Abstract
Rationale: Sputum neutrophil elastase and serum desmosine, which is a linked marker of endogenous elastin degradation, are possible biomarkers of disease severity and progression in bronchiectasis. This study aimed to determine the association of elastase activity and desmosine with exacerbations and lung function decline in bronchiectasis.
Methods: This was a single-center prospective cohort study using the TAYBRIDGE (Tayside Bronchiectasis Registry Integrating Datasets, Genomics, and Enrolment into Clinical Trials) registry in Dundee, UK. A total of 433 patients with high-resolution computed tomography–confirmed bronchiectasis provided blood samples for desmosine measurement, and 381 provided sputum for baseline elastase activity measurements using an activity-based immunosassay and fluorometric substrate assay. Candidate biomarkers were tested for their relationship with cross-sectional markers of disease severity, and with future exacerbations, mortality and lung function decline over 3 years.
Measurement and Main Results: Elastase activity in sputum was associated with the bronchiectasis severity index (r = 0.49; P < 0.0001) and was also correlated with the Medical Research Council dyspnea score (r = 0.34; P < 0.0001), FEV1% predicted (r = −0.33; P < 0.0001), and the radiological extent of bronchiectasis (r = 0.29; P < 0.0001). During a 3-year follow-up, elevated sputum elastase activity was associated with a higher frequency of exacerbations (P < 0.0001) but was not independently associated with mortality. Sputum elastase activity was independently associated with FEV1 decline (β coefficient, −0.139; P = 0.001). Elastase showed good discrimination for severe exacerbations with an area under the curve of 0.75 (95% confidence interval [CI], 0.72–0.79) and all-cause mortality (area under the curve, 0.70; 95% CI, 0.67–0.73). Sputum elastase activity increased at exacerbations (P = 0.001) and was responsive to treatment with antibiotics. Desmosine was correlated with sputum elastase (r = 0.42; P < 0.0001) and was associated with risk of severe exacerbations (hazard ratio 2.7; 95% CI, 1.42–5.29; P = 0.003) but not lung function decline.
Conclusions: Sputum neutrophil elastase activity is a biomarker of disease severity and future risk in adults with bronchiectasis.
Keywords: bronchiectasis, neutrophils, inflammation, biomarker, exacerbations
At a Glance Commentary
Scientific Knowledge on the Subject
There are no validated biomarkers of disease severity and progression in bronchiectasis. Studies in cystic fibrosis (CF) and pilot studies in non-CF bronchiectasis suggest that neutrophil elastase is associated with more severe disease and airway bacterial infection. We prospectively tested the hypothesis that exacerbations and lung function decline are associated with increased sputum neutrophil elastase activity and the related circulating biomarker desmosine.
What This Study Adds to the Field
Neutrophil elastase was associated with clinical and radiological extent of disease and with lung function. During follow-up, elevated levels of sputum neutrophil elastase activity identified patients at higher risk of exacerbations and severe exacerbations who required hospital admission over 3 years. Sputum elastase activity was also independently associated with lung function decline. Increased circulating desmosine was also associated with a higher risk of severe exacerbations. Because few clinical parameters have been shown to be associated with bronchiectasis outcomes, sputum neutrophil elastase and circulating desmosine may be useful adjuncts to clinical assessment or to patient evaluation in clinical trials.
Bronchiectasis is characterized by permanent bronchial dilatation associated with chronic neutrophilic airway inflammation (1). The pathogenesis of bronchiectasis is poorly understood, but activated neutrophils are believed to be a key component of the “vicious cycle” of lung damage (2). Neutrophil elastase (NE) is a 29-kD serine protease stored in azurophilic granules that may be released during degranulation, neutrophil extracellular trap formation, or cell death (3–8). NE is proinflammatory, slows ciliary beat frequency, and stimulates mucus secretion (9, 10). It is found in high concentrations in the sputum of patients with neutrophilic lung diseases, including bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis (CF) (5–7). It is believed that unopposed action of NE directly contributes to the pathogenesis and progression of these diseases.
NE activity is inhibited by antiproteases, including secretory leukoproteinase inhibitor produced by bronchial epithelium and by serum-derived alpha-1 antitrypsin (11). In addition, the presence of high concentrations of DNA released during neutrophil extracellular trap formation inhibits elastase activity, both directly and indirectly by modulating the response to NE inhibitors (12). Epithelial-derived factors (e.g., syndecan-1) also complex with elastase in the airway and reduce the inhibitory capacity of alpha-1 antitrypsin (7, 12).
Thus, the activity of NE within the inflamed airway is usually controlled by a range of inhibitors. However, in bronchiectasis, release of NE overwhelms the antiproteinase defense, which leads to detectable levels of NE proteolytic activity in sputum and bronchoalveolar lavage (13–15). This can be measured readily using assays that detect cleavage of chromogenic or fluorogenic peptide-based substrates in sputum, or downstream by measuring the endogenous degradation of mature elastin through the quantification of the unique covalent cross-linking amino acids desmosine and isodesmosine in serum and/or plasma (circulating desmosine [cDES]) (16, 17).
There are no widely accepted biomarkers of disease progression in bronchiectasis, but evidence is accumulating that sputum NE activity correlates with disease severity. In a study of 30 patients, Tsang and colleagues showed that NE activity correlated strongly with 24-hour sputum volume, extent of bronchiectasis, and FEV1 (13). In 385 patients with bronchiectasis from the UK, NE activity was correlated with airway bacterial load, the presence of Pseudomonas aeruginosa, and the extent of radiological bronchiectasis (14). No previous study has investigated the association of sputum NE activity or cDES with clinically relevant outcomes in bronchiectasis during long-term follow-up. In this study, we prospectively tested the hypothesis that elevated sputum NE activity or the related biomarker cDES is associated with increased frequency of exacerbations and lung function decline.
