Abstract
A central question in the evolution of brain development is whether species differ in rates of brain growth during fetal neurogenesis. Studies of neonatal data have found allometric evidence for brain growth rate differences according to physiological variables such as relative metabolism and placental invasiveness, but these findings have not been tested against fetal data directly. Here, we measure rates of exponential brain growth acceleration in 10 eutherian mammals, two marsupials, and two birds. Eutherian brain acceleration exhibits minimal variation relative to body and visceral organ growth, varies independently of correlated growth patterns in other organs, and is unrelated to proposed physiological constraints such as metabolic rate or placental invasiveness. Brain growth rates in two birds overlap with eutherian variation, while marsupial brain growth is exceptionally slow. Peak brain growth velocity is linked in time with forebrain myelination and eye opening, reliably separates altricial species born before it from precocial species born afterwards, and is an excellent predictor of adult brain size (r2 = 0.98). Species with faster body growth exhibit larger relative brain size in early ontogeny, while brain growth is unrelated to allometric measures. These findings indicate a surprising conservation of brain growth rates during fetal neurogenesis in eutherian mammals, clarify sources of variation in neonatal brain size, and suggest that slow body growth rates cause species to be more encephalized at birth.
Keywords: brain growth, fetal, prenatal, neurogenesis, evolution, encephalization
1. Introduction
Mammalian brains vary in size by five orders of magnitude, ranging from a fraction of a gram in some tree shrews [1] to nearly 10 kg in sperm whales [2]. How has evolution altered neurodevelopment to produce brains of such different size? It is well established that larger brains are grown by lengthening the duration of brain development [3–6], as reflected in extended neurodevelopmental schedules [7–9], longer periods of exponential growth [10], and later ages at which adult brain size is achieved [9]. However, it remains unclear whether mammals also differ in rates of brain growth, particularly during periods of fetal neurogenesis (e.g. as a consequence of altered cell cycle kinetics [11,12]). Several studies that estimate fetal brain growth from brain size at birth have suggested that growth rates might vary according to physiological and life-history variables (see below), but findings have differed according to how the data were analysed, and no effort has yet been made to test these hypotheses using fetal data directly.
Brain growth over ontogeny is nonlinear, following a sigmoid pattern that consists of an early phase of exponential growth (acceleration), an inflection point (peak growth velocity), and a decay curve (deceleration) (figure 1a,b) [14,15]. Growth velocity (e.g. g d−1) increases over fetal development until peak velocity is reached, generally around birth (but see below). Accordingly, the best measure of prenatal brain growth ‘rate’ is acceleration, or the pace of growth velocity increase as brains grow larger. Acceleration can be measured as a single variable by applying an exponential model (traditionally cubic; [16]) and comparing it against post-conception age (electronic supplementary material, figure S1a,b). Estimates of fetal acceleration from neonatal brain size and gestation length have suggested that brain growth is far less variable than whole-body growth [3] and may in fact be minimally variable within mammals [4].
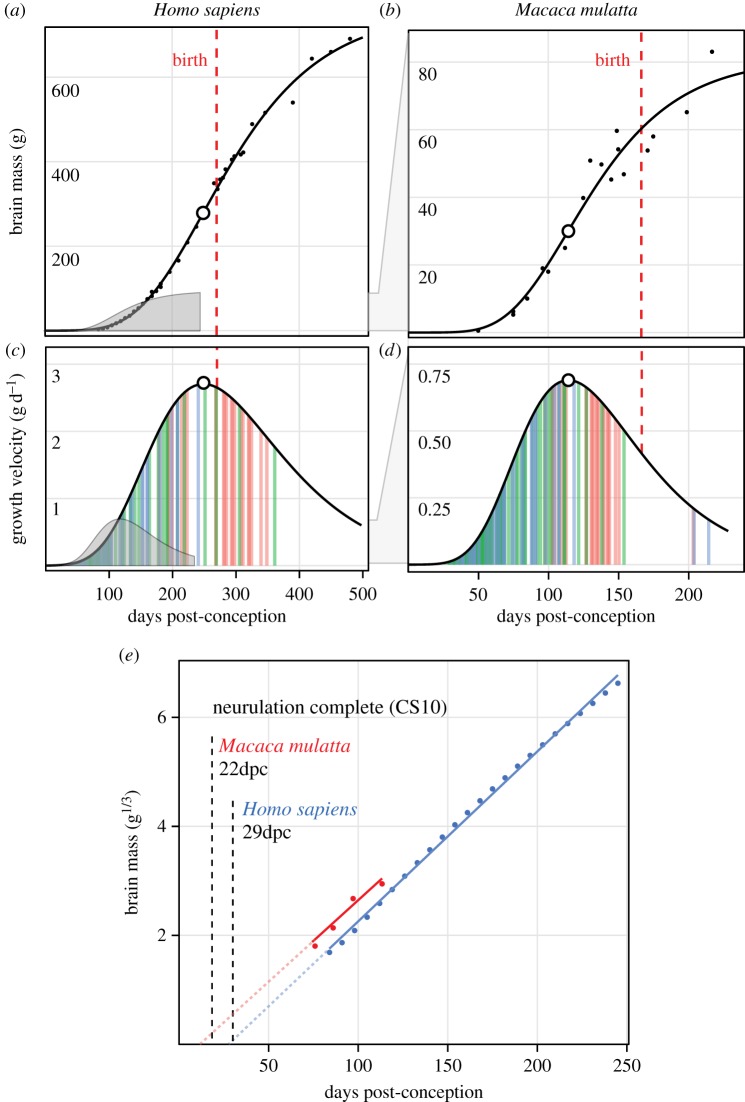
Figure 1.
