Abstract
It is widely assumed that organisms at low trophic levels, particularly microbes and plants, are essential to basic services in ecosystems, such as nutrient cycling. In theory, apex predators' effects on ecosystems could extend to nutrient cycling and the soil nutrient pool by influencing the intensity and spatial organization of herbivory. Here, we take advantage of a long-term manipulation of dingo abundance across Australia's dingo-proof fence in the Strzelecki Desert to investigate the effects that removal of an apex predator has on herbivore abundance, vegetation and the soil nutrient pool. Results showed that kangaroos were more abundant where dingoes were rare, and effects of kangaroo exclusion on vegetation, and total carbon, total nitrogen and available phosphorus in the soil were marked where dingoes were rare, but negligible where dingoes were common. By showing that a trophic cascade resulting from an apex predator's lethal effects on herbivores extends to the soil nutrient pool, we demonstrate a hitherto unappreciated pathway via which predators can influence nutrient dynamics. A key implication of our study is the vast spatial scale across which apex predators' effects on herbivore populations operate and, in turn, effects on the soil nutrient pool and ecosystem productivity could become manifest.
Keywords: trophic cascade, apex predator, arid ecology, predation, grazing exclosures, soil nutrients
1. Introduction
It is widely assumed that organisms at low trophic levels, particularly microbes and plants, are essential to basic services in ecosystems, such as nutrient cycling and carbon storage [1,2]. However, increasingly, it is being realized that terrestrial apex predators can influence nutrient dynamics, and vegetation structure and composition at small spatial scales where predators function as vectors of nutrients from one ecosystem to another [3] or on island ecosystems where predators suppress the abundances of species that function as nutrient vectors [4,5].
A key interaction pathway, via which large carnivores are hypothesized to shape ecosystems, is through trophic cascades arising from the suppressive effects they have on their herbivore prey [6–8]. According to trophic cascade theory, predators can limit herbivores' consumption of plants through the process of predation whereby they suppress herbivore populations by directly killing them [9,10]. Predators can also benefit plants if the fear that they instil causes herbivores to shift their patterns of habitat use and in so doing reduce herbivores' consumption of plants [11,12]. Thus, owing to the combined effects of predators' lethal and non-lethal effects on herbivores, trophic cascade theory predicts that there should be a greater biomass of the plant species preferred by herbivores in areas where predators are present than absent [6].
However, a criticism made of many studies reporting trophic cascades in terrestrial ecosystems is that the evidence is not experimental and thus, it remains possible that patterns attributed to the direct and indirect effects of large carnivores could be due to other underlying factors [13]. Although conducting manipulative experiments on terrestrial apex predators is often not possible due to legal and logistic constraints, strong evidence for trophic cascades can be compiled by providing mechanistic support for top-down control of herbivores by carnivores using observations of the behaviour, demography and survival of herbivores, the diets of carnivores and for herbivore–plant interactions by manipulating herbivores' access to plants [12–14].
In theory, apex predators' effects on ecosystems could extend to nutrient cycling and the soil nutrient pool by influencing the intensity and spatial organization of herbivory [15–18]. This could occur because herbivores influence the soil nutrient pool by modifying the composition of plant assemblages and quality of litter deposited, reducing the quantity of litter deposited and altering how plants distribute carbon and nutrients in their tissues [15,19–21]. Furthermore, herbivores can function as vectors that transport nutrients across landscapes [20] and fertilize soils through excretion of dung and urine [22] and by decomposition of their tissues following death [23].
The trigger–transfer–reserve–pulse model (TTRPM) for the function of arid and semi-arid ecosystems predicts that nutrients produced from vegetation growth in response to rainfall and deposition of plant material, positively feedback into reserves or resource sinks [24]. Furthermore, by causing the flow of water and wind across the land surface to be more tortuous, vegetation patches reduce the velocity of flows of wind and water near the soil surface and in turn the mobilization of materials such as leaf litter and soil particles across the landscape [24]. Vegetation patches also function as nutrient sinks because they are obstacles which trap and accumulate nutrients that are transferred across the landscape [2,24].
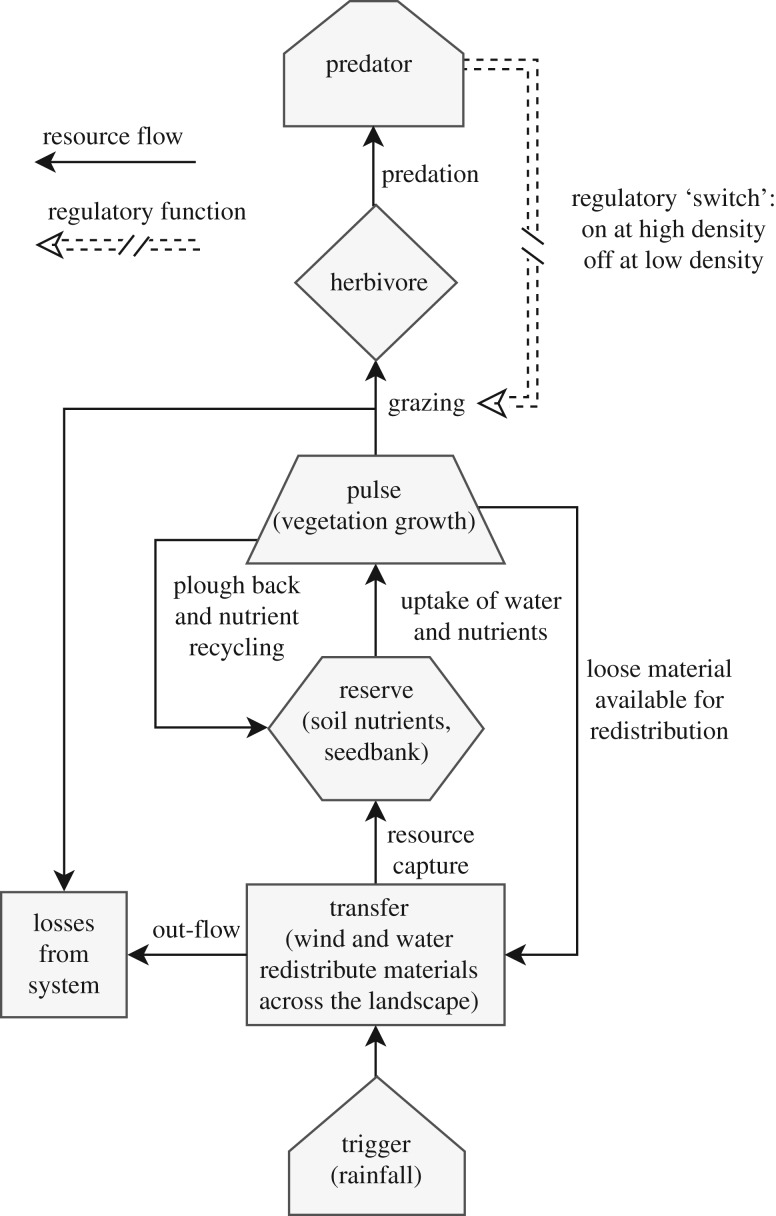
In its original form, the TTRPM did not explicitly incorporate the effects of predators [24]. We have modified the TTRPM by incorporating the predictions of trophic cascade theory (figure 1). Our modified TTRPM referred to hereafter as the top-down–TTRPM (TDTTRPM) proposes that predators have a moderating effect on grazing pressure by reducing herbivore numbers and altering their behaviour through fear, allowing for positive indirect effects on biomass accumulation. According to the TDTTRPM, excessive consumption of vegetation by herbivores in the absence of apex predators can decouple feedback loops between vegetation growth, litter-fall and the soil nutrient pool. In addition, reduced vegetation cover should reduce landscapes capacity to capture and retain nutrients because exports of nutrients by wind and water are greater when vegetation is denuded. Subsequently, the TDTTRPM predicts that heavily grazed landscapes should have diminished nutrient reserves and exhibit reduced ‘pulse’ growths of vegetation following rainfall [24].