Methods
This study was conducted and is reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (18). Patients were consecutively recruited to a prospective observational study (TAYBRIDGE [Tayside Bronchiectasis Registry Integrating Datasets, Genomics, and Enrolment into Clinical Trials] registry) at Ninewells Hospital, Dundee, UK 2012–2015. The study was approved by the East of Scotland Research Ethics committee (12/ES/0059), and all patients gave written informed consent. Inclusion criteria were age ≥18 years, high-resolution computed tomography–confirmed bronchiectasis, and clinical symptoms consistent with bronchiectasis. Exclusion criteria were inability to give informed consent, active nontuberculous mycobacterial infection, active allergic bronchopulmonary aspergillosis, active tuberculosis, active malignancy, CF, or pulmonary fibrosis with secondary traction bronchiectasis.
For inclusion in the present analysis, patients were asked to provide serum and sputum samples at the same baseline visit when clinically stable (defined as no antibiotic treatment within the preceding 4 weeks, excluding prophylactic oral or inhaled antibiotics).
Clinical Assessment
Full details of the clinical assessments are shown in the online supplement. The underlying cause of bronchiectasis was determined by standardized testing according to British Thoracic Society recommendations (19). The bronchiectasis severity index (BSI) was calculated as described (20). Quality of life was evaluated using the St. George’s Respiratory Questionnaire (SGRQ) (21). Chronic infection was defined as the isolation of pathogens on at least 2 occasions 3 months apart during the preceding 12 months (22). Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines (23). The severity of radiological bronchiectasis was evaluated using the Reiff score (24). Exacerbations were defined according to British Thoracic Society recommendations, and severe exacerbations were defined as those requiring hospital admission (19).
Sputum Sampling and Processing
Spontaneous sputum samples were split for microbiology and inflammatory marker measurement. For measurement of inflammatory markers, including NE, spontaneous sputum was ultracentrifuged at 50,000 × g for 90 minutes, and the soluble fraction carefully removed (14).
Methods of Measurement of NE Activity and Other Inflammatory Markers
Because previous studies have used several different methods of NE quantification, we simultaneously evaluated three methods in this study; two assays for sputum NE activity and one for cDES measurement.
Active NE was measured using an activity-based immunoassay (ProteaseTag Active NE Immunoassay referred to as the ABI-NE assay) (ProAxsis Ltd, Belfast, UK) in accordance with the manufacturer’s instructions (25, 26) and a fluorogenic substrate-based kinetic assay (referred to as kinetic-NE assay). The kinetic-NE assay used the substrate N-methoxysuccinyl-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin (Sigma-Aldrich, St. Louis, MO).
Sputum samples were assayed at dilutions ranging from 5× to 2,000×, and assays that remained below the lowest limit of detection (0.016 µg/ml) at 5× dilution were recorded as zero for the purposes of analysis.
Measurement of serum and sputum inflammatory markers (C-X-C ligand 8 [CXCL8], IL-1β, tumor necrosis factor-α [TNF-α], and extracellular newly identified receptor for advanced glycation end-product–binding protein [EN-RAGE]) were performed using commercially available ELISA. Before use, kits were validated for use in sputum according to the methods described by Woolhouse and colleagues (27).
Serum Desmosine Measurement
cDES was measured in serum using a validated liquid chromatography–mass spectrometry/mass spectrometry method as previously described (16, 17).
Exacerbation Study
Patients (n = 26) included in the main study who visited a hospital for a severe exacerbation of bronchiectasis were enrolled in a substudy of changes in NE during exacerbations (19). Spontaneous sputum samples were collected on day 1 before commencement of antibiotics and after treatment at day 14. Patients received standardized treatment for 14 days based on their previous sputum microbiology (19). These 26 patients subsequently had additional sampling 6 months post-exacerbation to determine dynamics of NE.
Statistical Analysis
Mean and SD were used to display continuous normally distributed data with median and interquartile range (IQR) for continuous nonnormally distributed data, and frequencies and percentages for categorical data. The association of biomarkers with linear variables was performed using Spearman’s correlation, whereas between group differences were evaluated by analysis of variance or Kruskal-Walis test. Frequency of exacerbations and severe exacerbations were evaluated using Poisson regression adjusted for duration of follow-up. Time to event data (time to first exacerbation, first hospital admission, and death) were analyzed using Kaplan-Meier survival analysis and Cox proportional hazard regression for multivariable analyses. Discrimination for mortality and severe exacerbations at 3 years was analyzed using the area under a receiver-operating characteristic curve (AUC). Analysis of FEV1 decline over 3 years was performed using multiple linear regression with appropriateness of the linear regression modeling evaluated by examining the distribution of residuals. In some analyses, patients were split into three groups based on low, intermediate, and high elastase levels, with cutoffs selected using Youden’s index. Patients with missing data were excluded from analysis of the specific test as outlined in the following. No imputation methods were used. Sample size was empirically based on previous studies with equivalent lengths of follow-up (14).
