Abstract
Oncolytic virotherapy is a treatment approach with increasing clinical relevance, as indicated by the marked survival benefit seen in animal models and its current exploration in human patients with cancer. The use of an adenovirus vector for this therapeutic modality is common, has significant clinical benefit in animals, and its efficacy has recently been linked to an anti-tumor immune response that occurs following tumor antigen presentation. Here, we analyzed the adaptive immune system’s response following viral infection by comparing replication-incompetent and replication-competent adenoviral vectors. Our findings suggest that cell death caused by replication-competent adenoviral vectors is required to induce a significant anti-tumor immune response and survival benefits in immunocompetent mice bearing intracranial glioma. We observed significant changes in the repertoire of immune cells in the brain and draining lymph nodes and significant recruitment of CD103+ dendritic cells (DCs) in response to oncolytic adenoviral therapy, suggesting the active role of the immune system in anti-tumor response. Our data suggest that the response to oncolytic virotherapy is accompanied by local and systemic immune responses and should be taken in consideration in the future design of the clinical studies evaluating oncolytic virotherapy in patients with glioblastoma multiforme (GBM).
Keywords: oncolytic virotherapy, replication-competent and replication-incompetent adenovirus, immune-competent and immune-deficient murine glioma model, tumor-specific cytotoxic T and dendritic cells
Introduction
Glioblastoma, the most common primary brain malignancy, is a devastating disease that is associated with poor patient outcomes and urgently needs more advanced and targeted therapies.1 One promising approach utilizes live viruses that have been designed to specifically infect and kill tumor cells, termed oncolytic virotherapy.2 Many studies have demonstrated a survival benefit for animals treated with oncolytic virotherapy when compared to the current standard of care, and multiple human clinical trials have repeatedly demonstrated the safety of virotherapy and are currently exploring the efficacy of this treatment modality.3, 4
Among the various oncolytic virotherapies explored clinically, Ad5Arg-Gly-Asp(RGD)-cytomegalovirus (CMV)-E1Δ24-based oncolytic virotherapies (e.g., ONXY-015, DNX2401, or Delta-24-RGD) have accumulated the most clinical data demonstrating their efficacy and safety in many different types of cancer.5, 6, 7, 8, 9, 10, 11 Furthermore, the data from clinical trials suggest that therapeutic efficacy of oncolytic therapies can be enhanced when the immune system response against tumor antigens is facilitated by the virotherapeutic treatment (i.e., oncolysis).5, 7, 9, 10, 11
Given the potential synergy between virotherapy and the immune system, it is important to understand the underlying mechanisms behind virotherapeutic treatment-mediated anti-tumor immune responses; however, it is difficult to pursue this analysis because a murine model, which is the best model system for immune-related analysis, is not permissive for the replication (i.e., progeny production) of human adenoviruses.12 However, human adenoviruses in murine cells are able to produce their necessary viral proteins via regulation of the transcription/translation machineries of infected cells,13, 14, 15, 16 even if they are not capable of producing their progenies efficiently (replication).13, 17
Jiang et al.18 recently showed that Delta-24-RGD infection in an immunocompetent murine glioma model can facilitate anti-tumor immune responses and immunity; this is the first mechanistic study of the immunological effects mediated by an oncolytic virus for glioma. However, it is not clear whether these immune responses were mediated by inflammation and cell death following viral entry/infection or by viral activities/viral protein production, which may be related to the replication efficiency of the virus that would occur in the human clinical setting.
To investigate this virotherapy-mediated immune response in an immunocompetent murine glioma model, we first selected modified adenoviral vectors that demonstrated enhanced infectivity of a murine glioma cell line, GL261. Two of the most commonly used adenoviral vectors for glioma virotherapy, Ad5RGD and Ad5PK7, were compared to the wild-type (WT) Ad5 virus for their murine glioma cell infectivity (i.e., entry). Next, we compared the cell death rate in vitro by adenoviruses with differential E1 gene product regulation (Survivin-E1 and CMV-E1Δ24; two different tumor-specific, replication-competent adenoviruses in humans) to that by an E1 gene-deleted adenoviral vector (i.e., replication-incompetent adenovirus in human).10, 12 Although murine glioma cells are not permissive for the replication (progeny production) of human adenoviral vectors, as mentioned above, differential transcriptional regulation of E1 gene products may change the efficiency of viral protein production, which could affect the viruses ability to cause cell death of infected murine cells, and, therefore, may alter how the adaptive immune response responds following virus administration.13, 14, 16, 17 As such, we investigated the impact on survival and changes in the cellular components of the adaptive immune responses by administrating either a replication-competent or -incompetent adenovirus in an immunocompetent murine glioma model.
In this study, we selected the adenoviral vector that is most suitable for investigation of virotherapeutic efficacy and virotherapy-mediated immune responses in a murine glioma model system. Furthermore, we investigated virotherapy-mediated changes in the cellular components of the immune system repertoire by using tumor antigen-specific CD8+ T cell tetramer staining to identify tumor antigen-specific immune cells in the brain of tumor-bearing animals as well as in cervical lymph nodes, one of the activation sites for immune cells. Our findings support the notion that efficient adenoviral replication is required for tumor-specific adaptive immune response against glioma, and this immune response is critical for the efficacy of the viral agent.
