Abstract
Water stress is known to cause xylem cavitation in the leaves, roots and stems of plants, but little is known about the vulnerability of flowers to xylem damage during drought. This is an important gap in our understanding of how and when plants become damaged by water stress. Here we address fundamental questions about if and when flowers suffer cavitation damage, using a new technique of cavitation imaging to resolve the timing of cavitation in water-stressed flower petals compared with neighbouring leaves. Leaves and flowers from a sample of two herbaceous and two woody eudicots were exposed to a severe water stress while the spatial and temporal propagation of embolism through veins was recorded. Although in most cases water potentials inducing 50% embolism of herbaceous flower veins were more negative than neighbouring leaves, there was no significant difference between the average vulnerability of leaves and petals of herbaceous species. In both woody species, petals were more vulnerable to cavitation than leaves, in one case by more than 3 MPa. Early cavitation and subsequent damage of flowers in the two woody species would thus be expected to precede leaf damage during drought. Similar cavitation thresholds of flowers and leaves in the herb sample suggest that cavitation during water shortage in these species will occur simultaneously among aerial tissues. Species-specific differences in the cavitation thresholds of petals provide a new axis of variation that may explain contrasting flowering ecology among plant species.
Keywords: flower, vein, hydraulic, water stress
1. Introduction
Angiosperm reproduction, evolution and diversity are all associated with the unique characteristics of their floral organs [1–3], yet there is a distinct lack of knowledge about the functioning of these critical structures, particularly in terms of physiological costs associated with maintaining flowers. An important potential cost is associated with the provision of water, which plays a role in flower opening and pollination success [4]. Flower maintenance may require a considerable amount of water [5] and, under dry conditions, the water loss from flowers can be more than from the leaves [6]. Therefore, understanding the physiological characteristics of flower water-relations is likely to provide new insights into the evolution of flowers and pollination ecology.
Water flows through the vessels and veins of plant organs to ensure that tissues exposed to the hostile evaporative environment of terrestrial earth do not desiccate. Daytime water fluxes through the xylem plumbing are high, driven by a tension gradient generated at air–water interfaces on evaporating surfaces of the plant [7]. Mass flow through xylem cells is the only means by which sufficient water can be carried internally to support transpiring leaves, but the water potential gradient that drives this flow can also be damaging because drying soils or high evaporation can generate tensions in the xylem that are sufficient to cause cavitation. Xylem cavitation occurs when air is pulled across interfaces between xylem water and air resident in the plant body [8]. This process leads to air blockages in the xylem that ultimately cut the plant off from its water supply in the soil. A large body of evidence clearly demonstrates the vulnerability of stems, roots and leaves to this cavitation process, and the detrimental impact it has on plant productivity and survival [9,10]. By contrast, nothing is known about the vulnerability of flowers to xylem cavitation. This is an important knowledge gap considering our reliance upon plant reproductive structures for our food.
The first step in understanding how flowers respond to water stress is to examine how flowers are hydrated by the plant body. A significant body of work studying the flow of water into flower petals shows considerable variation in the rates of water loss from flowers [11]. Traditionally, the maintenance of hydration in flowers has been attributed to water delivery from the phloem [4,12–14], but recent evidence also indicates that in species with petals supporting significant transpiration (typically those possessing stomatal pores) the majority of water is supplied to the flowers by the xylem, in the same way as leaves [15]. Indeed, it could be expected that variation in the water-relations of petals should be high considering the multiple independent origins of floral structures in the angiosperm phylogeny [16,17]. Not only are flowers developmentally independent from leaves, but they also appear to have followed different evolutionary trajectories in key traits such as water transport and venation properties [18]. Although there is enormous variation in the size and morphology of flowers among angiosperm species, it has been observed that the flowers of basal angiosperm clades are more like leaves in terms of transpiration and xylem water supply [19]. This concept is supported by observations that the flowers of extant relatives of the basal ANITA and Magnoliid clades transpire more, and are thus more dependent upon xylem water supply than the more derived eudicot clades [5].
The suggestion that flowers of modern eudicots and monocots function relatively independently of xylem-delivered water, due to lower transpiration rates and a greater dependence on stored water and phloem-delivered water [19], has significant implications for flower physiology during stress. Phloem-supplied flowers could theoretically maintain hydration independently of the soil or the rest of the plant, potentially allowing flowering to occur while other parts of the plant may be water stressed. However, it is well known that many crop species are highly susceptible to damage and yield losses when exposed to water stress during flowering [20], suggesting that flowers are at least as vulnerable as leaves to desiccation damage. One possible cause of this susceptibility to damage would be if flowers, like leaves and stems, were vulnerable to xylem cavitation during water stress. If flowers of eudicots were significantly xylem-connected, then water stress could cause water potentials in the petals to fall to the point of xylem cavitation, causing the flowers to be cut off from the soil, leading to lethal desiccation. Here we examine this hypothesis using four species of eudicots to determine whether flowers of recently evolved angiosperm families are exposed to cavitation during water stress, and if so, how the process of cavitation proceeds.
