Abstract
Rationale: The release of eosinophil granule proteins in the lungs of patients with asthma has been dogmatically linked with lung remodeling and airway hyperresponsiveness. However, the demonstrated inability of established mouse models to display the eosinophil degranulation occurring in human subjects has prevented a definitive in vivo test of this hypothesis.
Objectives: To demonstrate in vivo causative links between induced pulmonary histopathologies/lung dysfunction and eosinophil degranulation.
Methods: A transgenic mouse model of chronic T-helper cell type 2–driven inflammation overexpressing IL-5 from T cells and human eotaxin 2 in the lung (I5/hE2) was used to test the hypothesis that chronic histopathologies and the development of airway hyperresponsiveness occur as a consequence of extensive eosinophil degranulation in the lung parenchyma.
Measurement and Main Results: Studies targeting specific inflammatory pathways in I5/hE2 mice surprisingly showed that eosinophil-dependent immunoregulative events and not the release of individual secondary granule proteins are the central contributors to T-helper cell type 2–induced pulmonary remodeling and lung dysfunction. Specifically, our studies highlighted a significant role for eosinophil-dependent IL-13 expression. In contrast, extensive degranulation leading to the release of major basic protein-1 or eosinophil peroxidase was not causatively linked to many of the induced pulmonary histopathologies. However, these studies did define a previously unappreciated link between the release of eosinophil peroxidase (but not major basic protein-1) and observed levels of induced airway mucin.
Conclusions: These data suggest that improvements observed in patients with asthma responding to therapeutic strategies ablating eosinophils may occur as a consequence of targeting immunoregulatory mechanisms and not by simply eliminating the destructive activities of these purportedly end-stage effector cells.
Keywords: eosinophil peroxidase, major basic protein, asthma, lung, chronic inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
The roles of eosinophils and, more specifically, eosinophil secondary granule proteins in lung remodeling events in chronic inflammatory pulmonary diseases is unresolved.
What This Study Adds to the Field
Our use of a unique mouse model of chronic respiratory inflammation displaying the extensive eosinophil degranulation that occurs in human subjects with asthma demonstrated that the airway release of the granule proteins major basic protein-1 and eosinophil peroxidase was unable to elicit many induced pulmonary histopathologies and lung dysfunction (i.e., airway hyperresponsiveness), including progressive lung fibrosis and alveolar septa breakdown. These data suggest that the improvements observed in patients with asthma responding to biologic therapeutics targeting eosinophils may occur as a consequence of modulating one or more ongoing immune responses in the lung and not simply the monolithic targeting of tissue-destructive activities mediated by eosinophils. As such the study suggests we rethink how this class of therapeutics actually works.
Recent studies of patients have highlighted the diversity of immune/inflammatory phenotypes and patient responses to disease management approaches (reviewed in References 1 and 2). The long-held view of asthma as a quintessential chronic allergic disease (3, 4) accompanied by eosinophil-mediated airway inflammation linked with increased levels of T-helper cell type 2 (Th2)-derived cytokines is being reinterpreted in light of the realization that the underlying immune responses in these patients are much more diverse (reviewed in References 2, 5–8). Moreover, eosinophil targeting therapies have been efficacious only in a narrow subgroup of eosinophilic asthma patients with lower use of steroids and decreased exacerbation events as the most significant end point measures (9–12). Collectively, these studies suggested that instead of direct links with asthma symptoms, eosinophils seem to be one of several immunomodulatory leukocytes (e.g., natural killer cells [13], CD4+ T cells [14], and ILC2s [15]; reviewed in Reference 16) participating in allergic respiratory inflammation. Concurrent basic research studies investigating eosinophil activities in the lungs of mouse models following allergen provocation are providing support for this hypothesis and have led us and others to suggest that pulmonary eosinophils directly modulate allergic immune responses (17–23) and contribute to the remodeling/repair processes linked with the resolution of inflammation (see References 24 and 25). These studies thus suggest that eosinophil activities likely contribute to allergic pulmonary inflammation at many levels beyond their proposed role as mediators of tissue damage and cell death through the release of eosinophil secondary granule proteins (26).
The rationale for the use of biologic therapies targeting eosinophils (e.g., antibodies targeting IL-5 [9–12] or IL-5R [27]) to treat allergic asthma is based, in part, on the suppression of the proposed destructive capacities of eosinophils mediated predominantly by degranulation. A hallmark feature of chronic allergic severe asthma is the extensive deposition and extracellular accumulation of eosinophil secondary granule proteins in lung tissue and airways (28; reviewed in References 26, 29, 30). Moreover, the release of major basic protein-1 (MBP-1), eosinophil peroxidase (EPX), eosinophil-derived neurotoxin (EDN), and eosinophil cationic protein (ECP) have been suggested to mediate cell killing and tissue-damaging events occurring in the lung as part of the late phase response to allergen provocation (31; reviewed in Reference 32). This prospective is supported by a myriad of studies demonstrating that the introduction of purified granule proteins into the lungs of various animal models induced airway hyperresponsiveness (AHR) (33–35). Moreover, granule proteins were shown to mediate epithelial desquamation and cell killing in explant cultures (36), cytocidal activities using in vitro cell culture assays (37), and, on instillation in the lungs, induced deposition of extracellular matrix proteins (24).
Unfortunately, a definitive demonstration of this prospective has been out of reach using in vivo mouse models of allergen sensitization and aeroallergen challenge. That is, although these established models of allergen provocation replicate many of the immune responses and induced cellular infiltrates commonly observed in human subjects with asthma (reviewed in References 38 and 39), these models fail to induce the extensive eosinophil degranulation and release of individual granule proteins commonly occurring in patients (40, 41; reviewed in Reference 32). This lack of degranulation in allergen-challenged mice has uniquely denied the proof-of-concept studies necessary to link tissue destruction/remodeling events and lung dysfunction in response to allergic respiratory inflammation.
The capacity of eosinophils to more faithfully replicate the pulmonary pathologies occurring in response to chronic inflammation was demonstrated in a double transgenic model of severe asthma (I5/hE2 [41]) that constitutively expressed IL-5 from all mature peripheral T cells using an engineered CD3δ promoter and human eotaxin 2 from central airway epithelia cells using a CC10 promoter. This chronic model of Th2-driven inflammation displays age-dependent pulmonary histopathologies, induced remodeling events, and lung dysfunction. More importantly, these changes in the lung occur in the context of a significant airway eosinophilia uniquely accompanied by extensive eosinophil degranulation (41). Our objectives here were to use specific crosses with gene knockout mice and define the relative importance of immune regulatory activities mediated by eosinophils that polarize and/or amplify Th2 responses (17, 18, 20, 42) versus destructive pathology-causing mechanisms mediated by the release of eosinophil granule proteins (reviewed in Reference 32).
These studies surprisingly showed that the induced pulmonary pathologies occurring in this chronic model were unique responses to eosinophil-dependent IL-13 expression. Interestingly, these induced pathologies were also independent of CD4+ T-cell-mediated events, suggesting that pulmonary eosinophils, and not CD4+ T cells, are likely the dominant cellular source of IL-13 that, among other events, elicits cysteine leukotriene and transforming growth factor (TGF)-β-dependent pathways that accompany the fibrosis occurring in this chronic respiratory model (43). More importantly, using our available secondary granule protein gene knockout mice (i.e., MBP-1−/− [44] and EPX−/− [45]) showed that despite the extensive degranulation occurring in I5/hE2 mice, the release of either of these abundant granule proteins had only narrow contributory effects to the pathologies or lung dysfunction. These data highlight a more complex and sophisticated role for eosinophils in chronic inflammatory settings and suggest that earlier conclusions that eosinophils in patients contribute to induced lung pathologies and dysfunction exclusively via the tissue/cell destructive capacity of granule proteins MBP-1 and EPX may be overstated.