Methods for comparing brain growth. (a,b) Gompertz models fitted to fetal and early postnatal brain growth data in human and rhesus monkey show differences in birth timing and the age and size of peak velocity (open circles). The rhesus function is superimposed on the human curve (grey) to show earlier onset of exponential growth in rhesus. (c,d) Velocity curves (g d−1) derived from Gompertz functions for both species. Vertical colour bars correspond to neurodevelopmental event estimates related to neurogenesis (green), tract formation (blue), and myelination (red) [9]. Brain growth accelerates through the neurogenic period prior to peak velocity (open circle). Again, rhesus velocity function is superimposed (grey) for comparison with humans. (e) Exponential stages preceding peak velocity are fit with linear models of cube-root transformed brain mass to allow a comparison of growth acceleration (i.e. slope; electronic supplementary material, figure S1), which is similar in both species; the intercept shift is an artefact of temporal differences in the completion of neurulation (Carnegie Stage 10) [13].
By contrast, because brains are energetically expensive to grow and maintain [17], a number of theories have proposed that species with more energy to expend (either by increasing energy intake, reallocating available resources or both) should exhibit faster brain growth in utero. Evidence for differences in fetal brain growth come from more indirect measures than acceleration, such as neonatal brain/body allometric slopes and intercepts, or multiple regression models predicting neonatal brain size while including neonatal body size as a covariate. For example, Martin [18] noted that mammalian adult brain size and relative basal metabolic rate (BMR) both scale at a similar exponent (0.75) against adult body size, and proposed that smaller animals have relatively larger brains because their metabolism supports faster fetal brain growth. Barton & Capellini [6] found that precocial species have larger brains at birth than altricial species after controlling for neonatal body size, maternal body size, and gestation length, and proposed that faster fetal brain growth might support more precocial young. Finally, Elliot & Crespi [19] found that more invasive placental morphologies (which could more efficiently deliver resources to the fetus) were associated with steeper neonatal brain/body allometric slopes, as well as steeper allometric slopes of average brain growth velocity (g d−1) for whole litters against maternal body size.
However, both absolute and relative brain size at birth include two major artefacts that complicate their interpretation. First, species differ in the time from conception to the formation of the neural plate and closure of the neural tube (i.e. neurulation), a period ranging from 9.5 days in mouse [20] (40% gestation) to 29 days in humans [21] (11% gestation) and highly variable across species [13]. This makes total gestation length a poor marker for the variable of interest, namely the elapsed time from the onset of neurodevelopment (electronic supplementary material, figure S2a,b). Second, species differ in the timing of birth relative to peak velocity [10] (electronic supplementary material, figure S2c,d) and allometric brain/body growth [22] (electronic supplementary material, figure S3a), making birth a particularly unreliable proxy for fetal brain growth patterns. This has led several authors to directly caution against the use of birth as a temporal anchor in comparative neurodevelopment [23–25].
In this study, we collect a large, novel dataset on fetal and perinatal brain, body, and organ weight (n = 2 377) of known age post-conception in 14 species. Our goal is to characterize general prenatal brain growth patterns, compare rates of acceleration across organs and species, and test a number of proposed relationships that have been supported by neonatal data. We use two analytic techniques to re-examine these questions.
