Abstract
Rationale: Saline is the intravenous fluid most commonly administered to critically ill adults, but it may be associated with acute kidney injury and death. Whether use of balanced crystalloids rather than saline affects patient outcomes remains unknown.
Objectives: To pilot a cluster-randomized, multiple-crossover trial using software tools within the electronic health record to compare saline to balanced crystalloids.
Methods: This was a cluster-randomized, multiple-crossover trial among 974 adults admitted to a tertiary medical intensive care unit from February 3, 2015 to May 31, 2015. The intravenous crystalloid used in the unit alternated monthly between saline (0.9% sodium chloride) and balanced crystalloids (lactated Ringer’s solution or Plasma-Lyte A). Enrollment, fluid delivery, and data collection were performed using software tools within the electronic health record. The primary outcome was the difference between study groups in the proportion of isotonic crystalloid administered that was saline. The secondary outcome was major adverse kidney events within 30 days (MAKE30), a composite of death, dialysis, or persistent renal dysfunction.
Measurements and Main Results: Patients assigned to saline (n = 454) and balanced crystalloids (n = 520) were similar at baseline and received similar volumes of crystalloid by 30 days (median [interquartile range]: 1,424 ml [500–3,377] vs. 1,617 ml [500–3,628]; P = 0.40). Saline made up a larger proportion of the isotonic crystalloid given in the saline group than in the balanced crystalloid group (91% vs. 21%; P < 0.001). MAKE30 did not differ between groups (24.7% vs. 24.6%; P = 0.98).
Conclusions: An electronic health record–embedded, cluster-randomized, multiple-crossover trial comparing saline with balanced crystalloids can produce well-balanced study groups and separation in crystalloid receipt.
Clinical trial registered with www.clinicaltrials.gov (NCT 02345486).
Keywords: critical illness, crystalloid, intravenous fluid, acute kidney injury, saline
At a Glance Commentary
Scientific Knowledge on the Subject
Saline is the most commonly administered intravenous crystalloid globally. Previous studies of critically ill adults have suggested an association between saline receipt and risk of acute kidney injury and death, but results have been conflicting. Whether use of balanced crystalloids rather than saline improves patient outcomes requires evaluation in a large, randomized clinical trial.
What This Study Adds to the Field
This 974-patient pilot study found that a cluster-randomized, multiple-crossover trial using software tools within the electronic health record to compare saline with balanced crystalloids among critically ill adults produced well-balanced study groups and separation in crystalloid receipt, but no overall difference in patient outcomes.
Intravenous crystalloid administration is ubiquitous in critical care, yet whether choice of crystalloid affects patient outcomes remains unknown (1). The most commonly used crystalloid solution globally is 0.9% sodium chloride (saline) (2), with more than 200 million liters administered each year in the United States alone (1). However, the high chloride content of saline has been hypothesized to contribute to the development of acute kidney injury (AKI) in at-risk patients (3, 4). Alternatives to saline include crystalloids with electrolyte compositions that more closely approximate that of plasma (balanced crystalloids), such as lactated Ringer’s solution or Plasma-Lyte A. Emerging data suggest that the use of balanced crystalloids in critically ill adults may decrease rates of AKI (3, 4), renal replacement therapy (RRT) (3, 5), and death (6, 7).
To definitively evaluate the effect of balanced crystalloids versus saline on patient-centered outcomes, a large, randomized trial that carefully accounts for differences in baseline risk of AKI (8), volume of crystalloid received (7), and underlying pathophysiology (9–12) among patients in the intensive care unit (ICU) is required. Such a trial faces numerous logistical challenges, including the need to enroll thousands of patients, deliver the assigned crystalloid in a time-sensitive manner, and collect detailed data on the exact amounts of crystalloid administered, the physiological effects (e.g., serum chloride), and outcomes. We hypothesized that these challenges could be met by assigning crystalloid selection (saline vs. balanced crystalloids) at the ICU level and using software tools built into the electronic health record (EHR) to automatically enroll patients, steer ordering providers to the assigned crystalloid, and collect data. To test this hypothesis, we performed a 4-month EHR-embedded (13, 14), cluster-randomized, multiple-crossover trial that compared balanced crystalloids with saline among critically ill adults. Some of the results of this study have been previously reported in the form of an abstract (15).
Methods
Study Design and Oversight
The SALT (isotonic Solution Administration Logistical Testing) study was a prospective, open-label, cluster-randomized, multiple-crossover trial that compared the use of saline with balanced crystalloids among adults admitted to a tertiary medical ICU. The study protocol (see online supplement) was approved by the institutional review board at Vanderbilt University with waiver of informed consent (IRB#141349), and the trial was registered online before initiation (NCT 02345486).
Patient Population
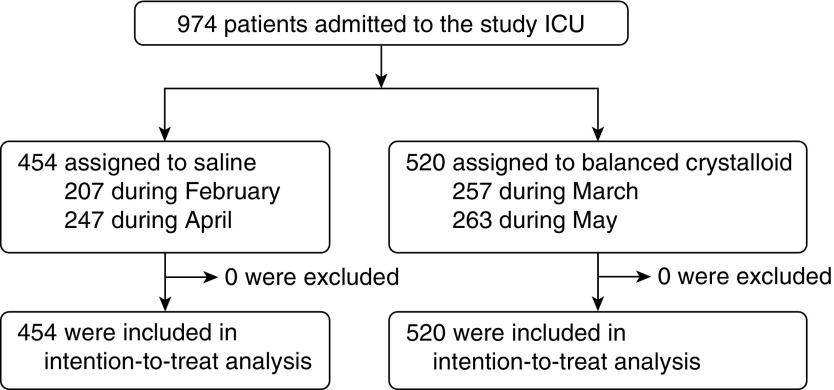
From February 3, 2015 to May 31, 2015, all adults (age ≥ 18 yr) admitted to the medical ICU at Vanderbilt University were automatically enrolled at the time of ICU admission (Figure 1). Enrolled patients who were discharged from the hospital were eligible again if they were readmitted to the medical ICU during the study period.