Figure 1.
Top-down–trigger–transfer–reserve–pulse model (adapted from the trigger–transfer–reserve–pulse model of Ludwig et al. [24]), describes the movement of materials in semi-arid and arid lands. Solid arrows indicate flows of materials between elements (boxes) in ecosystems. Rainfall events function as a ‘trigger’ for ‘pulses’ of plant growth. Flows of wind and water ‘transfer’ materials and nutrients across the landscape. Transported materials are lost through ‘out-flow’ or captured by obstacles in the landscape which interrupt fluid flows, such as vegetation patches, where they become incorporated into the soil which functions as a ‘reserve’. Nutrients and seeds stored in the soil promote pulses of vegetation of growth. Vegetation growth and resources are recycled through the deposition of senescent plant material and seeds back into the ‘reserve’ or consumed by herbivores. Loss of vegetation cover due to drought or excessive grazing by herbivores can decouple feedback loops between vegetation growth, litter-fall and the local soil nutrient pool. Predators limit consumption of plant biomass through predation and the fear they instil (dashed arrow) and thus promote the positive feedback loop between vegetation production, litter deposition and the soil nutrient pool and the capture of transported nutrients by vegetation.
Here, we take advantage of a long-term manipulation of dingo (Canis dingo) abundance in Australia's Strzelecki Desert to investigate the effects that removal of a large mammalian carnivore has on herbivore abundance, vegetation and the soil nutrient pool. In this region, the existence of a dingo-proof barrier fence enabled us to compare kangaroo abundances and dingo diets, and experimentally evaluate the effects of excluding kangaroos on vegetation and soil nutrients in nearby landscapes where dingoes were common and rare, thus facilitating assessment of dingoes' indirect effects on plants and soils. Applying the TDTTRPM (figure 1), we predicted, that: (i) kangaroo abundance would be greater where dingoes were rare, than common, (ii) plant cover would be greater in herbivore exclusion plots where dingoes were rare, but show a negligible response to herbivore exclusion where dingoes were common, and (iii) soil concentrations of available phosphorus, total carbon and total nitrogen would be greater in herbivore exclusion plots where dingoes were rare but show a negligible response to herbivore exclusion to where dingoes were common. In addition, to provide mechanistic support for prediction (i), we used scat analysis to quantify the diets of dingoes where they were common and rare in order to relate the occurrence of kangaroo remains in dingo scats to the frequency of alternative prey items and the abundance of kangaroos.
2. Methods
(a). Study site
Dingoes have destructive impacts on livestock, particularly sheep [25]. The dingo fence (electronic supplementary material, figure S1) was built for the purpose of preventing immigration of dingoes into sheep grazing lands that occupy the southeast of the continent [26]. The fence was constructed from 1900 to the 1960s and extends approximately 5600 km across South Australia, New South Wales (NSW) and Queensland [26]. The NSW section of the dingo fence follows the state boundaries of NSW/South Australia and NSW/Queensland (electronic supplementary material, figure S1). The state borders of NSW with South Australia and NSW with Queensland are arbitrary administrative boundaries that follow the meridians 29° S and 141° E, respectively. These borders were declared by royal decree in the nineteenth century prior to the area's exploration and settlement by colonists [27]. Therefore, the NSW section of the dingo fence does not represent any natural physical boundary.
In combination with the dingo fence, intensive population control of dingoes via shooting and use of baits laced with poison 1080 (sodium fluoroacetate) occurs on the NSW side of the fence [26]. Dingoes are only subject to intermittent control mostly by shooting on the South Australian side of the dingo fence [28,29]. Owing to differences in the intensity of control, dingoes are common north and west of the fence in Queensland and South Australia, which we term ‘outside’, but are rare south and east on the ‘inside’ of the fence [28,29].
The study was conducted in two conservation reserves, Sturt National Park (29°9′ S, 141°2′ E) and Strzelecki Regional Reserve (29°24′ S, 140°33′ E) and nearby pastoral properties which are situated on either side of the dingo fence in the Strzelecki Desert in NSW and South Australia (electronic supplementary material, figure S1). The landscape at all sites comprised longitudinal sand dunes and clay inter-dunal areas [27]. The study region receives less than 250 mm of annual rainfall [30]. Vegetation in the study region is classified as a Sand Plain Mulga Shrubland community [30] and is characterized by a sparse overstorey of perennial shrubs (Acacia aneura, Ac. ligulata, Dodonaea viscosa) and a short (less than 40 cm) understorey of ephemeral grasses (Aristida contorta, Eragrostis spp., Sporobolus actinocladus) and forbs (Sclerolaena spp., Portulaca oleracea, Salsola australis).