First, we directly measure and compare fetal brain growth acceleration in 10 eutherian, two marsupial, and two bird species. Our measure of acceleration is the slope of regression models predicting cube-root mass from age post-conception [16] (electronic supplementary material, figure S1a,b), which corrects for both the neurulation and birth timing artefacts described above (electronic supplementary material, figure S2a–d). Within the eutherian sample, we compare brain acceleration to that of whole body, liver, heart, lung, and kidney acceleration, testing for variation in growth patterns as well as correlations between different organs. This also allows a direct test of Sacher & Staffeldt's [3] theory that the brain is the slowest growing organ during prenatal development. Next, we test whether brain acceleration predicts adult brain size, and whether it differs according to relative BMR, placental morphology, or developmental state at birth (precocial/altricial). We also test whether patterns of fetal and neonatal encephalization described in earlier studies (e.g. [3,22]) are caused by faster brain growth (as is often assumed) or slower body growth. Finally, to examine how the current brain size of a growing fetus affects its growth velocity (g d−1), we plot instantaneous growth velocities (g d−1) against current brain size in early ontogeny (electronic supplementary material, figure S1c).
A second set of analyses uses sigmoid models of fetal and perinatal brain growth (Gompertz curves) to calculate three variables of interest in the eutherian sample: peak brain growth velocity (g d−1; i.e. the inflection point of the sigmoid curve), its associated age post-conception, and the brain size at which it is reached. We expect that peak velocity (g d−1) is highly correlated with adult brain size, since a longer period of exponential growth (and thus a higher maximum) is necessary to grow a larger brain. An additional set of tests is used to determine whether peak velocity is a better anchor for comparing species' neurodevelopment than birth is. We test whether adult brain size is better predicted by (i) neonatal brain size versus brain size at peak velocity and (ii) total gestation length versus age of peak velocity. To test whether birth or peak velocity is a better correlate of underlying neurodevelopmental events, we use the Translating Time model (an empirically derived tool for estimating and comparing the timing of species' neurogenesis, tract formation, myelination, etc.) [9] to compare the developmental events associated with birth versus peak velocity across our eutherian sample. Finally, we use the preceding findings to re-examine the timing of birth in precocial versus altricial species relative to neurodevelopment and growth. Together, these methods allow us to directly measure brain growth acceleration across species, link growth patterns to underlying neurodevelopmental events, and compare brain with whole body and other organ growth patterns.
2. Material and methods
(a). Data collection
Published data from the literature were collected on post-conception age (days) and weight (grams) of fetal brain, body, heart, liver, lung (×2), and kidney (×2) in as many species as possible. Additional details on dataset assembly are described in the electronic supplementary material. The final dataset includes 2 377 observations of organ (brain, liver, heart, lungs, kidneys) and whole-body size in 14 species, including three primates (Homo sapiens, Macaca mulatta, Callithrix jacchus), three ungulates (Ovis aries, Sus scrofa, Bos taurus), four glires (Oryctolagus cuniculus, Cavia porcellus, Mus musculus, Rattus rattus), two marsupials (Macropus eugenii, Monodelphis domestica), and two birds (Colinus virginianus, Melopsittacus undulatus). The dataset is accessible on the Dryad repository [26].
(b). Models
All data modelling was performed using R [27]. To characterize brain growth over time and measure peak velocity, brain size in grams versus age post-conception was modelled using nonlinear least-squares curve fitting ({stats}nls) [27] of a Gompertz equation in pre- and early postnatal stages of development. Exponential stages of growth were isolated from the total dataset for cube-root modelling, either by isolating data preceding peak velocity (brain) or by visually inspecting cube-root data and removing values that had decelerated (body and visceral organs). Growth acceleration was measured by cube-root transforming individual observations, predicting cube-root weight from age using ordinary least-squares (OLS) regression, and using model slope as a measure of acceleration. Cube-root models were calculated separately by data source for each organ and species to minimize artefacts introduced from differences in age estimation across studies (i.e. intercept shifts; see electronic supplementary material); model parameters were then averaged across available studies to produce a final slope estimate for statistical tests. Statistical tests use OLS bivariate regression models, comparing log-transformed data where listed. Instantaneous brain growth rates (g d−1) were calculated from adjacent raw data points according to increasing age for each species (electronic supplementary material, figure S1c).