Figure 1.
Flow of participants through the trial. All 974 patients admitted to the medical intensive care unit (ICU) during the 4-month study period were enrolled, assigned to a balanced crystalloid group, followed through hospital discharge, and included in the intention-to-treat analysis. A total of 51 patients assigned to saline remained in the ICU through a crossover to balanced crystalloids, and 53 patients assigned to balanced crystalloids remained in the ICU through a crossover to saline. Median days spent in the ICU after a crossover in crystalloid assignment were 0 (range, 0–0) overall and 3.0 (range, 2.3–6.4) among the 104 patients who remained in the ICU through a crossover.
Randomization and Allocation
The crystalloid used during the first month of the study (saline or balanced crystalloids) was selected by computer-generated simple randomization. Thereafter, the crystalloid assigned to the ICU alternated monthly for the duration of the study, following a cluster-randomized, cluster-crossover design (16).
Study Treatments
Study protocol governed only the choice of intravenous isotonic crystalloid: 0.9% sodium chloride (saline group) versus the treating clinician’s preference of lactated Ringer’s solution or Plasma-Lyte A (balanced crystalloid group). Composition of the study crystalloids is given in Table E1 in the online supplement. Decisions regarding frequency, rate, total volume, and additive content of the crystalloids were made by the treating clinician. No restrictions were placed on other fluids or therapies.
Delivery of the assigned crystalloid to patients was achieved via interventions in pharmacy supply and clinician order entry. Each month, the dispensing cabinets within the ICU were stocked with 1,000-ml bags of the assigned crystalloid. In addition, any order for intravenous crystalloid for a patient located in the ICU triggered an advisor application within the electronic order entry system. The advisor application informed providers about the study, asked about relative contraindications to the assigned crystalloid, and (if relative contraindications were not present) guided providers to order the assigned crystalloid. Accepted relative contraindications for patients assigned to balanced crystalloid included (1) hyperkalemia and (2) brain injury with a coexisting contraindication to Plasma-Lyte A. The severity of hyperkalemia and brain injury at which saline was used in favor of balanced crystalloids was determined by the treating clinician. The nonassigned crystalloid could also be provided by the pharmacy if a formal statement was submitted that the attending physician felt the nonassigned crystalloid was required for the safe treatment of a specific patient.
The type of fluid administered before ICU admission, during procedures performed outside the ICU, and after discharge from the ICU was not controlled by the study. Each day patients received the crystalloid to which the ICU was currently assigned. Because it was necessary that an intravenous crystalloid be clinically available at all times, there was no washout period, and patients who remained in the ICU through a crossover (i.e., from one calendar month to another) were potentially exposed to both types of crystalloid. Patients, clinicians, and investigators were not blinded to crystalloid assignment because available laboratory values overtly reflected the crystalloid being used, and previous studies showed high levels of provider awareness of crystalloid assignment despite attempts at blinding (17).
Data Collection
This pragmatic trial used data collected as part of routine clinical care and electronically extracted from the EHR (18). These data included: measures of prestudy renal function; demographic characteristics, admitting location and diagnosis, and severity of illness at enrollment; receipt of intravenous crystalloids, other fluids, and blood products; serum electrolyte and creatinine values; receipt of RRT, mechanical ventilation, and vasopressors; and vital status and serum creatinine at hospital discharge. A detailed description of how variables were electronically collected and classified has been published previously (18) and is available in the online supplement. For all patients who received new RRT, study personnel performed manual chart review to confirm the absence of previous RRT and to identify indications for RRT present at the time of RRT initiation.
Study Outcomes
As a pilot study (19), the primary outcome was the proportion of intravenous isotonic crystalloid administered in the ICU that was saline. This was a continuous variable calculated for each patient as the volume of saline received divided by volume of saline received plus volume of balanced crystalloids received with a range from 0.0 (no saline received) to 1.0 (only saline received). The secondary outcome was the proportion of patients meeting one or more criteria for major adverse kidney events within the 30 days after enrollment (MAKE30): in-hospital mortality, receipt of new RRT, or persistent renal dysfunction defined as a final inpatient serum creatinine value greater than or equal to 200% of baseline (see definitions of study variables in the online supplement) (20, 21). Patients who had received RRT before enrollment were ineligible to meet criteria for new RRT or persistent renal dysfunction, but could meet the MAKE30 endpoint if they experienced in-hospital mortality. Additional clinical outcomes included ICU and in-hospital mortality, ICU-free days, ventilator-free days, vasopressor-free days, and RRT-free days, all in the 28 days after enrollment. Additional renal outcomes included the incidence of stage II or greater AKI by Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria (22), highest serum creatinine value, change from baseline creatinine to highest creatinine, and duration of new RRT as an inpatient. Biochemical efficacy and safety outcomes included the first, highest, and lowest serum value for chloride, bicarbonate, and potassium each day from enrollment until hospital discharge or 30 days.
Statistical Analysis
The aims of this pilot study were to (1) test the logistics of the EHR-embedded, cluster-randomized, multiple-crossover design, and (2) examine the separation between study groups in saline administration (19). To observe the logistics of at least one crossover from each crystalloid assignment to the other while maintaining an equal number of saline and balanced crystalloid treatment blocks required a minimum study duration of 4 months. With approximately 250 ICU admissions per month, we anticipated a total enrollment of approximately 1,000 patients. Our goal separation between groups was a 60% absolute difference in the percentage of isotonic crystalloid administered as saline between the saline group and the balanced crystalloid group. Enrollment of 1,000 patients would enable detection of a 60% absolute difference between groups in the percentage of isotonic crystalloid administered as saline with a 95% confidence interval of 58.5 to 61.5% (see the online supplement for additional details).