(b). Fauna assessments
We indexed abundances of dingoes and kangaroos (Macropus rufus and M. giganteus) by conducting nocturnal spotlight transects from May 2012 to June 2016, at approximately four-month intervals, at two sites on each side of the dingo fence on each sampling occasion (figure 2). Surveys were conducted at Sturt National park and the pastoral property Winnathee (29°47′ S, 141°9′ E) on the ‘inside’ of the dingo fence and at Strzelecki Regional Reserve and the pastoral property Quinyambie (29°48′ S, 140°49′ E) ‘outside’ of the dingo fence.
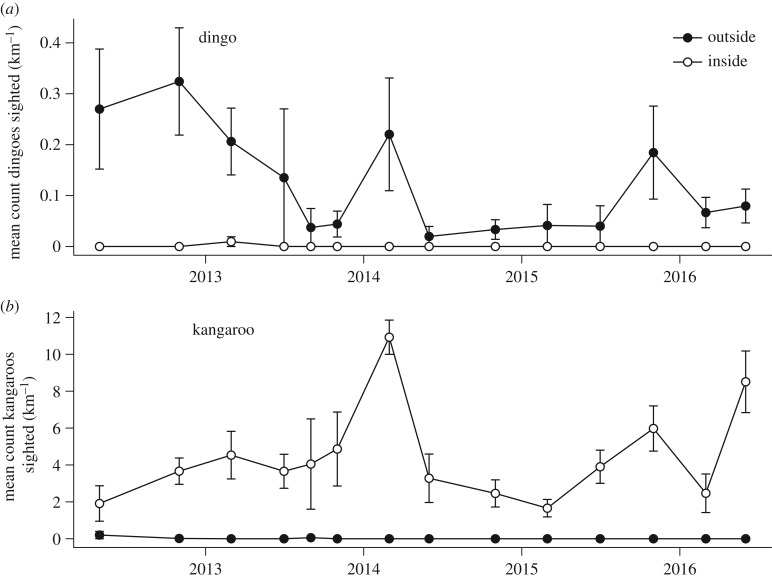
Figure 2.
The abundance of (a) dingoes and (b) kangaroos expressed as the mean number of individuals sighted per kilometre surveyed (±1 s.e.m.) during spotlight surveys conducted on each side of the dingo fence between May 2012 and June 2016. Open symbols indicate sites located inside the dingo fence where dingoes were rare and closed symbols indicate sites located outside the dingo fence where dingoes were common.
At each site, on each sampling occasion, we conducted approximately 30 km of spotlight surveys along single-lane dirt vehicle tracks. Counts were made by an observer using a 50 W spotlight while standing on the back of a vehicle moving at approximately 15 km h−1. Spotlight surveys were appropriate for assessing animal abundances in the study area as the flat landscape and low-lying vegetation provided an unobstructed view over a long distance [28]. Because the abundance of kangaroos was very low where dingoes were common (eight kangaroos were sighted in 786 km of spotlight survey), we were unable to estimate densities of kangaroos using distance sampling methods. Consequently, for analyses, the abundance of dingoes and kangaroos at each site was calculated as the mean number of individuals sighted per kilometre of survey on each sampling occasion.
(c). Dingo diet
Searches for dingo scats were undertaken by walking along the same vehicle tracks as the spotlight surveys for approximately 5 km on each sampling occasion. On collection, scats were placed into paper bags and air-dried. In the laboratory, scats were oven-dried overnight at 100°C, then placed individually in nylon bags and washed in a washing machine. Following washing, items present in the scats were identified to the lowest possible taxonomic level using microscopic analysis of diagnostic residues (i.e. hair cross-sections, teeth, claws) and comparison against known reference specimens. The frequency of occurrence of items in the categories kangaroo, rodent, rabbit, livestock, mammalian mesopredator, bird, arthropod and vegetation was calculated as the number of scats in which the dietary item was identified divided by the total number of scats sorted on each side of the dingo fence. We explored the dietary functional response of dingoes to kangaroos by plotting the abundance of kangaroos versus the frequency of kangaroo remains in dingo diets at sites on sampling occasions when more than five dingo scats were collected.
(d). Herbivore exclusion experiment
Because dingoes are common on one side of the fence and rare on the other, it provides a unique opportunity to measure trophic cascades on a large scale [31]. However, most of the landscape on each side of the dingo fence is used as rangeland to graze livestock at low densities. To remove the influence of livestock grazing, the herbivore exclusion experiment was conducted solely in conservation reserves where livestock were absent that were situated on either side of the dingo fence approximately 60 km apart with similar elevation, climate, landforms and vegetation. Another constraint imposed by using the dingo fence as an experimental manipulation is that the treatments are by necessity spatially segregated, so it is difficult to control for effects that could arise due to underlying gradients in physical variables. To reduce the influence of underlying physical gradients, we conducted identical herbivore exclosure experiments on each side of the dingo fence and asked whether the effects of herbivores on vegetation and soils differed where dingoes were common and rare, respectively. We hypothesized that if dingoes exert indirect effects on vegetation and soils as predicted by the TDTTRPM, herbivores should have negligible effects on vegetation and soil nutrients where dingoes were common. Conversely, we hypothesized that where dingoes were rare, herbivores should have suppressive effects on vegetation and soil nutrients.
Identical grazing exclosure experiments were established at the same time, on both sides of the dingo fence, at Sturt National Park and Strzelecki Regional Reserve in August 2013. At each reserve, four paired exclosure (‘ungrazed’) and control (‘grazed’) plots were established, six months following a wildfire event, and were matched for aspect, elevation and vegetation to reduce variability between treatments. We also attempted to establish a procedural control treatment consisting of a 30 cm wire fence made of the same mesh as the exclusion fences. However, we were unable to maintain these fences at sites inside the dingo fence due to damage caused by collisions with kangaroos that attempted to hurdle them. As a result, we were unable to measure the effects of a procedural control.
Each pair of grazing treatments (ungrazed/grazed) was positioned in a separate inter-dunal ‘swale’ 0.5–1 km apart between sand dunes, to capture vegetation and soil heterogeneity, characteristic of the landscape [24]. The kangaroo exclosure plots (11 m width × 11 m length × 2 m height) were constructed from wire mesh. Plot dimensions were 11 × 11 m to allow for a 10 × 10 m study area and 0.5 m along each side to mitigate fence effects in surveys. The exclosure plots had 2 m high wire mesh to prevent access by kangaroos. The wire mesh (10 × 10 cm openings) allowed access to rabbits and other small mammals. Therefore, grazing by rabbits was considered constant across both plot types. The control plots were marked by four metal posts.
