifications excluding detectable cognitive impairment∗ |
Classifications including detectable cognitive impairment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total group (=SCD) | SCDplus | SCDplusbio | NIA-AA stage 0 | NIA-AA stage 1 | NIA-AA stage 2 | Total group | NIA-AA stage 3 | MCI | Unclassified | ||
| N | 235 | 122 | 98 | 69 | 46 | 10 | 21 | 113 | 6 | 77 | 30 |
| Stable, n (%) | 118 (50%) | 74 (61%) | 56 (57%) | 36 (52%) | 33 (72%) | 5 (50%) | 4 (19%) | 44 (39%) | 0 | 23 (30%) | 21 (70%) |
| Declined at follow-up, n (%) | 117 (50%) | 48 (39%) | 42 (43%) | 33 (48%) | 13 (28%) | 5 (50%) | 17 (81%) | 69 (61%) | 6 (100%) | 54 (70%) | 9 (30%) |
| Dementia | 58 (25%) | 12 (10%) | 12 (12%) | 9 (13%) | 4 (9%) | 1 (10%) | 5 (24%) | 46 (41%) | 3 (50%) | 40 (52%) | 3 (10%) |
| Follow-up time, months, M (SD) | 39 (28) | 37 (33) | 39 (32) | 39 (35) | 37 (27) | 12 | 53 (42) | 39 (28) | 41 (23) | 39 (28) | 67 (4) |
| Cognitive decline, no dementia | 59 (25%) | 36 (30%) | 30 (31%) | 24 (35%) | 9 (20%) | 4 (40%) | 12 (57%) | 23 (20%) | 3 (50%) | 14 (18%) | 6 (20%) |
| Follow-up time, months, M (SD) | 54 (36) | 66 (35) | 63 (35) | 59 (33) | 57 (37) | 82 (40) | 55 (32) | 36 (29) | 24 (2) | 36 (29) | 23 (3) |
| Specific dementia diagnosis, n | |||||||||||
| AD dementia | 29 | 3 | 3 | 3 | 0 | 0 | 3 | 26 | 3 | 21 | 2 |
| Mixed dementia | 13 | 2 | 2 | 2 | 0 | 0 | 1 | 11 | 0 | 11 | 0 |
| Vascular dementia | 12 | 4 | 4 | 3 | 2 | 0 | 1 | 8 | 0 | 7 | 1 |
| Lewy body dementia | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Dementia NUD | 3 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 |
Abbreviations: AD, Alzheimer's disease; M, mean; MCI, mild cognitive impairment; NIA-AA, National Institute on Aging–Alzheimer's Association; NUD, nonultra descriptum; SCD, subjective cognitive decline; SD, standard deviation.
NIA-AA stages were by definition independent samples, but the SCDplus groups were partly overlapping with each other and with the NIA-AA stages (see also Supplementary Fig. 1). Follow-up times are presented as the mean and SD. Criteria used for dementia diagnostics (procedures specified in detail in Wallin et al. [19]): AD: the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) [34]; Lewy body dementia [35]; subcortical vascular dementia [36] and cortical vascular dementia: the National Institute of Neurological Disorders and Stroke (NINDS) and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (AIREN) criteria [37]. Mixed diagnosis refers to a combination of AD/subcortical vascular dementia or AD/cortical vascular dementia as previously described by Wallin et al. [19]. Unclassified patients = patients with subtle cognitive decline but at least one negative biomarker (=not fulfilling criteria for preclin-3). Dementia NUD = presence of dementia syndrome without hallmarks of specific etiology.
2.6. Statistical analyses
Two points of measurement were analyzed: baseline data and the last available follow-up for each individual. Baseline differences between SCD patients and patients with subtle cognitive decline/MCI were analyzed using chi-square or independent samples t tests.
To determine decline, we calculated normal delta value ranges using results from healthy control subjects with a matched follow-up time. Cutoff scores corresponded to the mean delta value for the lowest quartile of the control subjects. Control delta values were not affected by age, years of education, or follow-up time, hence cutoffs were not stratified. The cutoff delta values in raw scores for the neuropsychological tests were RAVLT immediate recall, −2.0; RCFT delayed recall, −2.5; VOSP silhouettes, −1.0; and BNT, −2.0. We defined cognitive decline as delta values less than the normal range for two or more tests.