Results
Ad5PK7 and Ad5RGD Efficiently Infect Both Human and Murine Glioma Cell Lines
Ad5PK7 and Ad5RGD are two fiber-modified adenoviral vectors that are among the most commonly used for human glioma virotherapy.12, 19 As viral infection is dictated by the fiber domain, both of these adenoviral vectors were designed to have enhanced infectivity of human glioma cells, either by adding seven poly(lysine) (PK7) residues (via binding anionic cell surface proteins) or the RGD motif (via binding abundant cell adhesion molecules, integrins), respectively, on the fiber knob domain.20
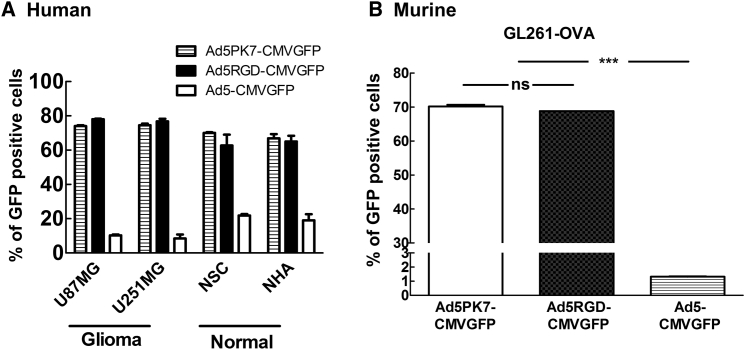
To determine the infectivity of these oncolytic adenoviral vectors (Ad5PK7-CMV-GFP and Ad5RGD-CMV-GFP) in both human and murine glioma model cell lines, we analyzed their infectivity by utilizing flow cytometric analysis for GFP expression. The E1 gene of these viruses was replaced with the GFP gene so that only initially infected cells were counted without replication/spreading of the viruses. Human glioma cell lines were used to ensure that the viral vectors explored with additional experiments in the murine models are also capable of translation into the human system. The GL261-ovalbumin (OVA) cell line was chosen on account of it being a well-characterized murine glioma cell line suitable for use in immunocompetent mice and also because it expresses a model antigen, OVA, which will be used to characterize antigen-specific immune responses in later analyses.21 Our findings revealed that both of these viral vectors efficiently infect (i.e., enter) human glioma cells (Figure 1A) as well as the murine glioma GL261-OVA cell line (Figure 1B) when compared to the WT Ad5 virus (Ad5-CMVGFP). Therefore, our infectivity analysis suggests that both of these vectors are suitable for further exploration in the murine glioma cell line.
Figure 1.
Infectivity of the Fiber-Modified Adenoviral Vectors in Both Human and Murine Glioma Cell Lines
(A and B) Cells were infected with Ad5PK7-CMVGFP, Ad5RGD-CMVGFP, or Ad5-CMVGFP at an MOI of 300 VP/cell for 2 hr in (A) human glioma cell lines (U87 and U25) and non-neoplastic cells (normal human astrocytes and neuronal stem cells) and (B) a murine glioma cell line (GL261-OVA). The virus-containing medium was then removed and replaced with fresh medium containing 2% FBS. After 72-hr incubation, flow cytometric analysis was performed. Each data point and column is the average of three independent replicates. The mean ± SE is plotted. ***p < 0.001. NHA, normal human astrocyte; ns, not significant; NSC, neuronal stem cell; VP, viral particle.
Both Ad5RGD-CMV-Δ24 and Ad5RGD-Sur-E1 Induce Cell Deaths of Both Human and Murine Glioma Cell Lines
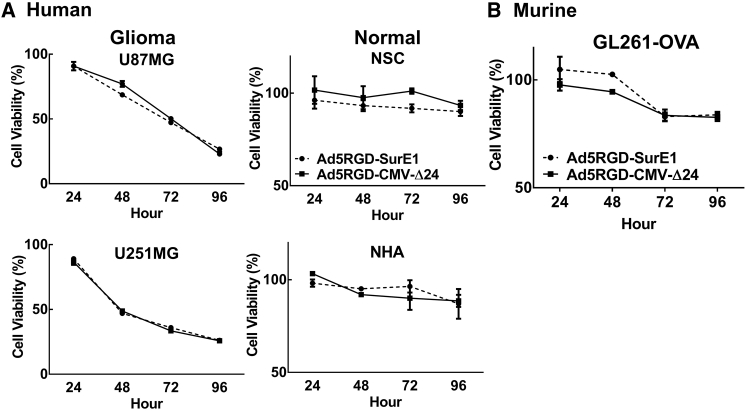
Because these two infectivity-enhanced vectors do not differentiate between neoplastic and non-neoplastic cells, their oncolytic replication must to be restricted by manipulating the expression of the replication essential early gene, E1. Two common approaches to restrict E1 expression or activity are (1) the Δ24 approach, which utilizes a 24-base-pair deletion in the E1 gene that removes its binding site for the retinoblastoma protein (Rb) and results in a truncated protein product that is only active in cells with an already inactive Rb (e.g., cancerous cells);9 or (2) by placing the expression of the E1 gene under the control of the glioma-specific promoter, Survivin (SurE1).19 To assess tumor-specific cytotoxic activity of these two different E1 regulation approaches, we used Ad5RGD-SurE1 and Ad5RGD-CMV-Δ24 and analyzed their cytotoxicity by utilizing an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Of note, the RGD-based fiber modification was chosen due to the slightly increased infectivity in human glioma cell lines compared to PK7 modification, although the difference is not statistically significant. We did not observe any difference in cytotoxic activity in either the murine glioma model cell line or in human glioma cell lines between the aforementioned Ad5RGD-Sur-E1 and Ad5RGD-CMV-Δ24 (Figure 2). However, given the slight increase observed in infectivity and cytotoxicity, the RGD fiber modification (Figure 1) and CMV-Δ24 E1 gene control approach (Figure 2) were used for further analysis.
Figure 2.
Cytotoxicity of Oncolytic Adenoviral Vectors with Differential E1 Gene Product Regulation in Both Human and Murine Glioma Cell Lines
(A and B) Cells were infected with Ad5PK7-Sur E1 or Ad5RGD-CMV-Δ24 at an MOI of 100 VP/cell for 2 hr in (A) human glioma cell lines (U87 and U251) and non-neoplastic cells (normal human astrocytes and neuronal stem cells) and (B) a murine glioma cell line (GL261-OVA). The virus-containing medium was removed and replaced with fresh medium containing 2% FBS. At time points of 24, 48, 72, and 96 hr, the MTT assay was performed according to the manufacturer’s instructions. Each data point and column is the average of three independent replicates. The mean ± SE is plotted. NHA, normal human astrocyte; ns, not significant; NSC, neuronal stem cell; VP, viral particle.