The theory of xylem segmentation proposes that during drought, plant organs that represent relatively small cost to a tree (such as leaves) should have more vulnerable xylem than more expensive (upstream) organs such as stems [21]. As a result, during acute water stress, leaves would be expected to become hydraulically isolated from the tree by cavitation and shed during drought, reducing water loss and protecting more carbon-dense tissues [22,23]. It is uncertain how flowers may be prioritized in such a segmentation framework, but we hypothesized that in perennial woody plants, flowers would be sacrificed due to their low cost, while in annual herbs, where flowering occurs only once, species may prioritize flower survival above leaves to minimize the risk of flower damage during drought. Here we compare leaf and petal xylem vulnerability in two herb and two woody species to determine where petals are located on the scale of xylem vulnerability of the individual plant.
We use a new method of cavitation detection called xylem optical vulnerability (XOV) technique [24]. This technique works on the principle that cavitation leads to the sudden production of air–water interfaces in the large lumen of xylem cells due to the phase change from liquid to gas inside the lumen of xylem conduits [25]. The XOV technique detects xylem cavitation optically by measuring changes in light transmission through xylem tissue caused by air propagation into the xylem tissue. Studies on leaves have shown very strong correlation between the loss of water transport function, as measured by hydraulic techniques, and the accumulation of xylem embolism as measured by the XOV technique across a diverse range of species [26]. Unlike previous hydraulic methods this technique can be used on delicate tissues such as flower petals, thus giving us a first view of xylem vulnerability in flowers.
2. Material and methods
(a). Plant material
Four species were chosen to examine cavitation in leaves and petals; Pologala myrtifolia (woody Fabaceae), Passiflora tarminiana (woody Passifloraceae), Pisum sativum (herbaceous Fabaceae) and Solanum lycopersicum (herbaceous Solanaceae). Pologala myrtifolia is a hardy shrub that blooms throughout the year with a peak in spring. Passiflora tarminiana (passionfruit) is a widespread woody vine species. Solanum lycopersicon and P. sativum are annual herbaceous species. The two woody species were sampled from plants growing in the university grounds, while the herbaceous species were grown under glasshouse conditions of 22°C D : 15°C N temperature. Three individuals of each species were sampled.
(b). Optical xylem vulnerability
In the case of woody species, branches longer than the longest vessels (more than 1.5 m) were cut from plants during the morning and transported to the laboratory in plastic bags to prevent desiccation. In the case of the herbaceous species, whole plants were removed from pots and soil removed from the roots before transporting to the laboratory to measure the vulnerability to cavitation of leaves and petals.
We used the XOV technique (see http://www.opensourceov.org for details) employed with a flatbed scanner (Canon V800) to view changes in air embolism in the veins of leaves and flowers [26]. This technique allowed us to simultaneously measure neighbouring leaves and petals on a single branch, thus providing the most robust comparison of the relative vulnerability of these two tissues. Petals and leaves were fixed in position using adhesive tape to prevent any movement between scans (scanning interval 3 min), and a stem psychrometer (ICT, Armidale, Australia) was attached to the stem adjacent to the scanned area to monitor branch water potential. Psychrometer readings were supplemented with occasional pressure bomb measurements to ensure similarity between these different techniques. We assumed that the xylem water potential measured in the branch xylem represented an equilibrated water potential of leaves and petals. This assumption is based on the general observation that stomata were closed prior to major cavitation and that water potential equilibrium should exist due to the very slow rates of drying (over a period of several days) until the downstream tissues become hydraulically separated by very high levels of embolism [27,28]. Owing to the very low rates of transpiration prior to the onset of cavitation, the only way petals could have been at a different water potential from the stems would be if they were completely isolated from the xylem, and uniquely phloem-hydrated. This possibility is addressed in §4.
All scans were made in transmission mode, such that images were measured in transmitted light, meaning that embolisms were seen as dark regions inside veins. For each species a total of three branches, or whole individuals in the case of herbs, were imaged on the scanner, with one leaf and adjacent petal scanned per branch. Samples were scanned every minute over 2–6 days until all embolism had ceased for a period of 12 h, at which point the tissues were considered completely embolized. Analysis of the resultant image sequence captured during desiccation was carried out to identify rapid changes in light transmission through petal and leaf veins that corresponded to air entry into the xylem conduits. This was done using an image subtraction method [24,25] that highlights rapid changes in light transmission caused by bubble expansion, while filtering all other slow movement associated with drying. Spatio-temporal maps of cavitation propagation were produced and finally scaled with water potential data taken from the same branches.
