Abstract
Rationale: Platelets are believed to contribute to acute respiratory distress syndrome (ARDS) pathogenesis through inflammatory coagulation pathways. We recently reported that leucine-rich repeat–containing 16A (LRRC16A) modulates baseline platelet counts to mediate ARDS risk.
Objectives: To examine the role of LRRC16A in ARDS survival and its mediating effect through platelets.
Methods: A total of 414 cases with ARDS from intensive care units (ICUs) were recruited who had exome-wide genotyping data, detailed platelet counts, and follow-up data during ICU hospitalization. Association of LRRC16A single-nucleotide polymorphisms (SNPs) and ARDS prognosis, and the mediating effect of SNPs through platelet counts were analyzed. LRRC16A mRNA expression levels for 39 cases with ARDS were also evaluated.
Measurements and Main Results: Missense SNP rs9358856G>A within LRRC16A was associated with favorable survival within 28 days (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.38–0.87; P = 0.0084) and 60 days (P = 0.0021) after ICU admission. Patients with ARDS who carried the variant genotype versus the wild-type genotype showed an attenuated platelet count decline (∆PLT) within 28 days (difference of ∆PLT, −27.8; P = 0.025) after ICU admission. Patients with ∆PLT were associated with favorable ARDS outcomes. Mediation analysis indicated that the SNP prognostic effect was mediated through ∆PLT within 28 days (28-day survival: HRIndirect, 0.937; 95% CI, 0.918−0.957; P = 0.0009, 11.53% effects mediated; 60-day survival: HRIndirect, 0.919; 95% CI, 0.901−0.936; P = 0.0001, 14.35% effects mediated). Functional exploration suggested that this SNP reduced LRRC16A expression at ICU admission, which was associated with a lesser ∆PLT during ICU hospitalization.
Conclusions: LRRC16A appears to mediate ∆PLT after ICU admission to affect the prognosis in patients with ARDS.
Keywords: SNP, platelets, ARDS outcome, causal mediation analysis
At a Glance Commentary
Scientific Knowledge on the Subject
Acute respiratory distress syndrome (ARDS) is a deadly, complex disease with an in-hospital mortality rate that exceeds 30%. Platelets are believed to contribute to the pathogenesis of ARDS and affect patients’ prognosis.
What This Study Adds to the Field
Leucine-rich repeat–containing 16A appears to mediate platelet count decline after admission to the intensive care unit, and, in turn, affect ARDS patients’ prognosis. The findings may aid in identifying novel therapeutic targets for ARDS within platelet cellular processes.
Acute respiratory distress syndrome (ARDS), which is characterized by acute hypoxemic respiratory failure and bilateral pulmonary infiltrates with an amplified inflammatory response, is a deadly complex disease with an in-hospital mortality rate that exceeds 30% (1–3). Several factors affect ARDS outcomes. For example, worsening oxygenation, multiple organ dysfunction, and progressive underlying diseases are common risk factors for ARDS mortality (4). Recently, platelets have been increasingly recognized for their roles in ARDS outcomes, which are likely mediated by their involvement in inflammatory responses and disseminated intravascular coagulation (5–7). Genetic risk factors may also determine ARDS outcomes (8); studies have identified a few potential prognostic biomarkers (9–13). However, the roles of genetic susceptibility in ARDS outcome and its interplay with micro-environmental factors remain incompletely understood (14).
Recently, we reported that a novel genetic variant in leucine-rich repeat–containing 16A (LRRC16A) is associated with reduced ARDS risk, and this phenomenon is partially mediated through platelet counts (15). These findings might aid in identifying novel therapeutic targets for ARDS within platelet cellular processes (16). However, no studies have yet examined the interplay of LRRC16A and platelets on ARDS outcomes. The Gene Nomenclature Committee of the Human Genome Organization might rename LRRC16A as a capping protein regulator and myosin 1 linker 1 (CARMIL1) to reflect its homology and functional similarity to the genes currently approved as CARMIL2 and CARMIL3.
We hypothesized that LRRC16A influences ARDS outcomes by regulating platelets after intensive care unit (ICU) admission, and we explored this association using mediation analysis. Mediation analysis is one of several causal inference approaches that can identify the potential mechanism by which an independent variable (e.g., genetic variant) affects the outcome (e.g., ARDS death) via an explanatory factor or mediator (e.g., platelet count decline [∆PLT] during ICU hospitalization). This approach has been widely applied in epidemiological studies and in lung disease research (15, 17–19). Using exome-wide genotyping data, we evaluated the association of genetic variants in LRRC16A with baseline platelet count at ICU admission and ∆PLT up to 28 days after ICU admission. Significant genetic variants were further evaluated for an association with ARDS survival and whether their effects were mediated through platelet counts using causal mediation analysis. Some of the results have been previously reported in the form of an abstract for the 2015 American Thoracic Society meeting (20).
Methods
Clinical and Genotyping Study Population
The original study titled “Molecular Epidemiology of ARDS Study (MEARDS)” was approved by the institutional review boards of Harvard T. H. Chan School of Public Health, Massachusetts General Hospital (MGH), and Beth Israel Deaconess Medical Center (BIDMC) (approval number: 1999P0086071/MGH). All participants or their surrogates gave written informed consent.