To measure vegetation cover, five 1 × 1 m quadrats were randomly allocated within each plot using a 10 × 10 m grid system. Two adjacent sides of the plot were numbered 1–10 and two randomly selected numbers were produced to determine quadrat location. Ground cover within the quadrat was classified as bare ground, litter, dung, live grass, dead grass and forb to the species name. Total vegetation cover comprised the sum of live grass, dead grass and forb cover. Ground cover was recorded as a percentage. Vegetation data, measured tri-annually in late summer, winter and late spring, were compiled from June 2014 to October 2016.
To assess whether soil characteristics varied across grazing treatments, nine soil cores were extracted within each plot in July 2015, November 2015 and June 2016. Cores were systematically extracted, with a soil auger (8 cm in diameter), in a 3 × 3 grid, at a depth of 5 cm. Cores were composited for each plot, with a total of 16 composite samples for each time period and stored in sealed bags. Compositing samples reduces short-range variability within each plot, as well as analytical costs [19].
Soil samples were oven-dried at 40°C and soil crushed to less than 2 mm in preparation for testing. Total carbon (%) and total nitrogen (%) were analysed by Dumas high-temperature combustion using a LECO CNS Analyser, following methods 6B2b and 7A5, respectively, in Rayment & Lyons [32]. Plant-available phosphorus (mg kg−1) was analysed by the Bray II extraction method (0.03 M NH4F in 0.1 M HCl) for soils greater than or equal to pH 7.5, as per methods 9E2 in Rayment & Lyons [32].
(e). Statistical analyses
All analyses were coded in the R Statistical Environment v. 3.3.2 [33]. Negative binomial generalized linear models were used to determine differences in fauna abundance either side of the dingo fence and over time. Abundance data were analysed using raw spotlight counts and a survey effort (survey length in kilometres) was included in the model as an offset variable to account for varying survey lengths. Negative binomial models were chosen to account for over-dispersion due to the large number of zeros experienced in the dataset [34]. Models were performed using the glm.nb function from the ‘MASS’ package [35] and significance of model terms were assessed with likelihood ratio tests.
We fitted a beta regression model to predict the proportion of dingo scats containing kangaroo as a function of kangaroo abundance [36]. Because the y variable for the beta regression model included zeroes, we transformed the y variable using the transformation (y×(n – 1) + 0.5)/ n, where n is the sample size [37].
We used linear mixed effects models to determine if there were differences in plant cover and soil nutrients between grazing treatments on either side of the dingo fence and over time. Models were individually conducted for each national park, on either side of the dingo fence, as we were interested in comparing within-site treatment effects and it was not possible to establish replicate study areas within conservation reserves on each side of the dingo fence. Models were performed using the lme and lmer functions from the ‘nlme’ package [38] and ‘lme4’ package [39], respectively. A square root transformation was used, when required, to meet model specifications. Significance of models terms was determined with likelihood ratio tests.
3. Results
(a). Dingo and kangaroo abundance
There was an inverse relationship between dingo and kangaroo abundance. During the 1500 km of spotlight surveying conducted over 14 sampling trips from 2012 to 2016, we sighted just one dingo inside the fence, compared with 85 dingoes outside the fence. During the same spotlight surveys, we sighted a total of 3245 kangaroos inside the fence compared with eight kangaroos outside the fence.
In accordance with the raw counts, generalized linear models showed that dingo abundance was on average higher outside than inside the dingo fence (χ2 = 108.62, d.f. = 1, 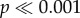 ; figure 2a; electronic supplementary material, table S2). Dingo sightings also varied between sampling trips (χ2 = 45.09, d.f. = 13, p < 0.001) but no ‘fence’ by ‘trip’ interaction was identified (χ2 = 3.29, d.f. = 13, p > 0.05). Kangaroo abundance was consistently higher inside the dingo fence (χ2 = 681.73, d.f. = 1,
; figure 2a; electronic supplementary material, table S2). Dingo sightings also varied between sampling trips (χ2 = 45.09, d.f. = 13, p < 0.001) but no ‘fence’ by ‘trip’ interaction was identified (χ2 = 3.29, d.f. = 13, p > 0.05). Kangaroo abundance was consistently higher inside the dingo fence (χ2 = 681.73, d.f. = 1, 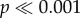 ; figure 2b; electronic supplementary material, table S3). Spotlight surveys revealed a significant ‘fence’ by ‘trip’ interaction for kangaroo numbers (χ2 = 36.27, d.f. = 13, p < 0.001), due to the fluctuations in kangaroo numbers between trips inside the fence compared with the consistently low or non-existence of kangaroos outside the fence.
; figure 2b; electronic supplementary material, table S3). Spotlight surveys revealed a significant ‘fence’ by ‘trip’ interaction for kangaroo numbers (χ2 = 36.27, d.f. = 13, p < 0.001), due to the fluctuations in kangaroo numbers between trips inside the fence compared with the consistently low or non-existence of kangaroos outside the fence.
(b). Dingo diet
Where dingoes were rare, the most frequently occurring species in dingo scats (n = 90) were kangaroos (32%) and rodents (32%; electronic supplementary material, table S1). Invertebrates and rabbits occurred in 29% and 21% of dingo scats where dingoes were rare, respectively. Where dingoes were common and kangaroos were rare, kangaroo remains occurred in just 1% of scats (n = 468). Small mammals and rabbits were the dominant dietary items of dingoes at sites where dingoes were common and occurred in 63% and 54% of scats, respectively (electronic supplementary material, table S1). There was a positive relationship between the frequency of kangaroo remains in dingo scats and abundance of kangaroos (z = 7.42, d.f. = 23, p < 0.001, pseudo-R2 = 0.72; electronic supplementary material, figure S2 and table S4). Notably, where dingoes were common, kangaroo remains occurred in dingo scats when kangaroo abundances were below detectable densities using the spotlight survey method.