Methods
Mice
Several unique lines of mice (C57BL/6 background, 8–12 wk of age) were used in these studies, including C57BL/6J wild-type animals purchased from the Jackson Laboratory (Jackson Research Laboratories, Bar Harbor, ME), I5/hE2 (41), IL-13−/− (a gift of Andrew McKenzie [46]), CD4+ T-cell-deficient mice (CD4tm1Knw; Jackson Laboratory), eosinophil-deficient mice (PHIL [21]), MBP-1−/− (44), and EPX−/− (45). Mice were maintained in ventilated microisolator cages housed in the specific pathogen-free animal facility. Protocols and studies involving animals were performed in accordance with National Institutes of Health and Mayo Foundation institutional guidelines.
Flow Cytometric Analyses
Single-cell suspensions of lung and lung-draining lymph nodes were generated as described previously (20, 42). Antibodies used to characterize T-cell populations include TCRβ (H57–597; BD Biosciences, San Jose, CA), CD4 (RM4–5; eBiosciences, San Diego, CA), CD44 (IM27; eBiosciences), IL-13 (eBio13A; eBiosciences), and CD62L (MEL-14; eBiosciences). Data acquisition and analysis were performed using Summit version 4.3 (Dako, Carpinteria, CA) software.
Collection and Cell Differentials of Bronchoalveolar Lavage Fluid–derived Leukocytes
The tracheas of individual mice were exposed and cannulated before bronchoalveolar lavage (BAL) of the lungs with 1 ml of 2% fetal calf serum in phosphate-buffered saline to obtain cell numbers and cell differentials as previously described (41). Cell differentials of each sample were performed on greater than or equal to 300 cells after staining cytospin preparations (Diff-Quik; Cardinal Health, Dublin, OH).
Cytokine Assays
Mouse IL-4, IL-13, IFN-γ, IL-17A, and TGF-β were assessed using immunoassay kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The limits of detection for each ELISA assay were 5–10 pg/ml.
Histology and Immunohistochemical Detection of Eosinophils and Individual Granule Proteins
Histopathologic changes of the airways were assessed as described previously (41). In brief, formalin-fixed and paraffin-embedded sections of mouse lungs were stained with hematoxylin and eosin for general pathologic assessments, periodic acid–Schiff staining to detect goblet cell metaplasia/epithelia cell mucin accumulation (GM/MA), or Masson’s trichrome for assessments of collagen deposition (i.e., fibrosis). All evaluations were performed using multiple parasagittal sections of the lung, oriented via structural “land markers” to ensure that identical regions of the lungs from each mouse were examined. Lung tissue eosinophilia and degranulation were determined as described in our earlier publications using both immunohistochemistry with a rat monoclonal antibody specific for MBP-1 (47) or a mouse monoclonal antibody specific for EPX (48).
ELISA-based Detection of Eosinophil Degranulation Occurring in the Airways
The levels of cell-free EPX in BAL fluid from mice were determined using a sandwich ELISA that was specific for EPX (49).
Assessments of GM/MA and Induced Pulmonary Fibrosis
GM/MA levels in the lungs of mice were determined using periodic acid–Schiff–stained formalin-fixed parasagittal sections (4 μm) analyzed by bright-field microscopy as described previously (21). Collagen deposition was determined by evaluation of Masson’s trichrome–stained parasagittal lung sections and in some cohorts of mice by analysis of picrosirius red–stained lung sections evaluated under polarized light (43).
Assessment of Methacholine Dose-Dependent Changes in Airway Resistance
AHR was determined as described previously (21, 41) with airway resistance presented as the percentage change from baseline measured following challenge with saline.
Statistical Analyses
All data are derived from cohort sizes of 5–14 mice depending of the assay performed (specific cohort sizes for individual studies are noted in the figure legends presenting these data). Comparative data between groups were analyzed by unpaired two-tailed Student’s t test using GraphPad Prism 5 (La Jolla, CA). Data are presented as mean ± SEM. Values of P less than 0.05 were considered statistically significant.
Results
Eosinophil-Dependent Pathologies Occurring in I5/hE2 Mice Are Accompanied by Significant Increases in CD4+ T Cells and Th2 Cytokines/Chemokines
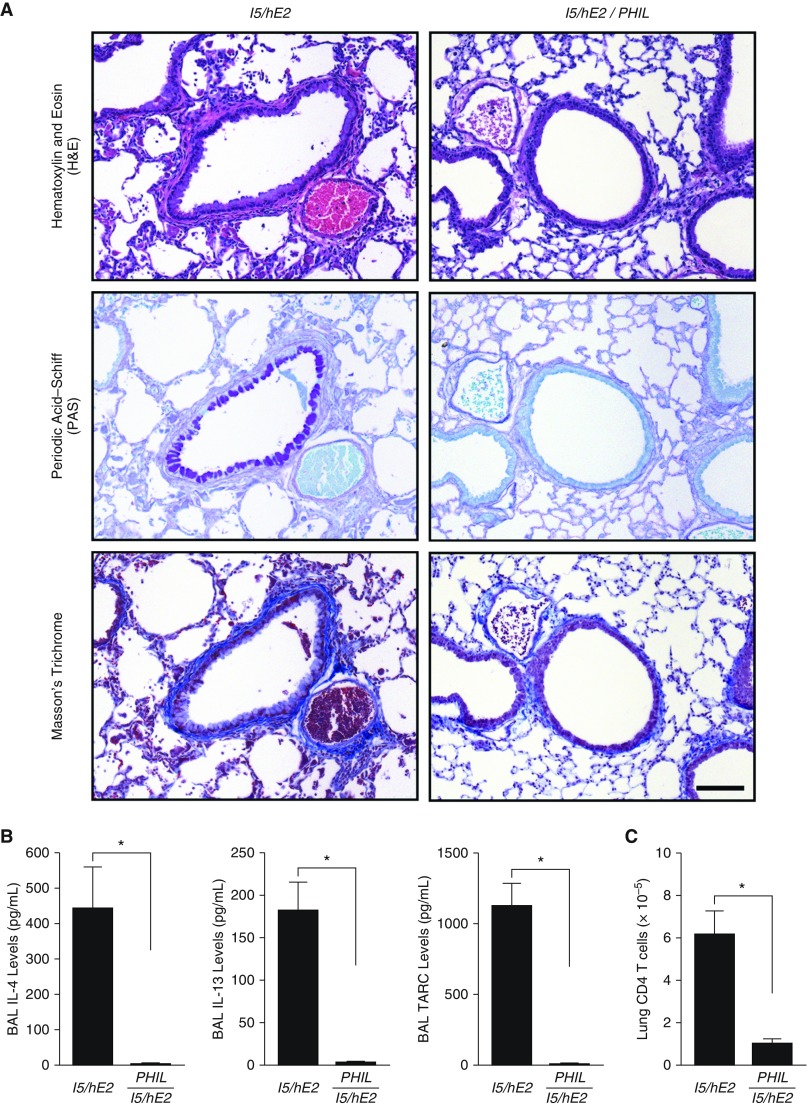
The extent by which pulmonary eosinophils modulate immune/inflammatory responses in the lungs of I5/hE2 mice was determined through a direct comparison of the parental double transgenic model I5/hE2 (41) with an eosinophil-less version of these animals (i.e., I5/hE2/PHIL). As shown in Figure 1A, whereas I5/hE2 mice develop eosinophil-dependent increases in airway smooth muscle, GM/MA, and collagen deposition/remodeling of the extracellular compartments of the lung, all these increases were abolished in I5/hE2/PHIL mice.
Figure 1.