Cases with ARDS were selected from the MEARDS study. As described previously (15), we screened each ICU admission at MGH and BIDMC for eligible subjects, who were defined as critically ill without any of the exclusion criteria (age younger than 18 yr, diffuse alveolar hemorrhage, chronic lung diseases other than chronic obstructive pulmonary disease or asthma, directive to withhold intubation, immunosuppression not secondary to corticosteroids, and treatment with granulocyte colony-stimulating factor) and with at least one predisposing condition for ARDS: sepsis, septic shock, trauma, pneumonia, aspiration, or massive transfusion of packed red blood cells (RBCs) (defined as >8 packed RBC units during the 24 h before admission). After enrollment, subjects were followed daily for the development of ARDS, as defined by the American-European Consensus Committee, which generally corresponds to moderate to severe ARDS by the Berlin definition (21, 22). Patient demographics and baseline clinical characteristics were collected at ICU admission. Patients who died or were discharged within 24 hours after ICU admission were excluded. All enrolled patients were followed until death, hospital discharge, or 28 days after ICU admission. Patients were also followed until 60 days after ICU admission for secondary outcomes. Lowest platelet counts were collected daily during ICU hospitalization for up to 28 days.
As described in the previous report (15), the genotyping was performed using the Infinium HumanExome BeadChip (Illumina, Inc, San Diego, CA) followed by standard quality control processes. The 414 cases with ARDS were selected from the existing exome-wide genotyping data, which had complete survival follow-up data and detailed platelet count data during ICU hospitalization. Five common single-nucleotide polymorphisms (SNPs) within LRRC16A of the 414 cases with ARDS were available, and they were included in the analysis.
Gene Expression Study Population
In addition, LRRC16A mRNA expression data of 39 cases with ARDS from the MEARDS cohort were included from an existing gene expression study and were further analyzed. The 39 cases were aged 64.2 ± 15.1 years old, 82% were men, 85% had declined platelet counts, and Acute Physiology and Chronic Health Evaluation (APACHE) III scores were averaged at 87.9 ± 22.7. Gene expression levels were evaluated by Custom TaqMan Arrays (probe #: Hs01084245_m1; Thermo Fisher Scientific, Inc, Waltham, MA). Protocols for sample collection, processing, and whole blood total RNA extraction were described previously (23). Each sample was run in triplicate, and cycle threshold (Ct) values were averaged (aCt) to represent Ct values for LRRC16A (aCtLRRC16A) and reference gene 18S (aCt18S), which was used to normalize LRRC16A expression by ∆CtLRRC16A = aCtLRRC16A – aCt18S. The 39 cases had complete platelet count data during ICU hospitalization and survival follow-up data, and were carried forward for further analysis. Of these, a subset of 27 cases had ExomeChip data and were used to explore the expression quantitative trait locus (eQTL) relationship.
Statistical Analyses
Baseline characteristics, including demographics and baseline measurements, were described as mean ± SD for continuous variables and number (%) for categorical variables. Student’s t-test or Fisher’s exact test was used for comparisons between groups for continuous or categorical variables, respectively. All SNPs were encoded in additive genetic format (0: wild type; 1: heterozygosity; 2: homozygosity; minor allele as effect allele), unless stated otherwise. Baseline platelet count was the lowest platelet count on the day of ICU admission (PLTbaseline). Maximal ∆PLT was estimated as PLTbaseline minus the lowest platelet count measured within 28 days (∆PLT28d, for primary purpose) or the lowest platelet count within 7 days (∆PLT7d, for sensitivity evaluation) after ICU admission. Primary prognostic outcome was ARDS survival (time-to-death) within 28 days (survival28d) after ICU admission, whereas the secondary prognostic outcome was survival within 60 days (survival60d) after ICU admission.
To test the association between LRRC16A variants and ARDS outcomes and the underlying causal mediation pathway through platelets, we split the analyses into four main scenarios in the predefined order of: (1) testing the associations between LRRC16A genetic variants and ARDS outcomes; (2) evaluating the associations between the identified genetic variant(s) and platelet count variables (PLTbaseline, ∆PLT28d, and ∆PLT7d); (3) analyzing the associations between the selected platelet count variable(s) and ARDS outcomes; and (4) causal inference analysis. A sequential testing strategy was used in which an analysis would move forward only if the previous one reached statistical significance (24).
Associations of LRRC16A SNPs on ARDS survival28d (primary outcome) and survival60d (secondary outcome) were evaluated by Cox regression with adjustment for common covariates, including age, sex, multiple transfusions, and APACHE III score. Results are described as hazard ratio (HR), 95% confidence interval (CI), and P value. False discovery rate (FDR) was used for multiple comparison correction (25). Any SNP with an FDR-q value ≤0.05 was carried forward. The identified SNP was further analyzed using Kaplan-Meier survival curves in additive and dominant (0: wild type, 1: heterozygosity or homozygosity) genetic models, respectively.
The identified SNP tested the association with platelet count variables (PLTbaseline, ∆PLT28d, and ∆PLT7d) using linear regression. For correlations between SNP and ∆PLT, sensitivity analysis was performed with additional adjustment for PLTbaseline. The platelet count variables with P values ≤0.05 were carried forward.
Cox regression was used to test the associations between platelet count variables and ARDS outcomes (survival28d and survival60d), respectively, with adjustment for common covariates. For the associations with ∆PLT28d and ∆PLT7d, models were additionally adjusted for PLTbaseline. Results are described as HR per 100 × 103/μl increment, 95% CI, and P value.