We report the proportion of stable, declining, and patients converting to specific dementia diagnoses in each group. The predictive ability of the classifications was analyzed by calculating sensitivity (true positives/[true positives + false negatives]), specificity (true negatives/[true negatives + false positives]), positive likelihood ratios (+LRs; sensitivity/[1 − specificity]), and negative LRs (−LRs; [1 − sensitivity]/specificity) of the classifications in relation to cognitive decline, dementia, and ADD, including pretest and post-test probability. Because of varying follow-up time, we divided the groups into shorter versus longer follow-up, using the median follow-up time, to subanalyze post-test probability for the classifications in relation to cognitive decline and dementia.
To investigate the individual contribution of each variable included in the classifications (CSF AD-markers; APOE ɛ4 ≥1 allele; informant-reported memory decline; SCD onset age ≥60 years; and SCD duration ≤5 years) in predicting cognitive decline, dementia, and ADD, we constructed binary logistic regression models with cognitive decline and dementia as dependent variables.
3. Results
3.1. Baseline characteristics
Baseline characteristics are presented in Table 1.
3.2. Description of cognitive outcome
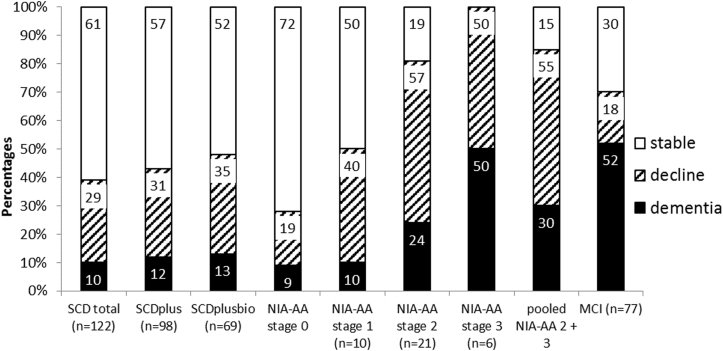
Cognitive outcomes are reported in Table 2 and Fig. 2. In the total patient sample (n = 235), 50% declined cognitively at follow-up and 25% converted to dementia. In SCD patients (n = 122), 39% declined cognitively and 10% converted to dementia. In patients with subtle cognitive decline/MCI at baseline (n = 113), 61% declined cognitively and 41% converted to dementia. Within the SCD group, the NIA-AA stage 2 group (n = 21) comprised the largest proportion of declining patients (81%) as well as dementia converters (24%). Of those categorized as SCDplusbio (n = 69), 48% declined cognitively and 13% converted to dementia. Three SCD patients converted to ADD, all of which fulfilled the criteria for NIA-AA stage 2, SCDplus, and SCDplusbio at baseline. NIA-AA stage 0 (n = 46) had the smallest proportion of dementia converters (9%), lower than the SCD total group (10%). When NIA-AA stages 2 + 3 were pooled, 85% (23/27) declined cognitively, compared with 70% (54/77) of MCI patients (Fig. 2).
Fig. 2.
Cognitive outcome at follow-up for the subclassification groups. Abbreviations: MCI, mild cognitive impairment; NIA-AA, National Institute on Aging–Alzheimer's Association; SCD, subjective cognitive decline.
3.3. Sensitivity, specificity, and likelihood ratios
Sensitivity, specificity, and likelihood ratios for the classifications in relation to cognitive decline, dementia, and ADD are reported in Table 3. Generally, sensitivity levels were highest for the SCDplusbio category, and specificity levels were highest in the NIA-AA preclinical AD groups. In the SCD group, positive likelihood ratios were highest for the NIA-AA stage 2 group for cognitive decline (+LR 6.3), dementia (+LR 3.4), and ADD (+LR 6.5). In the pooled NIA-AA stages 2 + 3, +LR increased for cognitive decline and dementia. The positive post-test probability for cognitive decline was highest for “pooled NIA-AA stages 2 to 3” (85%). Splitting the groups into shorter versus longer follow-up time resulted in a generally increased ability (higher post-test probability) for the classifications within the SCD total group to predict dementia in shorter compared with longer follow-up time. The ability to predict cognitive decline was fairly equal in groups of shorter versus longer follow-up time (Table 3).
Table 3.