Eosinophils and their associated effector functions are necessary for the induced histopathologies, remodeling, and the T-helper cell type 2 polarized immune microenvironment of the lungs of I5/hE2 mice. (A) Parasagittal lung sections from eosinophil-sufficient double transgenic I5/hE2 mice and eosinophil-deficient triple transgenic animals (i.e., I5/hE2/PHIL) were stained via multiple venues to determine the eosinophil dependency of the induced lung pathologies occurring in this chronic model: hematoxylin and eosin staining demonstrated a decrease in leukocyte tissue infiltration, tissue edema, and airway epithelial hypertrophy occurring in the absence of eosinophils (i.e., I5/hE2/PHIL vs. the parental I5/hE2 model); periodic acid–Schiff staining assessments of goblet cell metaplasia/epithelial cell mucin accumulation (dark-purple-staining cells) showed that the induced eosinophilia in I5/hE2 mice was required for any evidence of this pathology/remodeling event; and Masson’s trichrome staining demonstrated that eosinophils were also required for the enhanced collagen deposition that occurred around central airways of the parental I5/hE2 model (blue-staining extracellular matrix regions). Scale bar = 100 μm. (B) ELISA assessments of airway cytokines/chemokines revealed the characteristic eosinophil-dependent T-helper cell type 2 polarization of the pulmonary immune microenvironment of I5/hE2 mice. Specifically, the cytokines IL-4 and IL-13 and chemokine TARC were significantly elevated in I5/hE2 mice as compared with I5/hE2/PHIL animals. IFN-γ and IL-17A, and IP-10 were undetectable in both strains of mice. Error bars represent SEM. *P < 0.05. (C) Fluorescence-activated cell sorter assessments of single cell suspensions of lung leukocytes revealed the eosinophil-dependent accumulation of CD4+TCR-β+ T cells occurring in I5/hE2 that was effectively abolished in eosinophil-deficient triple transgenic mice (i.e., I5/hE2/PHIL). Error bars represent SEM; *P < 0.05. All studies were performed using cohort sizes (n) of three to five mice. BAL = bronchoalveolar lavage; TARC = thymus- and activation-regulated chemokine.
These eosinophil-dependent pulmonary changes led to concomitant airway increases in Th2 cytokines IL-4 and IL-13 (Figure 1B); IFN-γ and IL-17A levels were undetectable (data not shown). The Th2 polarized character of I5/hE2 lungs was also associated with an eosinophil-dependent increase in airway Th2 chemokine (TARC/CCL17) levels (Figure 1B) without similar increases in typical Th1 chemokines, such as IP-10 (see Figure E1A in the online supplement). This cytokine/chemokine expression was accompanied by an increase in CD4+ T cells in the lungs and BAL of I5/hE2 that did not occur in the absence of eosinophils (Figures 1C and E1B). This increase in CD4+ T cells was restricted to the pulmonary compartments as T-cell levels were unchanged in the spleens of the same mice. Moreover, most T cells recruited to the lung were of the effector cell phenotype (CD4+CD62LloCD44hi) (Figure E1C). Interestingly, the lung-draining lymph nodes of I5/hE2 also display differential accumulations of naive and memory T-cell subtypes that again were abolished in I5/hE2/PHIL mice (Figure E1C), suggesting that eosinophils, in part, promote T-cell proliferation by expanding these lymphatic lymphocyte populations.
Loss of IL-13 Resulted in a Significant Attenuation of the Induced Histopathologies and Lung Dysfunction That Was Only Partially Replicated by the Depletion of CD4+ T Cells
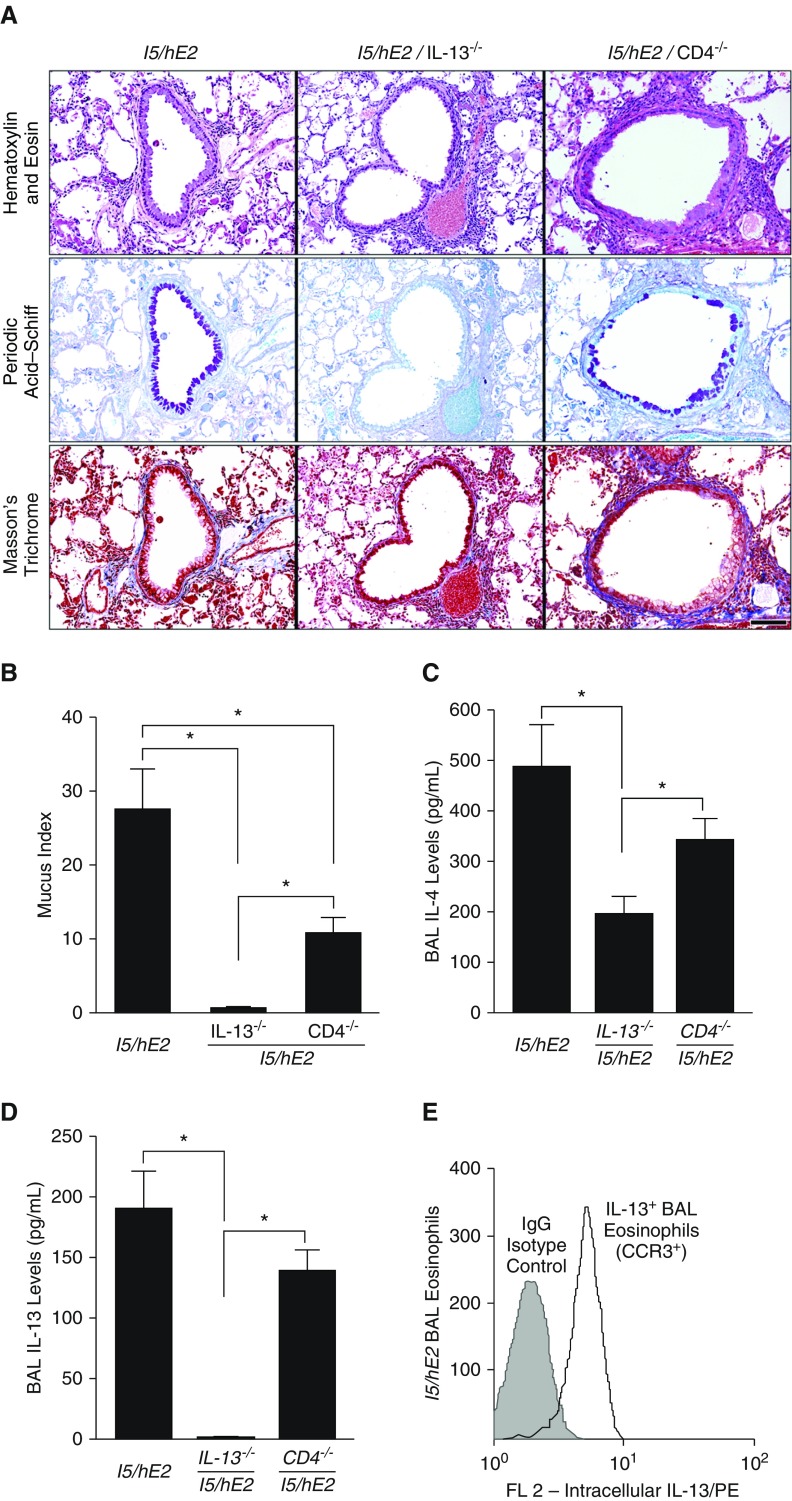
The elevated IL-13 expression levels and the accumulation of CD4+ effector T cells in the lungs of I5/hE2 mice were each targeted using compound transgenic/gene knockout mice to determine their relative contributions to the induced pathologies in this model. As expected, the targeted loss of either IL-13 (i.e., I5/hE2/IL-13−/−) or CD4+ T cells alone (i.e., I5/hE2/CD4−/−) had no effect on ectopic accumulation of pulmonary eosinophils resulting from concurrent constitutive expression of IL-5 and eotaxin-2 in these mice (see Figure E2A). The assessments of lung cellularity also showed that the loss of IL-13 from I5/hE2 mice had no significant effects on the accumulation of CD4+ T cells in the airway or lung-draining lymph nodes (see Figure E2B). However, the loss of IL-13 in the I5/hE2 model resulted in an almost total loss of GM/MA (Figures 2A and 2B). In contrast, although targeting CD4+ T cells alone in these mice reduced GM/MA, this decrease was only 50% of the levels observed in parental I5/hE2 mice (Figures 2A and 2B), suggesting the importance of other non-T-cell sources of IL-13. In agreement with this conclusion, I5/hE2/CD4−/− mice displayed levels of IL-4 (Figure 2C) and IL-13 (Figure 2D) in the airways that were only nominally lower than the levels observed in the parental I5/hE2 model. In addition, intracellular staining of pulmonary eosinophils accumulating in the airways of I5/hE2/CD4−/− mice demonstrated that eosinophils are a potentially important source of these elevated airway IL-13 levels (Figure 2E).