Results
Patient Cohort
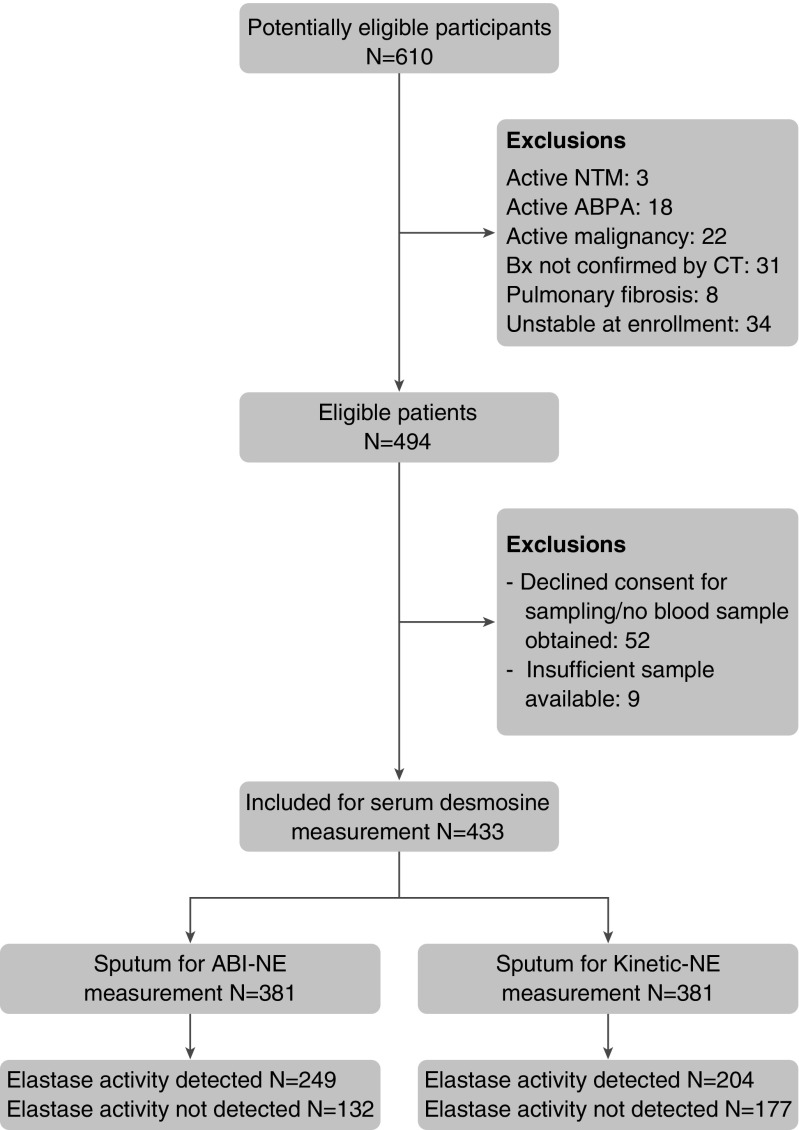
The study included 433 patients, of whom 381 patients were able to provide a sputum sample sufficient for measurement of NE activity. The flow of patients through the study is shown in the STROBE flowchart (Figure 1).
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology flowchart of study inclusion and exclusions. Patients with elastase levels below the lower limit of detection (“not detected”) were included in the analysis with values treated as zero. ABI-NE = activity-based immunoassay for neutrophil elastase; ABPA = allergic bronchopulmonary aspergillosis, Bx = bronchiectasis, CT = computed tomography; NTM = nontuberculous mycobacteria.
Characteristics of the included patients are shown in Table 1. The median (IQR) age was 67 (58–74) years; 60.7% of patients were women, and 45% of patients had idiopathic bronchiectasis. The median exacerbation frequency was 1 per year (IQR, 0–3). The median BSI score was 6, which indicated a population with moderate to severe bronchiectasis (range, 0–24).
Table 1.
Baseline Characteristics of the Cohort
| Baseline Characteristics | Full Cohort | Patients Providing Sputum |
|---|---|---|
| N | 433 | 381 |
| Age, yr | 67 (58–74) | 67 (58–74) |
| Sex, % female | 263 (60.7) | 225 (59.1) |
| Body mass index | 25.0 (22.3–28.5) | 25.1 (22.2–28.6) |
| Smoking status, never/ex/current | 266 (61)/151 (34.9)/16 (3.7) | 239 (62.7)/131 (34.4)/11 (2.9) |
| MRC dyspnea score | 2 (1–3) | 2 (1–3) |
| FEV1, L | 1.58 (1.10–2.20) | 1.58 (1.10–2.23) |
| FEV1% predicted | 71.9 (50.0–91.0) | 71.4 (49.4–90.9) |
| FVC, L | 2.45 (1.84–3.21) | 2.41 (1.85–3.19) |
| FVC, % predicted | 83.9 (68.4–99.5) | 83.2 (67.7–98.7) |
| Etiology of bronchiectasis | ||
| Idiopathic | 195 (45.0) | 169 (44.4) |
| Postinfective | 84 (19.4) | 78 (20.5) |
| Previous ABPA | 37 (8.5) | 34 (8.9) |
| Asthma | 15 (3.5) | 14 (3.7) |
| COPD | 22 (5.1) | 19 (5.0) |
| Rheumatoid arthritis | 21 (4.8) | 17 (4.4) |
| Connective tissue disease | 6 (1.4) | 4 (1.0) |
| Inflammatory bowel disease | 11 (2.5) | 11 (2.9) |
| Primary immunodeficiency | 18 (4.2) | 17 (4.5) |
| Previous NTM infection | 7 (1.6) | 4 (1.0) |
| Primary ciliary dyskinesia | 4 (0.9) | 3 (0.8) |
| Alpha-1 antitrypsin deficiency | 2 (0.5) | 1 (0.3) |
| Others | 11 (2.5) | 10 (2.6) |
| Exacerbations per year | 1 (0–3) | 1 (0–3) |
| Previous hospitalization for severe exacerbations | 107 (24.7) | 101 (26.5) |
| St. George’s Respiratory Questionnaire total score | 44.3 (24.6–62.7) | 46.1 (27.3–63.2) |
| Bronchiectatic on CT | 3 (2–4) | 3 (2–4) |
| Reiff score | 3 (2–6) | 3 (2–6) |
| Chronic colonization* | 236 (54.5) | 213 (55.9) |
| H. influenzae | 129 (29.8) | 116 (30.4) |
| P. aeruginosa | 63 (14.5) | 60 (15.7) |
| Moraxella catarrhalis | 51 (11.8) | 49 (12.9) |
| Streptococcus pneumoniae | 25 (5.8) | 23 (6.0) |
| Streptococcus aureus | 34 (7.9) | 30 (7.9) |
| Enterobacteriaceae | 39 (9.0) | 39 (10.2) |
| Bronchiectasis severity index | 6 (4–10) | 6 (4–11) |
| Mild | 126 (29.1) | 108 (28.3) |
| Moderate | 170 (39.3) | 144 (37.8) |
| Severe | 137 (31.6) | 129 (33.9) |
Definition of abbreviations: ABPA = allergic bronchopulmonary aspergillosis; COPD = chronic obstructive pulmonary disease; CT = computed tomography; MRC = Medical Research Council; NTM = nontuberculous mycobacteria.
Data are presented as median (interquartile range) or n (%).
Defined as isolation of a pathogenic microorganism in sputum when clinically stable on two occasions at least 3 months apart in a 12-month period.