Water stress was gradually induced in each sample by slow water loss from cut branches or exposed roots at 22°C under laboratory lighting (approx. 30 µmol quanta m−2 s−1) for between 48 and 144 h. Scanned leaves and petals received less light due to confinement in the scanner, but even here samples received 1 min of light during scans every 3 min.
(c). Leaf and petal anatomy and morphology
Petal area (PA) and leaf area (LA) were measured on scanned images of five leaves and petals. Petals and leaves were oven-dried at 75°C for 48 h to obtain dry weight. The petal dry mass per area (PMA, g m−2) and the leaf dry mass per area (LMA, g m−2) were calculated. Three to eight leaves and flowers were collected simultaneously and transported to the laboratory for sample processing. Sections of approximately 100 mm2 were taken from midway between the leaf midrib and margin; because of the high variability in vein density within a petal, we collected multiple 1 cm2 sections from all parts of the petals and sepals. For structures that were smaller than 100 mm2, we sampled the entire petal or sepal. Sections were then bleached in commercial heavy duty bleach (50 g l−1 sodium hypochlorite and 13 g l−1 sodium hydroxide) until clear. Bleach was removed by washing, after which sections were stained in 1% toluidine blue for 30 s to colour the lignin-rich veins. Stomatal density was measured directly from the paradermal sections. Finally, sections were mounted in phenol glycerine jelly and photographed with a Nikon Digital Sight DS-L1 camera (Melville, NY, USA) mounted on a Leica DM 1000 microscope (Nussloch, Germany) with a ×10 objective.
3. Results
(a). Leaf and petal morpho-anatomy
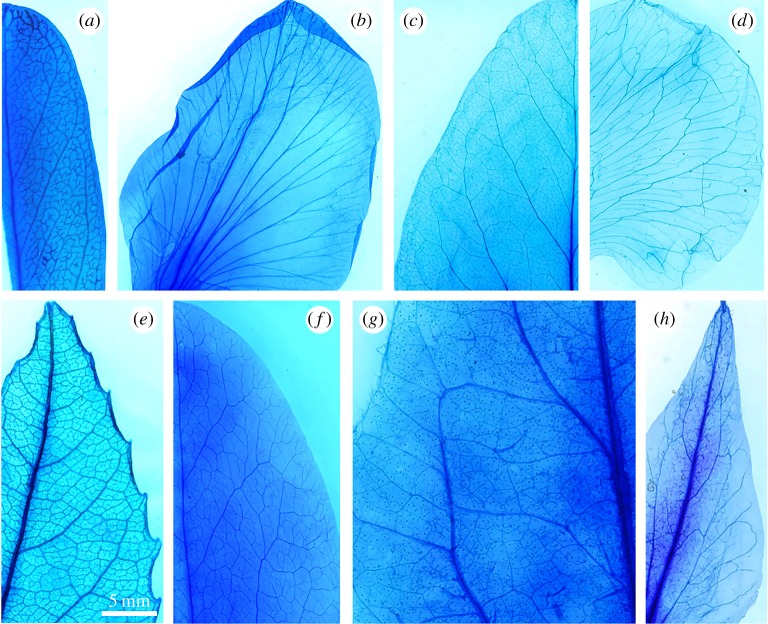
Vein densities in petals of all species were significantly lower than in leaves (table 1). On average, vein densities in petals were 34 ± 10% of those observed in leaves. Stomatal densities were also much lower in petals than in stems, with no stomata seen on the petals of P. sativum. Petals also exhibited a lower mass per area than the leaves, but there was some overlap in LMA and PMA overall, with the petals of P. myrtifolia found to have a higher mass per area than leaves of the other species. Vein topology in petals was different in both species of Fabaceae, with petals exhibiting a rather simple pattern of bifurcating veins compared with reticulate patterns in leaves. The other two species did not exhibit distinct differences in overall topology between leaves and petals other than reduced branching and lower vein density in the petals (figure 1; table 1).
Table 1.
The vein density and stomatal density of the studied species.
| species | family | organ type | vein density (mm mm−2) (mean ± s.e.) | stomatal density (mm−2) (mean ± s.e.) |
|---|---|---|---|---|
| Pologala myrtifolia | Fabaceae | leaf | 4.93 ± 0.20 | 88.56 ± 3.46 |
| petal | 2.24 ± 0.11 | 11.93 ± 2.43 | ||
| Passiflora tarminiana | Passifloraceae | leaf | 14.27 ± 0.03 | 533.35 ± 18.35 |
| petal | 3.12 ± 0.09 | 9.50 ± 0.85 | ||
| Pisum sativum | Fabaceae | leaf | 5.79 ± 0.23 | 154.92 ± 6.26 |
| petal | 2.25 ± 0.05 | 0 | ||
| Solanum lycopersicum | Solanaceae | leaf | 8.29 ± 0.19 | 259.39 ± 27.09 |
| petal | 2.48 ± 0.20 | 7.49 ± 4.87 |
Figure 1.