Causal inference analysis is detailed in the online supplement. Briefly, the mediation model by Valeri and VanderWeele was applied to evaluate the indirect effect of a SNP on ARDS survival that was mediated through ∆PLT (HRindirect) and the proportion of the effect mediated (26). Covariates included age, sex, multiple transfusion, APACHE III score, and baseline platelet count. The statistical P value for HRindirect was obtained by permutation test with 10,000 permutations.
Associations of the gene expression on ∆PLT variables (∆PLT28d and ∆PLT7d) were tested using linear regression, with adjustment for baseline platelet count. The eQTL relationship between SNP and gene expression was also analyzed using linear regression.
All analyses were performed in R Version 3.0.1 (The R Foundation, Vienna, Austria) or Stata Version 14 (StataCorp LP, College Station, TX).
Results
Distributions of demographic and baseline clinical characteristics among the 414 cases with ARDS are described in Table 1. Ninety-seven percent of cases were Caucasian. A total of 116 (28%) cases were dead within 28 days after ICU admission. Most of the patients had ∆PLT within 28 days (324 of 414; 78%) or the first 7 days (311 of 414; 75%) during ICU hospitalization. Seventeen SNPs in LRRC16A were genotyped on the Illumina ExomeChip; however, only five common SNPs (minor allele frequency ≥0.05) were included in the analysis to ensure adequate statistical power (see Figure E1 in the online supplement). The overall analysis workflow is described in Figure E2.
Table 1.
Study Population Demographic and Clinical Characteristics at Intensive Care Unit Admission
| ARDS Cases (n = 414) |
||
|---|---|---|
| Variable | Survivor | Nonsurvivor* |
| N |
298 (72.0) |
116 (28.0) |
| APACHE III score | 72.8 ± 21.3† | 91.2 ± 19.9 |
| Age, yr | 55.8 ± 17.5 | 68.0 ± 13.7 |
| Sex | ||
| Male | 191 (64.1) | 71 (61.2) |
| Female | 107 (35.9) | 45 (38.8) |
| Baseline platelet count‡, 103/μl | 216.1 ± 139.3§ | 189.2 ± 158.6 |
| ∆PLT28d‖, 103/μl | 57.5 ± 107.3§ | 85.8 ± 119.9 |
| ∆PLT7d¶, 103/μl | 40.6 ± 101.5** | 76.5 ± 113.8 |
| Ethnicity | ||
| Caucasian | 290 (97.3) | 113 (97.4) |
| African American | 3 (1.0) | 0 (0.0) |
| Hispanic | 2 (0.7) | 2 (1.7) |
| Asian/Pacific Islander | 2 (0.7) | 0 (0.0) |
| Other | 1 (0.3) | 1 (0.9) |
| Physiology | ||
| Heart rate, beats/min | ||
| Lowest†† | 79.6 ± 19.2 | 79.9 ± 17.9 |
| Highest‡‡ | 120.2 ± 22.9 | 120.9 ± 22.1 |
| Respiratory rate, breaths/min | ||
| Lowest†† | 15.7 ± 5.2§ | 16.9 ± 5.6 |
| Highest‡‡ | 30.3 ± 8.9 | 31.8 ± 8.8 |
| Systolic blood pressure, mmHg | ||
| Lowest†† | 81.8 ± 15.9 | 77.1 ± 14.6 |
| Highest‡‡ | 146.8 ± 24.4 | 143.9 ± 21.9 |
| Mean arterial pressure, mmHg | ||
| Lowest†† | 57.9 ± 10.0† | 54.1 ± 8.3 |
| Highest‡‡ | 99.8 ± 16.6 | 96.5 ± 13.9 |
| ABG testing in 24 h after ICU admission | ||
| PaO2, mmHg | 118.0 ± 73.6 | 118.1 ± 73.0 |
| FiO2, mmHg | 0.79 ± 0.23 | 0.80 ± 0.23 |
| P/F ratio | 157.4 ± 87.0 | 156.9 ± 93.3 |
| ARDS predispositions | ||
| Sepsis | 261 (87.6)† | 113 (97.4) |
| Pneumonia | 207 (69.5) | 82 (70.7) |
| Aspiration | 30 (10.1) | 8 (6.9) |
| Multiple transfusion | 25 (8.4) | 8 (6.9) |
| Pulmonary contusion | 12 (4.0) | 1 (0.9) |
| Multiple fractures | 12 (4.0) | 1 (0.9) |
| Complications | ||
| Diabetes mellitus | 57 (19.3) | 27 (23.3) |
| Immune suppression§§ | 21 (7.1) | 14 (12.1) |
| Chronic dialysis or peritoneal dialysis | 25 (8.5) | 12 (10.3) |
| Cirrhosis | 10 (3.4) | 16 (13.8) |
| Hepatic failure with coma or encephalopathy | 9 (3.1) | 8 (6.9) |
| Solid tumor with metastasis | 4 (1.4) | 5 (4.3) |
| Non-Hodgkin’s lymphoma | 0 (0.0) | 2 (1.7) |
Definition of abbreviations: ABG = arterial blood gas; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; ICU = intensive care unit; P/F = PaO2/FiO2; ∆PLT = platelet count decline.
Continuous variables are described as mean ± SD and compared by t test or Kruskall-Wallis rank sum test (platelet variables); categorical variables are described as number (%) and compared by Fisher’s exact test.
Patients dead within 28 days after ICU admission.
P < 0.001.
Lowest platelet count at ICU admission.
P < 0.05.