(c). Vegetation
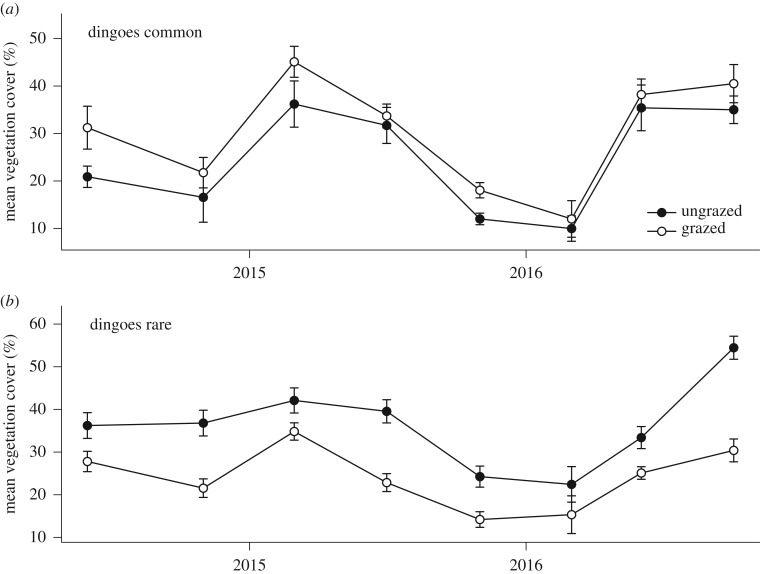
Grazing by kangaroos suppressed vegetation cover where dingoes were rare but not where dingoes were common (figure 3; electronic supplementary material, figure S3; tables S5 and S6). Where dingoes were common and kangaroos rare, vegetation cover did not differ between grazed and ungrazed plots (χ2 = 3.46, d.f. = 1, p > 0.05; figure 3a). Vegetation cover fluctuated through time in response to rainfall events (χ2 = 27.11, d.f. = 7, p < 0.001) in similar ways in both grazed and ungrazed plots, but there was no ‘treatment’ by ‘trip’ interaction (χ2 = 2.08, d.f. = 7, p > 0.05).
Figure 3.
Mean percentage vegetation cover (±1 s.e.m.) in ‘ungrazed exclosures’ (closed symbols) and grazed control plots (open symbols) located (a) outside the dingo fence where dingoes were common and (b) located inside the fence where dingoes were rare. Data presented are for surveys conducted between June 2014 and October 2016.
Where dingoes were rare and kangaroos abundant, vegetation cover was consistently greater in ‘ungrazed’ exclosure plots compared with control ‘grazed’ plots (χ2 = 18.02, d.f. = 1, p < 0.001; figure 3b) and fluctuated through time with increases in vegetation cover occurring after significant rainfall events (χ2 = 17.79, d.f. = 7, p < 0.05). However, the interaction between ‘treatment’ and ‘trip’ was not significant (χ2 = 8.16, d.f. = 7, p > 0.05), indicating that fluctuations in vegetation cover through time were similar in both grazed and ungrazed plots.
(d). Soil nutrients
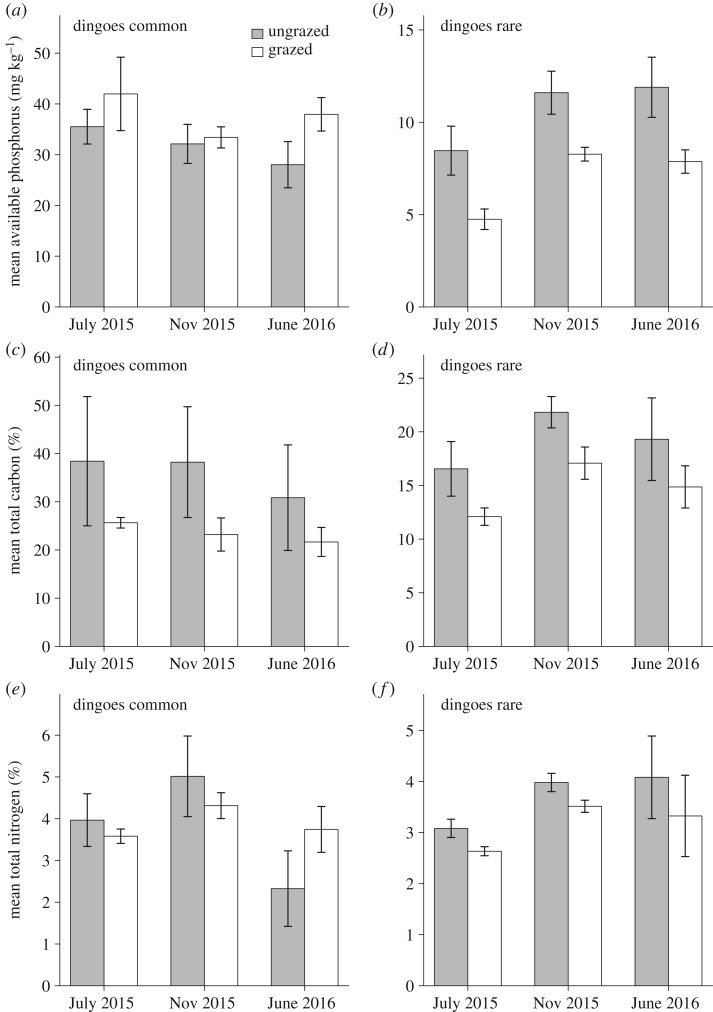
Grazing by kangaroos suppressed levels of available phosphorous where dingoes were rare but not where dingoes were common (figure 4a,b; electronic supplementary material, tables S7 and S8). Where dingoes were common, no differences were identified in phosphorus levels between grazing treatments (χ2 = 0.24, d.f. = 1, p > 0.05; figure 4a;) or between sampling periods (χ2 = 4.49, d.f. = 2, p > 0.05) and there was no ‘treatment’ by ‘trip’ interaction (χ2 = 4.86, d.f. = 2, p > 0.05). Where dingoes were rare, levels of available phosphorus were greater in ungrazed plots than grazed plots (χ2 = 10.35, d.f. = 1, p < 0.01; figure 4b) and varied across the three sampling periods (χ2 = 8.85, d.f. = 2, p < 0.05), with lower levels in the first sampling trip, July 2015. However, treatment effects were consistent over time with no ‘treatment’ by ‘trip’ interaction (χ2 = 0.54, d.f. = 2, p > 0.05).
Figure 4.
Mean soil nutrient levels (±1 s.e.m.), in ‘ungrazed exclosure’ plots (grey bars) and grazed control plots (white bars). (a) Mean available phosphorus, by Bray 2 method, in milligrams per mass of soil (mg kg−1), outside the fence where dingoes were common and (b) inside the fence where dingoes were rare. (c) Mean per cent total carbon outside the dingo fence where dingoes were common and (d) inside the fence where dingoes were rare. (e) Mean per cent total nitrogen outside the dingo fence where dingoes were common and (f) inside the fence where dingoes were rare.