Micrographs of cleared and xylem-stained leaves and petals of the four eudicot species examined. (a) Leaf of P. myrtifolia, (b) petal of P. myrtifolia, (c) leaf of P. sativum, (d) petal of P. sativum, (e) leaf of P. tarminiana, (f) petal of P. tarminiana, (g) leaf of S. lycopersicum and (h) petal of S. lycopersicum.
(b). Leaf and petal cavitation
Leaves and petals dried for long periods of time before the initial cavitation events were observed propagating within the venation. The shortest period between initiation of scans and the recording of the first cavitation was 4.5 h in the leaf of S. lycopersicum and the longest period was 39 h in leaf of P. myrtifolia. Patterns of cavitation were easily captured using the image-difference analysis of image sequences, thus enabling maps of embolism spread to be constructed for neighbouring leaves and petals connected to a common stem subjected to desiccation (figures 2 and 3). A similar pattern of cavitation was observed in both leaves and petals whereby the lower order (larger) veins cavitated first followed by higher order veins, though the differences between vein orders were noticeably smaller in petals (figure 2). Large differences in vulnerability were observed between species and organs. The most vulnerable organ measured was the leaf of S. lycopersicum, which was found to cavitate 50% of its venation at a water potential (P50) of −1.18 MPa. At the other end of the spectrum, the most resistant organ was a leaf of Pologala with a P50 of −6.85 MPa (figure 4).
Figure 2.
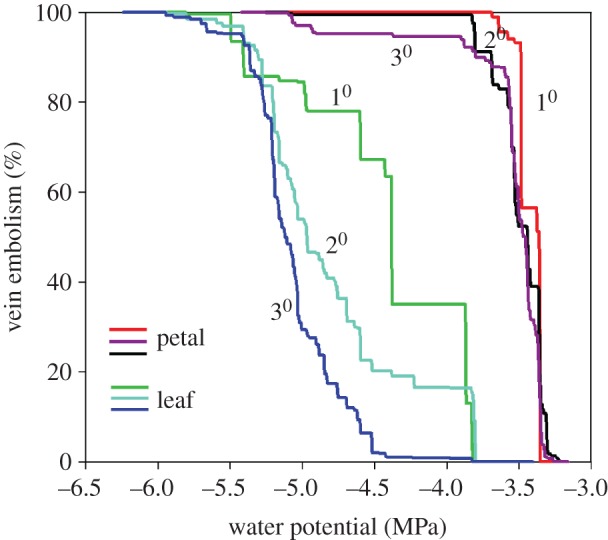
Cumulative area of cavitated 1st, 2nd and 3rd order veins of an adjacent leaf (green, cyan, blue lines) and petal (red, black and purple lines) of Passiflora tarminiana, expressed as a function of water potential. The resultant accumulation of air embolism is shown as a per cent of the maximum area. Step increases in %embolism indicate large cavitations that propagate among multiple veins.
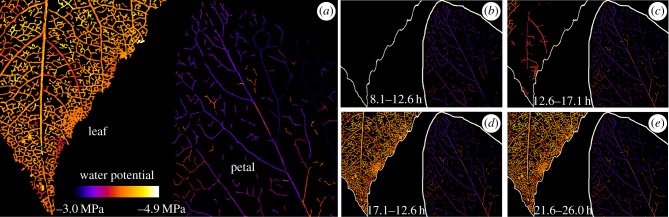
Figure 3.
Spatio-temporal maps showing the initiation and propagation of cavitation in an adjacent leaf and petal of Passiflora tarminiana attached to a single branch. (a) Shows a map of all vein cavitation using a colour scale to show the water potential at which different cavitation events occurred. The blue colours in the petal indicate much earlier cavitation (close to −3 MPa) in the petal compared with −4 to −5 MPa in the leaf. (b–e) The progression of cavitation in time starting with the first cavitations observed in the petal at around 8 h after branch excision. Colours follow the same water potential scale as (a). Time ranges after excision are shown in each panel.