Maximal platelet count decline within 28 days (∆PLT28d) after ICU admission compared with baseline platelet count.
Maximal platelet count decline within 7 days (∆PLT7d) after ICU admission compared with baseline platelet count.
P < 0.01.
Minimal value in first 24 hours of ICU admission.
Maximal value in first 24 hours of ICU admission
Immune suppression within the past 6 months including radiation or chemotherapy with ≥0.3 mg/kg/d prednisone or equivalent.
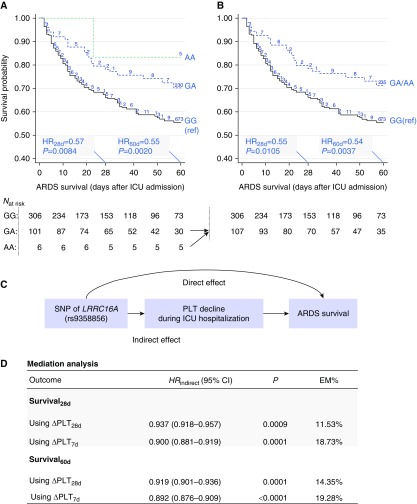
First, associations between five common SNPs and outcomes of ARDS patients were analyzed (Table 2). Only missense SNP rs9358856 (p.Val77Ile) showed a significant association with both the primary outcome: 28-day survival (HR, 0.57; 95% CI, 0.38–0.87; P = 0.0084; FDR-q = 0.04) (Figure 1A), and the secondary outcome: 60-day survival (HR, 0.55; 95% CI, 0.38−0.81; P = 0.0021; FDR-q = 0.01) (Figure 1A). The results retained statistical significance with additional adjustment for sepsis (see Table E1 in the online supplement). Because only a small number of patients were homozygous (AA) for SNP rs9358856, we also tested the association between SNP rs9358856 in a dominant genetic model and ARDS survival, which gave consistent results (Figure 1B). SNP rs7766874, which was previously reported to be associated with ARDS risk (15) was only modestly associated with 28-day survival, with borderline significance (HR, 0.80; P = 0.0610) (Table 2). Thus, SNP rs9358856 was analyzed further.
Table 2.
Association between LRRC16A SNPs and Survival of Patients with Acute Respiratory Distress Syndrome
| Survival28d* |
Survival60d† |
|||
|---|---|---|---|---|
| SNP‡ | HR (95% CI) | P Value (FDR-q) | HR (95% CI) | P Value (FDR-q) |
| rs9358856 | 0.57 (0.38–0.87) | 0.0084 (0.04) | 0.55 (0.38–0.81) | 0.0020 (0.01) |
| rs2274089 | 0.63 (0.38–1.04) | 0.0714 (0.12) | 0.69 (0.42–1.11) | 0.1260 (0.25) |
| rs7766874 | 0.80 (0.63–1.01) | 0.0610 (0.12) | 0.85 (0.68–1.06) | 0.1528 (0.25) |
| rs1012899 | 1.12 (0.82–1.52) | 0.4762 (0.48) | 1.10 (0.81–1.50) | 0.5344 (0.67) |
| rs742132 | 1.12 (0.87–1.45) | 0.3684 (0.46) | 1.05 (0.82–1.36) | 0.6816 (0.68) |
Definition of abbreviations: CI = confidence interval; FDR-q = false discovery rate–adjusted P value; HR = hazard ratio per minor/effect allele; LRRC16A = leucine-rich repeat–containing 16A; SNP = single-nucleotide polymorphism.
The model was adjusted for age, sex, blood transfusion, baseline platelet count, and Acute Physiology and Chronic Health Evaluation III score.
Primary outcome.
†Secondary outcome.
Analyzed in additive genetic model.
Figure 1.
Association between single-nucleotide polymorphism (SNP) rs9358856 and acute respiratory distress syndrome (ARDS) survival and the effects mediated through platelet count. (A) Kaplan-Meier survival curves stratified by SNP rs9358856 genotypes in additive genetic model—wild type (GG; black line), heterozygous (GA; blue dashed line), and homozygous (AA; green dashed line). (B) Due to the small sample size of the AA group, AA was merged with GA group. Numbers on the Kaplan-Meier curves represent censored cases. Hazard ratios (HRs) per minor allele, 95% confidence intervals (95% CIs), and statistical P values were estimated by Cox regression with adjustment for age, sex, blood transfusion, baseline platelet count, and Acute Physiology and Chronic Health Evaluation III score. (C) The mediation model. (D) Results of mediation analyses for ARDS 28-day survival and 60-day survival. Results are described as observed indirect prognostic effect (indirect hazard ratio [HRindirect]) of the SNP that was mediated through platelet count decline, 95% CI, P value, and the proportion of effect mediated (EM%). Two mediators were considered: maximal platelet count decline within 28 days (∆PLT28d) and within 7 days (∆PLT7d) after intensive care unit (ICU) admission compared with the baseline platelet count. LRRC16A = leucine-rich repeat–containing 16A.