Grazing by kangaroos suppressed soil carbon concentrations where dingoes were rare but not where dingoes were common (figure 4c,d; electronic supplementary material, tables S9 and S10). Where dingoes were common, total soil carbon concentrations did not differ between ungrazed and grazed plots (χ2 = 1.47, d.f. = 1, p > 0.05; figure 4c), but did vary between sampling trips (χ2 = 6.34, d.f. = 2, p < 0.05). The interaction between ‘treatment’ and ‘trip’ was not significant, indicating that fluctuations in soil carbon through time were similar in both grazed and ungrazed plots (χ2 = 0.80, d.f. = 2, p > 0.05). By contrast, where dingoes were rare, total soil carbon concentrations were greater in ungrazed than grazed plots (χ2 = 8.25, d.f. = 1, p < 0.01; figure 4d) and varied across sampling trips (χ2 = 10.23, d.f. = 2, p < 0.01). Lack of a ‘treatment’ by ‘trip’ interaction (χ2 = 0.03, d.f. = 2, p > 0.05) indicated that treatment effects on soil carbon were consistent across sampling trips.
Grazing by kangaroos suppressed soil total nitrogen concentrations where dingoes were rare but not where dingoes were common (figure 4e,f; electronic supplementary material, tables S11 and S12). Where dingoes were common, total nitrogen concentrations did not differ between grazing treatments (χ2 = 0.002, d.f. = 1, p > 0.05; figure 4e) and total nitrogen concentrations varied across sampling periods (χ2 = 11.67, d.f. = 2, p < 0.01). The ‘treatment’ by ‘trip’ interaction was significant (χ2 = 16.11, d.f. = 2, p < 0.001), due to a drop in nitrogen levels in ungrazed plots during June 2016, compared with previous sampling trips. Where dingoes were rare, total nitrogen concentrations were greater in ungrazed plots compared with grazed plots (χ2 = 9.72, d.f. = 1, p < 0.01; figure 4f). Total nitrogen concentrations varied significantly across sampling periods (χ2 = 14.70, d.f. = 2, p < 0.001). Lack of a ‘treatment’ by ‘trip’ interaction indicated that treatment effects for soil nitrogen were consistent over time (χ2 = 0.09, d.f. = 2, p > 0.05).
4. Discussion
In accordance with our predictions generated from the TDTTRPM, kangaroos were more abundant where dingoes were rare, and the effects of kangaroo exclusion on vegetation were marked where dingoes were rare, but negligible where dingoes were common. Furthermore, kangaroo exclusion had a strong effect on total carbon, total nitrogen and available phosphorus where dingoes were rare, but negligible effect where dingoes were common. Taken together, these results provide evidence that a trophic cascade resulting from an apex predator's top-down effects on herbivores can extend to vegetation and in turn the soil nutrient pool.
The marked differences in dingo and kangaroo abundances that we observed on either side of the dingo fence correspond with the findings of previous studies that have investigated the effects of dingo culling on kangaroo populations [40–42]. The presence of kangaroo remains in dingo scats outside the fence, despite kangaroo abundances there being below detectable densities using spotlight surveys, supports the hypothesis that kangaroo populations outside the dingo fence were trapped in a ‘predation pit’ [43]. This scenario could occur if dingoes are abundant and the dynamics of dingo and kangaroo populations are decoupled because kangaroos are secondary rather than primary prey of dingoes [43]. Consistent with the ‘predation pit’ hypothesis, dingoes were more abundant and rabbits and rodents were more important components of dingo diets than kangaroos at sites outside the dingo fence where dingoes were common. Moreover, because dingoes prey primarily on female and juvenile kangaroos [44], even occasional predation could maintain kangaroo populations outside the dingo fence at low densities by limiting recruitment [43]. Conversely, on the ‘inside’ of the dingo fence, dingoes frequently preyed upon kangaroos but appear to have a low impact on kangaroo populations because they occur at very low population densities.
The strong effect that kangaroo grazing had on vegetation cover where dingoes were rare, and the absence of a kangaroo grazing effect where dingoes were common provides compelling evidence that suppression of dingoes triggers a trophic cascade. On average, vegetation cover in grazing-allowed control plots was 12% less than in kangaroo exclosure plots at Sturt National Park where dingoes were rare. That the suppressive effect that kangaroo grazing had on vegetation cover in Sturt National Park was consistent through time, despite fluctuations in rainfall suggest that that kangaroos' and dingoes' respective direct and indirect effects on vegetation were not influenced by temporal fluctuations in primary productivity. These results accord with previous studies which have shown that high-density kangaroo populations at sites where dingoes are rare can have considerable impacts on vegetation, particularly grass cover [31,45,46].
Previous studies examining evidence for trophic cascades between large mammalian carnivores, mammalian herbivores and plants have to a large extent focused on behaviourally mediated trophic cascades [13,47]. These studies have provided levels of support, ranging from acceptance through to rejection [11,12,14], of the hypothesis that fear of predators can protect plants by causing herbivores to shift their patterns of habitat use and or the species they consume. Although fear of dingoes almost certainly causes kangaroos to change their behaviour [48], we contend that the trophic cascade reported in this study was due primarily to the difference in grazing pressure (the total metabolic demand of herbivore populations) resulting from the contrast in dingoes' lethal, suppressive effects on kangaroo populations on either side of the dingo fence. However, we add the caveat that we did not assess kangaroo behaviour on either side of the dingo fence, and thus highlight the need for further studies to parse out the effects that dingoes' lethal and non-lethal effects on kangaroos have on vegetation.
The increase in total carbon, total nitrogen and available phosphorus concentrations identified in ungrazed plots, where kangaroos were abundant and dingoes rare, was consistent with the TDTTRPM for soil nutrient dynamics in arid landscapes [24]. According to the TDTTRPM (figure 1), greater vegetation growth in ungrazed plots where dingoes are rare should allow for greater litter build-up and more opportunity for patches of vegetation to intercept and accumulate nutrients transported by wind and water [49]. We hypothesize that by suppressing kangaroo numbers, dingoes function as a limiting factor on grazing, thus promoting the positive feedback loop between vegetation growth, deposition of litter and the soil nutrient pool and also by promoting greater vegetation cover which in turn promotes the capture and retention of water and matter. Conversely, lower nutrient concentrations in plots that had been heavily grazed by kangaroos is consistent with the hypotheses that removal of vegetation by kangaroos decouples the positive feedback loop between vegetation growth, litter-fall and the soil nutrient pool and that sparse vegetation cover also facilitates higher velocity flows of wind and water which export nutrients out of the plots [2,24]. At larger spatial and temporal scales, we predict that export of nutrients away from areas grazed heavily by kangaroos will shift the distribution of nutrients in the landscape towards low-lying areas where they are transported by gravity and towards the shade trees under which kangaroos rest during the daytime and deposit their dung and urine.