Sensitivity, specificity, and likelihood ratios for classifications in relation to cognitive decline, dementia, and AD dementia
| Sensitivity, specificity, likelihood ratios, and post-test probabilities | Classifications excluding detectable cognitive impairment (=SCD total group)∗ |
Classifications including detectable cognitive impairment† |
Pooled analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| SCDplus (n = 98) | SCDplusbio (n = 69) | NIA-AA stage 0 (n = 46) | NIA-AA stage 1 (n = 10) | NIA-AA stage 2 (n = 21) | NIA-AA stage 3 (n = 6) | MCI (n = 77) | NIA-AA stages 2–3 pooled‡ (n = 27) | |
| Cognitive decline | ||||||||
| Sensitivity | 83 | 69 | 27 | 10 | 35 | 5 | 46 | 37 |
| Specificity | 22 | 51 | 54 | 93 | 94 | 100 | 81 | 96 |
| Positive likelihood ratio | 1.1 | 1.4 | 0.6 | 1.5 | 6.3§ | — | 2.4 | 8.7§ |
| Negative likelihood ratio | 0.8 | 0.6 | 1.4 | 1.0 | 0.7 | 0.9 | 0.7 | 0.7 |
| Positive (pre) post-test probability | (39) 40% | (39) 48% | (39) 28% | (39) 50% | (39) 81% | (51) NA | (50) 70% | (49) 85% |
| Shorter follow-up¶ | 37% | 52% | 17% | NA# | 80% | NA# | 70% | 85% |
| Longer follow-up¶ | 46% | 44% | 41% | 82% | 74% | 85% | ||
| Dementia | ||||||||
| Sensitivity | 100 | 90 | 20 | 10 | 50 | 6 | 67 | 50 |
| Specificity | 21 | 46 | 60 | 92 | 84 | 98 | 76 | 87 |
| Positive likelihood ratio | 1.3 | 1.7 | 0.5 | 1.2 | 3.4 | 3.6 | 2.8 | 3.7 |
| Negative likelihood ratio | 0 | 0.2∗∗ | 1.3 | 1.0 | 0.6 | 1.0 | 0.4 | 0.6 |
| Positive (pre) post-test probability | (8) 10% | (8) 13% | (8) 4% | (8) 10% | (8) 24% | (21) 50% | (21) 43% | (10) 30% |
| Shorter follow-up¶ | 16% | 21% | 9% | NA# | 30% | NA# | 48% | 29% |
| Longer follow-up¶ | 4% | 6% | 0 | 18% | 38% | 31% | ||
| AD dementia | ||||||||
| Sensitivity | 100 | 100 | 0 | 0 | 100 | 10 | 72 | 75 |
| Specificity | 20 | 45 | 60 | 91 | 85 | 99 | 73 | 86 |
| Positive likelihood ratio | 1.3 | 1.8 | 0 | 0 | 6.5§ | 6.9§ | 2.7 | 5.4§ |
| Negative likelihood ratio | 0 | 0†† | 1.7 | 1.1 | 0†† | 0.9 | 0.4 | 0.3∗∗ |
| Positive (pre) post-test probability | (3) 3% | (3) 4% | (3) 0% | (3) 0% | (3) 14% | (13) 50% | (13) 27% | (5) 22% |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; NIA-AA, National Institute on Aging–Alzheimer's Association; SCD, subjective cognitive decline.
SCD subgroup analyses were performed within the SCD group, not including patients in NIA-AA stage 3 or MCI.
Analyses for NIA-AA stage 3 and MCI were performed within the total sample, including also the SCD total group.
Analyses of pooled NIA-AA groups 2 to 3 were performed for SCD patients + patients with subtle cognitive decline.
Positive likelihood ratio 5 to 10 (indicating moderately increased likelihood; ≈30% to 45% increase).
Shorter versus longer follow-up was split by the median follow-up time in the SCD group (57 months), in the MCI group (25 months), and in the SCD + subtle cognitive impairment group (27 months).
Subanalyses were only performed for group sizes ≥n = 20.
Negative likelihood ratio 0.1 to 0.2 (indicating moderately decreased likelihood; ≈30% to 45% decrease).
Negative likelihood ratio <0.1 (indicating largely decreased likelihood; >45% decrease). Interpretation guide for likelihood ratios was adapted from Jaeschke et al. [38].