Figure 2.
The loss of IL-13, but not CD4+ T cells, differentially attenuates multiple remodeling events linked with the pulmonary eosinophilia of I5/hE2 mice. (A) Parasagittal lung sections of transgenic/compound gene knockout mice were stained to evaluate the dependency of pulmonary histopathologies occurring in the parental I5/hE2 model on IL-13 (i.e., I5/hE2/IL-13−/−) and CD4+ T cells (i.e., I5/hE2/CD4−/−). Hematoxylin and eosin staining demonstrated that neither the loss of IL-13 or CD4+ T cells had effects on the induced pulmonary leukocyte infiltrate. Masson’s trichrome staining demonstrated that the loss of IL-13 (i.e., I5/hE2/IL-13−/−) resulted in a reduction of lung structural changes, such as fibrosis (blue-staining extracellular matrix regions) that was not observed in CD4+ T-cell–deficient mice (i.e., I5/hE2/CD4−/−). Periodic acid–Schiff staining of sections demonstrated that although the loss of CD4+ T cells fractionally reduced goblet cell metaplasia/epithelial cell mucin accumulation (GM/MA), the absence of IL-13 in I5/hE2 mice nearly abolished this remodeling metric (dark-purple-staining cells). Scale bar = 100 μm. (B) Quantitative group mean assessments of GM/MA were determined as described in the Methods and showed that although GM/MA significantly decreased in the absence of CD4+ T cells (i.e., I5/hE2/CD4−/−), it was not abolished, unlike the virtual loss of this histopathology metric in IL-13–deficient mice (i.e., I5/hE2/IL-13−/−). Cohort sizes: I5/hE2 (n = 7); I5/hE2/CD4−/− (n = 12); I5/hE2/IL-13−/−(n = 7). Error bars represent SEM; *P < 0.05. Bronchoalveolar lavage (BAL) levels of T-helper cell type 2 cytokines IL-4 (C) and IL-13 (D) were determined by ELISA, demonstrating a significant reduction in IL-4 and a complete loss of IL-13 in I5/hE2/IL-13−/− mice, whereas the targeting of CD4+ T cells (i.e., I5/hE2/CD4−/−) only partially reduced both cytokines as compared with parental I5/hE2 model (neither achieving statistical significance). IFN-γ and IL-17A were undetectable in both strains of mice. *P < 0.05. (E) A representative flow cytometric plot demonstrating intracellular IL-13 staining in BAL eosinophils recovered from I5/hE2 mice. Cohort sizes: I5/hE2, n = 4; I5/hE2/CD4−/−, n = 6; I5/hE2/IL-13−/−, n = 7. Error bars represent SEM; *P < 0.05. CCR3 = C-C chemokine receptor type 3.
These studies also highlight the differential downstream consequences of these induced IL-13 levels on remodeling events, such as increased airway collagen deposition. The loss of IL-13 expression reduced staining for collagen by Masson’s trichrome, whereas a CD4+ T-cell deficiency did not (Figure 2A). This was quantitatively confirmed by picrosirius red staining of lung sections that showed a nearly 60% drop in collagen deposition in the absence of IL-13 (I5/hE2: 152.1 ± 55.0 vs. I5/hE2/IL-13−/− 60.6 ± 9.4 [collagen-positive pixels/mm of basement membrane × 10−3]). The data also demonstrated that the decrease in fibrosis occurred with a concurrent reduction in BAL TGF-β1 levels in the airways of these mice (Figure E3), confirming an earlier study suggesting that the eosinophil-dependent pulmonary pathologies and remodeling events occurring in I5/hE2 mice are a response to one or more IL-13-induced downstream events (43).
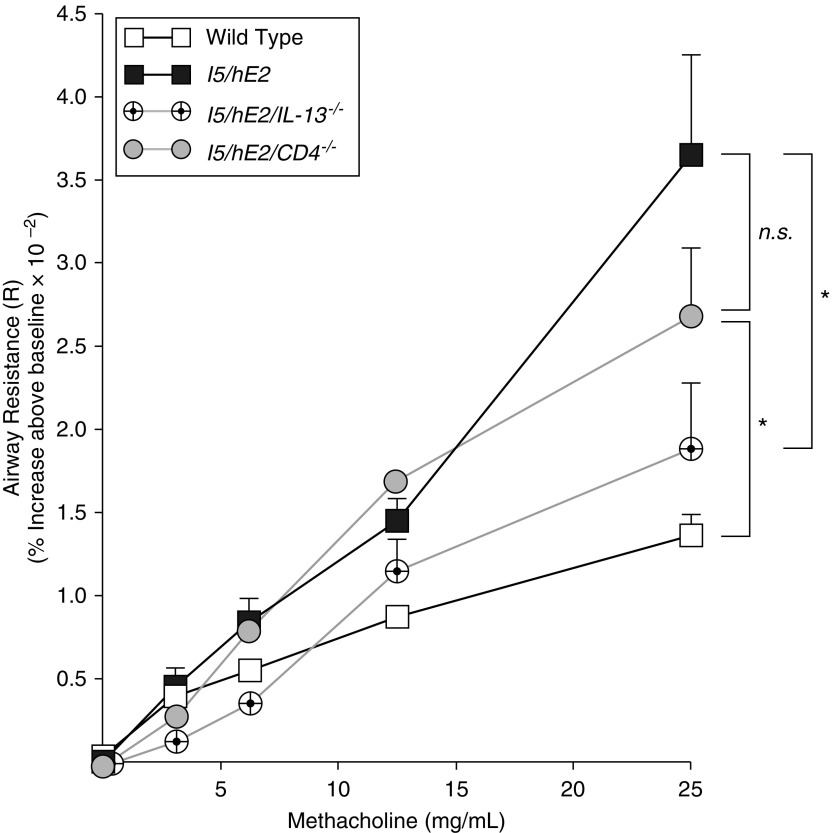
Invasive ventilator-based measures of lung function (Figure 3) demonstrated that the significant AHR in response to methacholine challenge displayed by I5/hE2 mice did not occur in the absence of IL-13 (i.e., I5/hE2/IL-13−/− mice). This effect was not seen in mice targeting CD4+ T cells alone (i.e., I5/hE2/CD4−/− mice) suggesting that this airways dysfunction occurs by an eosinophil-dependent mechanism linked to induced IL-13 expression and not necessarily the presence of CD4+ T cells.
Figure 3.
The induced airway hyperresponsiveness linked with the pulmonary eosinophilia of I5/hE2 mice is dependent on IL-13 expression and not the presence/absence of CD4+ T cells. Aerosolized methacholine-induced airway resistance was assessed using an invasive ventilator-based technique (flexi-Vent; SCIREQ). I5/hE2 mice displayed an increase in airway resistance (relative to wild-type C57BL/6J mice) as a function of exposure to increasing concentrations of aerosolized methacholine. In contrast, the absence of IL-13 expression (i.e., I5/hE2/IL-13−/−) resulted in a significant reduction in methacholine responses, which became comparable wild-type C57BL/6J mice. A deficiency in CD4+ T cells (i.e., I5/hE2/CD4−/−) resulted only in a small decrease in airway resistance compared with the parental I5/hE2 model at the highest dose of methacholine exposure, but this reduction did not achieve statistical significance. Baseline airway resistance (cm H2O⋅s/ml) of each experimental cohort (mean ± SEM): C57BL/6J wild-type, 0.95 ± 0.05; I5/hE2, 0.95 ± 0.04; I5/hE2/IL-13−/−, 0.94 ± 0.07; and I5/hE2/CD4−/−, 0.82 ± 0.05. Cohort sizes: C57BL/6J, n = 11; I5/hE2, n = 12; I5/hE2/CD4−/−, n = 7; I5/hE2/IL-13−/−, n = 7. Error bars represent SEM. *P < 0.05; n.s. = not significant.