A limitation of our herbivore exclusion experiment was that we were unable to maintain a procedural control due to damage caused by kangaroos. The gauge of the mesh that we used for the exclusion fences was large (10 × 10 cm) and therefore unlikely to have a great effect on the movement of nutrients by wind and water. Moreover, our experimental approach accounted for such fence effects by having identical exclosures on each side of the dingo fence and asking if the effects of herbivore exclusion differed where dingoes were common and rare. Thus, if fence effects did occur, they would have been expected to have occurred on both sides of the dingo fence. However, our results showed that the exclosures had different effects on vegetation and the soil nutrient pool on each side of the dingo fence. Hence, the results suggest that the differential effects that the kangaroo exclosures had on vegetation and nutrients where dingoes were common and rare were due largely to differences in the intensity of kangaroo grazing and not due to fence effects.
Our finding that a trophic cascade between dingoes, kangaroos and vegetation in a desert ecosystem translates to the soil nutrient pool demonstrates a hitherto unappreciated interaction pathway via which apex predators can influence nutrient dynamics. A key implication of this study is the vast spatial scale across which the presence/absence of apex predators' effects on herbivore populations operate and, in turn, effects on vegetation and the soil nutrient pool could become manifest. For example, in Australia, kangaroos have irrupted across approximately one-third of the continent from which dingoes are now rare or extinct [41]. According to the TDTTRPM, heavily grazed landscapes, where dingoes are rare, will exhibit muted growth pulses following rainfall events due to their smaller nutrient pools [24]. If the indirect positive effects that dingoes have on the soil nutrient pool translate to enhanced primary productivity, it would blur the distinction between top-down and bottom-up control of ecosystems and could be a driver of ecosystem productivity and the composition of ecological assemblages at a continental scale.
Supplementary Material
Acknowledgements
James Rees, Charlotte Mills, Ben Feit, Anna Feit, Nick Tong, Dave Forsyth and the Ogilvy family.
Data accessibility
Primary data collected from the field and the R script, used herein for coding of statistical analysis and graphing, can be accessed online from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.5bn81) [50].
Authors' contributions
Both authors contributed to the design of the study, collection of field data and writing of the manuscript. T.M. conducted the statistical analysis. M.L. conceived the study.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Australian Research Council, Linnean Society of New South Wales, Joyce Vickery Research Fund and the UNSW School of Biology, Earth and Environmental Sciences.
References
- 1.Dobson A, et al. 2006. Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87, 1915–1924. ( 10.1890/0012-9658(2006)87%5B1915:HLTCAT%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 2.Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141, 221–235. ( 10.1007/s00442-004-1519-1) [DOI] [PubMed] [Google Scholar]
- 3.Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC. 1999. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121, 546–550. ( 10.1007/s004420050961) [DOI] [PubMed] [Google Scholar]
- 4.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961. ( 10.1126/science.1108485) [DOI] [PubMed] [Google Scholar]
- 5.Fukami T, et al. 2006. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol. Lett. 9, 1299–1307. ( 10.1111/j.1461-0248.2006.00983.x) [DOI] [PubMed] [Google Scholar]
- 6.Schmitz OJ, Hambäck PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153. ( 10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 7.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 8.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 9.Estes JA, Duggins DO. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr. 65, 75–100. ( 10.2307/2937159) [DOI] [Google Scholar]
- 10.Ripple WJ, et al. 2016. What is a trophic cascade? Trends Ecol. Evol. 31, 842–849. ( 10.1016/j.tree.2016.08.010) [DOI] [PubMed] [Google Scholar]
- 11.Donadio E, Buskirk SW. 2016. Linking predation risk, ungulate antipredator responses, and patterns of vegetation in the high Andes. J. Mammal. 97, 966–977. ( 10.1093/jmammal/gyw020) [DOI] [Google Scholar]
- 12.Ford AT, Goheen JR, Otieno TO, Bidner L, Isbell LA, Palmer TM, Ward D, Woodroffe R, Pringle RM. 2014. Large carnivores make savanna tree communities less thorny. Science 346, 346–349. ( 10.1126/science.1252753) [DOI] [PubMed] [Google Scholar]
- 13.Ford AT, Goheen JR. 2015. Trophic cascades by large carnivores: a case for strong inference and mechanism. Trends Ecol. Evol. 30, 725–735. ( 10.1016/j.tree.2015.09.012) [DOI] [PubMed] [Google Scholar]
- 14.Kauffman MJ, Brodie JF, Jules ES. 2010. Are wolves saving Yellowstone's aspen? A landscape-level test of a behaviorally mediated trophic cascade. Ecology 91, 2742–2755. ( 10.1890/09-1949.1) [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Wu J, Clark CM, Pan Q, Zhang L, Chen S, Wang Q, Han X. 2012. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 49, 1204–1215. ( 10.1111/j.1365-2664.2012.02205.x) [DOI] [Google Scholar]
- 16.Frank DA. 2008. Evidence for top predator control of a grazing ecosystem. Oikos 117, 1718–1724. ( 10.1111/j.1600-0706.2008.16846.x) [DOI] [Google Scholar]
- 17.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209. ( 10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 18.Flagel D, Belovsky G, West W. 2016. Digging further into wolf–deer interactions: food web effects on soil nitrogen availability in a Great Lakes Forest. Am. Midl. Nat. 176, 147–151. ( 10.1674/0003-0031-176.1.147) [DOI] [Google Scholar]
- 19.Allen DE, Pringle MJ, Page KL, Dalal RC. 2010. A review of sampling designs for the measurement of soil organic carbon in Australian grazing lands. Rangeland J. 32, 227–246. ( 10.1071/RJ09043) [DOI] [Google Scholar]
- 20.Giese M, et al. 2013. N balance and cycling of Inner Mongolia typical steppe: a comprehensive case study of grazing effects. Ecol. Monogr. 83, 195–219. ( 10.1890/12-0114.1) [DOI] [Google Scholar]