Replication-Competent Ad5RGD-CMV-Δ24 Results in Enhanced Cytotoxicity in the GL261-OVA Cell Line as Compared to Replication-Incompetent Ad5RGD-CMV-GFP
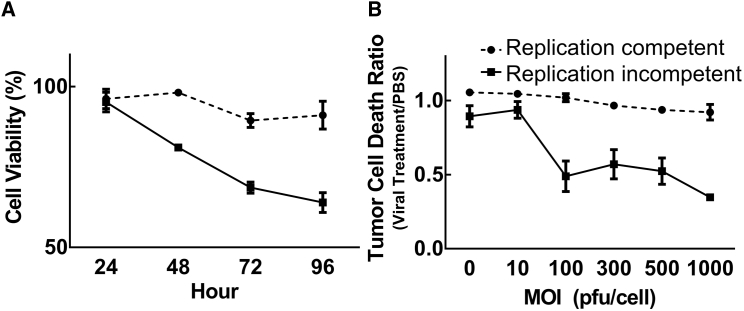
All adenovirus-based vectors are derived from the human adenovirus serotype 5, making these viruses unable to produce progeny in murine cells.13, 17 Because of this, it is expected that viral replication-induced oncolytic effects will be considerably minimized in murine glioma cells. However, even in the absence of viral progeny production, the excessive production of viral proteins can still result in cell death of infected murine cells, possibly through an autophagy mechanism.22, 23 Hence, we hypothesized that a replication-competent adenoviral vector would be more cytotoxic in murine cells via E1-derived viral protein production compared to that of a replication-incompetent vector. To investigate our hypothesis, we compared the cytotoxic activity of replication-competent adenovirus (Figures 1 and 2) to a replication-incompetent vector (which has the same fiber modification but the E1 gene is replaced with GFP) by performing cell MTT (Figure 3A) and crystal violet viability assays (Figure 3B) in the murine glioma cell line. As shown in Figure 3, the replication-incompetent adenoviral vector, Ad5RGD-CMV-GFP, was very minimally cytotoxic (approximately 10% of cells died). However, the replication-competent vector, Ad5RGD-CMV-Δ24, was significantly cytotoxic in GL261-OVA cells (Figure 3), although the efficacy of this oncolytic activity was notably lower when compared to human glioma cell lines, as predicted (Figure 2A).
Figure 3.
Comparison of Replication-Competent and Replication-Incompetent Adenoviral Vector Cytotoxicity in a Murine Glioma Cell Line
Murine glioma GL261-OVA cells were infected with replication-incompetent Ad5RGD-CMV-GFP or replication-competent Ad5RGD-CMV-Δ24. (A) For the MTT assay, the virus-containing medium was removed and replaced with fresh medium containing 2% FBS. At time points of 24, 48, 72, and 96 hr, the MTT assay was performed according to the manufacturer’s instructions. (B) An MOI of 100 PFU/cell was used. For the crystal violet staining assay, viral vectors were infected at a serially diluted MOI from 0 to 1,000 PFU/cell and the virus-containing medium was removed and replaced with fresh medium containing 2% FBS. Eight days after viral infection, cells were fixed and stained with crystal violet, followed by dissolution with methanol, and read with a plate reader at OD570. Relative ratios of viral treatments to PBS treatments were calculated and plotted. Each data point and column is the average of three independent replicates. The mean ± SE is plotted. OD570, optical density 570; PFU, plaque-forming unit.
Replication-Competent Ad5RGD-CMV-Δ24 Significantly Prolongs Survival in an Immunocompetent, but Not in Immunodeficient, Murine Glioma Model
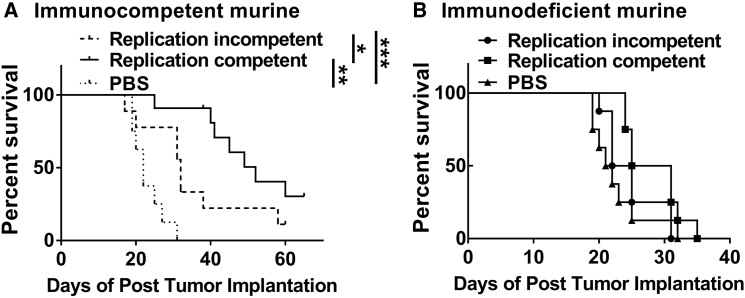
As demonstrated above, although the replication-competent adenoviral vector leads to enhanced tumor cell death in comparison to the replication-incompetent vector in vitro, the replication-incompetent vector still induced tumor cell death. Importantly, it is not known whether the therapeutic efficacy of virotherapy is simply due to pronounced oncolytic virus-mediated cytotoxicity or whether a cell death-stimulated immune response plays a key role in the therapeutic benefit of the treatment. To investigate this, we performed the survival analysis comparing replication-competent versus replication-incompetent viral treatment in both immunocompetent and immunodeficient murine glioma models. In the immunocompetent murine model, mice injected with the replication-competent virus had a significantly (median survival = 52 days) prolonged survival relative to the group treated with the replication-incompetent virus (median survival = 32 days), indicating that efficient cytotoxicity following viral infection and viral protein production is an important factor for achieving a survival benefit (Figure 4A). However, this survival benefit was abolished when the viruses were injected into immunodeficient Rag1 knockout (KO) mice, implying that virotherapy-mediated adaptive immune responses are required to achieve therapeutic efficacy (Figure 4B). Of note, there was no difference in the growth rate of GL261-OVA in these two different murine models (Figure S1). Thus, in order to achieve a survival benefit following virotherapy administration in a murine glioma model, it is necessary to have both a strong oncolytic effect and an adaptive immune response.