First-order derivatives of Gompertz equations were used to generate velocity curves, and model estimates of neurodevelopmental event timing [6] were applied to demonstrate neurogenic, tract formation and myelination sequences in relation to velocity curves. Gompertz functions were used to calculate peak brain growth velocity (g d−1) and its associated post-conception age and brain size. Neurodevelopmental ‘event scores’ (a 0–1 scale of relative progression along highly conserved sequences of neurodevelopment) at peak velocity and birth were calculated from equations described in Workman et al. [9].
(c). Statistical tests
Bivariate OLS regression models were used in all statistical tests, using log-transformed data where listed (electronic supplementary material, tables S4 and S5). We repeated these tests using phylogenetic generalized least squares (PGLS), incorporating the supertree from Bininda-Emonds et al. [28], pruned using the {geiger} package [29] and performed using the pgls function in the {caper} package for R (electronic supplementary material, tables S6 and S7) [30]. BMR (ml O2 g−1 h−1) were taken from the published literature (Homo [31], Macaca [32], Callithrix [33], Ovis, Sus, Bos, Mus [34], Oryctolagus, Cavia, Rattus [35]). Data on placental invasiveness (haemochorial/epitheliochorial) and developmental state at birth (altricial/precocial) come from Sacher & Staffeldt [3] and are dummy coded for inclusion in regression models.
3. Results
Rates of organ acceleration (i.e. cube-root regression slope) in each species are summarized in electronic supplementary material, table S1. Gompertz, velocity, and cube-root plots of brain growth are presented in electronic supplementary material, figures S6–S17; whole-body and visceral organ cube-root plots are shown in electronic supplementary material, figures S18–S22. Details on slope estimates from individual data sources are detailed in electronic supplementary material, figures S6–S17 (brain) and electronic supplementary material, tables S8–S12 (whole body and visceral organs). With one exception (see below), incorporating phylogeny (electronic supplementary material, tables S6 and S7) did not change the findings of this study, and so the uncorrected measures are described here.
(a). Brain, body, and visceral organ growth rates
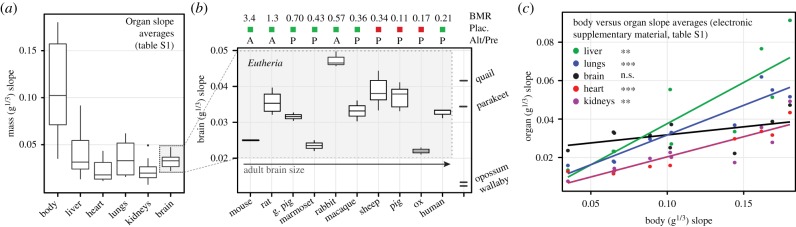
Measures of brain growth acceleration calculated from different published datasets (electronic supplementary material, figures S6–S17) largely overlap in eutherians, though high slopes are observed in rabbit, and low slopes in ox, mouse, and marmoset (figure 2b). Primate brain growth rates fall within the range of non-primate eutherian mammals (electronic supplementary material, tables S1 and S2). Opossum and wallaby brain acceleration is exceptionally slower than in eutherian mammals, and supports previous descriptions of decelerated marsupial brain growth [36,37] and neurodevelopment [9,38]. Parakeet and quail values fall within the range of eutherian variation (figure 2b). Among eutherians, the brain is the fastest growing organ in humans, macaque, marmoset, pig, and guinea pig. The brain grows slower than liver in sheep, ox, rabbit, mouse, and rat; slower than lungs in sheep, ox, rabbit, and rat; and slower than kidneys in rabbit. No species exhibits faster heart growth than brain growth (electronic supplementary material, table S1). These findings do not support Sacher & Staffeldt's [3] theory that the brain is the slowest growing organ, which has never been directly tested to our knowledge. Data on early embryonic brain growth in mouse and rat [39] reveal faster acceleration between E9–E12 in mouse and between E12–E15 in rat (electronic supplementary material, figure S5); as these periods likely reflect symmetric proliferative cell division in the cortex [12], only later neurogenic periods are used in these species. Finally, using a complementary analytic technique that calculates instantaneous brain growth rates (g d−1) from adjacent raw data points (see electronic supplementary material, figure S1c, Material and methods), we show that the brain growth velocity is primarily a function of current fetal brain size at any given moment over several orders of magnitude (figure 3).
Figure 2.