There were no significant differences between patients who were able and unable to produce sputum. A total of 42 patients were receiving long-term inhaled antibiotics, and 129 were receiving long-term oral antibiotic treatments at baseline.
Sputum NE Activity Is Associated with Disease Severity
The ABI-NE assay detected activity above the lower limit of detection in 249 patients (65.4%), whereas the kinetic-NE assay detected active NE in 204 (53.5%) samples. The two assay methods were highly correlated (see Figure E1 in the online supplement).
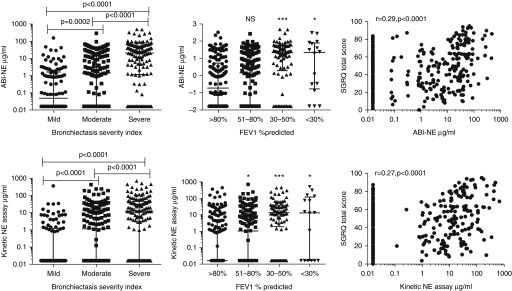
Sputum NE activity as measured by the ABI-NE assay showed a univariate association with cross-sectional markers of disease severity, including the Medical Research Council dyspnea score (r = 0.34; P < 0.0001), SGRQ score (r = 0.28; P < 0.0001), absolute FEV1 (r = −0.31; P < 0.0001), FEV1% predicted (r = −0.33; P < 0.0001), Reiff score (r = 0.29; P < 0.0001), and the BSI (r = 0.49; P < 0.0001). Similar results were obtained with the kinetic-NE assay (Figure 2 and see Figure E2).
Figure 2.
Association between neutrophil elastase (NE) and severity of disease. The activity-based immunoassay (ABI)-NE assay (upper panels) and kinetic NE assay (lower panels) are significantly different between bronchiectasis severity index and FEV1% predicted groups and correlate with the St. George’s Respiratory Questionnaire (SGRQ). *P < 0.05 compared with >80% predicted FEV1; ***P < 0.0001 compared with >80% FEV1% predicted. Across-group comparisons for FEV1, P < 0.0001 by Kruskal-Walis test. Bronchiectasis severity index and FEV1 cutoffs were chosen as those used in Reference 20. The median and interquartile range are shown. NS = no significant difference compared with FEV1 >80% predicted.
Both ABI-NE and kinetic-NE assays were correlated with sputum myeloperoxidase activity (P < 0.0001), but there was no correlation with sputum CXCL8, IL-1β, TNF-α, or EN-RAGE (data not shown).
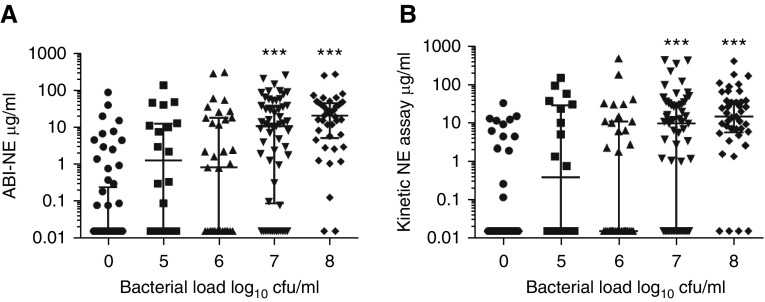
There was a relationship between NE activity and airway bacterial load (at bacterial load >107 cfu/ml) (Figures 3A and 3B). Patients chronically infected with P. aeruginosa, Enterobacteriaceae, and Haemophilus influenzae had increased levels of NE using both assays (P < 0.0001) compared with patients without chronic bacterial infection.
Figure 3.
Association between neutrophil elastase (NE) and sputum bacterial load. (A) Data for the activity-based immunoassay (ABI)-NE assay. (B) Data for the kinetic assay. ***P < 0.0001 compared with no organism isolated. The median and interquartile range are shown.
Sputum NE Activity and Longitudinal Clinical Outcomes
In the sputum producing cohort, the mortality rate was 8.7% and 25.5% of patients who had hospital admissions for severe exacerbations during follow-up. The median frequency of exacerbations was 1 per patient per year (IQR, 0–3).
Because the ABI-NE assay was more sensitive and had stronger correlations with the most clinical outcomes for clarity, we only presented the results for the ABI-NE assay here. Using receiver-operating characteristic analysis, ABI-NE activity was associated with hospital admissions during follow-up (AUC, 0.75; 95% CI, 0.72–0.79) and mortality (AUC, 0.70; 95% CI, 0.67–0.73). Entering NE activity as a continuous variable, after multivariable adjustment, including the BSI, a 1 µg/ml increase in NE activity was independently associated with a 0.5% increased risk of hospital admission (hazard ratio [HR], 1.005; 95% confidence interval [CI], 1.002–1.008; P < 0.0001). No independent relationship with mortality was identified. Additional models are shown in Table E1 in the online supplement.
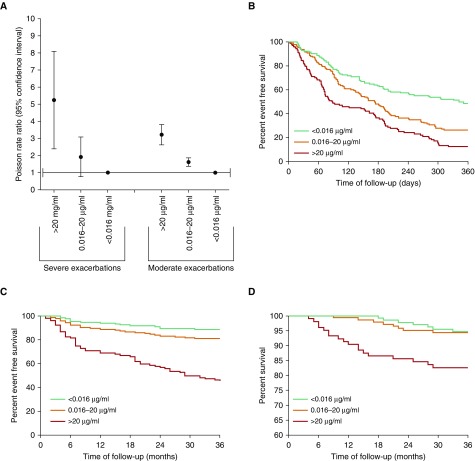
Using receiver-operating characteristic analysis, we determined candidate clinically meaningful cutoff values of sputum NE. A total of 132 patients had values below the lower limit of detection (<0.016 µg/ml [low NE]), 143 patients had values between 0.016 and 20 µg/ml (intermediate NE), and106 had values >20 µg/ml (high NE).