- 21.Wardle D, Bonner K, Barker G. 2002. Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct. Ecol. 16, 585–595. ( 10.1046/j.1365-2435.2002.00659.x) [DOI] [Google Scholar]
- 22.Murray BD, Webster CR, Bump JK. 2013. Broadening the ecological context of ungulate–ecosystem interactions: the importance of space, seasonality, and nitrogen. Ecology 94, 1317–1326. ( 10.1890/12-1582.1) [DOI] [PubMed] [Google Scholar]
- 23.Bump JK, Webster CR, Vucetich JA, Peterson RO, Shields JM, Powers MD. 2009. Ungulate carcasses perforate ecological filters and create biogeochemical hotspots in forest herbaceous layers allowing trees a competitive advantage. Ecosystems 12, 996–1007. ( 10.1007/s10021-009-9274-0) [DOI] [Google Scholar]
- 24.Ludwig J, Tongway D, Freudenberg J, Noble KH. 1997. Landscape ecology, function and management: principles from Australia’s rangelands. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 25.Short J, Kinnear JE, Robley A. 2002. Surplus killing by introduced predators in Australia—evidence for ineffective anti-predator adaptations in native prey species? Biol. Conserv. 103, 283–301. ( 10.1016/S0006-3207(01)00139-2) [DOI] [Google Scholar]
- 26.McKnight TL. 1969. Barrier fencing for vermin control in Australia. Geogr. Rev. 59, 330–347. ( 10.2307/213480) [DOI] [Google Scholar]
- 27.Gordon CE, Eldridge DJ, Ripple WJ, Crowther MS, Moore BD, Letnic M. 2016. Shrub encroachment is linked to extirpation of an apex predator. J. Anim. Ecol. 86, 147–157. ( 10.1111/1365-2656.12607) [DOI] [PubMed] [Google Scholar]
- 28.Letnic M, Koch F. 2010. Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Austral Ecol. 35, 167–175. ( 10.1111/j.1442-9993.2009.02022.x). [DOI] [Google Scholar]
- 29.Newsome A, Catling P, Cooke BD, Smyth R. 2001. Two ecological universes separated by the dingo barrier fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes. Rangeland J. 23, 71–98. ( 10.1071/RJ01015) [DOI] [Google Scholar]
- 30.Keith DA. 2006. Ocean shores to desert dunes: the native vegetation of New South Wales and the ACT. Hurstville, Australia: Department of Environment and Conservation (NSW). [Google Scholar]
- 31.Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc. R. Soc. B 276, 3249–3256. ( 10.1098/rspb.2009.0574). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayment GE, Lyons DJ. 2011. Soil chemical methods: Australasia. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 33.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 34.Warton DI. 2005. Many zeros does not mean zero inflation: comparing the goodness-of-fit of parametric models to multivariate abundance data. Environmetrics 16, 275–289. ( 10.1002/env.702) [DOI] [Google Scholar]
- 35.Venables WN, Ripley BD. 2013. Modern applied statistics with S-PLUS. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 36.Cribari-Neto F, Zeileis A. 2010. Beta regression in R. J. Stat. Softw. 34, 1–24. ( 10.18637/jss.v034.i02) [DOI] [Google Scholar]
- 37.Smithson M, Verkuilen J. 2006. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11, 54 ( 10.1037/1082-989X.11.1.54) [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2016. nlme: linear and nonlinear mixed effects models. R package version 3.1-128 Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Bates D. et al 2016. Package ‘lme4’. Obtenido de http://pbil.univlyon1.fr/CRAN/web/packages/lme4/lme4.pdf.
- 40.Caughley G, Grigg G, Caughley J, Hill G. 1980. Does dingo predation control the densities of kangaroos and emus? Wildl. Res. 7, 1–12. ( 10.1071/WR9800001) [DOI] [Google Scholar]
- 41.Letnic M, Ritchie EG, Dickman CR. 2012. Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol. Rev. 87, 390–413. ( 10.1111/j.1469-185X.2011.00203.x) [DOI] [PubMed] [Google Scholar]
- 42.Pople A, Grigg G, Cairns S, Beard L, Alexander P. 2000. Trends in the numbers of red kangaroos and emus on either side of the South Australian dingo fence: evidence for predator regulation? Wildl. Res. 27, 269–276. ( 10.1071/WR99030) [DOI] [Google Scholar]
- 43.Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE. 1998. Predicting effects of predation on conservation of endangered prey. Conserv. Biol. 12, 564–575. ( 10.1046/j.1523-1739.1998.97030.x) [DOI] [Google Scholar]
- 44.Shepherd N. 1981. Predation of red kangaroos, Macropus rufus, by the dingo, Canis familiaris dingo (Blumenbach) in North-Western New South Wales. Wildl. Res. 8, 255–262. ( 10.1071/WR9810255) [DOI] [Google Scholar]
- 45.Wilson A. 1991. The influence of kangaroos and forage on sheep productivity in the semi-arid woodlands. Rangeland J. 13, 69–80. ( 10.1071/RJ9910069) [DOI] [Google Scholar]
- 46.Leigh J, Wood D, Holgate M, Slee A, Stanger M. 1989. Effects of rabbit and kangaroo grazing on two semi-arid grassland communities in central–western New South Wales. Aust. J. Bot. 37, 375–396. ( 10.1071/BT9890375) [DOI] [Google Scholar]
- 47.Ripple WJ, Beschta RL. 2012. Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol. Conserv. 145, 205–213. ( 10.1016/j.biocon.2011.11.005) [DOI] [Google Scholar]
- 48.Mella VS, Cooper CE, Davies SJ. 2014. Behavioural responses of free-ranging western grey kangaroos (Macropus fuliginosus) to olfactory cues of historical and recently introduced predators. Austral Ecol. 39, 115–121. ( 10.1111/aec.12050) [DOI] [Google Scholar]
- 49.Tongway DJ, Ludwig JA. 1994. Small-scale resource heterogeneity in semi-arid landscapes. Pac. Conserv. Biol. 1, 201–208. ( 10.1071/PC940201) [DOI] [Google Scholar]
- 50.Morris T, Letnic M. 2017. Data from: Removal of an apex predator initiates a trophic cascade that extends from herbivores to vegetation and the soil nutrient pool. Dryad Digital Repository. ( 10.5061/dryad.5bn81) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Morris T, Letnic M. 2017. Data from: Removal of an apex predator initiates a trophic cascade that extends from herbivores to vegetation and the soil nutrient pool. Dryad Digital Repository. ( 10.5061/dryad.5bn81) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Primary data collected from the field and the R script, used herein for coding of statistical analysis and graphing, can be accessed online from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.5bn81) [50].