Targeting of IL-13 or CD4+ T Cells Had No Effects on the Extensive Eosinophil Degranulation Occurring in I5/hE2 Mice
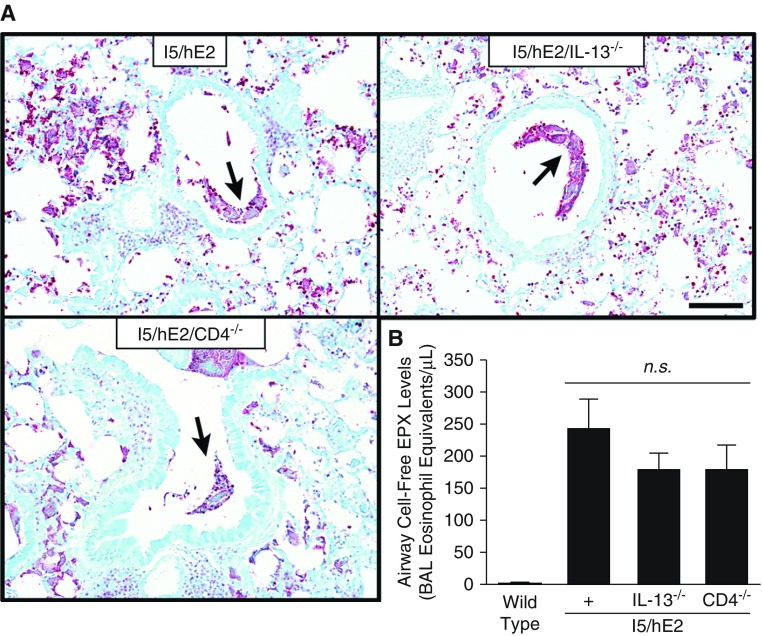
Eosinophil degranulation in the interstitial regions of the lung and the airways of I5/hE2 mice was previously described in detail in our earlier studies (41, 43). These studies had showed evidence of granule protein released predominantly by eosinophils undergoing cytolysis but also evidence of piecemeal release of granule proteins by otherwise intact eosinophils. These studies also used multiple venues to assess degranulation including electron microscopy, immunohistochemistry, and single-dimension immunoblot assays. The extensive degranulation displayed by IL-13 deficient I5/hE2 mice and transgenic animals devoid of CD4+ T cells were assessed here by two independent methods: immunohistochemistry of tissue sections using an antimouse MBP-1 monoclonal antibody (21), and a novel ELISA assessment of BAL fluid using an EPX-specific assay (49). In both strains of compound transgenic/gene knockout mice, MBP-based immunohistochemistry detected the presence of MBP-1 within otherwise intact infiltrating eosinophils and released cell-free MBP-1 bound to extracellular matrix within the lung parenchyma to an extent equal to what was observed in parental I5/hE2 mice (Figures 4A and E4). A more quantitative assessment of the degranulation occurring in the airways using a EPX-based ELISA confirmed these immunohistochemistry tissue assessments showing that the observed levels of EPX in BAL fluid from I5/hE2/IL-13−/− and I5/hE2/CD4−/− mice were equivalent to the released EPX levels observed in the lungs of parental I5/hE2 control mice, suggesting that degranulation occurs independent of IL-13 or activities mediated by CD4+ T cells (Figure 4B).
Figure 4.
The significant and extensive eosinophil degranulation occurring in I5/hE2 mice is unaffected by the loss of IL-13 (i.e., I5/hE2/IL-13−/−) or the absence of CD4+ T cells (i.e., I5/hE2/CD4−/−). (A) Parasagittal lung sections stained by immunohistochemistry with a monoclonal antibody targeting major basic protein-1 demonstrated that the induced lung parenchymal tissue eosinophilia (dark-red-staining cells) and eosinophil degranulation (diffuse extracellular red-staining areas) characteristic of I5/hE2 mice occurred to the same extent in mice deficient for either IL-13 (i.e., I5/hE2/IL-13−/−) or CD4+ T cells (i.e., I5/hE2/CD4−/−). Arrows indicate mucus plug positive for extracellular major basic protein-1. Scale bar = 50 μm. (B) Airway eosinophil degranulation as determined by ELISA, assessing the levels of released eosinophil peroxidase in the bronchoalveolar lavage (eosinophil equivalents per milliliter). These data showed that the loss of either IL-13 (i.e., I5/hE2/IL-13−/−) or CD4+ T cells (i.e., I5/hE2/CD4−/−) did not significantly affect the extent of eosinophil degranulation occurring in each of these mouse cohorts relative to the parental I5/hE2 model. Cohort sizes: I5/hE2, n = 7; I5/hE2/CD4−/−, n = 10; I5/hE2/IL-13−/−, n = 9. Error bars represent SEM; BAL = bronchoalveolar lavage; EPX = eosinophil peroxidase; n.s. = not significant.
Loss of Either EPX or MPB-1 Had No Effects on the Eosinophil Degranulation, Induced Cellular Infiltrate, or Polarization of Th2 Pulmonary Environment Occurring in I5/hE2 Mice
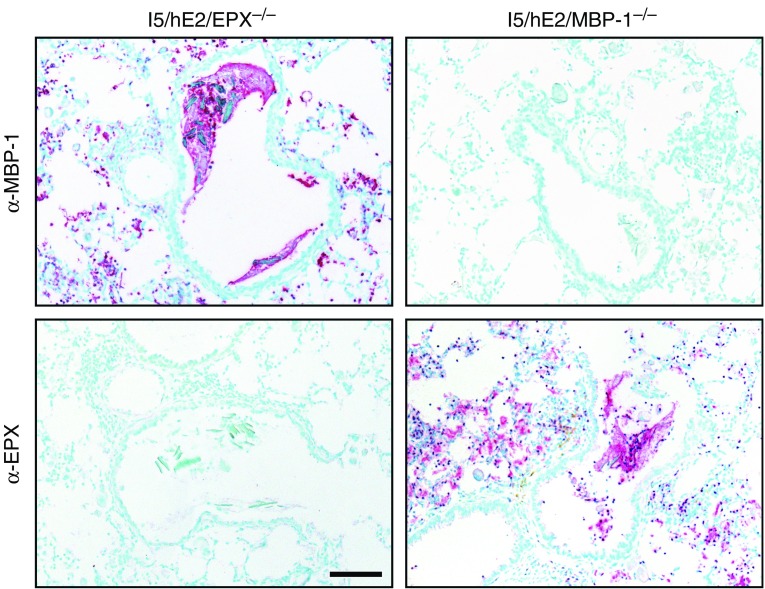
We crossed I5/hE2 mice to animals deficient for MBP-1 (44) or EPX (45) to generate compound transgenic/gene knockout mice deficient for each of these abundant granule proteins (i.e., I5/hE2/EPX−/− or I5/hE2/MBP-1−/−, respectively). Immunohistochemical staining using our granule protein-specific monoclonal antibodies showed that the loss of EPX or MBP-1 had no effects on the induced eosinophil degranulation and deposition of the remaining abundant granule protein (Figure 5). In either setting, the release of the remaining granule protein also occurred at a level equivalent to that observed in the parental I5/hE2 model with both granule proteins present. In addition, the loss of either abundant granule protein did not alter the increased accumulation of BAL leukocytes (dominated by eosinophils) that occurs in the parental I5/hE2 model (Figure E5). This lack of an affect also extended to the induced Th2 polarization in these compound transgenic/gene knockout mice, which displayed no reductions in the typical Th2 cytokine profile displayed by parental I5/hE2 model (IL-13: 175.5 ± 28.5 pg/ml [I5/hE2]; 162.8 ± 47.8 pg/ml [I5/hE2/EPX−/−]; 130.7 ± 42.1 pg/ml [I5/hE2/MBP-1−/−]) (IL-4: 570.6 ± 218.6 pg/ml [I5/hE2]; 412.6 ± 221.9 pg/ml [I5/hE2/EPX−/−]; 315.6 ± 88.9 pg/ml [I5/hE2/MBP-1−/−]).