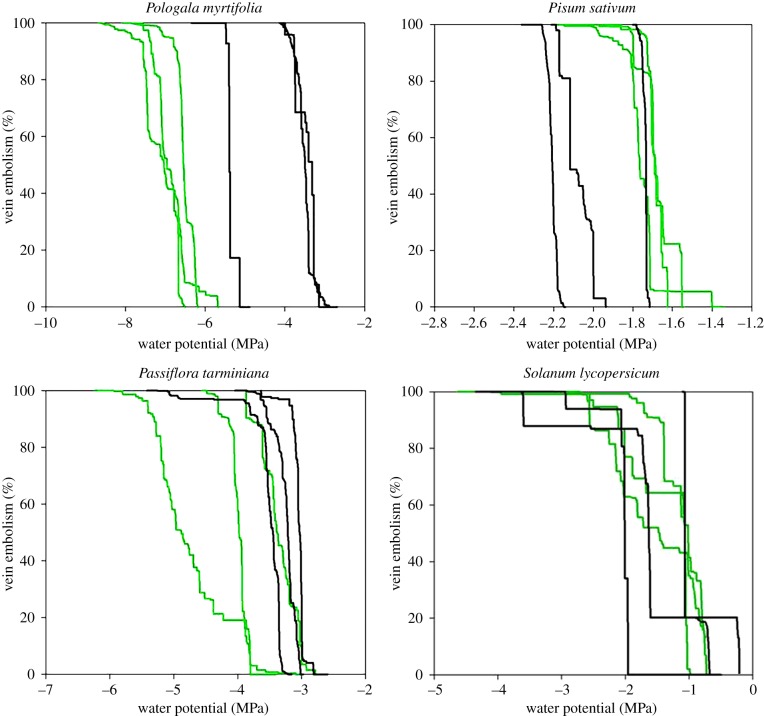
Figure 4.
Cumulative embolism curves for all veins in the leaves (green) and petals (black) of the three replicates of each species.
In both woody species, petals were found to be more vulnerable than leaves, but differences in mean P50 were only significant between leaves and petals of P. myrtifolia, where petals cavitated on average 3 MPa before the leaves. Within individual branches of the two woody species there was little overlap in the timing of petal and leaf embolism, with petals becoming completely cavitated before the initiation of cavitation in neighbouring leaves in most branches (figure 4).
In both herbaceous species, we found very small differences in the timing of leaf and petal cavitation, and no significant difference in mean P50 between petals and leaves of either species. In five out of six of the herbaceous samples, we found that leaves reached P50 before petals, contrasting with the pattern in woody species (figure 5).
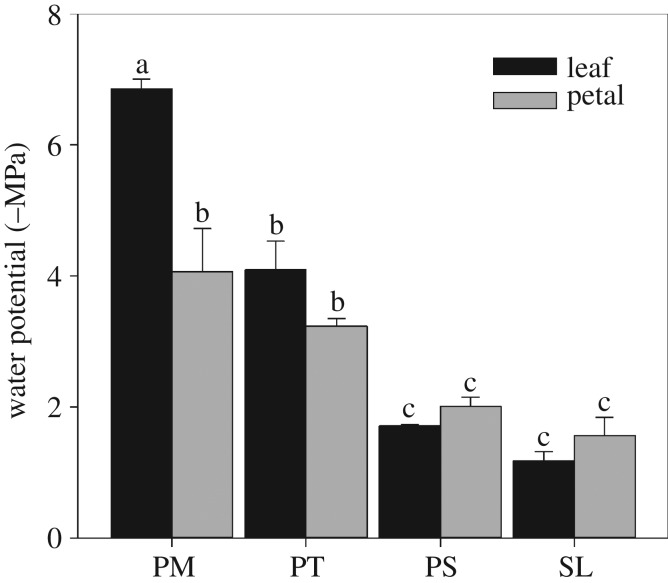
Figure 5.
Mean (with standard deviation; n = 3) water potential at 50% loss of xylem function in P. myrtifolia (PM), P. tarminiana (PT), P. sativum (PS) and S. lycopersicum (SL). Different letters denote significant differences between means.
The overlap between leaf and petal cavitation profiles among species followed a trend associated with tissue density, with higher LMA or PMA associated with more negative P50 (table 2). This trend was only significantly correlated across the whole dataset, but not within petal or leaf subgroups.
Table 2.
Leaf and petal dry mass per unit area of the studied species.
| species | family | LMA (g m−2) (mean ± s.e.) | PMA (g m−2) (mean ± s.e.) |
|---|---|---|---|
| Pologala myritifolia | Fabaceae | 97.15 ± 3.62 | 71.46 ± 1.99 |
| Passiflora tarminiana | Passifloraceae | 50.94 ± 1.65 | 19.17 ± 2.03 |
| Pisum sativum | Fabaceae | 24.59 ± 1.01 | 10.48 ± 0.37 |
| Solanum lycopersicum | Solanaceae | 42.79 ± 2.31 | 5.54 ± 0.26 |
4. Discussion
Our observations of cavitation in the xylem of petals of water stressed plants provided some answers to important questions about the plumbing and vulnerability of flowers to water stress. These first observations of xylem cavitation in flower petals demonstrate that flower petals behave similarly to leaves in terms of being vulnerable to cavitation damage of the xylem hydraulic system. By constructing spatio-temporal maps of embolism propagation within individual branches, we found that petals generally had similar or higher vulnerability to xylem cavitation than leaves. However, considerable variation was found to occur between species, and within our small sample we found greater convergence between leaf and petal P50 in the two herb species sampled than the woody plant sample.