All analyses were conducted at the level of the individual patient in an intention-to-treat fashion, unless otherwise specified. Continuous variables were reported as mean ± SD or median and interquartile range (IQR); categorical variables were reported as frequencies and proportions. Between-group comparisons were made with the Mann-Whitney rank-sum test for continuous variables, Fisher’s exact test, or χ2 test for categorical variables, generalized estimating equations for repeatedly measured variables, and multivariable regression for adjusted analyses and tests of interaction. To facilitate interpretation of the results, we present univariate comparisons of baseline characteristics, fluid receipt and laboratory values, and clinical outcomes between the saline and balanced crystalloid groups among all patients enrolled (intention-to-treat) and all patients who received at least 250 ml of intravenous crystalloid in the first 72 hours (modified intention-to-treat).
The primary analysis compared the proportion of intravenously administered isotonic crystalloid that was saline between patients assigned to saline and balanced crystalloids. The secondary analysis compared the proportion of patients who experienced MAKE30 in the saline and balanced crystalloid groups, accounting for patients’ overall volume of isotonic crystalloid received. For this analysis, we constructed a logistic regression model with MAKE30 as the outcome and independent variables of the study group, total isotonic crystalloid received between enrollment and 30 days, and the interaction between the two (as a cross-product term). Other analyses included (1) univariate comparison of additional clinical and renal outcomes between study groups, (2) effect modification by severity of illness and prespecified subgroups (source of admission, primary diagnosis, mechanical ventilation, vasopressors, and baseline renal function), and (3) sensitivity analyses excluding patients admitted in the week before a crossover (washout) and excluding patients who were transferred between ICUs or remained in the ICU through a crossover (per protocol).
Each patient’s baseline creatinine was considered to be the lowest serum creatinine value between 12 months and 24 hours before hospital admission, when available. For patients without an available serum creatinine value during this period, the lowest value between 24 hours before hospital admission and enrollment was used. Patients without a measured serum creatinine value between 12 months before hospital admission and enrollment had baseline creatinine values estimated using a previously described three-variable formula (23). Multiple alternative approaches to missing baseline creatinine data were explored in sensitivity analyses, including use of complete cases, multivariable single imputation, and use of the first creatinine after enrollment or the highest or lowest creatinine during the study (see the online supplement).
All secondary analyses were considered hypothesis-generating, and no statistical corrections were made for multiple comparisons. All analyses were performed using R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Enrollment and Baseline Characteristics
All 974 patients admitted to the medical ICU during the study period were enrolled (Figure 1). Patients assigned to receive saline (n = 454) were similar at baseline to those assigned to receive balanced crystalloids (n = 520) (Table 1 and see Tables E2 and E3). Patients’ median age was approximately 60 years, approximately one-half were men, and most were admitted from the emergency department. Sepsis and respiratory failure were the most common admitting diagnoses, with one-quarter of patients receiving vasopressors and more than one-third on mechanical ventilation.
Table 1.
Patient Characteristics at Baseline
| Patient Characteristics | Saline (n = 454) | Balanced (n = 520) |
|---|---|---|
| Age, median (IQR), yr | 58 (46–70) | 57 (44–68) |
| Men, n (%) | 246 (54.2) | 268 (51.5) |
| White, n (%) | 358 (78.9) | 376 (72.3) |
| Weight, median (IQR), kg | 77.6 (63.0–95.3) | 77.1 (63.5–95.3) |
| Body mass index, median (IQR), kg/m2 | 26.4 (22.4–32.8) | 26.7 (22.5–32.5) |
| Renal comorbidities, n (%) | ||
| Chronic kidney disease, stage III or greater* | 104 (22.9) | 119 (22.9) |
| Previous renal replacement therapy receipt | 44 (9.7) | 42 (8.1) |
| Source of admission to ICU, n (%) | ||
| Emergency department | 256 (56.4) | 310 (59.6) |
| Transfer from another hospital | 93 (20.5) | 106 (20.4) |
| Hospital ward | 80 (17.6) | 79 (15.2) |
| Another ICU within the hospital | 15 (3.3) | 14 (2.7) |
| Operating room | 6 (1.3) | 4 (0.8) |
| Outpatient | 4 (0.9) | 7 (1.3) |
| Admitting diagnosis, n (%) | ||
| Sepsis or septic shock | 130 (28.6) | 130 (25.0) |
| Respiratory failure | 53 (11.7) | 41 (7.9) |
| Gastrointestinal bleeding | 25 (5.5) | 19 (3.7) |
| Liver failure | 21 (4.6) | 24 (4.6) |
| Ingestion | 22 (4.8) | 32 (6.2) |
| Malignancy | 19 (4.2) | 27 (5.2) |
| Diabetic ketoacidosis | 20 (4.4) | 21 (4.0) |
| Pneumonia | 14 (3.1) | 16 (3.1) |
| Acute kidney injury | 5 (1.1) | 18 (3.5) |
| Other | 115 (25.3) | 150 (28.8) |
| Mechanical ventilation, n (%) | 155 (34.1) | 174 (33.5) |
| Vasopressors, n (%) | 111 (24.4) | 114 (21.9) |
| UHC expected mortality, mean (95% CI), %† | 14.7 (12.7–16.7) | 13.1 (11.4–14.9) |
| Serum creatinine, median (IQR), mg/dl | ||
| Lowest in 12 mo before hospitalization | 0.78 (0.64–1.10) | 0.76 (0.62–1.05) |
| n (%) of patients | 271 (59.7) | 324 (62.3) |
| Lowest between hospitalization and ICU admission | 0.97 (0.76–1.51) | 0.95 (0.75–1.61] |
| n (%) of patients | 122 (26.9) | 137 (26.3) |
| Estimated by three-variable formula‡ | 0.91 (0.88–0.96) | 0.91 (0.88–0.95) |
| n (%) of patients | 61 (13.4) | 59 (11.3) |
| Study baseline | 0.86 (0.69–1.12) | 0.83 (0.67–1.09) |
| Acute kidney injury, stage II or greater§ | 87 (19.2) | 96 (18.5) |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; IQR = interquartile range; UHC = University HealthSystem Consortium.
Data are presented as median (25th–75th percentile) or number (percentage). Comparison of the saline and balanced crystalloid groups using the Mann-Whitney rank sum test for continuous variables and the Fisher’s exact test or χ2 test for categorical variables found no difference between groups in any baseline characteristic except white race (P = 0.02).