Figure 4.
Survival Analysis in Immunocompetent and Immunodeficient Murine Glioma Models Infected with Either Replication-Competent or Replication-Incompetent Adenoviral Vectors
(A) Immunocompetent or (B) immunodeficient mice bearing intracranial GL261-OVA were treated intracranially (i.c.) with either replication-incompetent Ad5RGD-CMV-GFP or replication-competent Ad5RGD-CMV-Δ24 5 days after tumor implantation. Kaplan-Meier survival curves of treated mice were calculated. *p < 0.05, **p < 0.01, and ***p < 0.001.
Tumor-Specific CD8+ Cells Were Increased in Both the Brains and Lymph Nodes in the Group Treated with the Replication-Competent Ad5RGD-CMV-Δ24
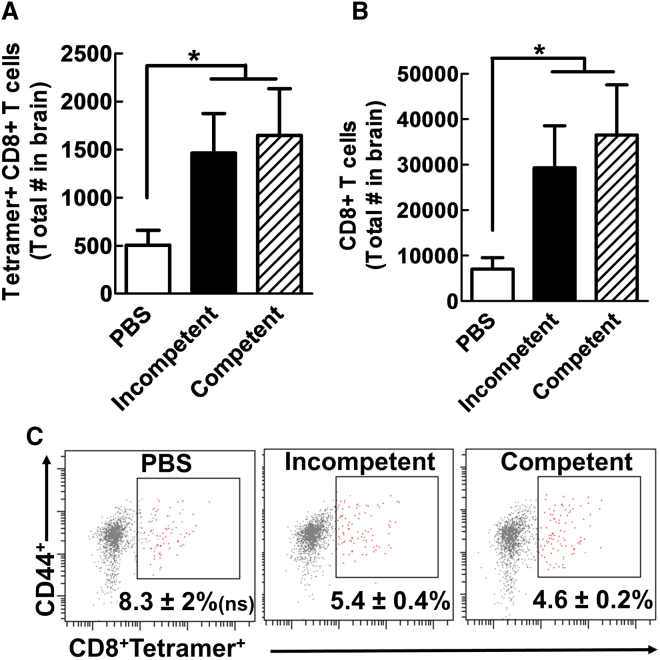
It has been suggested that oncolytic activity of virotherapy induces the release of tumor antigens, which likely facilitate the activation of a tumor antigen-specific immune response.18 As we observed, virotherapy-mediated survival benefit was only in the immunocompetent model, so it was of interest to evaluate how different viral vectors influence the immune system response following viral treatment. To investigate this, we analyzed tumor antigen-specific cytotoxic (CD8+) T cells in the tumor implantation site and lymph nodes, which are the sites of antigen-specific T cell recruitment and activation, respectively. Five days after tumor implantation with GL261-OVA cells, three different agents (PBS, Ad5RGD-CMV-GFP, and Ad5RGD-CMV-Δ24) were administered intratumorally. The brains and lymph nodes of the animals were examined to analyze the change in immune repertoire 5 days following the administration of the viral vector. In the brain, the number of tumor antigen-specific CD8+ cells (OVA-specific, tetramer-positive staining) (Figure 5A) and total CD8+ cells (Figure 5B) was significantly increased in both the groups treated with replication-incompetent or replication-competent viruses when compared to the PBS group (Figure 5) (p < 0.01). The specificity of our tetramer staining was validated by both fluorescence minus one (FMO) analysis and the use of OT1 mice as a positive control (Figure S2). Interestingly, although we observed a change in the total number of CD8+ T cells and tetramer-positive CD8+ cells in mice treated with a viral agent, there was not a significant difference in the percentage of tetramer-specific T cells among the CD44+ cell population in the brains of virally treated animals compared to the group treated with PBS (Figure 5C). Additionally, there was no change in the total number of CD8+ T cells and tetramer-positive CD8+ T cells in the brains of mice treated with the replication-incompetent virus compared to the mice treated with the replication-competent virus. These findings suggest that while the administration of a viral vector itself can induce tumor cell death and facilitate an increase in the total number of tumor-specific T cells and total cytotoxic CD8+ T cells, additional components of the immune system must be responsible for the survival benefit observed in immunocompetent mice treated with replication-competent adenovirus.
Figure 5.
Increase of Antigen-Specific CD8+ within the Tumor Sites of Mice Treated with Adenoviral Vector
Five days after GL261-OVA implantation, mice were injected intracranially (i.c.) with PBS, replication-incompetent Ad5RGD-CMV-GFP, or replication-competent Ad5RGD-CMV-Δ24. Flow cytometric analyses of the brain of treated mice were performed 5 days after viral administration. (A and B) Both GFP and Delta-24 viruses recruit more tetramer-positive CD8 T cells and total CD8 T cells to the tumor, respectively. (C) The percentage of CD8+ T cells expressing the T cell receptor (TCR) for OVA in class I as determined by tetramer staining analyses is shown. Error bars are calculated as the mean ± SEM, with n = 5 per group. Significance was calculated using a one-way ANOVA followed by a Tukey’s post hoc test to compare individual groups. *p < 0.05.