Comparing the frequency of exacerbation between the three ABI-NE cutoffs using Poisson regression, patients with the highest elastase values (>20 µg/ml) had a rate ratio (RR) of 3.18 (95% CI, 2.65–3.18; P < 0.0001), and patients with intermediate NE values had a RR of 1.61 (95% CI, 1.39–1.86; P < 0.0001) compared with the low elastase (reference) group, which indicated that high sputum NE activity was associated with a greatly increased frequency of future exacerbations. For severe exacerbations, the corresponding values were RR 1.69 (95% CI, 0.90–3.17; P = 0.1) for intermediate NE values and 4.73 (95% CI, 2.67–8.33; P < 0.0001) for the highest NE group (Figure 4A). Consistent with this, elevated NE activity was associated with a shorter time to next exacerbation (P < 0.0001) (Figure 4B), shorter time to next severe exacerbation (P < 0.0001) (Figure 4C), and increased all-cause mortality (Figure 4D) (P < 0.0001). An analysis of exacerbation frequency according to different severity groups using the BSI is shown in Table E2. Prediction statistics are shown in Table E3.
Figure 4.
Association between neutrophil elastase and longitudinal clinical outcomes. (A) Rate ratio from Poisson regression; elastase levels >0.016 µg/ml are associated with significantly increased risk of moderate exacerbations (P < 0.0001), and levels >20 µg/ml are associated with increased severe exacerbations (P < 0.0001). (B) Time to next exacerbation. Elevated neutrophil elastase is associated with shorter time to next exacerbation (P < 0.0001 by log rank test). (C) Time to next hospitalization for severe exacerbation over 36 months (P < 0.0001 by log rank test). (D) All-cause mortality over 3 years (P < 0.0001 by log rank test; comparison between <0.016 and 0.016–20 µg/ml, not significant). Less than 0.016 µg/ml, n = 132; 0.016 to 20 µg/ml, n = 143; >20 µg/ml, n = 106.
FEV1 decline over 3 years was normally distributed. The mean FEV1 decline was 48.2 ml/yr (SD 83.7). Across the defined three elastase groups, mean FEV1 decline was 35.6 ml (SD 81.1) for NE activity <0.016 µg/ml, 49.5 ml (SD 92.5) for intermediate elastase levels, and 56.4 ml (SD 67.4) for those with NE >20 µg/ml. On univariate regression, there was a weak but statistically significant relationship between NE activity and FEV1 decline (P = 0.004). After adjustment for BSI, sex, and baseline FEV1, increasing NE-ABI elastase was associated with more rapid lung function decline (β coefficient, −0.139; P = 0.001; model fit r = 0.7).
Serum Desmosine Is Associated with Age and Disease Severity
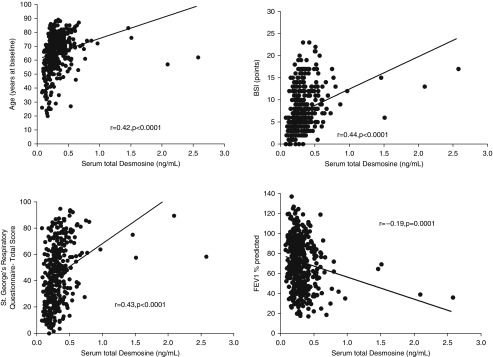
cDES was most strongly correlated with age (r = 0.48; P < 0.0001 (Figure 5A). Additional univariate correlations were observed between cDES and Medical Research Council dyspnea score (r = 0.32; P < 0.0001), SGRQ (r = 0.40; P < 0.0001), absolute FEV1 (r = −0.39; P < 0.0001), and Reiff score (r = 0.15; P = 0.002). cDES was also significantly higher in patients who had P. aeruginosa (P < 0.0001). There was an association between cDES and BSI (r = 0.46; P < 0.0001). Correlations were demonstrated with sputum NE activity (see Figure E1). Removing the outliers at >1 ng/ml showed similar correlations with markers of disease severity as described in the online supplement.
Figure 5.
Spearman rank correlation analysis of the relationship between serum total desmosine and age, bronchiectasis severity index (BSI), St. George’s Respiratory Questionnaire, and FEV1% predicted.
In the total cohort, mortality was 9.5% and 22.6% of patients admitted to hospital for severe exacerbations. Median exacerbation frequency was 1 per patient per year (IQR, 0–3).
In analysis of longitudinal clinical outcomes, there was no relationship between cDES and FEV1 decline over 3 years (P = 0.1), but there was a strong relationship between cDES and severe exacerbations (HR, 6.0; 95% CI, 3.61–10.0; P < 0.0001), which persisted after adjustment for BSI (HR, 2.7; 95% CI, 1.42–5.29; P = 0.003). There was no significant association between cDES and moderate exacerbations (P = 0.2), but after combining moderate and severe exacerbations, a statistically significant association more than 0.4 ng/ml was observed (RR, 1.96; 95% CI, 1.61–2.39; P < 0.0001).
There was similar association between cDES and all-cause mortality (HR, 2.60; 95% CI 1.24–5.45; P = 0.01), but this relationship was not statistically significant after adjustment for BSI (HR, 1.15; 95% CI, 0.45–2.91; P = 0.8). Additional models are shown in Table E2. The AUC values for biomarkers compared with individual recognized predictors of outcome in bronchiectasis is shown in Table E4.
A sensitivity analysis conducted in patients taking long-term antibiotics demonstrated that sputum NE and cDES had similar associations with long-term outcomes compared with the overall cohort (Table E5).
Changes in NE at Exacerbation and after Antibiotic Therapy
To determine whether sputum NE was responsive to treatment, we studied 26 patients during an acute exacerbation that required intravenous antibiotic therapy. Characteristics of the included patients compared with the overall population are shown in Table E6.