Next, we evaluated the association between SNP rs9358856 and platelet count–related variables, including PLTbaseline, ∆PLT28d, and ∆PLT7d (see Figure E3). There was no significant association between SNP rs9358856 and the baseline platelet count (P = 0.335) (see Figure E3A). However, patients carrying the A allele of rs9358856 (GA or AA genotype) showed a significantly less decline of platelet count versus the wild-genotype (GG) group within 28 days (difference of ∆PLT28d between GA/AA and GG groups, βGA/AA-vs.-GG = −27.8; 95% CI −52.0 to −3.6; P = 0.025), which retained statistical significance with further adjustment for PLTbaseline (P = 0.036) (see Figure E3B). Similar results were observed for ∆PLT within 7 days after ICU admission (βGA/AA-vs.-GG = –29.9; P = 0.018; adjustment for PLTbaseline, P = 0.030) (see Figure E3C). ∆PLT variables further tested the associations with ARDS outcomes. Both ∆PLT28d and ∆PLT7d were significantly associated with ARDS outcomes (Table 3), which retained statistical significance after additional adjustment for sepsis (see Table E2).
Table 3.
Platelet Count Decline and Prognosis of Patients with Acute Respiratory Distress Syndrome
| Survival28d* |
Survival60d† |
|||
|---|---|---|---|---|
| Variable | HR (95%CI) | P Value | HR (95%CI) | P Value |
| ∆PLT28d‡ | 1.53 (1.08–2.16) | 0.0172 | 1.73 (1.25–2.39) | 8.4 × 10−4 |
| ∆PLT7d§ | 1.73 (1.26–2.39) | 7.3 × 10−4 | 1.82 (1.36–2.44) | 6.8 × 10−5 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio per 100 × 103/μl increment; PLT = platelet count.
Cox model was used with adjustment for age, sex, blood transfusion, and baseline platelet count and Acute Physiology and Chronic Health Evaluation III score.
Primary outcome.
Secondary outcome.
Maximal platelet count decline within 28 days (∆PLT28d) after intensive care unit admission compared with baseline platelet count.
Maximal platelet count decline within 7 days (∆PLT7d) after intensive care unit admission compared with baseline platelet count.
Furthermore, because SNP rs9358856 was a significant QTL marker of ∆PLT and was associated with ARDS survival, ∆PLT after ICU admission might act as an important mediator of the genetic effect of LRRC16A on ARDS outcomes. To test this hypothesis, we performed causal mediation analysis (Figure 1C), which detected a significant indirect effect for SNP rs9358856 on favorable ARDS 28-day survival that was mediated through ∆PLT28d (HRIndirect, 0.937 per effect allele; 95% CI, 0.918−0.957; P = 0.0009, 11.53% effects mediated) (Figure 1D). Sensitivity analysis using ∆PLT7d revealed a higher proportion of indirect effects (HRindirect, 0.900; 95% CI, 0.881−0.919; P < 0.0001, 18.73% effects mediated) (Figure 1D). Causal mediation analysis on 60-day survival showed similar results (Figure 1D). The indirect effects were also tested with additional adjustment for sepsis, which retained statistical significance (see Table E3).
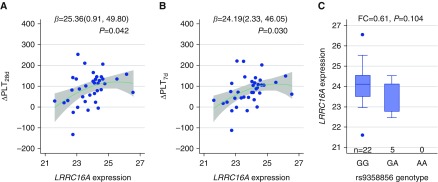
Furthermore, to explore the functionality of SNP rs9358856 and the gene, we evaluated LRRC16A mRNA expression levels at ICU admission of 39 cases with ARDS who had complete survival follow-up and platelet count data during ICU hospitalization. A higher LRRC16A gene expression at ICU admission was significantly correlated with a higher ∆PLT within both 28 days (P = 0.042) (Figure 2A) and 7 days (P = 0.030) (Figure 2B) after ICU admission. Interestingly, 27 of the 39 cases with ARDS had both gene expression and genotyping data, which showed a trend between SNP and reduced gene expression (P = 0.104) (Figure 2C).
Figure 2.
Relationship among single-nucleotide polymorphism (SNP) rs9358856, leucine-rich repeat–containing 16A (LRRC16A) gene expression, and platelet count. LRRC16A mRNA expression levels at intensive care unit (ICU) admission of 39 cases with acute respiratory distress syndrome were used to analyze the associations with platelet count decline within (A) 28 days (∆PLT28d) and (B) 7 days (∆PLT7d) after ICU admission, respectively. Unilinear relationship (solid line and confidence band) was demonstrated. Linear regression was used to estimate β, 95% confidence interval, and P value with adjustment for the baseline platelet count. (C) A subset of 27 cases had both expression and genotyping data. Sample size (n) of each genotyping group: 22 were wild type (GG), 5 were heterozygous (GA), and none were homozygous (AA). Box plot and expression fold change (FC) were used to explore the relationship between SNP rs9358856 and expression level.
Discussion
As previously reported, genetic variants in LRRC16A are associated with platelet counts among relatively healthy populations and critically ill patients in the ICU (15, 27). Interestingly, in this study, we identified a novel missense SNP in LRRC16A that downregulated the gene expression and was associated with attenuated ∆PLT after ICU admission among cases with ARDS. The previously identified SNP rs7766874 is located in the intron of LRRC16A,, but little is understood about its function. However, the newly identified SNP rs9358856 is a missense SNP located in exon #5 that causes valine-to-isoleucine amino acid change. It is also located at the exonic splicing enhancer, which indicates SNP rs9358856 may affect the process of LRRC16A pre-messenger RNA splicing (28). In contrast, although the intron SNP rs7766874 was associated with baseline platelet count at ICU admission as reported in our previous study (15), the presently identified missense SNP rs9358856 was associated with ∆PLT after ICU admission rather than the platelet count at ICU admission. The two SNPs are not linked among Caucasians (r2 = 0.02; D′ = 0.31) (see Figure E2), which suggests different underlying mechanisms that affect the gene and warrants further well-designed functional experiments. Taken together, these studies suggest that LRRC16A plays a role in the platelets of critically ill patients in the ICU.