3.4. Predictive value of individual variables
Binary logistic regression analyses of the individual predictive values are presented in Table 4. Model 1 contained features included in SCD plus/SCDplusbio, and in model 2 we included only CSF biomarkers as per the NIA-AA stages. In both models, CSF Aβ42 ≤ 482 ng/L was the only significant predictor of cognitive decline (odds ratio [OR]: model 1 = 3.7; model 2 = 5.4), and also predicted dementia using model 2 (OR = 5.6). No variables predicted dementia when using model 1 (data not shown). Model 2 correctly classified 92% of SCD patients converting to dementia. The outcome did not change if sex, baseline age, years of education, and follow-up time (months from baseline to last follow-up) were entered as covariates (data not shown), or if patients classified as NIA-AA stage 3 were included in the analysis.
Table 4.
Binary logistic regression. Baseline predictors of cognitive decline in SCD patients at follow-up
| Wald | P | OR | 95% CI for OR Lower-upper |
|
|---|---|---|---|---|
| Prediction of cognitive decline∗ Model 1a |
||||
| Aβ42 ≤ 482 | 3.9 | <.047 | 3.7 | 1.0–13.7 |
| T-tau ≥320 | 0.2 | ns | 0.7 | 0.1–3.6 |
| p-tau ≥52 | 2.0 | ns | 3.3 | 0.6–17.4 |
| APOE ɛ4 ≥1 allele | 0.1 | ns | 0.8 | 0.3–2.6 |
| Informant-reported memory decline (CIMP-QUEST memory scale) | 0.0 | ns | 1.0 | 0.1–6.9 |
| Informant-reported subjective symptom onset age ≥60 years | 2.6 | ns | 2.4 | 0.8–6.9 |
| Informant-reported subjective symptom duration ≤5 years | 0.2 | ns | 1.5 | 0.2–10.7 |
| Prediction of cognitive decline∗ Model 2a |
||||
| Aβ42 ≤ 482 | 12.5 | <.001 | 5.4 | 2.1–13.8 |
| T-tau ≥ 320 | 0.1 | ns | 1.3 | 0.3–4.7 |
| p-tau ≥ 52 | 0.1 | ns | 1.2 | 0.3–4.3 |
| Prediction of dementia† Model 2b |
||||
| Aβ42 ≤ 482 | 4.9 | .027 | 5.6 | 1.2–25.6 |
| T-tau ≥ 320 | 0.1 | ns | 1.4 | 0.1–22.3 |
| p-tau ≥ 52 | 0.0 | ns | 1.2 | 0.1–18.0 |
Abbreviations: CI, confidence interval; CIMP-QUEST, Cognitive Impairment Questionnaire; OR, odds ratio; SCD, subjective cognitive decline.
NOTE. Results from the logistic regression models are reported as OR with 95% CI. Years of education, sex, and follow-up time were tested as covariates. All variables except age and years of education were dichotomous.
Prediction of cognitive decline: Model 1a: Omnibus test of model coefficients: P = .017 (Χ2 = 17.1; df = 7). Hosmer and Lemeshow test: P = .49 (Χ2 = 6.5; df = 7). Correctly classified SCD patients when including seven variables: 73.8%. Model 2a: Omnibus test of model coefficients: P < .001 (Χ2 = 17.1; df = 3). Hosmer and Lemeshow test: P = .64 (Χ2 = 1.7; df = 3). Correctly classified declining patients when including three variables: 70.8%.
Prediction of dementia: Model 2b: Omnibus test of model coefficients: P = .07 (Χ2 = 7.0; df = 3). Hosmer and Lemeshow test: P = .79 (Χ2 = 1.0; df = 3). Correctly classified patients with dementia when including all three variables: 92.0%.
4. Discussion
The main finding of the present study was that the NIA-AA stage 2 classification successfully predicted cognitive decline, dementia, and ADD specifically. Furthermore, the predictive values (i.e., +LR) increased for all three outcomes when NIA-AA stages 2 and 3 were pooled. The SCDplus and SCDplusbio subgroups had lower +LR for the prediction of cognitive decline and dementia. When NIA-AA stages 2 + 3 were pooled, the proportion of cognitive decliners was higher than in the MCI group, which offers a perspective to the potential benefits of adding biomarkers in SCD. Overall, these results support the usefulness of the NIA-AA preclinical AD stages 2 + 3 in predicting cognitive decline, dementia, and ADD, in memory clinic patients with SCD or subtle cognitive decline.