Figure 5.
Targeted deficiencies of the abundant granule proteins eosinophil peroxidase (EPX) or major basic protein-1 (MBP-1) did not alter the extent of eosinophil infiltration occurring in parental I5/hE2 mice and, more importantly, did not change the extent or spatial pattern of degranulation observed in each transgenic/gene knockout strain of mice. Parasagittal lung sections were stained by immunohistochemistry with monoclonal antibodies targeting each of the abundant eosinophil secondary granule proteins (i.e., α-EPX and α-MBP-1). The loss of either abundant granule protein abolished staining with the cognate antibody (i.e., α-EPX and α-MBP-1 did not stain lung sections from I5/hE2/EPX−/− and I5/hE2/MBP-1−/− mice, respectively). Moreover, these data showed that the induced eosinophilia (dark-red-staining cells), the extent of degranulation (diffuse extracellular red-staining areas), and the spatial deposition of the remaining granule protein were not altered relative to events occurring in the parental I5/hE2 model (Figures 4 and E3). Scale bar = 50 μm.
Loss of EPX or MBP-1 Had No Significant Effects on the Induced Lung Structural Changes in I5/hE2 Mice That Are Typically Associated with Eosinophilic Inflammatory Remodeling
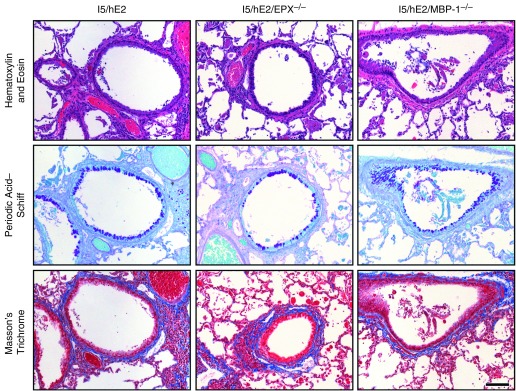
The loss of either EPX (I5/hE2/EPX−/−) or MBP-1 (i.e., I5/hE2/MBP-1−/−) unexpectedly resulted in no observable effects on many of the lung structural changes that collectively represent induced remodeling events. Specifically, hematoxylin and eosin and Masson’s trichrome staining of sections showed that granule protein–deficient mice each displayed the cellular infiltrates and induced histopathologic changes in lung structure (i.e., airway epithelial hypertrophy, tissue edema, airway smooth muscle hyperplasia, disruption of alveolar structure, appearance cellular debris, and collagen deposition around the airways [fibrosis]) characteristic of the parental I5/hE2 model (Figure 6).
Figure 6.
The loss of either of the abundant secondary granule proteins (i.e., eosinophil peroxidase [EPX] or major basic protein-1 [MBP-1]) has nominal but differential effects on the induced histopathologies/remodeling occurring in I5/hE2 mice. Parasagittal lung sections of transgenic/compound granule protein gene knockout mice were stained to evaluate the dependency of the pulmonary histopathologies occurring in the parental I5/hE2 model on EPX (i.e., I5/hE2/EPX−/−) and MBP-1 (i.e., I5/hE2/MBP-1−/−). Hematoxylin and eosin and Masson’s trichrome staining demonstrated that the loss of either granule protein had little to no effect on the leukocyte tissue infiltration or the induced lung structural changes (including pulmonary fibrosis [blue-staining extracellular matrix regions in Masson’s trichrome–stained lung sections]) occurring in the parental I5/hE2 model. In contrast, periodic acid–Schiff staining of lung sections for goblet cell metaplasia/epithelial cell mucin accumulation (dark-purple-staining cells) demonstrated a differential effect regarding the loss of these granule proteins with the loss of EPX and not MBP-1, resulting in a greater than 50% reduction in goblet cell metaplasia/epithelial cell mucin accumulation relative to the levels observed in I5/hE2 mice. Scale bar = 100 μm. The cohort size of each group (i.e., I5/hE2, I5/hE2/EPX−/−, and I5/hE2/MBP-1−/−) was n = 3–5 mice.
Specific Loss of EPX but Not MBP-1 in I5/hE2 Mice Uniquely Targets the Induced GM/MA Occurring in Parental I5/hE2 Model
Periodic acid–Schiff–stained lung sections demonstrated that a reproducible change in GM/MA was evident in the absence of EPX (Figure 6). Specifically, the loss of EPX in I5/hE2/EPX−/− mice resulted in a greater than 50% decrease in the GM/MA that occurred in the parental I5/hE2 model (see Figure E5). This phenomenon was EPX-specific and did not occur in I5/hE2/MBP-1−/− mice (Figures 6 and E6).
Presence of EPX or MBP-1 Does Not Significantly Contribute to the Induced AHR Occurring in I5/hE2 Mice
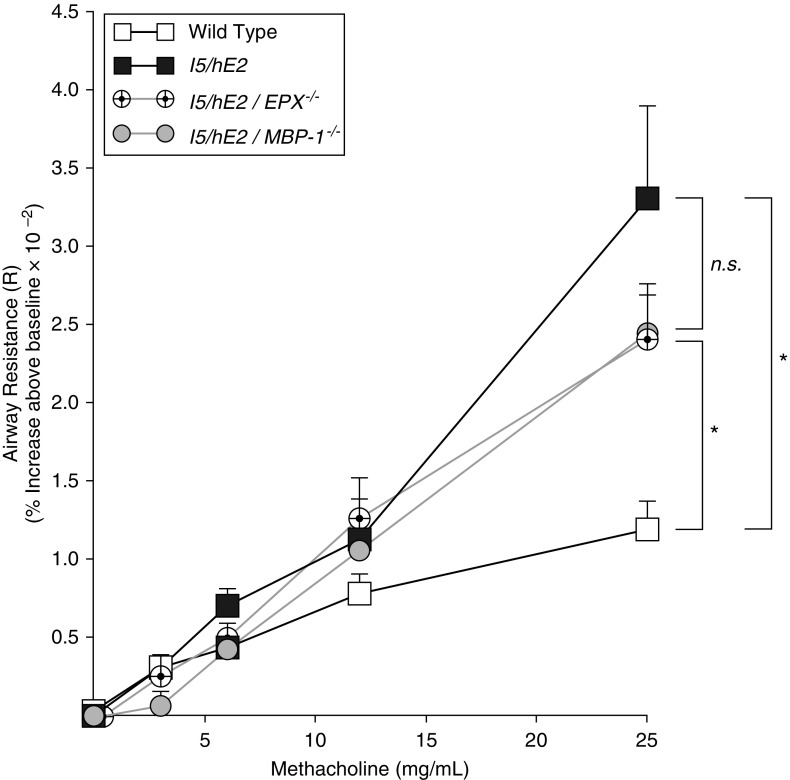
Invasive measurements of lung function were performed to define any potential contribution that eosinophil degranulation has on the methacholine-induced AHR occurring in I5/hE2 mice. Surprisingly, despite the extensive degranulation occurring in I5/hE2 mice, lung function assessments demonstrated that the loss of either EPX (i.e., I5/hE2/EPX−/−) or MBP-1 (i.e., I5/hE2/MBP-1−/−) did not significantly attenuate AHR relative to the parental I5/hE2 model (Figure 7). Thus, unlike the effects the expression of IL-13 had on the induced AHR of this chronic model, the individual release of either EPX or MBP-1 had only a limited effect on AHR, suggesting that neither protein alone was a “driver” of this phenomenon.