Two possible conclusions could be drawn from the observations here that petal cavitation typically occurred before, or simultaneous with, cavitation in leaves. The most parsimonious explanation seems to be that petals were equilibrated with the branch water supply in our sample of eudicots, and that the petal xylem was similar, or more vulnerable to cavitation than leaves. The second possibility is that petals may be highly disconnected from the stem xylem, allowing them to remain more hydrated than the leaves during desiccation; although in this case the petal xylem would have to be far more vulnerable than the leaf xylem to account for the simultaneous or early cavitation of the petals compared to leaves. We certainly cannot rule out this second hypothesis due to the difficult task of measuring petal water potential; however, in the light of the temporal cavitation data presented here, this seems the less likely of the two explanations. If petals of these species were supplied with water from the phloem and disconnected from the xylem, as has been suggested for some other eudicots based on the presence of higher water potentials in the petals than in the neighbouring leaves [14], then it would be expected that petal cavitation might be delayed relative to leaves due to a hydraulic isolation of petals from low water potentials occurring in the leaf and stems of our samples. The concept of hydraulic isolation of reproductive organs is supported by observations in the fruits of many species [29], including tomato, one of the species sampled here [30]. However, this view has also been challenged by direct measurements of flow into tomato fruits using nuclear magnetic resonance imaging, which suggest that tomato fruits are well connected with the xylem and import most of their water from the xylem [31]. Our data indicate that flowers of our eudicot sample do not experience a hydraulic isolation that might shield them from declining leaf water potential during drought. It could possibly be argued that the rapid cavitation of flower xylem that we observed during water stress may itself provide a hydraulic isolation mechanism that could enable phloem to take over in supplying water under stress. This did not appear to be the case in the petals measured here because we observed that flower petals at the time of more than 50% xylem cavitation were highly desiccated and damaged (T. J. Brodribb 2017, unpublished observations). As has been observed with leaves [32], it appears that xylem cavitation in the petals leads rapidly to damage, probably due to a rapid decline in leaf water potential as tissue downstream of cavitated xylem becomes hydraulically isolated and rapidly desiccates.
An important goal of our research was to identify where flowers were located on the xylem vulnerability spectrum, both within and between species, with particular reference to the hydraulic segmentation theory predicting that low cost structures such as flower petals should cavitate and be lost before more expensive structures [33]. In the two woody species sampled, we found that flower petals were more vulnerable than leaves, as might be expected considering the low cost of petal production compared with the relatively large and carbon-dense stem and leaves. In the case of both leaves and flowers, it should be noted that even in the most vulnerable species stomatal closure during water stress precedes cavitation by a considerable margin [32]. However, upon rewatering, droughted plants have been shown to recover from between 50 and 88% loss of xylem function due to cavitation [10,34], meaning that damage to the xylem can be a significant cost to the recovering plant after drought, in terms of lost productivity or investment in repair [35]. Considering the large transpirational load often attributed to flowers [36], sacrificing flowers in a perennial plant makes sense as a mechanism for reducing water loss, thereby potentially avoiding damage to longer-lived tissues, conserving their function and delaying flowering until more favourable moisture conditions exist. It was interesting to note that flowers of the dry forest shrub P. myrtifolia were highly resistant to cavitation (more so than leaves and petals of all other species), possibly explaining why this species is capable of flowering even under substantial water stress [37]. Notably, we found that there was a large range of petal vulnerability among species and that the petals of both woody species were more resistant to cavitation than both the leaves and petals of the two herbaceous species. This may suggest either, that plants are exposed to selection for petal xylem vulnerability, or that selection for generally greater cavitation resistance in the xylem of woody plants leads to their flowers becoming secondarily more cavitation resistant.