Furthermore, we provided evidence that a lower baseline platelet count or larger decline of platelet count after ICU admission was associated with worse outcomes in patients with ARDS in the ICU, which was consistent with a previous report (29). Thrombocytopenia is the most common coagulation problem in the ICU, and is generally caused by the following mechanisms: increased destruction or consumption of platelets, decreased production, dilution, and sequestration of platelets (30, 31). Clinical events related to these mechanisms, such as disseminated intravascular coagulation, trauma, or massive transfusions, are considered risk factors that affect ARDS outcomes (7, 32, 33). Thus, treatments for thrombocytopenia might have benefits for patients with ARDS, whereas the treatments should be given in respect to the etiology (34). For example, systemic activation of blood coagulation results in consumption and subsequent exhaustion of coagulation proteins and platelets that causes thrombocytopenia (30). For this etiology, anticoagulant therapy is an option for thrombocytopenia, which also has a potential benefit for ARDS outcomes (35). However, in this study, we could not distinguish among the potential roles of platelet production, consumption, or functional disorders in ARDS outcomes; therefore, future experimental studies are warranted.
The primary finding of this study suggested that LRRC16A is associated with ARDS outcomes, which appear to be partially mediated through a declining platelet count after ICU admission. The protective effect of SNP rs9358856 for ARDS outcomes might be mediated through reduced LRRC16A expression, which attenuates declines in platelet count. Capping protein ARP2/3 and myosin-I linker (CARMIL), encoded by LRRC16A, binds capping protein Z, which acts as an inhibitor of F-actin polymerization (36). Animal models demonstrated that the binding of the CARMIL protein to capping protein decreases its affinity for the actin filament barbed end, which leads to F-actin polymerization (37). F-actin polymerization is crucial during platelet activation through involvement in the formation of dynamic structures such as filopodia and lamellopodia (38, 39). The missense SNP that reduces LRRC16A expression might result in moderate F-actin polymerization, which is related to decreased platelet consumption after ICU admission, which leads to a favorable prognosis. Although this mediation pathway is statistically significant, only 10 to 20% of the prognostic effect of the SNP rs9358856 is mediated through ∆PLT, which implies that LRRC16A has an effect beyond ∆PLT during ICU stay. LRRC16A might therefore affect other pathways that contribute to ARDS survival. However, the mechanisms underlying the effects of the missense SNP on CARMIL protein quantity and/or activity, and, in turn, on dynamic platelet counts (or function) after ICU admission, are not well understood, and therefore, highlight the need for further well-designed functional studies.
Accumulating evidence demonstrates a role of platelets in ARDS morbidity and mortality (5, 40, 41). Platelets promote lung injury via inflammatory signaling and contribute to basal barrier integrity of alveolar capillaries (42, 43). Thus, reduced or inactivated platelets might reduce lung inflammation and permeability. As a result, there is emerging interest in therapies that target platelet function among at-risk patients with ARDS. Aspirin treatment in mouse models promotes increased levels of lipoxin (ATL; 15-epi-lipoxin A4) and blocking of the lipoxin A4 receptor, which prevents neutrophil-platelet aggregation and attenuates acute lung injury (44). Accordingly, prehospital aspirin use in patients at high risk for ARDS is associated with reduced ARDS risk (45–49). Even patients in the ICU with ARDS potentially receive therapeutic benefits from aspirin usage to reduce ICU mortality (50). However, a recent published LIPS-A (Lung Injury Prevention Study with Aspirin) randomized clinical trial did not demonstrate a benefit to administration of aspirin to either reduce the risk of ARDS at 7 days or improved secondary outcomes (51). Interestingly, as commented by Reilly and Christie, one possible explanation for the inefficacy is timing of delivery (52). Identifying biomarkers for uncovering patient subtypes might predict treatment responsiveness and prognosis, which could be incorporated into the design of clinical trials (53). Enhancing our understanding of the mechanisms of how platelets mechanisms contribute to ARDS outcome, by integrating with genetic information, might aid in identifying novel therapeutic targets or identifying more sensitive subgroups for antiplatelet therapy.
We acknowledge some limitations in our study. First, statistical power was insufficient to study the association of rare functional SNPs, which could be examined using target sequencing in larger cohorts. We also were aware that the mRNA study population (39 cases) did show, to some extent, imbalances in terms of age, sex, severity of disease and platelet decline, and comorbidities compared with the genotyping study population, which might be partially due to sampling and small sample size. For this reason, the results of the present gene expression study should be taken with caution and validated using a more comparable population and larger sample size. Second, functional studies are needed to evaluate the mechanisms that underlie the associations between LRRC16A genetic variants and ARDS outcome and the mediating pathway through platelets. Third, we only evaluated platelet count as a potential causal mediator, whereas platelet function likely plays a comparable mediation role in this pathway. Therefore, more detailed platelet information should be measured in future studies, including immature platelet fractions, platelet function, aggregation, and neutrophil adhesion (16). Fourth, thrombocytopenia is a marker for common conditions in the ICU (e.g., disseminated intravascular coagulation, hemodilution, or advanced liver disease) and could result in clinical consequences (e.g., bleeding, immunomodulation) that might be the biologic drivers for ARDS poor outcomes. Thus, related underlying causal pathways should be explored in the future to provide better understanding of ARDS etiology. In addition, our cases with ARDS were recruited from an ICU at-risk population with a high proportion of sepsis, and all cases with ARDS corresponded to moderate to severe ARDS according to the Berlin definition, which showed a higher mortality than those with mild ARDS (2). Therefore, the findings in the study were mainly pertinent to patients with septicemia in the ICU who developed ARDS, and should be validated before generalization in cohorts with ARDS with different precipitating conditions.