Figure 7.
The induced airway hyperresponsiveness linked with the pulmonary eosinophilia of I5/hE2 mice is independent of the eosinophil degranulation leading to the release of either eosinophil peroxidase (EPX) or major basic protein-1 (MBP-1). Aerosolized methacholine-induced airway resistance was assessed using an invasive ventilator-based technique (flexi-Vent; SCIREQ). I5/hE2 mice display an increase in airway resistance as a function of exposure to increasing concentrations of aerosolized methacholine (responses of wild-type C57BL/6J mice are shown as a negative control). Surprisingly, the absence of either EPX (i.e., I5/hE2/EPX−/−) or MBP-1 (i.e., I5/hE2/MBP-1−/−) expression had no significant effects on the methacholine responses displayed by the parental I5/hE2 model. Baseline airway resistance (cm H2O⋅s/ml) of each experimental cohort (mean ± SEM): C57BL/6J wild-type, 0.89 ± 0.25; I5/hE2, 1.03 ± 0.07; I5/hE2/EPX−/−, 0.93 ± 0.07; and I5/hE2/MBP-1−/−, 0.87 ± 0.09. Cohort sizes: C57BL/6J, n = 9; I5/hE2, n = 10; I5/hE2/EPX−/−, n = 13; I5/hE2/MBP-1−/−, n = 10. Error bars represent SEM. *P < 0.05; n.s. = not significant.
Discussion
Clearly the most commonly suggested activity linking pulmonary pathologies with infiltrating lung eosinophils in chronic allergic asthma has been the activation/degranulation of these granulocytes, leading to the release of the cationic secondary granule proteins (reviewed in Reference 26), the most abundant of which are MBP-1 and EPX. This link found support in detailed descriptive studies of patients that correlated tissue damage, cell death, and pathophysiologic changes in lung function with the appearance of degranulating airway eosinophils (see for example Reference 50). Moreover, ex plant/in vitro studies demonstrating that these proteins were capable of promoting cell death and tissue destruction provided potential mechanisms leading to lung pathologies (36, 37). Thus, together with potential cell agonist activities on lung structural cells (24) and other inflammatory leukocytes (51), the release of these secondary granule proteins have become indirect targets of therapeutic strategies targeting eosinophils. Indeed, the recent favorable reviews, and in some cases approval by the Food and Drug Administration, of antibody-mediated (i.e., biologic) approaches directed against the eosinophil-agonist cytokine IL-5 (i.e., mepolizumab [11, 12], reslizumab [9], and the IL-5 receptor benralizumab [10]) have been, in part, based on targeting these eosinophil-mediated activities (reviewed in Reference 52).
In spite of the commonly held perspective proposing that eosinophil secondary granule proteins are significant contributors to histopathologies and lung dysfunction, the definition of the cellular and molecular events surrounding degranulation has been severely limited by the inability of established mouse models of aeroallergen challenge to display the extensive eosinophil degranulation found in human subjects with asthma (38, 40, 53). This lack of concordance has typically been explained as either a limitation of mouse models (reviewed in Reference 54), shortcomings linked with available allergens administered to mice (55), and/or the allergen challenge protocols themselves (56, 57). The data presented here provocatively showed that the loss of either MBP-1 or EPX had only nominal (if any) effects on many of the age-dependent pulmonary pathologies occurring in I5/hE2 mice, including the breakdown of alveolar septa and pulmonary fibrosis. Given the extensive literature (see for example References 29 and 30), it was equally surprising that the loss of each of these proteins did not have a significant effect on the AHR associated with this model.
These data suggest that eosinophil degranulation and the release of either MBP-1 or EPX is not a driving causative event leading to many of the pulmonary pathologies in chronic settings. However, it is also noteworthy that these data do not rule out additive or synergistic events that the concurrent loss of both proteins may have on these pathologies following their release from activated eosinophils. Unfortunately, mice with targeted mutations of both granule protein genes display an inherent defect in eosinophilopoiesis that abrogates the production of mature peripheral eosinophils (58). As a result, although it is possible to generate I5/hE2/MBP-1−/−/EPX−/− animals, instead of providing I5/hE2 mice deficient of both MBP-1 and EPX these compound transgenic/gene knockout mice are simply another eosinophil-deficient version of the I5/hE2 model.
Significantly, our studies showed a differential effect in the development of GM/MA with the loss of EPX versus MBP-1 from I5/hE2 mice. The data demonstrated that although levels of IL-13 in the airways of these compound transgenic/knockout mice are equivalent, the loss of EPX, but not MBP-1, attenuated GM/MA by 50% relative to the parental I5/hE2 model. This observation is significant because it offers a translational insight as to the role of eosinophils in the development of GM/MA that could not have been obtained using established allergen challenge models. That is, because established allergen challenge mouse models fail to display eosinophil degranulation, allergen-induced GM/MA in these models is exclusively an IL-13-mediated event that was independent of EPX activities (59–61). This also provides a likely explanation as to why the loss of EPX in knockout mice had no effect on the development of allergen-induced GM/MA using these same established allergen models (45). However, the extensive degranulation in I5/hE2 mice leading to the release of EPX overcomes this limitation and in doing so demonstrated that the release of EPX is actually a modifier of IL-13-mediated events that enhances GM/MA. Our recent mechanistic studies demonstrating that the peroxidase activities of EPX mediate carbamylation of lung proteins provide a mechanistic explanation of this effect (62). Specifically, these carbamylated proteins were shown to have direct agonist activities on airway epithelia leading to goblet cell metaplasia and increased mucin gene expression. This observation highlights a potential link between EPX activities and IL-13-mediated events and suggests that the release of EPX by pulmonary eosinophils is likely to be part of a positive feedback loop that augments the GM/MA levels in the lung promoted by local IL-13 expression. In addition, this observed effect linking eosinophil degranulation and GM/MA was granule protein–specific, confirming previously formulated hypotheses suggesting the potential of differential activities mediated by each of the released secondary granule proteins (see for example Reference 63).
In summary, the transgenic expression of IL-5 and eotaxin-2 in I5/hE2 mice affords ectopic pressures that both maintain a robust constitutive airway eosinophilia and surprisingly high levels of airway IL-13. The data also show that similar to established models of allergen provocation (59–61), this pulmonary IL-13 was critical to the GM/MA and the AHR that develops in the I5/hE2 model. Moreover, the IL-13 expression in I5/hE2 mice was also absolutely dependent on the induced eosinophilia in I5/hE2 mice irrespective of resident CD4+ T-cell levels. Indeed, the targeted loss of eosinophils, but not CD4+ T cells, significantly reduced GM/MA and the induced AHR occurring in these mice. Recognizing that these are mouse model studies whose translation to human subjects may be problematic, the observations reported here provide an alternative hypothesis to the commonly accepted perspective that eosinophils are exclusively destructive end-stage effector cells whose toxic pathology-driving activities directly account for induced pathologies and/or symptoms. That is, if eosinophils essentially act as destructive end-stage effector cells, then anti-IL-5 therapies would have been expected to have direct significant impacts on a variety of functional endpoints. Moreover, these therapies would also have been likely to be efficacious in a broad collection of patients and directly correlated with eosinophil numbers in airways.
In contrast, our conclusion that eosinophils contribute to inflammatory events in the lung primarily through immunoregulative events provides an explanation for the current outcomes of IL-5 therapies in subsets of patients. Specifically, eosinophils may become prominent (even perhaps dominant) immune modulatory agents in the lungs in only a subset of patients, accounting for the narrow efficacy window. If they are primarily contributing to lung pathologies through immunoregulative activities, then targeting airway eosinophils in these patients would reduce the inflammatory milieu, lowering the frequency that secondary extrinsic events would lead to a disequilibrium resulting in hospitalization (i.e., exacerbation events). Thus, if our mouse model studies translate to human patients the implications are significant because they would suggest that the targeting of eosinophil immune regulatory pathways is likely to be a more successful approach in the management of many patients with allergic asthma.