Chronic kidney disease stage III or greater is defined as a glomerular filtration rate <60 ml/min per 1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (41) using the patient’s baseline creatinine value.
UHC expected mortality is an estimated probability of death before hospital discharge generated for each patient based on age, sex, comorbidities, admission source, race, and principal diagnosis (details at www.uhc.edu).
Definitions for baseline serum creatinine are: “Lowest in 12 months before hospitalization” = lowest available serum creatinine between 12 months and 24 hours before hospital admission; “Between hospitalization and ICU admission” = lowest available serum creatinine between 24 hours before hospital admission and ICU admission; “Estimated by three-variable formula” = creatinine value calculated using a previously described three-variable formula (creatinine [mg/dl] = 0.74 − 0.2 [if female] + 0.08 [if African American] + 0.003 × age [in years]) (23); and “Study baseline” = lowest in 12 months before hospitalization if available, otherwise between hospitalization and ICU admission, using the estimated creatinine only for patients without an available creatinine between 12 months before hospitalization and the time of ICU admission.
Acute kidney injury, stage II or greater is defined according to Kidney Disease Improving Global Outcomes creatinine criteria (22) as a first creatinine value after enrollment at least 200% of the baseline value or both (1) >4.0 mg/dl and (2) increased at least 0.3 mg/dl from the baseline value.
Fluid Therapy
The volume of saline and balanced crystalloids received by patients in each group is displayed in Figure 2. Saline made up 91.2% of the isotonic crystalloid given in the ICU to patients in the saline arm compared with 21.2% in the balanced arm (mean proportion of total isotonic crystalloid: 0.91 vs. 0.21; absolute difference 0.70; 95% confidence interval, 0.66–0.74; P < 0.001). Patients in the saline and balanced crystalloid groups received a similar total volume of intravenous crystalloid by 7 days (median [IQR], 1,250 ml [390–3,000] vs. 1,320 ml [435–3,139]; P = 0.38) and 30 days (1,424 ml [500–3,377] vs. 1,617 ml [500–3,628]; P = 0.40) (see Table E4 and Figure E1).
Figure 2.
Crystalloid receipt in the intensive care unit. For patients assigned to the saline group (left) and balanced crystalloid group (right), the cumulative volume of intravenous 0.9% sodium chloride (diamonds) and balanced crystalloid (circles) is displayed over time. Cumulative volume (mean and 95% confidence interval) includes fluids given in the intensive care unit as a bolus, as maintenance, or to accompany medications. Day 0 is the day of study enrollment. Fluid received before the time of enrollment on Day 0 includes fluid received between hospital admission and intensive care unit admission but does not include fluid given before hospital admission by the emergency medical system, emergency department, or transferring facility.
Among 854 orders for crystalloid during months assigned to balanced crystalloids, 788 (92.2%) were for balanced crystalloids. Saline was selected in 45 (5.3%) cases for hyperkalemia, 5 (0.6%) cases for brain injury and a contraindication to Plasma-Lyte A, and 16 (1.9%) cases because the attending physician felt saline was required for the safe treatment of the patient (see Table E5). In months assigned to saline, 95.2% of orders were for saline, whereas 4.8% were for balanced crystalloid because the attending physician felt balanced crystalloid was required for safe treatment of the patient (see Table E5). Receipt of nonstudy intravenous fluids and blood products did not differ between the saline and balanced crystalloid groups (see Table E4).
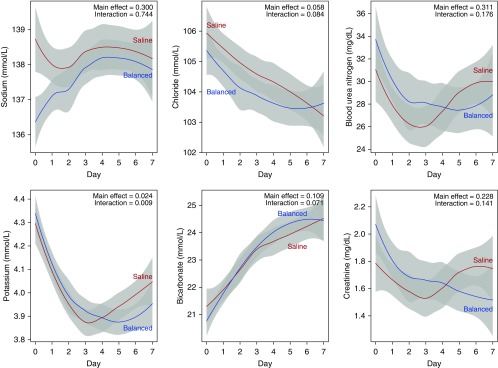
Electrolytes
Serum electrolyte values are given in Figure 3. The highest serum chloride between enrollment and day 30 was greater in the saline group than in the balanced crystalloid group (median [IQR], 109 mmol/L [105–113] vs. 108 mmol/L [104–112]; P = 0.03). Serum potassium values >5.0 mmol/L and <3.0 mmol/L appeared to be slightly more common with balanced crystalloids, but sodium and bicarbonate levels did not differ between groups (see Table E6 and Figures E2 and E3 in the online supplement).
Figure 3.
Daily laboratory values by saline (red) and balanced crystalloid (blue) group. The first value each day is displayed as mean (95% confidence interval) for each study group using locally weighted scatterplot smoothing. Values on Day 0 represent the first available laboratory measurement after enrollment and may not precede the receipt of the assigned crystalloid. A P value for the difference between groups in the laboratory value overall (main effect) and the difference between groups in the change in the laboratory value over time (interaction) were generated using generalized estimating equations. The number of patients with laboratory values available declined from 882 on Day 1 to 311 on Day 7. Note that the highest and lowest serum values each day are available in Figures E3 and E4 of the online supplement.
Renal Function
Daily values for blood urea nitrogen and serum creatinine were similar between groups (Figure 3). There were no differences in the highest serum creatinine value, change from baseline to highest value, or final creatinine value before discharge or 30 days (Table 2). The incidence of stage II or greater AKI by KDIGO creatinine criteria did not differ between groups. Receipt of new RRT was similar between saline and balanced crystalloids (3.1% vs. 4.6%, P = 0.22), with no difference in the indications for RRT (see Table E7).
Table 2.