The strength of the present study is the simultaneous evaluation of several classification models, permitting comparisons. The performance of symptom versus biomarker classifications within one clinical sample is of clinical interest, even if the constructs are not intended to compete. Furthermore, our outcome measures encompass cognitive decline as well as dementia diagnoses, and the follow-up time is longer than in previous similar studies. The present study also has some limitations. Follow-up length varied and group sizes were small, especially for NIA-AA stages 1 and 3, limiting the conclusions we can draw about these groups. In addition, some of the features that have been suggested as SCDplus criteria were not available in our data set, and specific type of subjective symptoms was not assessed.
We are only aware of three previous memory clinic–based studies that have investigated the predictive value of CSF AD biomarkers in relation to clinical progression in SCD patients. A multicenter study reported that SCD patients with a CSF AD profile (n = 30) had worse baseline cognition than those without a CSF AD profile (n = 28) but did not deteriorate within 1 to 3 years [39]. The present study findings are more in line with a second study of similar sample size reporting that the preclinical AD stages predicted an incremental cognitive decline for each stage over a mean (SD) of 2 (1) years [40]. In a third recent study, 27% (n = 3) of patients with SCD and a pathologic CSF ratio (Aβ42/p-tau) developed ADD within 5 years [41]. The most comparable diagnostic category in our study would be the NIA-AA stage 2 that had a similar proportion of dementia converters (24%) although only 14% to ADD. The lower rate of ADD converters in our study may be explained by the use of CSF markers individually, not analyzed as a ratio. Our study had a longer follow-up period than the previous available studies and a larger sample than two-thirds of studies.
Comparing SCD categories and NIA-AA stages, the SCD/SCDplus/SCDplusbio categories did not convincingly improve the ability to identify true positive cases and were clearly outperformed by the NIA-AA stage 2. The SCDplusbio classification performed well in identifying true negative cases, compared with the NIA-AA stages 1 to 3. The results remained largely the same when the various groups were analyzed separately for those with shorter and longer follow-up time. However, within the SCD group the ability to predict dementia was better in those with shorter follow-up time compared with longer follow-up time indicating that some SCD patients have a high pathologic burden. This may be associated with the relatively young age of the patient group [42]. Consistent with previous research [40], [41] CSF Aβ42 was the only significant single predictor of cognitive decline. This is also in line with the model proposed by Jack et al. [43], describing a temporal evolution of AD with changes in CSF Aβ42 occurring first.
Few SCD patients in our sample (10/122) fulfilled the criteria for NIA-AA stage 1. It has been proposed that SCD is present approximately 15 years before the MCI stage [7], [44]. Thus, SCD should occur parallel to early amyloid changes, in which case it would be expected that more help-seekers would have met criteria for NIA-AA stage 1. Rather, our results may reflect the suggested time-slope in which individuals in preclinical AD stage 1 still have not developed cognitive symptoms [43], but that SCD triggering help-seeking may be concurrent with NIA-AA stage 2. The notion of neuronal injury markers representing more advanced stages of the AD disease trajectory was also supported by our findings of an increased rate of decline when tau markers were added to classifications (i.e., NIA-AA stage 2), which is in accordance with previous findings of increased subjective difficulties in stage 2 compared with stage 1 [45]. Possibly, patients meeting criteria for NIA-AA stage 1 are thus less frequent in populations of active help-seekers. NIA-AA stages 2 and 3 should be explored further in larger clinical samples.
In a meta-analysis of longitudinal studies with >4 years of follow-up, 14.1% of SCD patients developed dementia [46]. The slightly lower conversion rate in our study (10%) may result from the relatively young SCD sample (mean 62 years). Results from a community-based study reported lower rates of cognitive decline in SCD patients followed for 13 months (18.9% for SCDplus and 5.6% for SCD) than what was found in our study (56% for SCDplus and 39% for SCD) [47]. The higher proportion of decliners in our sample is likely because of longer follow-up time and generally higher conversion rates in samples consisting of active help-seekers [48]. The shorter follow-up time for MCI patients was expected, as advanced neuropsychological testing was not continued for patients with very poor cognitive functioning, because of ethical reasons.
5. Conclusions
Although group sizes were small, the results from this study offer some support for the use of biomarker-based classifications—specifically the NIA-AA preclinical AD stage 2 or stage 2 + 3—to predict cognitive decline, dementia, and ADD, in memory clinic patients with subjective or subtle cognitive decline. Our findings also support that SCD serious enough to trigger help-seeking likely occurs concurrent with preclinical stage 2, that is, when tau markers have become positive. We additionally found CSF Aβ42 to be the most important predictor of cognitive decline and dementia in these SCD patients. SCDplus categories may also be valid categories to identify at-risk patients, especially when neurobiological methods are not available, although in this study to a lesser extent than the preclinical AD categories.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources, focusing on studies presenting classification models to improve prediction of future objective cognitive decline in patients without current objective cognitive impairment. We address, and properly cite, classification models that were presented recently (i.e., “subjective cognitive decline” and National Institute on Aging–Alzheimer's Association (NIA-AA) “preclinical Alzheimer's disease stages”).