Acknowledgments
Acknowledgment
The authors thank members of Lee Laboratories who reviewed various drafts of this manuscript and in many ways helped in the data collection and organization/infrastructure needed to complete the studies presented. They also acknowledge the invaluable assistance of the Mayo Clinic Arizona medical graphic artist, Marv Ruona, and the excellent administrative support provided to Lee Laboratories by Linda Mardel, Stefanie Brendle, and Shirley (“Charlie”) Kern.
Footnotes
Author Contributions: N.A.L. had full access to all of the data reported in this study and had final responsibility for the decision to submit this report for publication. E.A.J., S.I.O., A.D.D., H.H.H.S., C.G.I., J.J.L., and N.A.L. designed the research study. E.A.J., S.I.O., A.D.D., W.E.L., W.L., C.A.P., D.C., and K.R.Z. performed research. E.A.J., S.I.O., A.D.D., W.L., H.H.H.S., C.G.I., J.J.L., and N.A.L. analyzed data. E.A.J., J.J.L., and N.A.L. wrote the initial draft of the manuscript. S.I.O., A.D.D., H.H.H.S., C.G.I., J.J.L., and N.A.L. provided critical assessments during the revision process leading to the final submitted manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201606-1129OC on December 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gonem S, Desai D, Siddiqui S, Brightling CC. Evidence for phenotype-driven treatment in asthmatic patients. Curr Opin Allergy Clin Immunol. 2011;11:381–385. doi: 10.1097/ACI.0b013e328348a8f9. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–857. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Byrne PM. What is asthma? An update on the mechanisms. J Investig Allergol Clin Immunol. 1995;5:6–11. [PubMed] [Google Scholar]
- 5.Orihara K, Dil N, Anaparti V, Moqbel R. What’s new in asthma pathophysiology and immunopathology? Expert Rev Respir Med. 2010;4:605–629. doi: 10.1586/ers.10.57. [DOI] [PubMed] [Google Scholar]
- 6.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA, Jr, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129(Suppl. 3):S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel SE. Eosinophils in asthma: closing the loop or opening the door? N Engl J Med. 2009;360:1026–1028. doi: 10.1056/NEJMe0900334. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 10.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, et al. CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;338:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 11.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 13.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlström J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131:1267–1274, quiz 1275. doi: 10.1016/j.jaci.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2015;136:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen EA, Lee NA, Lee JJ. Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin Exp Allergy. 2014;44:1119–1136. doi: 10.1111/cea.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immunol. 2001;107:945–957. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- 24.Pégorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–4869. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 25.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin K, Janson C, Bystrom J. Role of eosinophil granulocytes in allergic airway inflammation endotypes. Scand J Immunol. 2016;84:75–85. doi: 10.1111/sji.12448. [DOI] [PubMed] [Google Scholar]
- 27.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015;3:167–174. doi: 10.1016/j.jaip.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- 29.Hamid Q. Pathogenesis of small airways in asthma. Respiration. 2012;84:4–11. doi: 10.1159/000339550. [DOI] [PubMed] [Google Scholar]
- 30.Persson C, Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014;189:628–633. doi: 10.1164/rccm.201311-2069OE. [DOI] [PubMed] [Google Scholar]
- 31.Venge P. Eosinophil activity in bronchial asthma. Allergy Proc. 1994;15:139–141. doi: 10.2500/108854194778702937. [DOI] [PubMed] [Google Scholar]
- 32.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 33.Lefort J, Nahori MA, Ruffie C, Vargaftig BB, Pretolani M. In vivo neutralization of eosinophil-derived major basic protein inhibits antigen-induced bronchial hyperreactivity in sensitized guinea pigs. J Clin Invest. 1996;97:1117–1121. doi: 10.1172/JCI118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation [review] Am J Respir Crit Care Med. 1994;150:S63–S71. doi: 10.1164/ajrccm/150.5_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 35.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980;42:35–43. [PubMed] [Google Scholar]
- 37.Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925–2927. [PubMed] [Google Scholar]
- 38.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lötvall J, Pauwels RA, Plopper CG, et al. Murine models of asthma. Eur Respir J. 2003;22:374–382. doi: 10.1183/09031936.03.00026403. [DOI] [PubMed] [Google Scholar]
- 39.Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Waltham, MA: Elsevier; 2012. [Google Scholar]
- 40.Stelts D, Egan RW, Falcone A, Garlisi CG, Gleich GJ, Kreutner W, Kung TT, Nahrebne DK, Chapman RW, Minnicozzi M. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am J Respir Cell Mol Biol. 1998;18:463–470. doi: 10.1165/ajrcmb.18.4.2957. [DOI] [PubMed] [Google Scholar]
- 41.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen EA, Doyle AD, Colbert DC, Zellner KR, LeSuer WE, Protheroe CA, Lee NA, Lee JJ. Differential activation of airway eosinophils induces IL-13 mediated allergic Th2 pulmonary responses in mice. Allergy. 2015;70:1148–1159. doi: 10.1111/all.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochkur SI, Protheroe CA, Li W, Colbert DC, Zellner KR, Shen HH, Luster AD, Irvin CG, Lee JJ, Lee NA. Cys-leukotrienes promote fibrosis in a mouse model of eosinophil-mediated respiratory inflammation. Am J Respir Cell Mol Biol. 2013;49:1074–1084. doi: 10.1165/rcmb.2013-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 45.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 46.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 47.Macias MP, Welch KC, Denzler KL, Larson KA, Lee NA, Lee JJ. Identification of a new murine eosinophil major basic protein (mMBP) gene: cloning and characterization of mMBP-2. J Leukoc Biol. 2000;67:567–576. doi: 10.1002/jlb.67.4.567. [DOI] [PubMed] [Google Scholar]
- 48.Protheroe CA, Woodruff SA, DePetris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, et al. A novel histological scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, Lee JJ, Lee NA. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods. 2012;375:138–147. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 51.Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol. 1984;133:2180–2185. [PubMed] [Google Scholar]
- 52.Fajt ML. Blood eosinophils: the holy grail for asthma phenotyping? Ann Allergy Asthma Immunol. 2016;116:90–91. doi: 10.1016/j.anai.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Clark K, Simson L, Newcombe N, Koskinen AM, Mattes J, Lee NA, Lee JJ, Dent LA, Matthaei KI, Foster PS. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J Leukoc Biol. 2004;75:1001–1009. doi: 10.1189/jlb.0803391. [DOI] [PubMed] [Google Scholar]
- 54.Persson CG, Erjefält JS, Korsgren M, Sundler F. The mouse trap. Trends Pharmacol Sci. 1997;18:465–467. doi: 10.1016/s0165-6147(97)01142-5. [DOI] [PubMed] [Google Scholar]
- 55.DiGiovanni FA, Ellis R, Wattie J, Hirota JA, Southam DS, Inman MD. Concurrent dual allergen exposure and its effects on airway hyperresponsiveness, inflammation and remodeling in mice. Dis Model Mech. 2009;2:275–282. doi: 10.1242/dmm.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999;21:480–489. doi: 10.1165/ajrcmb.21.4.3659. [DOI] [PubMed] [Google Scholar]
- 58.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DTC, Protheroe C, Colbert D, Kloeber J, Neely J, et al. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 60.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 61.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, DiDonato JA, Buffa J, Comhair SA, Aronica MA, Dweik RA, Lee NA, Lee JJ, Thomassen MJ, Kavuru M, et al. Eosinophil peroxidase catalyzed protein carbamylation participates in asthma. J Biol Chem. 2016;291:22118–22135. doi: 10.1074/jbc.M116.750034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torpier G, Colombel JF, Mathieu-Chandelier C, Capron M, Dessaint JP, Cortot A, Paris JC, Capron A. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin Exp Immunol. 1988;74:404–408. [PMC free article] [PubMed] [Google Scholar]