It was interesting to note that, contrary to the woody species, the flowers of both herbaceous species studied here tended to cavitate after the leaves as water stress intensified. This observation aligns with ecology of these short-lived species whereby plants would be greatly penalized if their flowers were damaged prematurely by water stress. Constrained by a short plant lifespan, herbaceous species would derive little benefit from prioritizing protection of the vegetative plant body if this meant jeopardizing reproductive output. In perennial woody plants, the early cavitation of petals makes sense in terms of xylem segmentation to protect more costly tissues, but this strategy makes little sense for herbaceous plants. In this respect, data showing that stems, roots and leaves of tomato all share similar vulnerability to cavitation [32] aligns well with observations here that petals of tomato also fell into the same vulnerability range. More work is needed to determine whether the petals of herbaceous species are generally similar in xylem vulnerability to the leaves, and also to investigate whether some species may prioritize the xylem connections of reproductive organs above leaves. Our data suggested this may be the case in the two herbaceous species sampled here, but differences were too small to be significant.
5. Conclusion
Petals of eudicot species were found to experience cavitation damage to xylem tissue in their veins in a similar fashion to leaves. Herb flowers were similar to leaves in terms of vulnerability to cavitation, but in the two woody plants measured flowers were more vulnerable than leaves. These results suggest that a specific knowledge of flower vulnerability is necessary to determine the types of water stress likely to result in flower loss in trees and shrubs. Future research into the vulnerability of flowers to cavitation is needed to understand how this new dimension in plant water-relations may affect plant competition.
Data accessibility
More details about the methods used will be available after April 2017 at the following website: http://www.opensourceov.org.
Authors' contributions
T.J.B. conceived the study, T.J.B. and F.-P.Z. carried out the experiments, T.J.B. wrote introduction results and discussion, F.-P.Z. wrote the methods. Both authors contributed equally to figure preparation.
Competing interests
Authors declare no competing interests
Funding
T.J.B. received funding from an Australian Research Council Grant. F.-P.Z. received a postdoctoral grant from the Chinese Academy of Sciences.
References
- 1.Sargent RD, Ackerly DD. 2008. Plant–pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130. ( 10.1016/j.tree.2007.11.003) [DOI] [PubMed] [Google Scholar]
- 2.van der Niet T, Johnson SD. 2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol. Evol. 27, 353–361. ( 10.1016/j.tree.2012.02.002) [DOI] [PubMed] [Google Scholar]
- 3.Memmott J, Waser NM. 2002. Integration of alien plants into a native flower–pollinator visitation web. Proc. R. Soc. Lond. B 269, 2395–2399. ( 10.1098/rspb.2002.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galen C. 2005. It never rains but then it pours: the diverse effects of water on flower integrity and function. In Reproductive allocation in plants (eds Reekie EG, Bazzaz FA), pp. 75–93. New York, NY: Academic Press. [Google Scholar]
- 5.Roddy AB, Brodersen CR, Dawson TE. 2016. Hydraulic conductance and the maintenance of water balance in flowers. Plant Cell Environ. 39, 2123–2132. ( 10.1111/pce.12761) [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht SC. 2013. Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). Int. J. Plant Sci. 174, 74–84. ( 10.1086/668230) [DOI] [Google Scholar]
- 7.Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin, Germany: Springer. [Google Scholar]
- 8.Sperry JS, Tyree MT. 1988. Mechanism of water stress-induced xylem embolism. Plant Physiol. 88, 581–587. ( 10.1104/pp.88.3.581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choat B, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. ( 10.1038/nature11688) [DOI] [PubMed] [Google Scholar]
- 10.Brodribb TJ, Cochard H. 2009. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 149, 575–584. ( 10.1104/pp.108.129783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Doorn WG. 1997. Water relations of cut flowers. In Horticultural Reviews 18 (ed. J. Janick), pp. 1–85. Oxford, UK: John Wiley & Sons Inc. ( 10.1002/9780470650608.ch1) [DOI] [Google Scholar]
- 12.Nobel PS, Andrade JL, Wang N, North GB. 1994. Water potentials for developing cladodes and fruits of a succulent plant, including xylem-versus-phloem implications for water movement. J. Exp. Bot. 45, 1801–1807. ( 10.1093/jxb/45.12.1801) [DOI] [Google Scholar]
- 13.Trolinder N, McMichael B, Upchurch D. 1993. Water relations of cotton flower petals and fruit. Plant Cell Environ. 16, 755–760. ( 10.1111/j.1365-3040.1993.tb00496.x) [DOI] [Google Scholar]