-
2.
Interpretation: Our findings provide some support for the biomarker-based NIA-AA stages 2 and 3 and less support for the subjective cognitive decline (SCD)plus variants (as operationalized in the present study) to improve prediction of cognitive decline, dementia, and Alzheimer's disease dementia specifically, in patients with subjective or subtle cognitive decline.
-
3.
Future directions: NIA-AA stages 2 and 3 should be explored further in larger clinical samples, for example, in combination with onset age of SCD.
Acknowledgments
Financial support for this study was provided by the Sahlgrenska University Hospital, Swedish Medical Research Council, Swedish Alzheimer Foundation, Swedish Dementia Foundation (AF-452271), Swedish Psychiatric Research Foundation, Hjalmar Svensson Foundation, Wilhelm and Martina Lundgren Foundation, Konung Gustaf V:s och Drottning Viktorias Frimurarestiftelse, and the Gothenburg Foundation for Neurological Research.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.04.006.
Supplementary data
References
- 1.Morris J.C. Mild cognitive impairment and preclinical Alzheimer's disease. Geriatrics. 2005;Suppl:9–14. [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drachman D.A. The amyloid hypothesis, time to move on: amyloid is the downstream result, not cause, of Alzheimer's disease. Alzheimers Dement. 2014;10:372–380. doi: 10.1016/j.jalz.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hessen E., Nordlund A., Stalhammar J., Eckerstrom M., Bjerke M., Eckerstrom C. T-Tau is associated with objective memory decline over two years in persons seeking help for subjective cognitive decline: a report from the Gothenburg-Oslo MCI Study. J Alzheimers Dis. 2015;47:619–628. doi: 10.3233/JAD-150109. [DOI] [PubMed] [Google Scholar]
- 6.Wirth M., Madison C.M., Rabinovici G.D., Oh H., Landau S.M., Jagust W.J. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisberg B., Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer's disease. Int Psychogeriatr. 2008;20:1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 8.Prichep L.S., John E.R., Ferris S.H., Rausch L., Fang Z., Cancro R. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006;27:471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Glodzik-Sobanska L., Reisberg B., De Santi S., Babb J.S., Pirraglia E., Rich K.E. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- 10.Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Oijen M., de Jong F.J., Hofman A., Koudstaal P.J., Breteler M.M. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dement. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Jessen F., Wiese B., Bachmann C., Eifflaender-Gorfer S., Haller F., Kolsch H. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 13.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrotin A., Mormino E.C., Madison C.M., Hayenga A.O., Jagust W.J. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinuevo J.L., Rabin L.A., Amariglio R., Buckley R., Dubois B., Ellis K.A., for the Subjective Cognitive Decline Initiative (SCD-I) Working Group Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13:296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colijn M.A., Grossberg G.T. Amyloid and tau biomarkers in subjective cognitive impairment. J Alzheimers Dis. 2015;47:1–8. doi: 10.3233/JAD-150180. [DOI] [PubMed] [Google Scholar]
- 18.Lista S., Molinuevo J.L., Cavedo E., Rami L., Amouyel P., Teipel S.J. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis. 2015;48:S171–S191. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- 19.Wallin A., Nordlund A., Jonsson M., Lind K., Edman A., Gothlin M. The Gothenburg MCI study: design and distribution of Alzheimer's disease and subcortical vascular disease diagnoses from baseline to 6-year follow-up. J Cereb Blood Flow Metab. 2016;36:114–131. doi: 10.1038/jcbfm.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Reisberg B., Ferris S.H., de Leon M.J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 22.Astrand R., Rolstad S., Wallin A. Cognitive Impairment Questionnaire (CIMP-QUEST): reported topographic symptoms in MCI and dementia. Acta Neurol Scand. 2010;121:384–391. doi: 10.1111/j.1600-0404.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery S.A., Smeyatsky N., de Ruiter M., Montgomery D.B. Profiles of antidepressant activity with the Montgomery-Asberg Depression Rating Scale. Acta Psychiatr Scand Suppl. 