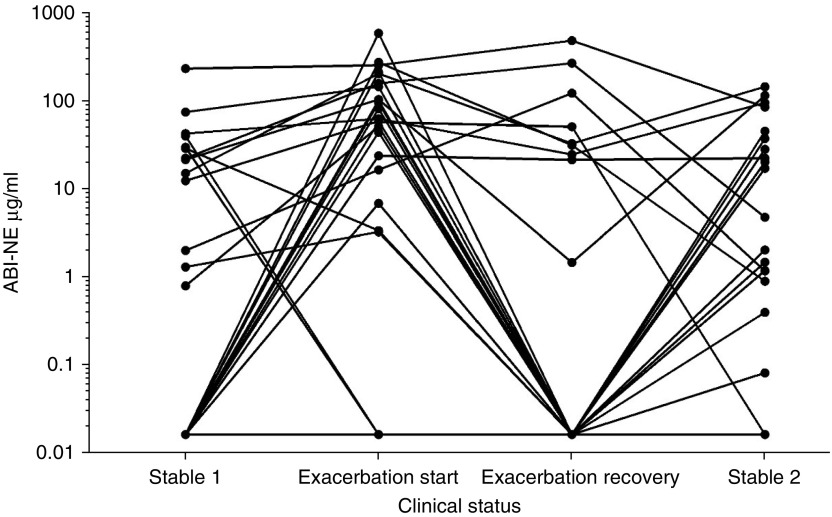
Median ABI-NE levels were 0.39 µg/ml (IQR, 0–23.5) at baseline, 57.0 µg/ml (IQR, 3.3–145 µg/ml) at onset of exacerbation, 0 µg/ml (IQR, 0–25.8) after 14 days of antibiotic therapy, and 1.3 µg/ml (IQR, 0–29.9) at the second stable measurement 6 months later (Figure 6). Although NE activity was generally higher at exacerbations than at baseline (P = 0.0002) and at recovery (P < 0.0001), the assay did not discriminate between exacerbation and disease quiescence because of the high baseline activity in some individuals. The ABI-NE assay level >50 µg/ml was associated with a sensitivity of 57.7% and specificity of 92.3%. An increase from baseline was present at exacerbation in 20 of 26 patients at exacerbation.
Figure 6.
Changes in sputum neutrophil elastase (NE) activity at exacerbation and recovery. ABI = activity-based immunoassay.
Remarkably, even with this small sample size, failure to return to NE baseline levels after completion of antibiotics was associated with a shorter time to the next exacerbation (HR, 2.92; 95% CI, 1.16–7.38; P = 0.02). Data on the correlation between elastase measurements at two stable visits more than 6 months apart are shown in Figure E3.
Discussion
This study indicated a role for sputum NE activity as a biomarker of disease severity and disease progression in bronchiectasis, while also providing the first data on the linked biomarker cDES. NE activity in sputum was independently associated with risk of exacerbations, severe exacerbations, and lung function decline, even after adjustment for underlying severity of the disease. This suggested that NE is a useful marker that might identify patients at future risk. Elastase is dynamic and responded to treatment, and we showed that a failure to improve elastase with treatment predicted a shorter time to the next exacerbation. To the best of our knowledge, NE activity is the first biomarker to be associated with this range of clinically relevant outcomes in bronchiectasis. This confirmed and extended previous observations in diverse bronchiectasis populations in Hong Kong, Belgium, and the UK (13–15).
Tsang and colleagues previously showed that 24-hour NE output was correlated with 24-hour sputum volume, radiological severity of bronchiectasis, and FEV1 (13). NE is not the only airway protease found in the bronchiectasis lung, but Goeminne and colleagues, in a study of 63 patients, showed that NE accounted for 82% of the total gelatinolytic activity of sputum, making a greater contribution than matrix metalloproteinases (15). Goeminne and colleagues also showed a statistically significant association between NE and FEV1% predicted, which was not seen for matrix metalloproteinase-9 (MMP-9) (15). In the largest previous study on 385 patients with bronchiectasis, sputum NE activity measured using a kinetic assay was found to be associated with bacterial load, P. aeruginosa infection, radiological severity, and lung function (14). However, previous studies used a variety of different assays and a limited number of bronchiectasis severity indexes without longitudinal follow-up.
It is essential that candidate biomarkers undergo independent validation because markers typically perform better in their “discovery” or derivation cohort than in subsequent independent cohorts (28). Our study therefore validated these previous findings in a large cohort, because we demonstrated a clear association between elastase activity and a variety of markers of disease severity, including breathlessness, quality of life, and FEV1. There was a strong relationship between elastase activity and the multidimensional BSI (20).
We observed strong relationships between NE activity and bacterial load. NE activity was also highest in patients with P. aeruginosa infection, and this was consistent with previous studies in bronchiectasis that showed that bacteria, and P. aeruginosa in particular, were the key drivers of airway neutrophilic inflammation (22), and that that P. aeruginosa infection represented a distinct clinical phenotype associated with earlier mortality, more frequent exacerbations, and worse quality of life (14, 22, 29, 30).
Although a biomarker that identifies patients with more severe disease is of interest, it is most important to find biomarkers that can identify patients at highest risk of future exacerbations and disease progression. Our study showed that NE activity was independently associated with lung function decline over 3 years, and identified elastase as the first biomarker associated with disease progression in bronchiectasis. In addition, elastase was independently associated with future exacerbations. Although patients with the highest elastase levels were at an increased risk of early death, this association was not independent of disease severity using the BSI, and only the highest levels of elastase were associated with increased mortality, which indicated that airway inflammation itself was not likely to be the primary driver of mortality in this population.
Our data were consistent with those seen in CF, in which NE has been shown to be a key biomarker (31–33). In a pooled analysis of 4 multicenter studies, Mayer-Hamblett and colleagues showed a clear relationship between elastase and FEV1 (31). Sagel and colleagues extended these observations and demonstrated that elastase was the strongest predictor of lung function decline over 3 years (32). Sly and colleagues also demonstrated that elastase activity present in bronchoalveolar lavage was the strongest predictor of the early development of bronchiectasis in infants with CF (33).
Bronchiectasis has been a neglected disease and so, in contrast, biomarker studies are in their infancy. Markers identified in bronchiectasis include sputum MMP-8 and MMP-9, which were shown in a Chinese cohort to correlate with radiological severity, FEV1, and the BSI (34). These findings were extended by Taylor and colleagues who showed that MMP-8 and MMP-9 were higher with P. aeruginosa or H. influenzae colonization and inversely associated with lung function (34, 35). These studies included 102 and 86 patients, respectively, and included only 1 year of follow-up; therefore, further large validation studies are required.