Clinical Outcomes
| Outcome | n | Saline (n = 454) | Balanced (n = 520) | P Value |
|---|---|---|---|---|
| Secondary outcome | ||||
| Major adverse kidney event within 30 d, n (%)* | 974 | 112 (24.7) | 128 (24.6) | 0.98 |
| Additional clinical outcomes | ||||
| In-hospital mortality, n (%) | ||||
| Before ICU discharge | 974 | 44 (9.7) | 45 (8.7) | 0.57 |
| Before 30 d | 974 | 68 (15.0) | 72 (13.8) | 0.62 |
| Before 60 d | 974 | 83 (18.3) | 87 (16.7) | 0.53 |
| ICU-free days, median (IQR) | 969 | 25.1 (22.1 to 26.2) | 25.2 (21.8 to 26.4) | 0.58 |
| Mean ± SD | 21.0 ± 9.3 | 21.1 ± 9.1 | ||
| Ventilator-free days, median (IQR) | 974 | 28.0 (25.0 to 28.0) | 28.0 (26.0 to 28.0) | 0.85 |
| Mean ± SD | 22.9 ± 9.9 | 23.2 ± 9.6 | ||
| Vasopressor-free days, median (IQR) | 974 | 28.0 (27.0 to 28.0) | 28.0 (27.0 – 28.0) | 0.78 |
| Mean ± SD | 23.5 ± 9.9 | 23.9 ± 9.6 | ||
| Renal replacement therapy-free days, median (IQR) | 974 | 28.0 (28.0 to 28.0) | 28.0 (28.0 to 28.0) | 0.42 |
| Mean ± SD | 23.7 ± 10.0 | 24.0 ± 9.7 | ||
| Additional renal outcomes | ||||
| Serum creatinine, mg/dl | ||||
| Highest before discharge or day 30, median (IQR), mg/dl | 950 | 1.19 (0.81 to 2.30) | 1.19 (0.80 to 2.62) | 0.51 |
| Change from baseline to highest value, median (IQR), mg/dl | 950 | 0.07 (−0.10 to 0.50) | 0.07 (−0.10 to 0.50) | 0.65 |
| Final value before discharge or 30 d, median (IQR), mg/dl | 950 | 0.89 (0.69 to 1.54) | 0.87 (0.70 to1.45) | 0.90 |
| Among survivors, median (IQR), mg/dl | 808 | 0.85 (0.68 to 1.40) | 0.82 (0.69 to 1.30) | 0.48 |
| Final creatinine >200% baseline, n (%) | 974 | 59 (13.0) | 76 (14.6) | 0.47 |
| Among survivors to hospital discharge | 834 | 42 (10.9) | 46 (10.3) | 0.71 |
| Among survivors to hospital discharge without new RRT | 814 | 39 (10.2) | 41 (9.5) | 0.39 |
| Acute kidney injury, stage II or greater, n (%)† | 974 | 129 (28.4) | 135 (26.0) | 0.39 |
| Developing after enrollment‡ | 974 | 87 (19.2) | 97 (18.7) | 0.84 |
| Receipt of new renal replacement therapy, n (%) | 974 | 14 (3.1) | 24 (4.6) | 0.22 |
| Duration of in-hospital receipt, median (IQR), d | 38 | 5.5 (3.0 to 8.2) | 3.0 (0.5 –to4.5) | 0.04 |
| Continued receipt after hospital discharge, n (%) | 38 | 2 (0.4) | 5 (1.0) | 0.68 |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; RRT = renal replacement therapy.
Data are presented as median (25th–75th percentile) or number (percentage). ICU-free, ventilator-free, vasopressor-free, and renal replacement therapy free days refer to days alive and free from the specified therapy in the first 28 days after enrollment.
Major adverse kidney events within 30 days is the presence of any of the following before discharge from the hospital or 30 days after enrollment: death, receipt of new renal replacement therapy, or a final serum creatinine value ≥200% of baseline.
Acute kidney injury, stage II or greater is defined according to Kidney Disease Improving Global Outcomes creatinine criteria (22) as any creatinine value between enrollment and discharge or 30 days at least 200% of the baseline value or both (1) >4.0 mg/dl and (2) increased at least 0.3 mg/dl from the baseline value. This includes both acute kidney injury present at the time of enrollment and developing after enrollment.
Stage II or greater acute kidney injury developing after enrollment is defined as any creatinine value between enrollment and discharge or 30 days that is (1) increased at least 0.3 mg/dl from a preceding postenrollment value and (2) at least 200% of the baseline value, at least 200% of a preceding postenrollment value, or at least 4.0 mg/dl.
MAKE30 Outcome
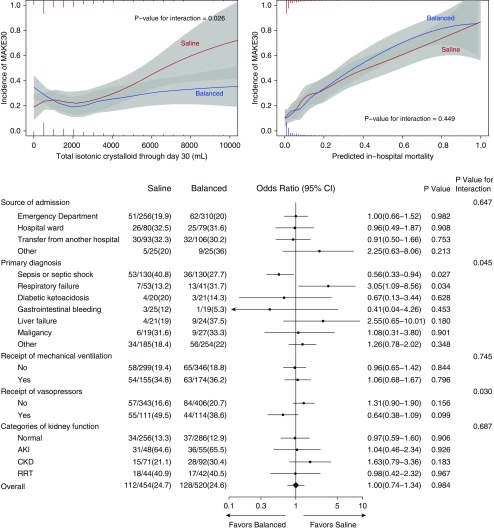
In the saline group, 112 of 454 patients (24.7%) experienced the MAKE30 outcome compared with 128 of 520 patients (24.6%) in the balanced crystalloid group (P = 0.98) (Table 2 and see Table E8). Results were similar in (1) prespecified subgroups (Figure 4); (2) modified intention-to-treat analysis of patients who received at least 250 ml of isotonic crystalloid in the first 72 hours (see Table E9); (3) sensitivity analyses that addressed missing baseline serum creatinine values (n = 120) using imputations, extreme scenarios, or complete cases; (4) analyses that excluded patients admitted in the week before a crossover (washout) or who remained in the ICU through a crossover (per protocol); and (5) multivariable regression analyses accounting for prespecified covariates (see Table E10).
Figure 4.