In summary, our findings suggest a role of LRRC16A in ARDS outcomes, and its prognostic effect appears to be partially mediated through a progressive decline of platelet counts after ICU admission.
Acknowledgments
Acknowledgment
The authors thank all the participants and their family members. They also thank Nancy Diao, S.M., for data management, Andrea Shafer, M.P.H., and all of the nurses and staff for research assistance.
Footnotes
Supported by NHLBI grant R01HL060710 (D.C.C.); National Natural Science Foundation of China grants 81402764 (Y.W.), 81402763 (R.Z.), and 81473070 and 81530088 (F.C.); and Natural Science Foundation of Jiangsu, China, grant BK20140907 (Y.W.). The work was also partially supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Outstanding Young Teachers Training Program of Nanjing Medical University. The sponsors had no role in study design, collection and analysis of data, or preparation of the manuscript.
Author Contributions: Conception and design: Y.W. and D.C.C. Statistical analysis and interpretation: Y.W., P.T., Z.W., R.Z., F.C., X.L., and D.C.C. Drafting the manuscript: Y.W. Assembling of study samples, laboratory experiments, and quality control: L.S. Assembly of study subjects and phenotyping of patients: E.K.B. and B.T.T. Manuscript revision: all coauthors. All coauthors approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201605-0946OC on October 21, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 4.Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol. 2012;4:159–169. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuebler WM. Selectins revisited: the emerging role of platelets in inflammatory lung disease. J Clin Invest. 2006;116:3106–3108. doi: 10.1172/JCI30664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Sheng L, Wang S, Li Q, Zhang M, Xu S, Gan J. Analysis of clinical risk factors associated with the prognosis of severe multiple-trauma patients with acute lung injury. J Emerg Med. 2012;43:407–412. doi: 10.1016/j.jemermed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Liu Z, Wang Z, Duan M, Li G, Wang S, Li W, Zhu Z, Wei Y, Christiani DC, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One. 2014;9:e94124. doi: 10.1371/journal.pone.0094124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer NJ, Feng R, Li M, Zhao Y, Sheu CC, Tejera P, Gallop R, Bellamy S, Rushefski M, Lanken PN, et al. IL1RN coding variant is associated with lower risk of acute respiratory distress syndrome and increased plasma IL-1 receptor antagonist. Am J Respir Crit Care Med. 2013;187:950–959. doi: 10.1164/rccm.201208-1501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortt K, Chaudhary S, Grigoryev D, Heruth DP, Venkitachalam L, Zhang LQ, Ye SQ. Identification of novel single nucleotide polymorphisms associated with acute respiratory distress syndrome by exome-seq. PLoS One. 2014;9:e111953. doi: 10.1371/journal.pone.0111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahasic AM, Zhao Y, Su L, Sheu CC, Thompson BT, Christiani DC. Adiponectin gene polymorphisms and acute respiratory distress syndrome susceptibility and mortality. PLoS One. 2014;9:e89170. doi: 10.1371/journal.pone.0089170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azevedo ZM, Moore DB, Lima FC, Cardoso CC, Bougleux R, Matos GI, Luz RA, Xavier-Elsas P, Sampaio EP, Gaspar-Elsas MI, et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) single nucleotide polymorphisms: importance in ARDS in septic pediatric critically ill patients. Hum Immunol. 2012;73:661–667. doi: 10.1016/j.humimm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Gardinali M, Borrelli E, Chiara O, Lundberg C, Padalino P, Conciato L, Cafaro C, Lazzi S, Luzi P, Giomarelli PP, et al. Inhibition of CD11-CD18 complex prevents acute lung injury and reduces mortality after peritonitis in rabbits. Am J Respir Crit Care Med. 2000;161:1022–1029. doi: 10.1164/ajrccm.161.3.9901066. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Yang KY, Chen MY, Chen HY, Lin MT, Lee YC, Perng RP, Hsieh SL, Yang PC, Chou TY. Decoy receptor 3 levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:751–760. doi: 10.1164/rccm.200902-0222OC. [DOI] [PubMed] [Google Scholar]
- 14.Devaney J, Contreras M, Laffey JG. Clinical review: gene-based therapies for ALI/ARDS: where are we now? Crit Care. 2011;15:224. doi: 10.1186/cc10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Wang Z, Su L, Chen F, Tejera P, Bajwa EK, Wurfel MM, Lin X, Christiani DC. Platelet count mediates the contribution of a genetic variant in LRRC16A to ARDS risk. Chest. 2015;147:607–617. doi: 10.1378/chest.14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly JP, Christie JD. Linking genetics to ARDS pathogenesis: the role of the platelet. Chest. 2015;147:585–586. doi: 10.1378/chest.14-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Yang X, Ji W, Deng J, Qiu F, Yang R, Fang W, Zhang L, Huang D, Xie C, et al. Effects of a functional variant c.353T>C in snai1 on risk of two contextual diseases: chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med. 2014;189:139–148. doi: 10.1164/rccm.201307-1355OC. [DOI] [PubMed] [Google Scholar]