Inconsistent results were seen with cytokines such as CXCL8, TNF-α, and IL-1β in previous studies; in the present study, these were not significantly associated with clinical outcomes (14, 36).
No blood biomarkers have been studied in detail in bronchiectasis, which makes the identification of cDES as a potential marker of severity of significant interest. The results here are similar to those recently described in a large cohort with chronic obstructive pulmonary disease, in which cDES was associated with age and quality of life (17).
There was overwhelming evidence that elastase is involved in the pathophysiology of bronchiectasis. Destruction of elastin, basement membrane collagen, and proteoglycans by proteases contributes to disease progression and might explain the relationship between elastase and FEV1 decline observed in this study (37). NE induces neutrophil dysfunction through multiple mechanisms, including cleavage of FcγRIIIb, and has also been shown to cleave complement receptor 1 in patients with CF (3, 38). NE can also cleave the opsonin iC3b from the surface of pathogens, leading to opsonin/receptor mismatch (39), although Vandivier and colleagues showed that elastase cleaved phosphatidylserine, which prevented the phagocytosis and clearance of apoptotic cells (40). Therefore, not surprisingly, therapeutic manipulation of elastase has been proposed in bronchiectasis. In a proof of concept study, Stockley and colleagues tested an oral NE inhibitor for 4 weeks in 38 patients with bronchiectasis (41). Although the primary outcome of a reduction in sputum neutrophils was not achieved, the study showed a clinically important and significant improvement in FEV1 of 100 ml versus placebo, and a more than 4-point improvement in the SGRQ, which did not reach statistical significance (41).
Although NE activity appears able to stratify patients as high and low risk of disease progression, we cannot currently recommend management decisions based on elastase measurement. The next step would be implementation of such a strategy in a controlled clinical trial. Sputum biomarkers are not currently in routine use, and implementation would be greatly enhanced by the availability of a point-of-care device that could make the assay more rapid and accessible.
Because we and others have shown that elastase is responsive to change, and that changes in elastase correlate with clinical outcomes, measurement of NE might be particularly useful in clinical trials, where it could be used as an early “signal searching” or “early efficacy” endpoint for new antibiotics or anti-inflammatory therapies (14, 42). Such endpoints are essential to identify candidates in smaller clinical trials before embarking on large definitive phase 3 studies. It has been suggested that the absence of such an early response endpoint has contributed to the failure of a number of large phase 3 programs to reach their primary endpoints (43). NE should be further evaluated for this purpose.
There are a wide range of commercially available NE assays, and our data only demonstrated the validity of the ABI-NE, kinetic NE, and cDES assays in bronchiectasis. Additional studies with alternative assays, including those that quantify total elastase rather than elastase activity and urinary desmosine, may not give the same results. This was a single-center study, although the external validity of our results were strengthened by the similarity of the characteristics of these patients with other cohorts across Europe and the previous validation of findings from our center across multiple European centers (20, 44). We did not obtain multiple elastase measurements over time, except in a subset, and it will be important in the future to determine if repeated measurement of elastase could provide improved predictive accuracy. The cutoffs that we proposed here for intermediate and high levels of NE were not independently validated and should be tested in future cohort studies. The use of expectorated sputum for NE measurement might introduce sampling bias because only patients able to produce sputum could be included.
Conclusions
Sputum NE activity is associated with the future risk of exacerbations, including severe exacerbations and lung function decline in bronchiectasis. Elastase is therefore a marker of disease progression in bronchiectasis that may complement clinical assessment of multidimensional clinical scoring systems. NE levels reflect clinical status, and its response is associated with future risk of exacerbations. Future interventional studies should therefore evaluate whether elastase reduction can be used as a surrogate of efficacy in clinical trials.
Footnotes
Supported by the Wellcome Trust (Postdoctoral Fellowship for Clinician Scientists to J.D.C.) and Tenovus Scotland. Additional support from the European Bronchiectasis Network (EMBARC), a European Respiratory Society Clinical Research Collaboration. EMBARC has received financial support from Bayer Healthcare and Aradigm Corporation. K.L.M. was supported by a grant to S.L.M., J.S.E., and B.W. from Cystic Fibrosis Foundation Therapeutics Inc. (MARTINXX130).