Heterogeneity of treatment effect. The incidence of major adverse kidney events within 30 days (MAKE30) is compared between patients assigned to the saline (red) and balanced crystalloid (blue) groups across the spectrum of exposure to the study crystalloids (upper left), baseline risk of death (upper right), and prespecified subgroups (bottom). Colored vertical bars display a histogram of the proportion of patients in each group who received a given volume of crystalloid (upper left) or possessed a given predicted in-hospital mortality (upper right). For each prespecified subgroup, the lower panel displays the unadjusted number and percentage of patients in each study arm who experienced MAKE30, the unadjusted odds ratio for experiencing MAKE30 with 95% confidence interval (CI), and the P values within the subgroup and for the test of interaction. Normal kidney function at enrollment is defined as the absence of acute kidney injury (AKI), chronic kidney disease (CKD), or renal replacement therapy (RRT) before enrollment. AKI refers to patients without CKD whose first creatinine after enrollment was at least 200% of the baseline value or both (1) >4.0 mg/dl and (2) increased at least 0.3 mg/dl from the baseline value (22). CKD refers to patents with a glomerular filtration rate <60 ml/min per 1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation using the patient’s baseline creatinine value (41). RRT refers to patients who received any form of RRT before enrollment.
Outcomes by Volume of Crystalloid Received
Although the proportion of patients who experienced MAKE30 was similar between groups overall, among patients who received larger volumes of isotonic crystalloid, those assigned to the saline group experienced a higher incidence of MAKE30 (P value for interaction = 0.026) (Figure 4). Among patients who received larger volumes of isotonic crystalloid, there appeared to be greater separation between arms in serum chloride concentration and higher peak serum creatinine, greater incidence of AKI, and more frequent receipt of new RRT for those patients assigned to saline (see Figures E4–E8).
Discussion
Our findings demonstrate that a cluster-randomized, multiple-crossover trial using software tools within the EHR to compare saline with balanced crystalloids among critically ill adults can produce well-balanced study groups and separation in crystalloid receipt. Although we observed no difference between groups in the overall incidence of AKI or major adverse kidney events, among patients exposed to larger volumes of isotonic crystalloid, those assigned to saline appeared to experience more major adverse kidney events. These findings have important implications for future studies of crystalloid choice in critical illness and for the application of bioinformatics in clinical trials.
Fluid resuscitation with intravenous saline has been shown to cause hyperchloremic metabolic acidosis (24, 25) and might predispose to AKI via chloride-mediated renal vasoconstriction (26, 27). Observational studies among critically ill adults have suggested higher rates of AKI (3, 4), RRT (3, 5), and death (6, 7, 28) with use of saline compared with balanced crystalloids. Inconsistency across studies (29) and the limitations of observational research have prompted calls for prospective clinical trials (1).
Our findings in the SALT trial add to those of previous observational studies (3, 6, 7) and the only previous prospective trial, the SPLIT (0.9% Saline versus Plasma-Lyte 148 [PL-148] for ICU fluid Therapy) trial (17). SPLIT was a cluster-randomized, double-crossover trial that enrolled nearly all patients admitted to four ICUs. Both SALT and SPLIT were designed as pilot studies to assist with the planning of definitive trials (30). Both enrolled broad populations of patients in the ICU, delivered relatively small overall volumes of intravenous crystalloid (median 1.5 L in SALT vs. 2.0 L in SPLIT), and observed no overall difference between saline and balanced crystalloids in rates of AKI, RRT, or death. Despite these similarities, the studies differ in important ways. Compared with the largely postoperative population of SPLIT, patients in SALT were admitted predominantly for sepsis or respiratory failure, and experienced higher rates of AKI (27.1% in SALT vs. 9.9% in SPLIT) and in-hospital mortality (17.4% in SALT vs. 8.0% in SPLIT). The measures of the physiological effects available in SALT (e.g., daily chloride concentration) permitted clearer characterization of the proposed mechanistic pathway between crystalloid administration and outcomes. Perhaps the most intriguing addition of SALT to the previous findings was that the relationship between crystalloid choice and development of major adverse kidney events differed based on the amount of crystalloid received, suggesting a “dose–response” relationship. Among patients who received small volumes of crystalloid, the incidence of MAKE30 was similar between groups. In contrast, among patients exposed to larger volumes of crystalloid, the incidence of MAKE30 was significantly higher in the saline arm. Although mechanistically plausible, this finding relies on a relatively small number of patients using a variable that emerged after randomization and should be considered hypothesis-generating. Future studies might benefit from restricting enrollment to patients predicted to receive large volumes of crystalloid in the ICU (although such prediction might prove challenging) or carefully accounting for heterogeneity in crystalloid exposure during study design and analysis (31).
The present study had important limitations. It was designed as a pilot study and was not powered to detect small, but potentially meaningful, differences in patient outcomes. Enrollment from a single medical ICU limits generalizability. The study population was extremely broad, and specific subgroups of patients whose underlying physiology might alter their response to intravenous crystalloids might have been inadequately represented. Although the impact of crystalloid composition on laboratory values precluded blinding (17), the study endpoints were objective, and the co-interventions received were similar between groups. Based on the hypothesized mechanism of chloride-mediated AKI (26, 27), we evaluated lactated Ringer’s solution or Plasma-Lyte A together as a single balanced crystalloids group, despite differences between these solutions in electrolyte concentration, buffers, and osmolality. Importantly, treating clinicians in the study ICU chose lactated Ringer’s for more than 90% of the balanced crystalloid administered (see Table E4), limiting the conclusions that could be drawn from our study about Plasma-Lyte A.