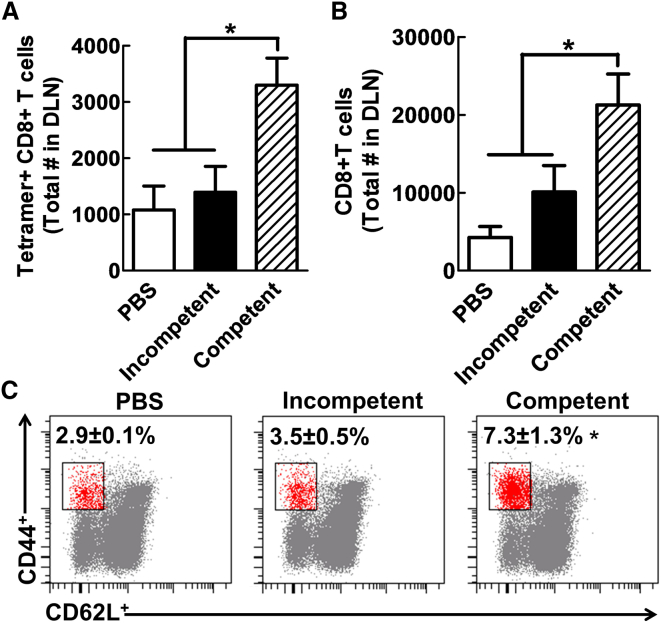
Importantly, in the draining lymph nodes where cytotoxic T cells are activated against the tumor-specific antigen (OVA), only the group that received the replication-competent Ad5RGD-CMVΔ24 had a statistically significant increase in both total number of CD8+ T cells (p < 0.01, Figure 6B) and tumor antigen-specific CD8+ T cells (p < 0.01, Figure 6A), and in the relative percentage of tumor-specific antigen T cells to total CD44+ cells when compared to the group treated with PBS and the replication-incompetent virus treatment group (Figure 6C). Therefore, an increase in tumor-specific CD8+ cells was seen in the lymph nodes exclusively in the group treated with the replication-competent virus.
Figure 6.
Increase in T Cell CD8+ Activation in the Draining Lymph Nodes of Mice with Replication-Competent Virus
The draining lymph nodes of GL-261OVA tumor-bearing mice were analyzed 5 days after viral vector administration. (A and B) The total numbers of the tetramer-positive CD8+ (A) and total CD8+ population (B), respectively, compared across all three groups. (C) The percentage of CD8+ with an activated phenotype CD44+CD62L− in the lymph nodes of analyzed mice. Error bars are calculated as the mean ± SEM, with n = 5 per group. Significance was calculated using a one-way ANOVA followed by a Tukey’s post hoc test to compare individual groups. *p < 0.05. DLN, draining lymph node.
Administration of Replication-Competent Ad5RGD-CMV-Δ24 Robustly Increases Antigen Cross-Presenting CD103+ Dendritic Cell Population at the Tumor Site
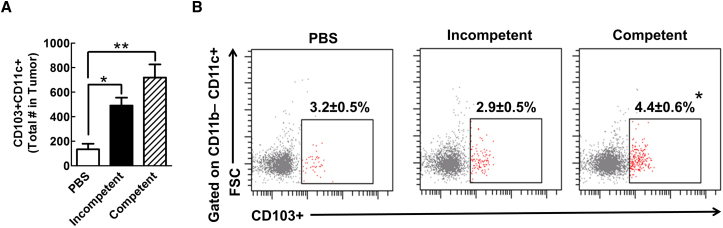
The profound accumulation of cytotoxic T cells observed in the lymph nodes of mice 5 days after treatment with the replication-competent Ad5RGD-CMV-Δ24 likely signals an increase in tumor antigen presentation to this T cell population (Figure 6). One possibility is that antigen-presenting cells, such as dendritic cells (DCs), relocate to the draining lymph nodes after tumor antigen uptake and activate the cytotoxic T cells in a tumor antigen (i.e., OVA)-specific manner. To investigate the contribution of antigen-presenting DCs to the anti-tumor response, we analyzed CD103+ DC subsets (CD11b−/CD11C+/CD103+), which are known for presenting antigen in the tumor24 and are a critical player in the anti-tumor immune response. As shown in the Figure 7, when compared to the PBS-treated control group, the administration of the replication-incompetent Ad5RGD-GFP results in more recruitment of CD103+ DCs into the tumor site (p = 0.01) and, more importantly, the replication-competent Ad5RGD-CMV-Δ24 group has an even more robust recruitment of this DC population at the tumor site (p = 0.006). Therefore, the total number of antigen cross-presenting and lymph-node tropic DCs was increased when replication-competent Ad5RGD-CMV-Δ24 was administered in the tumor. Furthermore, this accumulation of DCs at the tumor site is significantly correlated with increased antigen-specific CD8+ T cells in the lymph nodes (Figures 5, 6, and S3), as well as the survival benefit seen in immunocompetent mice treated with replication-competent virus (Figure 4).
Figure 7.
CD103+ DCs Accumulate within Brain Tumors of GL261 Mice
GL-261 OVA-bearing mice had flow cytometric analysis of their myeloid compartment performed 5 days after viral vector injection. (A) The total number of CD11c+CD103+ DCs within the tumors of mice is displayed. (B) The percentage of CD11b−CD11c+ cells expressing CD103 in the brain is shown. Error bars are calculated as the mean ± SEM, with n = 5 per group. Significance was calculated using a one-way ANOVA followed by a Tukey’s post hoc test to compare individual groups. *p < 0.05; **p < 0.01. FSC, forward scatter.
Discussion
Our results expand on previous work demonstrating the importance of the immune system for the therapeutic efficacy of oncolytic virotherapy in murine gliomas.21, 25, 26 Our findings indicate that even the replication-incompetent adenovirus has some cytotoxicity in murine glioma cells in vitro, and accompanying this cytotoxicity was an increase in tumor-infiltrating CD8+ T cells and tumor antigen-specific CD8+ T cells in vivo; albeit, this influx of lymphocytes was not associated with a corresponding increase in survival for animals treated with this virus. Perhaps the limited tumor cell death achieved by the replication-incompetent virus does not provide enough stimuli to the immune system to overcome the immunosuppression of the glioblastoma multiforme (GBM) microenvironment. In contrast, the replication-competent virus, which is capable of producing more robust tumor cell death in murine glioma cells, provided a survival benefit only in mice with an intact adaptive immune system, indicating that the anti-tumor response to the oncolytic activity of a human adenovirus is complemented by the activity of immune system in murine glioma and likely assists the oncolytic virus in the efficient anti-tumor response.