Cube-root slopes are averaged across available studies to produce robust measures of relative organ growth in 10 eutherian species (electronic supplementary material, table S1). (a) Eutherian variation in average whole body and organ cube-root slopes; brain slopes exhibit the lowest degree of variation among the organs studied. (b) Brain cube-root slopes in 10 eutherians, two marsupials, and two birds. Species are ordered by increasing adult brain size, which is unrelated to brain acceleration. Relative basal metabolic rate in ml O2 g−1 h−1 (BMR), placental invasiveness (green, haemochorial; red, endotheliochorial) and precociality/altriciality (P/A) are unrelated to brain acceleration. Eutherian ranges indicate variation in estimates from individual studies (electronic supplementary material, figures S6–S17). (c) OLS regression models predicting organ slopes from whole-body slope in the eutherian sample (solid lines; n = 10). Body acceleration significantly predicts acceleration in every organ except the brain. In phylogenetic models (electronic supplementary material, tables S6), brain acceleration is predicted by body acceleration (p = 0.043), but much more weakly than other organs are (all p < 0.001).
Figure 3.
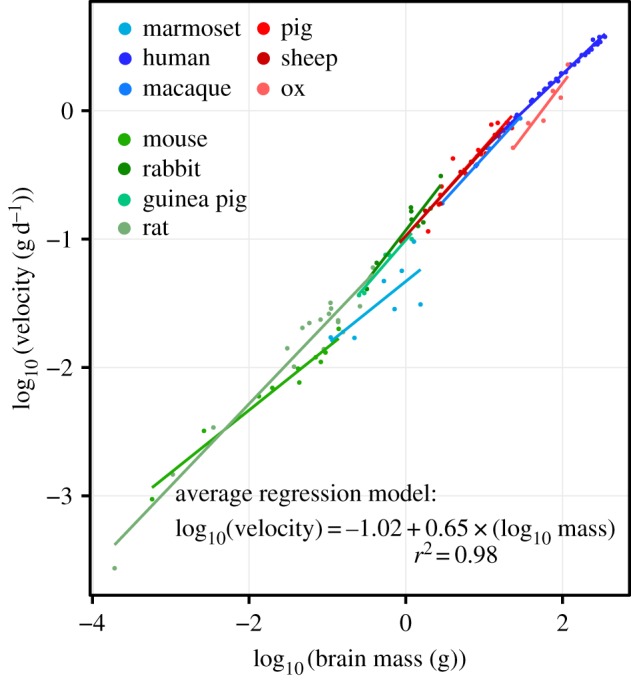
Instantaneous velocity (g d−1) scales with brain size at an approximately linear rate over six orders of magnitude. The rate at which brains grow during fetal neurogenesis in any given species is primarily a function of how large the brain already is. Growth velocity is negatively allometric with increasing brain size. Velocities outside of a 95% CI were removed from species datasets prior to model fitting. See electronic supplementary material, figure S1c for methods.
The fastest rates of whole-body acceleration are observed in rabbit, sheep, and rat; these species also exhibit the fastest rates of liver, heart, lung, and kidney growth (electronic supplementary material, table S1). The slowest body acceleration rates are observed in the three primate species (human, macaque, and marmoset), which also have the slowest rates of liver, heart, lung, and kidney growth (electronic supplementary material, table S1). To test how brain growth variation compares against other organs, we performed F-tests comparing brain versus whole body and organ acceleration. Brain growth exhibits the lowest variation in the organs studied within eutherians (figure 2a; electronic supplementary material, table S1); brain slope variation is significantly lower than whole body, liver, and lungs; heart and liver variation are not significantly larger (electronic supplementary material, table S2). To test whether species with faster brain acceleration also exhibit rapid body and organ growth, we ran inter-organ correlations (electronic supplementary material, table S3) as well as regression models predicting organ acceleration from whole-body acceleration (figure 2c; electronic supplementary material, table S4). Eutherian body acceleration positively predicts rates in liver (r2 = 0.70; p < 0.01), heart (r2 = 0.89; p < 0.001), lung (r2 = 0.89; p < 0.001), and kidney (r2 = 0.73; p < 0.01), but not with brain growth (r2 = 0.30; p = 0.103). When phylogenetic regression was used, brain acceleration was predicted by body acceleration (r2 = 0.42; p = 0.043), but as with OLS, this association was much weaker than those of other organs (all p < 0.001) (electronic supplementary material, table S6).