Author Contributions: Conception and design: J.D.C., E.F., S.E.M., and T.C.F. Recruited patients: J.D.C., S.F., S.E.M., and T.C.F. Performed experiments and sample processing: J.D.C., K.L.M., G.S.-C., O.S., S.F., E.F., A.D., K.W., S.L.M., and J.T.-J.H. Analysis and interpretation: J.D.C., K.L.M., G.S.-C., O.S., J.S.E., B.W., S.L.M., S.E.M., J.T.-J.H., and T.C.F. Drafting the manuscript: J.D.C., J.S.E., S.E.M., J.T.-J.H., and T.C.F. All authors participated in writing and revising the article before submission.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201605-1027OC on December 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015;45:1446–1462. doi: 10.1183/09031936.00119114. [DOI] [PubMed] [Google Scholar]
- 2.Dente FL, Bilotta M, Bartoli ML, Bacci E, Cianchetti S, Latorre M, Malagrino L, Nieri D, Roggi MA, Vagaggini B, et al. Neutrophilic bronchial inflammation correlates with clinical and functional findings in patients with noncystic fibrosis bronchiectasis. Mediators Inflamm. 2015;2015:642503. doi: 10.1155/2015/642503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voglis S, Quinn K, Tullis E, Liu M, Henriques M, Zubrinich C, Peñuelas O, Chan H, Silverman F, Cherepanov V, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med. 2009;180:159–166. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol. 2014;21:16–22. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27–34. doi: 10.1016/j.molimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 8.Amitani R, Wilson R, Rutman A, Read R, Ward C, Burnett D, Stockley RA, Cole PJ. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Chan SC, Shum DK, Ip MS. Sputum sol neutrophil elastase activity in bronchiectasis: differential modulation by syndecan-1. Am J Respir Crit Care Med. 2003;168:192–198. doi: 10.1164/rccm.200208-829OC. [DOI] [PubMed] [Google Scholar]
- 10.Roghanian A, Drost EM, MacNee W, Howie SE, Sallenave JM. Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am J Respir Crit Care Med. 2006;174:1189–1198. doi: 10.1164/rccm.200605-632OC. [DOI] [PubMed] [Google Scholar]
- 11.Weldon S, McGarry N, Taggart CC, McElvaney NG. The role of secretory leucoprotease inhibitor in the resolution of inflammatory responses. Biochem Soc Trans. 2007;35:273–276. doi: 10.1042/BST0350273. [DOI] [PubMed] [Google Scholar]
- 12.Dubois AV, Gauthier A, Bréa D, Varaigne F, Diot P, Gauthier F, Attucci S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 2012;47:80–86. doi: 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 13.Tsang KW, Chan K, Ho P, Zheng L, Ooi GC, Ho JC, Lam W. Sputum elastase in steady-state bronchiectasis. Chest. 2000;117:420–426. doi: 10.1378/chest.117.2.420. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 15.Goeminne PC, Vandooren J, Moelants EA, Decraene A, Rabaey E, Pauwels A, Seys S, Opdenakker G, Proost P, Dupont LJ. The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology. 2014;19:203–210. doi: 10.1111/resp.12219. [DOI] [PubMed] [Google Scholar]
- 16.Albarbarawi O, Barton A, Miller D, McSharry C, Chaudhuri R, Thomson NC, Palmer CN, Devereux G, Huang JT. Characterization and validation of an isotope-dilution LC-MS/MS method for quantification of total desmosine and isodesmosine in plasma and serum. Bioanalysis. 2013;5:1991–2001. doi: 10.4155/bio.13.164. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich RA, Miller BE, Wrobel K, Ranjit K, Williams MC, Drost E, Edwards LD, Lomas DA, Rennard SI, Agustí A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur Respir J. 2016;47:1365–1373. doi: 10.1183/13993003.01824-2015. [DOI] [PubMed] [Google Scholar]
- 18.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasteur MC, Bilton D, Hill AT British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–i58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 20.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quittner AL, O’Donnell AE, Salathe MA, Lewis SA, Li X, Montgomery AB, O’Riordan TG, Barker AF. Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax. 2015;70:12–20. doi: 10.1136/thoraxjnl-2014-205918. [DOI] [PubMed] [Google Scholar]
- 22.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611. doi: 10.1513/AnnalsATS.201506-333OC. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 25.Martin SL, Moffitt KL, Elborn JS, Walker B. Development of a novel tool for the rapid detection of neutrophil elastase as a marker of inflammation with in the clinic. J Cyst Fibros. 2011;10:S46. [Google Scholar]
- 26.Moffitt KL, McNally P, Linnane B, Walker B, Martin SL. Utilisation of a novel ProteaseTag activity immunoassay for the specific measurement of neutrophil elastase in BAL from children with cystic fibrosis. Pediatr Pulmonol. 2015;50:266–267. [Google Scholar]
- 27.Woolhouse IS, Bayley DL, Stockley RA. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax. 2002;57:667–671. doi: 10.1136/thorax.57.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller BE, Tal-Singer R, Rennard SI, Furtwaengler A, Leidy N, Lowings M, Martin UJ, Martin TR, Merrill DD, Snyder J, et al. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:607–613. doi: 10.1164/rccm.201509-1722PP. [DOI] [PubMed] [Google Scholar]
- 29.Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, Fardon TC, Rutherford R, Pesci A, Restrepo MI, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016;47:1113–1122. doi: 10.1183/13993003.01899-2015. [DOI] [PubMed] [Google Scholar]
- 30.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, Li ZM, Zheng JP, Chen RC, Zhong NS. Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China. J Thorac Dis. 2015;7:625–636. doi: 10.3978/j.issn.2072-1439.2015.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 34.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Gu YY, Liu GH, Li HM, Chen RC, Zhong NS. Sputum matrix metalloproteinase-8 and -9 and tissue inhibitor of metalloproteinase-1 in bronchiectasis: clinical correlates and prognostic implications. Respirology. 2015;20:1073–1081. doi: 10.1111/resp.12582. [DOI] [PubMed] [Google Scholar]
- 35.Taylor SL, Rogers GB, Chen AC, Burr LD, McGuckin MA, Serisier DJ. Matrix metalloproteinases vary with airway microbiota composition and lung function in non-cystic fibrosis bronchiectasis. Ann Am Thorac Soc. 2015;12:701–707. doi: 10.1513/AnnalsATS.201411-513OC. [DOI] [PubMed] [Google Scholar]
- 36.Chen AC, Martin ML, Lourie R, Rogers GB, Burr LD, Hasnain SZ, Bowler SD, McGuckin MA, Serisier DJ. Adult non-cystic fibrosis bronchiectasis is characterised by airway luminal Th17 pathway activation. PLoS One. 2015;10:e0119325. doi: 10.1371/journal.pone.0119325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J. 2008;31:396–406. doi: 10.1183/09031936.00069007. [DOI] [PubMed] [Google Scholar]
- 38.Berger M, Sorensen RU, Tosi MF, Dearborn DG, Döring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J Clin Invest. 1989;84:1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockley R, De Soyza A, Gunawardena K, Perrett J, Forsman-Semb K, Entwistle N, Snell N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med. 2013;107:524–533. doi: 10.1016/j.rmed.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, Greening AP, Haslett C, Hill AT. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2011;183:491–499. doi: 10.1164/rccm.201005-0756OC. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers JD, Loebinger M, Aliberti S. Challenges in the development of new therapies for bronchiectasis. Expert Opin Pharmacother. 2015;16:833–850. doi: 10.1517/14656566.2015.1019863. [DOI] [PubMed] [Google Scholar]
- 44.Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, Polverino E, Van de Kerkhove C, Rutherford R, Davison J, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12:1764–1770. doi: 10.1513/AnnalsATS.201507-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]