The inability of adenoviral vectors to produce progeny in murine cell lines imposes limitations to all studies investigating the therapeutic effect of virus in a syngeneic model of glioma. Here we demonstrated that the replication-competent, but not replication-deficient, virus was able to cause more cytotoxicity of a murine glioma cell in vitro without replication, as it would replicate and induce more cell death in human tumor cells. Interestingly, the replication-competent virus produces a survival benefit only in immunocompetent mice bearing murine gliomas, with a median survival of 52 days in WT mice versus 32 days in Rag−/− mice. An increase in the total number of CD8+ T cells and tumor antigen-specific CD8+ T cells in the brain was observed in mice treated with both the replication-competent and the replication-incompetent virus. However, only the treatment of mice with the replication-competent virus resulted in an increase in activated CD8+ T cells and tumor antigen-specific activated CD8+ T cells in the draining cervical lymph nodes. Finally, there was a corresponding increase in CD103+ dendritic cells, a subpopulation of DCs that has been shown to play a critical role in the presentation of tumor antigens to T cells and in the production of proinflammatory cytokines,24, 27, 28 in the brain of mice treated with the replication-competent virus. Our data corroborate prior analyses of murine tumor models that have established a correlation between the immune system’s anti-tumor activity, specifically tumor antigen-specific CD8+ T cells, and prolonged animal survival.29, 30, 31 Furthermore, reducing the intrinsic immunosuppression in the glioma microenvironment reliably improves overall survival and augments the anti-tumor immune response.30, 32 Given these findings, therapeutic approaches capable of increasing the presence of infiltrating tumor antigen-specific CD8+ T cells and other effector cells of the adaptive immune system are likely to result in a reduction in tumor burden and therapeutic efficacy. Additionally, these approaches are also intriguing options for exploration in combinatorial treatment strategies that utilize other immunotherapeutic agents. Our findings further endorse the likely benefit of this approach, and we encourage further investigation of the synergistic effects of virotherapy and immunotherapy.33, 34
In conclusion, we have expanded on previous data demonstrating the importance of a tumor-specific immune response following treatment with virotherapy in murine gliomas.26 We also show that an adaptive immune system is necessary for an improvement in survival in mice bearing syngeneic murine gliomas following treatment with a replication-competent adenovirus, and that a replication-incompetent virus is not capable of providing the same stimulus for the immune system to create an effective anti-tumor response. The intratumoral treatment of replication-competent adenovirus increases the tumor antigen-specific CD8+ T cells in the brain and draining lymph nodes and also leads to an increase in CD103+ DCs, which likely contribute to the therapeutic effect of the virus.
Materials and Methods
Cell Lines and Culture Condition
HEK293 cells, two human glioma cell lines (U87MG and U251MG), two non-neoplastic cell lines (normal human astrocytes [NHAs] and neuronal stem cells [NSCs], HB1.F3 CD), and a murine glioma cell line (GL261-OVA, which stably expresses ovalbumin) were cultured in DMEM (Mediatech) with 10% fetal bovine serum (FBS; HyClone), 100 U/mL penicillin, and 100 mg/mL streptomycin (Mediatech) and incubated at 37°C in 5% CO2 in humidified conditions.
Virus Production and Purification
All of the viruses were propagated in HEK293 and the last propagation in 20 × 175 mL flasks was performed in HEK293 cells. Viruses were purified by two rounds of CsCl gradient ultracentrifugation and their titer was determined at 260 nm.35 The titers of all viruses used in this study were equivalent to each other.
Virus Infectivity Analysis
3 × 105 cells were plated in 24-well plates and incubated overnight. Each virus sample was diluted to an MOI of 300 viral particles (VPs)/cell in 500 μL infection media containing 2% FBS in DMEM. After 2 hr at 37°C, the virus-containing medium was replaced with fresh medium containing 2% FBS and the virus-infected cells were maintained at 37°C in atmospheric humidification containing 5% CO2 for 3 days until flow cytometry analysis.
Cytopathic Efficacy
Virus infection was conducted in the same way as described above, except an MOI of 100 VP/cell was used instead. The MTT assay was performed using cell proliferation kit I (MTT) according to the manufacturer’s instructions (Roche). For crystal violet staining, cells were infected with serial dilutions of virus (MOI ranging from 0 to 100 VP/cell) and incubated at 37°C in atmospheric humidification containing 5% CO2. After 8 days of incubation, cells were stained with 500 μL 0.1% crystal violet solution in distilled water. Following several washings with water, the plates were dried at room temperature. Crystal violet staining was dissolved with 500 μL 100% methanol and read with a plate reader at optical density 570 (OD570).
Animal Experiments
For intracranial glioma xenograft implantation, GL261-OVA (4 × 105 cells) murine glioma cells were implanted via cranial guide screws as described previously.36 Mice were cared for in accordance with a study-specific animal protocol approved by Northwestern University Institutional Animal Care and Use Committee. Five days after tumor implantation, mice were randomly separated into three groups and were then injected with either 2.5 μL PBS or 109 VPs. Mice injected with VPs either received the replication-incompetent Ad5RGD-CMV-GFP or replication-competent Ad5RGD-CMV-Δ24 in 2.5 μL PBS. Wild-type C57BL/6 and Bl/6 rag1 KO mice were used as immunocompetent and immunodeficient mouse models, respectively. All animals were cared for in accordance with a study-specific animal protocol approved by the Northwestern University Institutional Animal Care and Use Committee.