The most important potential concern was whether the difference in exposure to saline and balanced crystalloids between groups was large enough to influence outcomes. Including all ICU patients in the intention-to-treat analysis protected against selection bias in a design where group assignment preceded enrollment, but resulted in (1) inclusion of some patients who never received any intravenous crystalloid, and (2) relatively small volumes of administered crystalloid overall. However, similar average fluid volumes in the CHEST (Crystalloid versus Hydroxyethyl Starch) Trial were sufficient to demonstrate a difference between groups in the incidence of AKI requiring RRT, albeit in a considerably larger study population (32). Similarly, the differences between groups in serum chloride concentration and hyperchloremia in our trial, although small overall, were comparable to those associated with increased rates of AKI and RRT in previous observational studies (3, 33). Moreover, the difference between groups in serum chloride concentration was greatest among patients who received large volumes of crystalloids—in whom rates of RRT and MAKE30 also appeared to be higher in the saline group than the balanced crystalloid group (see Figures E4–E8). Contamination (exposure to the nonassigned crystalloid) was a related concern. This study did not collect or attempt to control fluid given in the emergency department or operating room before ICU admission, and future trials should address these important sources of fluid exposure. The multiple-crossover design without a washout period guaranteed that some patients would be exposed to both types of crystalloid. However, because most intravenous fluid was given shortly after ICU admission, the volume of nonassigned fluid administered as a result of the study structure was relatively small (<125 ml per patient; see Table E4), and sensitivity analysis that excluded patients who experienced a crossover did not change the findings in a statistically significant manner. Despite enrolling a population with high rates of relative contraindications to the study crystalloids (e.g., 30% incidence of hyperkalemia), almost 95% of provider orders were for the assigned crystalloid, and the separation between groups in the type of crystalloid received (70%) exceeded our target separation (60%). Despite relatively low overall volumes of intravenous fluid and some exposure to the nonassigned crystalloid, the separation between groups appeared to be sufficient to suggest a difference in RRT and MAKE30 among patients who received large volumes of crystalloid—a key finding for the planning of a definitive trial.
Despite these limitations, our study also had important strengths. Group assignment was random. Large size provided outstanding preliminary data on which to base a definitive trial. Enrolling all patients admitted to the study ICU precluded selection bias and improved generalizability. Enrollment immediately upon ICU admission, earlier than many trials of fluid management in critical illness, captured a peak period of fluid administration and risk for AKI development. Our study used MAKE30, a patient-centered outcome recommended for phase III trials by the National Institute of Diabetes and Digestive and Kidney Diseases workgroup on Clinical Trials in AKI (18, 20, 34). Finally, although previous studies have examined informatics-based interventions (35–37) or leveraged the EHR to facilitate aspects of trial conduct (37–39), to our knowledge, this is the first large, critical care clinical trial to be conducted entirely within the EHR. By successfully enrolling nearly 1,000 critically ill adults, accurately delivering the assigned intervention in a time-sensitive manner, and collecting detailed data on patient characteristics, fluid receipt, laboratory values, and clinical outcomes, SALT demonstrated that the EHR-embedded clinical trial (13, 14, 40) is a powerful new tool for generating evidence to guide clinical practice in critical care.
In summary, an EHR-embedded, cluster-randomized, multiple-crossover trial comparing saline and balanced crystalloids among critically ill adults can achieve well-balanced study groups and separation in crystalloid receipt. Larger trials examining patient-centered outcomes are warranted.
Acknowledgments
Acknowledgment
This trial was conducted within the Vanderbilt Learning Healthcare System. The authors thank the patients, nurses, nurse practitioners, residents, fellows, and attending physicians of the Vanderbilt Medical Intensive Care Unit for making this study possible. In particular, the authors recognize the mentorship of Arthur P. Wheeler, M.D., Vanderbilt University.
SALT Investigators: Vanderbilt University Medical Center, Nashville, TN: Gordon R. Bernard*, Matthew W. Semler*, Michael J. Noto, and Todd W. Rice* (Division of Allergy, Pulmonary, and Critical Care Medicine); Daniel W. Byrne*, Henry J. Domenico, and Li Wang* (Department of Biostatistics); Jonathan P. Wanderer* and Jesse M. Ehrenfeld* (Department of Biomedical Informatics and Department of Anesthesiology); Andrew D. Shaw*, Antonio Hernandez, and Avinash B. Kumar (Department of Anesthesiology); Wesley H. Self* (Department of Emergency Medicine); Edward D. Siew* (Vanderbilt Center for Kidney Disease and Integrated Program for AKI, Division of Nephrology and Hypertension); Debra F. Dunlap, Joanna L. Stollings*, Mark Sullivan, and Molly Knostman (Department of Pharmaceutical Services); and David P. Mulherin and Fred R. Hargrove (Department of Health Information Technology). Louisiana State University School of Medicine, New Orleans, LA: David R. Janz (Section of Pulmonary/Critical Care and Allergy/Immunology). American Society of Health-System Pharmacists, Bethesda, MD: Seth Strawbridge (Clinical Informatics).
*Writing committee members.
Footnotes
A complete list of SALT Investigators may be found before the beginning of the References.
Biostatistical support was provided by the Vanderbilt Institute for Clinical and Translational Research (UL1 TR000445 from the National Center for Advancing Translational Sciences/National Institutes of Health). M.W.S. was supported by an NHLBI T32 award (HL087738 09). W.H.S. was supported in part by the National Institute of General Medical Sciences (K23GM110469). E.D.S. was supported by the Vanderbilt Center for Kidney Disease and Veterans Affairs HS and RD IIR 13-073. T.W.R. was supported in part by the National Institutes of Health (R34HL105869). The funding institutions had no role in (1) conception, design, or conduct of the study; (2) collection, management, analysis, interpretation, or presentation of the data; or (3) preparation, review, or approval of the manuscript.