(b). Correlates of brain, body, and organ growth
We next tested whether faster brain or body growth results in larger adult brain or body size. We found no relationship between adult body size and fetal body acceleration (t = 0.589, p = 0.572), and no relationship between adult brain size and fetal brain acceleration (t = −0.324, p = 0.754), indicating that larger brains and bodies are not grown more quickly in utero. To test whether brain acceleration is associated with physiological variables (as proposed from studies of neonatal brain size [5,6,18,19]), we performed regression models predicting brain acceleration from relative BMR, degree of placental invasiveness (haemochorial versus epitheliochorial), and developmental state at birth (altricial versus precocial). We found no relationship between any variable and brain acceleration, either when using OLS (electronic supplementary material, table S5) or phylogenetic models (electronic supplementary material, table S7).
Higher brain/body ratios at birth can be produced by either faster brain or slower body growth in utero. To distinguish between these possibilities, we tested whether brain or body acceleration predicts neonatal brain/body proportions in our subsample of 10 eutherians. Neonatal brain/body ratios were negatively predicted by body acceleration (p < 0.01), while brain acceleration failed to predict neonatal brain/body ratios (electronic supplementary material, table S5). This indicates that neonatal brain/body proportions reflect variation in body acceleration. We also tested whether brain or body acceleration predicts the slope of fetal brain/body allometric growth (i.e. the rapid growth phase (RGP) of ontogenetic allometry; measures taken from Halley [22]). Briefly, species with higher RGP slopes have faster brain or slower body growth (electronic supplementary material, figure S3a,b). We found that body acceleration negatively predicts RGP slope (p < 0.01), meaning that species with faster body growth exhibit decreasing relative brain size as they grow. Again, brain acceleration failed to predict RGP slope (electronic supplementary material, table S5). In sum, these tests indicate that brain/body allometry during early ontogeny reflects differences in body, but not brain growth.
(c). Peak brain growth velocity and birth timing
Among eutherians, peak brain growth velocity (g d−1) does an excellent job of predicting adult brain size (p < 0.001; r2 = 0.98) (electronic supplementary material, figure S4a), and is predicted by age of peak velocity (PV age; p < 0.001; r2 = 0.91) (electronic supplementary material, figure S4b). Because birth is highly variable relative to neurodevelopment and brain growth, a number of tests were performed to compare total gestation length versus PV age. PV age predicts adult brain size better than total gestation length does (r2 = 0.85 versus 0.77) (electronic supplementary material, figure S4c); similarly, brain size at peak velocity predicts adult brain size better than neonatal brain size does (r2 = 0.99 versus 0.94) (electronic supplementary material, figure S4d). Age and brain size at peak brain growth velocity is, therefore, a much better correlate of adult brain size than these variables at birth.
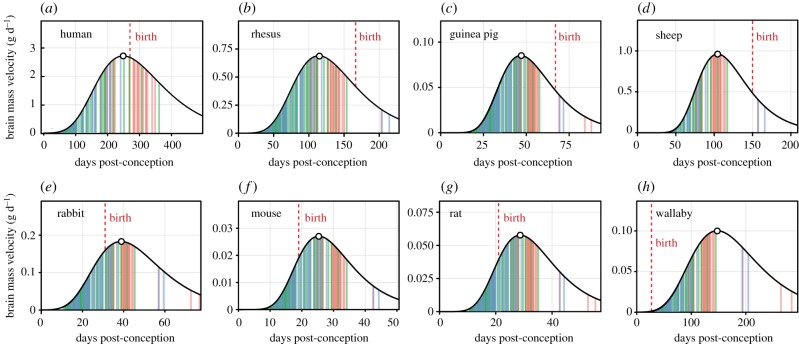
We also tested whether altricial and precocial species differ in birth timing relative to brain growth curves. Every precocial species in our sample (human, macaque, marmoset, guinea pig, sheep, pig, and ox) was born after peak velocity; every altricial species (mouse, rat, rabbit, wallaby, and opossum) was born prior to peak velocity (see birth timing in figure 4).
Figure 4.
Velocity models of fetal and early postnatal brain growth in four precocial (a–d) and four altricial species (e–h). Colour bars indicate model estimates for the age of events associated with neurogenesis (green), tract formation (blue), and myelination (red) taken from www.translatingtime.net [9]. Birth timing is highly variable relative to growth. Altricial species tend to be born earlier along growth trajectories and correlated patterns of neurodevelopment, given that peak velocity corresponds well to eye opening (a common proxy for altriciality) during neurodevelopment (see text).