Flow Cytometric Analyses
Mice harboring 4 × 105 GL261-OVA had their brain tumors, spleen, draining lymph nodes, and non-draining lymph nodes harvested into complete RPMI (Mediatech) 5 days after adenoviral vector injection. Single cell suspensions were prepared by processing organs through a 70-μm nylon cell strainer (Thermo Fisher). Leukocytes were isolated from the tumor tissue using a 30%/70% discontinuous Percoll gradient centrifugation (GE). Red blood cells were lysed using 1 mL ammonium-chloride-potassium (ACK) buffer (Lonza) followed by inactivation, centrifugation, and resuspension. All antibody staining was performed in 2% PBS/FBS except where noted.
For tetramer analysis, single cell suspensions were co-incubated with 1:100 SIINFEKL-H-2K(b) class I tetramer conjugated to Alexa 488 (NIH Tetramer Core Facility) and anti-CD16/32 (BioLegend) for 1 hr at 37°C in complete RPMI. Cells were then washed two times and stained for 15 min at 4°C with fluorescently labeled antibodies for the following surface markers (and their dilution): anti-CD3 (Alexa 700, 1:100), anti-CD4 (allophycocyanin [APC], 1:200), anti-CD8 (phycoerythrin [PE], 1:100), anti-CD44 (Percp-Cy5.5, 1:100), and anti-CD62L (APC-Cy7, 1:100) (all purchased from BioLegend except for CD8-PE, which was purchased from MBL). After surface staining was performed, cells were fixed/permeabilized and stained with anti-Foxp3 eFluor-450 (1:100; eBioscience) per the manufacturer’s best protocol. Samples were acquired using a LSR-Fortessa flow cytometer (Becton Dickinson).
For myeloid cell flow cytometric analysis, single cell suspensions were blocked with anti-CD16/32 blocking antibodies for 15 min at 4°C. Cells were then directly stained for 15 min at 4°C with fluorescently labeled antibodies for the following surface markers (and their dilutions): anti-CD45 (APC-Cy7, 1:100), anti-CD11b (PE, 1:200), anti-CD11c (APC, 1:100), anti-Ly6G (Percp-Cy5.5, 1:400), and anti-Ly6C (Alexa 700, 1:400) (all purchased from BioLegend). Anti-CD103 eFluor-450 (1:100) was purchased from eBioscience. Samples were acquired using a LSR-Fortessa flow cytometer (Becton Dickinson). All flow cytometry gating and data analysis was performed using FACS Diva software from BD.
Author Contributions
Conceptualization, J.W.K. and J.M.; Methodology, J.W.K., J.M., and D.K.; Investigation, J.W.K., J.M., J.S.Y., A.R., W.K.P., and Y.H.; Writing – Original Draft, J.W.K., J.S.Y. J.M., and J.R.K; Writing – Review & Editing, J.W.K., J.M., J.S.Y., A.R., W.K.P., D.K. and I.V.B.; Funding Acquisition, M.S.L.; Supervision, J.W.K. and M.S.L.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work is supported by NIH (grants R01CA122930, R35CA197725, and R01NS093903).
Footnotes
Supplemental Information includes three figures and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2017.05.001.
Supplemental Information
References
- 1.Spencer D.A., Young J.S., Kanojia D., Kim J.W., Polster S.P., Murphy J.P., Lesniak M.S. Unlocking the promise of oncolytic virotherapy in glioma: combination with chemotherapy to enhance efficacy. Ther. Deliv. 2015;6:453–468. doi: 10.4155/tde.14.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane J.R., Miska J., Young J.S., Kanojia D., Kim J.W., Lesniak M.S. Sui generis: gene therapy and delivery systems for the treatment of glioblastoma. Neuro-oncol. 2015;17(Suppl 2):ii24–ii36. doi: 10.1093/neuonc/nou355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffinger B., Ahmed A.U., Lesniak M.S. Oncolytic virotherapy for malignant glioma: translating laboratory insights into clinical practice. Front. Oncol. 2013;3:32. doi: 10.3389/fonc.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann J.K., Chiocca E.A. Oncolytic virotherapy for gliomas: steps toward the future. CNS Oncol. 2013;2:389–392. doi: 10.2217/cns.13.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemunaitis J., Senzer N., Sarmiento S., Zhang Y.A., Arzaga R., Sands B., Maples P., Tong A.W. A phase I trial of intravenous infusion of ONYX-015 and Enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–893. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- 6.Au T., Thorne S., Korn W.M., Sze D., Kirn D., Reid T.R. Minimal hepatic toxicity of Onyx-015: spatial restriction of coxsackie-adenoviral receptor in normal liver. Cancer Gene Ther. 2007;14:139–150. doi: 10.1038/sj.cgt.7700988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opyrchal M., Aderca I., Galanis E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. Methods Mol. Biol. 2009;542:705–717. doi: 10.1007/978-1-59745-561-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanis E., Okuno S.H., Nascimento A.G., Lewis B.D., Lee R.A., Oliveira A.M., Sloan J.A., Atherton P., Edmonson J.H., Erlichman C. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 9.Pesonen S., Diaconu I., Cerullo V., Escutenaire S., Raki M., Kangasniemi L., Nokisalmi P., Dotti G., Guse K., Laasonen L. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int. J. Cancer. 2012;130:1937–1947. doi: 10.1002/ijc.26216. [DOI] [PubMed] [Google Scholar]
- 10.Kimball K.J., Preuss M.A., Barnes M.N., Wang M., Siegal G.P., Wan W., Kuo H., Saddekni S., Stockard C.R., Grizzle W.E. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin. Cancer Res. 2010;16:5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nokisalmi P., Pesonen S., Escutenaire S., Särkioja M., Raki M., Cerullo V., Laasonen L., Alemany R., Rojas J., Cascallo M. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.W., Auffinger B., Spencer D.A., Miska J., Chang A.L., Kane J.R., Young J.S., Kanojia D., Qiao J., Mann J.F. Single dose GLP toxicity and biodistribution study of a conditionally replicative adenovirus vector, CRAd-S-pk7, administered by intracerebral injection to Syrian hamsters. J. Transl. Med. 2016;14:134. doi: 10.1186/s12967-016-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair G.E., Dixon S.C., Griffiths S.A., Zajdel M.E. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989;14:339–346. doi: 10.1016/0168-1702(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 14.Younghusband H.B., Tyndall C., Bellett A.J. Replication and interaction of virus DNA and cellular DNA in mouse cells infected by a human adenovirus. J. Gen. Virol. 1979;45:455–467. doi: 10.1099/0022-1317-45-2-455. [DOI] [PubMed] [Google Scholar]