Author Contributions: Study concept and design: M.W.S, A.D.S., G.R.B., and T.W.R. Acquisition of data: M.W.S, J.P.W., J.M.E., J.L.S., and T.W.R. Analysis and interpretation of data: M.W.S, W.H.S., E.D.S., L.W., D.W.B., and T.W.R. Drafting of the manuscript: M.W.S, J.P.W., and T.W.R. Critical revision of the manuscript for important intellectual content: M.W.S, J.P.W., J.M.E., J.L.S., W.H.S., E.D.S., L.W., D.W.B., A.D.S., G.R.B., and T.W.R. Statistical analysis: M.W.S, W.H.S., L.W., D.W.B., and T.W.R. Study supervision: M.W.S, J.L.S., A.D.S., G.R.B., and T.W.R. Had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis: M.W.S, J.P.W., and L.W. Conducted and are responsible for the data analysis: L.W. and D.W.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201607-1345OC on October 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Gordon R. Bernard, Matthew W. Semler, Michael J. Noto, Todd W. Rice, Daniel W. Byrne, Henry J. Domenico, Li Wang, Jonathan P. Wanderer, Jesse M. Ehrenfeld, Andrew D. Shaw, Antonio Hernandez, Avinash B. Kumar, Wesley H. Self, Edward D. Siew, Debra F. Dunlap, Joanna L. Stollings, Mark Sullivan, Molly Knostman, David P. Mulherin, Fred R. Hargrove, David R. Janz, and Seth Strawbridge
References
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J SAFE TRIPS Investigators. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 4.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, Kellum JA. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 6.Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–1905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192:1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS. ARDS subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. doi: 10.1164/rccm.201603-0645OC. [online ahead of print] 11 Aug 2016; DOI: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semler MW, Wheeler AP, Bernard GR, Thompson BT. Conservative fluid management decreases mortality in acute respiratory distress syndrome patients with low central venous pressure [abstract] Am J Respir Crit Care Med. 2014;189:A5094. [Google Scholar]
- 12.Semler MW, Marney AM, Rice TW, Nian H, Yu C, Wheeler AP, Brown NJ NIH NHLBI ARDS Network. B-type natriuretic peptide, aldosterone, and fluid management in ARDS. Chest. 2016;150:102–111. doi: 10.1016/j.chest.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semler MW, Rice TW, Ehrenfeld JM. Leveraging clinical informatics in the conduct of clinical trials. J Med Syst. 2015;39:112. doi: 10.1007/s10916-015-0317-0. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC. Fusing randomized trials with big data: the key to self-learning health care systems? JAMA. 2015;314:767–768. doi: 10.1001/jama.2015.7762. [DOI] [PubMed] [Google Scholar]
- 15.Semler MW, Noto MJ, Stollings J, Ehrenfeld JM, Wanderer JP, Plante M, Mulherin D, Strawbridge S, Wheeler AP, Rice TW The SALT Investigators; the Pragmatic Critical Care Research Group. Effect of saline versus balanced crystalloids on major adverse kidney events in the medical intensive care unit: the SALT randomized trial[abstract] Am J Respir Crit Care Med. 2016;193:A4290. [Google Scholar]
- 16.Crespi CM. Improved designs for cluster randomized trials. Annu Rev Public Health. 2016;37:1–16. doi: 10.1146/annurev-publhealth-032315-021702. [DOI] [PubMed] [Google Scholar]
- 17.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, McGuinness S, Mehrtens J, Myburgh J, Psirides A, et al. SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 18.Semler MW, Rice TW, Shaw AD, Siew ED, Self WH, Kumar AB, Byrne DW, Ehrenfeld JM, Wanderer JP. Identification of major adverse kidney events within the electronic health record. J Med Syst. 2016;40:167. doi: 10.1007/s10916-016-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kistin C, Silverstein M. Pilot studies: a critical but potentially misused component of interventional research. JAMA. 2015;314:1561–1562. doi: 10.1001/jama.2015.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844–850. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 21.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 23.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA AKI6 investigators. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 24.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 28.Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, Mbuagbaw L, Szczeklik W, Alshamsi F, Altayyar S, et al. Fluids in Sepsis and Septic Shock Group. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–355. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 29.Rochwerg B, Alhazzani W, Gibson A, Ribic CM, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, Mbuagbaw L, Szczeklik W, et al. FISSH Group (Fluids in Sepsis and Septic Shock) Fluid type and the use of renal replacement therapy in sepsis: a systematic review and network meta-analysis. Intensive Care Med. 2015;41:1561–1571. doi: 10.1007/s00134-015-3794-1. [DOI] [PubMed] [Google Scholar]
- 30.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, Mehrtens J, Myburgh J, McGuinness S, Psirides A, et al. The statistical analysis plan for the 0.9%Saline vs Plasma-Lyte 148 for Intensive care fluid Therapy (SPLIT) study2014 [accessed 2016 Feb 18]Available from: http://wellingtonicu.com/Data/Trials/SPLITSAP.pdf
- 31.Semler MW, Rice TW. Saline is not the first choice for crystalloid resuscitation fluids. Crit Care Med. 2016;44:1541–1544. doi: 10.1097/CCM.0000000000001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 33.Yunos NM, Kim IB, Bellomo R, Bailey M, Ho L, Story D, Gutteridge GA, Hart GK. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 34.Weisbord SD, Gallagher M, Kaufman J, Cass A, Parikh CR, Chertow GM, Shunk KA, McCullough PA, Fine MJ, Mor MK, et al. Prevention of contrast-induced AKI: a review of published trials and the design of the prevention of serious adverse events following angiography (PRESERVE) trial. Clin J Am Soc Nephrol. 2013;8:1618–1631. doi: 10.2215/CJN.11161012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, Decruyenaere J. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med. 2012;40:1164–1170. doi: 10.1097/CCM.0b013e3182387a6b. [DOI] [PubMed] [Google Scholar]
- 36.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35:1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semler MW, Weavind L, Hooper MH, Rice TW, Gowda SS, Nadas A, Song Y, Martin JB, Bernard GR, Wheeler AP. An electronic tool for the evaluation and treatment of sepsis in the ICU: a randomized controlled trial. Crit Care Med. 2015;43:1595–1602. doi: 10.1097/CCM.0000000000001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooper MH, Weavind L, Wheeler AP, Martin JB, Gowda SS, Semler MW, Hayes RM, Albert DW, Deane NB, Nian H, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med. 2012;40:2096–2101. doi: 10.1097/CCM.0b013e318250a887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodcock A, Bakerly ND, New JP, Gibson JM, Wu W, Vestbo J, Leather D. The Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in asthma. BMC Pulm Med. 2015;15:160. doi: 10.1186/s12890-015-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiore LD, Lavori PW. Integrating randomized comparative effectiveness research with patient care. N Engl J Med. 2016;374:2152–2158. doi: 10.1056/NEJMra1510057. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]