- 18.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanderWeele TJ, Asomaning K, Tchetgen EJ, Han Y, Spitz MR, Shete S, Wu X, Gaborieau V, Wang Y, McLaughlin J, et al. Genetic variants on 15q25.1, smoking, and lung cancer: an assessment of mediation and interaction. Am J Epidemiol. 2012;175:1013–1020. doi: 10.1093/aje/kwr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wei Y, Tejera P, Su L, Bajwa E, Christiani DC.A functional missense SNP in LRRC16A contributes to an improved prognosis of ARDS patients mediated through attenuated platelet count decline in ICU[abstract]Am J Respir Crit Care Med 2016D93A7489 [Google Scholar]
- 21.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 22.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Beach D, Su L, Zhai R, Christiani DC. A genome-wide expression analysis in blood identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol Biol. 2008;38:724–732. doi: 10.1165/rcmb.2007-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dmitrienko A, Wiens BL, Tamhane AC, Wang X. Tree-structured gatekeeping tests in clinical trials with hierarchically ordered multiple objectives. Stat Med. 2007;26:2465–2478. doi: 10.1002/sim.2716. [DOI] [PubMed] [Google Scholar]
- 25.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–e24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 27.Qayyum R, Snively BM, Ziv E, Nalls MA, Liu Y, Tang W, Yanek LR, Lange L, Evans MK, Ganesh S, et al. A meta-analysis and genome-wide association study of platelet count and mean platelet volume in African Americans. PLoS Genet. 2012;8:e1002491. doi: 10.1371/journal.pgen.1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew SL, Liu HX, Mayeda A, Krainer AR. Evidence for the function of an exonic splicing enhancer after the first catalytic step of pre-mRNA splicing. Proc Natl Acad Sci USA. 1999;96:10655–10660. doi: 10.1073/pnas.96.19.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau D, Timsit JF, Vesin A, Garrouste-Orgeas M, de Lassence A, Zahar JR, Adrie C, Vincent F, Cohen Y, Schlemmer B, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 30.Rice TW, Wheeler AP. Coagulopathy in critically ill patients: part 1: platelet disorders. Chest. 2009;136:1622–1630. doi: 10.1378/chest.08-2534. [DOI] [PubMed] [Google Scholar]
- 31.Greinacher A, Selleng K.Thrombocytopenia in the intensive care unit patient Hematology Am Soc Hematol Educ Program 2010. 2010:135–143 [DOI] [PubMed]
- 32.Housinger TA, Brinkerhoff C, Warden GD. The relationship between platelet count, sepsis, and survival in pediatric burn patients. Arch Surg. 1993;128:65–66, discussion 66–67. doi: 10.1001/archsurg.1993.01420130073011. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Delgado JC, Rovira A, Esteve F, Rico N, Mañez Mendiluce R, Ballús Noguera J, Berrade J. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly. 2013;143:w13788. doi: 10.4414/smw.2013.13788. [DOI] [PubMed] [Google Scholar]
- 34.Izak M, Bussel JB. Management of thrombocytopenia. F1000Prime Rep. 2014;6:45. doi: 10.12703/P6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27:860–873. doi: 10.1592/phco.27.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol. 1996;135:169–179. doi: 10.1083/jcb.135.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, Pring M, Wear MA, Huang M, Cooper JA, Svitkina TM, Zigmond SH. Mammalian CARMIL inhibits actin filament capping by capping protein. Dev Cell. 2005;9:209–221. doi: 10.1016/j.devcel.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerecedo D. Platelet cytoskeleton and its hemostatic role. Blood Coagul Fibrinolysis. 2013;24:798–808. doi: 10.1097/MBC.0b013e328364c379. [DOI] [PubMed] [Google Scholar]
- 39.Safdar Z, Wang P, Ichimura H, Issekutz AC, Quadri S, Bhattacharya J. Hyperosmolarity enhances the lung capillary barrier. J Clin Invest. 2003;112:1541–1549. doi: 10.1172/JCI18370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871–1876. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 41.Dengler V, Downey GP, Tuder RM, Eltzschig HK, Schmidt EP. Neutrophil intercellular communication in acute lung injury: emerging roles of microparticles and gap junctions. Am J Respir Cell Mol Biol. 2013;49:1–5. doi: 10.1165/rcmb.2012-0472TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124:2625–2634. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Janz DR, Bastarache JA, May AK, O’Neal HR, Jr, Bernard GR, Ware LB. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43:801–807. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palakshappa JA, Christie JD. Prehospital aspirin use and acute respiratory distress syndrome: a case for aspirin in the drinking water? Crit Care Med. 2015;43:916–917. doi: 10.1097/CCM.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139:289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neal HR, Jr, Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kor DJ, Erlich J, Gong MN, Malinchoc M, Carter RE, Gajic O, Talmor DS U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators. Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Crit Care Med. 2011;39:2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle AJ, Di Gangi S, Hamid UI, Mottram LJ, McNamee L, White G, Cross LJ, McNamee JJ, O’Kane CM, McAuley DF. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A) Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-A Randomized Clinical Trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly JP, Christie JD. Is it possible to prevent ARDS? JAMA. 2016;315:2403–2405. doi: 10.1001/jama.2016.5988. [DOI] [PubMed] [Google Scholar]
- 53.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]