- 15.Katze M.G., Persson H., Johansson B.M., Philipson L. Control of adenovirus gene expression: cellular gene products restrict expression of adenovirus host range mutants in nonpermissive cells. J. Virol. 1983;46:50–59. doi: 10.1128/jvi.46.1.50-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevins J.R. Control of cellular and viral transcription during adenovirus infection. CRC Crit. Rev. Biochem. 1986;19:307–322. doi: 10.3109/10409238609082543. [DOI] [PubMed] [Google Scholar]
- 17.Jogler C., Hoffmann D., Theegarten D., Grunwald T., Uberla K., Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J. Virol. 2006;80:3549–3558. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H., Clise-Dwyer K., Ruisaard K.E., Fan X., Tian W., Gumin J., Lamfers M.L., Kleijn A., Lang F.F., Yung W.K. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE. 2014;9:e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulasov I.V., Zhu Z.B., Tyler M.A., Han Y., Rivera A.A., Khramtsov A., Curiel D.T., Lesniak M.S. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum. Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 20.Contreras J.L., Wu H., Smyth C.A., Eckstein C.P., Young C.J., Seki T., Bilbao G., Curiel D.T., Eckhoff D.E. Double genetic modification of adenovirus fiber with RGD polylysine motifs significantly enhances gene transfer to isolated human pancreatic islets. Transplantation. 2003;76:252–261. doi: 10.1097/01.TP.0000066361.02042.CA. [DOI] [PubMed] [Google Scholar]
- 21.Qiao J., Dey M., Chang A.L., Kim J.W., Miska J., Ling A., M Nettlebeck D., Han Y., Zhang L., Lesniak M.S. Intratumoral oncolytic adenoviral treatment modulates the glioma microenvironment and facilitates systemic tumor-antigen-specific T cell therapy. OncoImmunology. 2015;4:e1022302. doi: 10.1080/2162402X.2015.1022302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H., White E.J., Ríos-Vicil C.I., Xu J., Gomez-Manzano C., Fueyo J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J. Virol. 2011;85:4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein S.R., Jiang H., Hossain M.B., Fan X., Gumin J., Dong A., Alonso M.M., Gomez-Manzano C., Fueyo J. Critical role of autophagy in the processing of adenovirus capsid-incorporated cancer-specific antigens. PLoS ONE. 2016;11:e0153814. doi: 10.1371/journal.pone.0153814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broz M.L., Binnewies M., Boldajipour B., Nelson A.E., Pollack J.L., Erle D.J., Barczak A., Rosenblum M.D., Daud A., Barber D.L. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleijn A., Kloezeman J., Treffers-Westerlaken E., Fulci G., Leenstra S., Dirven C., Debets R., Lamfers M. The therapeutic efficacy of the oncolytic virus Delta24-RGD in a murine glioma model depends primarily on antitumor immunity. OncoImmunology. 2014;3:e955697. doi: 10.4161/21624011.2014.955697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleijn A., Kloezeman J., Treffers-Westerlaken E., Fulci G., Leenstra S., Dirven C., Debets R., Lamfers M. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE. 2014;9:e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon H., Idoyaga J., Rahman A., Leboeuf M., Remark R., Jordan S., Casanova-Acebes M., Khudoynazarova M., Agudo J., Tung N. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L., Kroemer G. CD103+ dendritic cells producing interleukin-12 in anticancer immunosurveillance. Cancer Cell. 2014;26:591–593. doi: 10.1016/j.ccell.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Boissonnas A., Fetler L., Zeelenberg I.S., Hugues S., Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J. Exp. Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda R., Fujita M., Zhu X., Sasaki K., Kastenhuber E.R., Kohanbash G., McDonald H.A., Harper J., Lonning S., Okada H. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin. Cancer Res. 2009;15:6551–6559. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu A., Oh S., Gharagozlou S., Vedi R.N., Ericson K., Low W.C., Chen W., Ohlfest J.R. In vivo vaccination with tumor cell lysate plus CpG oligodeoxynucleotides eradicates murine glioblastoma. J. Immunother. 2007;30:789–797. doi: 10.1097/CJI.0b013e318155a0f6. [DOI] [PubMed] [Google Scholar]
- 32.Wainwright D.A., Chang A.L., Dey M., Balyasnikova I.V., Kim C.K., Tobias A., Cheng Y., Kim J.W., Qiao J., Zhang L. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koks C.A., De Vleeschouwer S., Graf N., Van Gool S.W. Immune suppression during oncolytic virotherapy for high-grade glioma; yes or no? J. Cancer. 2015;6:203–217. doi: 10.7150/jca.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woller N., Gürlevik E., Fleischmann-Mundt B., Knocke S., Geffers R., Manns M.P., Kubicka S., Kühnel F. Virotherapy overcomes tumor resistance to PD-1-immunotherapy by broad mutanome-directed T cell responses in mice. Z Gastroenterol. 2015;53 doi: 10.1038/mt.2015.115. A4_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maizel J.V., Jr., White D.O., Scharff M.D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.W., Young J.S., Solomaha E., Kanojia D., Lesniak M.S., Balyasnikova I.V. A novel single-chain antibody redirects adenovirus to IL13Rα2-expressing brain tumors. Sci. Rep. 2015;5:18133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.