1985;320:38–42. doi: 10.1111/j.1600-0447.1985.tb08073.x. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage J.A. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 25.Gottfries G.G., Noltorp S., Norgaard N. Experience with a Swedish version of the Geriatric Depression Scale in primary care centres. Int J Geriatr Psychiatry. 1997;12:1029–1034. doi: 10.1002/(sici)1099-1166(199710)12:10<1029::aid-gps683>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Blennow K., Ricksten A., Prince J.A., Brookes A.J., Emahazion T., Wasslavik C. No association between the alpha2-macroglobulin (A2M) deletion and Alzheimer's disease, and no change in A2M mRNA, protein, or protein expression. J Neural Transm (Vienna) 2000;107:1065–1079. doi: 10.1007/s007020070052. [DOI] [PubMed] [Google Scholar]
- 27.Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 28.Eckerstrom C., Olsson E., Bjerke M., Malmgren H., Edman A., Wallin A. A combination of neuropsychological, neuroimaging, and cerebrospinal fluid markers predicts conversion from mild cognitive impairment to dementia. J Alzheimers Dis. 2013;36:421–431. doi: 10.3233/JAD-122440. [DOI] [PubMed] [Google Scholar]
- 29.Binetti G., Cappa S.F., Magni E., Padovani A., Bianchetti A., Trabucchi M. Visual and spatial perception in the early phase of Alzheimer's disease. Neuropsychology. 1998;12:29–33. doi: 10.1037//0894-4105.12.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Geffen G.M., Butterworth P., Geffen L.B. Test-retest reliability of a new form of the auditory verbal learning test (AVLT) Arch Clin Neuropsychol. 1994;9:303–316. [PubMed] [Google Scholar]
- 31.Kaplan E., Goodglass H., Weintraub S. 2nd ed. Lea & Febiger; Philadelphia: 1983. The Boston Naming Test. [Google Scholar]
- 32.Meyers J.K. Psychological Assessment Resources Inc; Odessa, Florida: 1995. Rey Complex Figure Test and Recognition Trial. [Google Scholar]
- 33.Edmonds E.C., Delano-Wood L., Galasko D.R., Salmon D.P., Bondi M.W., Alzheimer's Disease Neuroimaging Initiative Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. J Alzheimers Dis. 2015;47:231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 35.McKeith I.G., Perry E.K., Perry R.H. Report of the second dementia with Lewy Body International Workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53:902–905. doi: 10.1212/wnl.53.5.902. [DOI] [PubMed] [Google Scholar]
- 36.Erkinjuntti T., Inzitari D., Pantoni L., Wallin A., Scheltens P., Rockwood K. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 37.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke R., Guyatt G.H., Sackett D.L. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 39.Visser P.J., Verhey F., Knol D.L., Scheltens P., Wahlund L.O., Freund-Levi Y. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 40.van Harten A.C., Smits L.L., Teunissen C.E., Visser P.J., Koene T., Blankenstein M.A. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81:1409–1416. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 41.Sierra-Rio A., Balasa M., Olives J., Antonell A., Iranzo A., Castellvi M. Cerebrospinal fluid biomarkers predict clinical evolution in patients with subjective cognitive decline and mild cognitive impairment. Neurodegener Dis. 2016;16:69–76. doi: 10.1159/000439258. [DOI] [PubMed] [Google Scholar]
- 42.Marshall G.A., Fairbanks L.A., Tekin S., Vinters H.V., Cummings J.L. Early-onset Alzheimer's disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol. 2007;20:29–33. doi: 10.1177/0891988706297086. [DOI] [PubMed] [Google Scholar]
- 43.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reisberg B., Ferris S.H., Shulman E., Steinberg G., Buttinger C., Sinaiko E. Longitudinal course of normal aging and progressive dementia of the Alzheimer's type: a prospective study of 106 subjects over a 3.6 year mean interval. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:571–578. doi: 10.1016/0278-5846(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Amariglio R.E., Mormino E.C., Pietras A.C., Marshall G.A., Vannini P., Johnson K.A. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Blazquez M.A., Avila-Villanueva M., Maestu F., Medina M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis. 2016;52:271–281. doi: 10.3233/JAD-150956. [DOI] [PubMed] [Google Scholar]
- 48.Farias S.T., Mungas D., Reed B.R., Harvey D., DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.