- 14.Chapotin S, Holbrook NM, Morse SR, Gutierrez M. 2003. Water relations of tropical dry forest flowers: pathways for water entry and the role of extracellular polysaccharides. Plant Cell Environ. 26, 623–630. ( 10.1046/j.1365-3040.2003.00998.x) [DOI] [Google Scholar]
- 15.Feild TS, Chatelet DS, Brodribb TJ. 2009. Giant flowers of southern Magnolia are hydrated by the xylem. Plant Physiol. 150, 1587–1597. ( 10.1104/pp.109.136127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanis MJ, Soltis PS, Qiu YL, Zimmer E, Soltis DE. 2003. Phylogenetic analyses and perianth evolution in basal angiosperms. Ann. Mo. Bot. Garden 90, 129–150. ( 10.2307/3298579) [DOI] [Google Scholar]
- 17.Irish VF. 2009. Evolution of petal identity. J. Exp. Bot. 60, 2517–2527. ( 10.1093/jxb/erp159) [DOI] [PubMed] [Google Scholar]
- 18.Roddy A, Guilliams M, Lilittham T, Farmer J, Wormser V, Pham T, Fine P, Field T, Dawson T. 2013. Uncorrelated evolution of leaf and petal vein densities across the angiosperm tree of life. J. Exp. Bot. 64, 4081–4088. ( 10.1093/jxb/ert247) [DOI] [PubMed] [Google Scholar]
- 19.Feild TS, Chatelet DS, Brodribb TJ. 2009. Ancestral xerophobia: a hypothesis on the whole plant ecophysiology of early angiosperms. Geobiology 7, 237–264. ( 10.1111/j.1472-4669.2009.00189.x) [DOI] [PubMed] [Google Scholar]
- 20.Saini HS, Westgate ME. 1999. Reproductive development in grain crops during drought. Adv. Agron. 68, 59–96. ( 10.1016/S0065-2113(08)60843-3) [DOI] [Google Scholar]
- 21.Tyree MT, Ewers FW. 1991. The hydraulic architecture of trees and other woody plants. New Phytol. 119, 345–360. ( 10.1111/j.1469-8137.1991.tb00035.x) [DOI] [Google Scholar]
- 22.Alder N, Sperry J, Pockman W. 1996. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia 105, 293–301. ( 10.1007/BF00328731) [DOI] [PubMed] [Google Scholar]
- 23.Choat B, Lahr EC, Melcher PJ, Zwieniecki MA, Holbrook NM. 2005. The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant Cell Environ. 28, 1082–1089. ( 10.1111/j.1365-3040.2005.01336.x) [DOI] [Google Scholar]
- 24.Brodribb TJ, Bienaimé D, Marmottant P. 2016. Revealing catastrophic failure of leaf networks under stress. Proc. Natl Acad. Sci. USA 113, 4865–4869. ( 10.1073/pnas.1522569113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponomarenko A, Vincent O, Pietriga A, Cochard H, Badel E, Marmottant P. 2014. Ultrasonic emissions reveal individual cavitation bubbles in water-stressed wood. J. R. Soc. Interface 11, 20140480 ( 10.1098/rsif.2014.0480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodribb TJ, Skelton RP, McAdam SA, Bienaimé D, Lucani CJ, Marmottant P. 2016. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol. 209, 1403–1409. ( 10.1111/nph.13846) [DOI] [PubMed] [Google Scholar]
- 27.Bartlett MK, Klein T, Jansen S, Choat B, Sack L. 2016. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl Acad. Sci. USA 113, 13 098–13 103. ( 10.1073/pnas.1604088113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodribb TJ, Holbrook NM. 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 132, 2166–2173. ( 10.1104/pp.103.023879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews MA, Shackel KA. 2005. Growth and water transport in fleshy fruit. In Vascular transport in plants (eds Holbrook NM, Zwieniecki MA), pp. 181–197. New York, NY: Academic Press. [Google Scholar]
- 30.Ho LC, Grange RI, Picken AJ. 1987. An analysis of the accumulation of water and dry matter in tomato fruit. Plant Cell Environ. 10, 157–162. ( 10.1111/1365-3040.ep11602110) [DOI] [Google Scholar]
- 31.Windt CW, Gerkema E, Van As H. 2009. Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study. Plant Physiol. 151, 830–842. ( 10.1104/pp.109.141044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelton RP, Brodribb TJ, Choat B. 2017. Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytol. 214, 561–569. ( 10.1111/nph.14450) [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann MH. 1983. Xylem structure and the ascent of sap. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 34.Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S. 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 33, 672–683. ( 10.1093/treephys/tpt030) [DOI] [PubMed] [Google Scholar]
- 35.Brodribb TJ, Bowman DJMS, Nichols S, Delzon S, Burlett R. 2010. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 188, 533–542. ( 10.1111/j.1469-8137.2010.03393.x) [DOI] [PubMed] [Google Scholar]
- 36.Blanke MM, Lovatt CJ. 1993. Anatomy and transpiration of the avocado inflorescence. Ann. Bot. 71, 543–547. ( 10.1006/anbo.1993.1070) [DOI] [Google Scholar]
- 37.Palgrave KC. 2002. Trees of Southern Africa. Cape Town, South Africa: Struik. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
More details about the methods used will be available after April 2017 at the following website: http://www.opensourceov.org.