(d). Brain growth patterns versus neurogenic event timing
Finally, to test how the timing of peak brain growth velocity versus birth compares to models of neurodevelopmental event timing, we compared event scores (see Material and methods) for PV age versus gestation length for eight species in which models are described in [9]. Figure 4 shows colour-coded neurodevelopmental event types (neurogenesis, tract formation, and myelination) against velocity curves in these species. Eutherian neurodevelopmental event scores for age of peak velocity fell between 0.57 and 0.69 (average = 0.62; s.d. = 0.04). This range occurs after the vast majority of neurogenesis and tract formation are complete, and overlaps with myelination events in a variety of forebrain tracts (e.g. fornix, anterior commissure, external capsule; see [9] for additional events). Notably, the event score for eye opening (0.663) falls within this range [9], which helps to explain why altricial and precocial species (as defined by eye opening) are so reliably born before and after peak velocity, respectively (figure 4; see above). The range of event scores for birth calculated here (0.42–0.83; average = 0.64, s.d. = 0.18; see also [9]) is much larger than that of peak brain velocity timing (F = 0.042, p < 0.01). In sum, the age of peak brain growth velocity is a reliable marker of underlying neurodevelopmental events, while birth is highly variable.
4. Discussion
The central finding of this study is that eutherian brain growth acceleration varies minimally during fetal neurogenesis and is largely uncorrelated to differences in the growth rates of whole body and visceral organs (figure 2). This means that brain growth velocity (g d−1) is fairly conserved at any given moment of eutherian prenatal development, once growth is aligned according to the onset of brain growth (i.e. neurulation) (figure 3). We found no evidence that larger adult brains, with correspondingly larger neocortices, are grown faster in utero; similarly, we found no evidence for a relationship between body acceleration and adult body size. Instead, larger brains are grown primarily by extending the duration of brain growth, a pattern that closely tracks extended schedules of neurodevelopmental events [9] (figure 4). We are unaware of any variable that predicts the acceleration rates of brain, organ, or whole-body growth described here.
All three primate species exhibit exceptionally slow rates of whole-body and visceral organ growth when compared with non-primate eutherians. Slow primate post-cranial growth helps to explain primate-shared prenatal encephalization, a robust and poorly understood fetal growth pattern that makes primates exceptionally encephalized across prenatal development [22,40–42]. Previous work has shown that the slope of prenatal primate brain/body allometric growth (RGP, see above) is higher than in non-primate mammals, implying either faster brain or slower body acceleration in primates [22]. The present results confirm that slower body growth causes primates to be more encephalized in utero (see also [41–43]), and may indicate that patterns of encephalization shared across primate radiations are partially a consequence of body size reduction [25,40]. Finally, the data on Tammar wallaby and short-tailed opossum support previous observations that marsupial brain development is exceptionally slow [9,36–38], a developmental feature that remains poorly understood.
Finally, peak brain growth velocity serves as a reliable marker in diverse eutherian species for a similar period of neurodevelopment, corresponding to forebrain myelination and eye opening (figure 4). As such, it perfectly distinguishes altricial species born prior to peak velocity from precocial species born after it. Adult brain size is also highly predictable in our sample from peak brain growth velocity (g d−1; r2 = 0.98) and brain size at peak velocity (r2 = 0.99). One application of these models is to predict peak velocity and its corresponding brain size in species for which adult brain size is known but for which growth data are unavailable.
(a). Analytic confounds and birth timing
The evidence presented here for conserved rates of eutherian fetal growth confirm previous studies that have noted minimal variation in fetal brain growth rates by comparing cube-root neonatal brain size [3,4], but fail to support theories that fetal growth rates vary according to physiological and life-history variables [6,18,19]. What produces these different findings between studies? The central limitation to the present study is the small number of eutherian species (n = 10) for which direct fetal brain growth data are available in the literature, and additional data may reveal relationships that were not detected due to our limited sample size. This is particularly true for placental invasiveness and developmental state at birth, since only three endotheliochorial and three altricial species are represented in our eutherian sample (n = 10). However, we believe that several analytic confounds may explain the discrepancies between our results and those of previous researchers.
The principal confound concerns predicting raw neonatal brain size from gestation length. For example, Elliot & Crespi [19] calculate ‘brain growth rate’ (i.e. velocity in g d−1) in a large sample of mammals by dividing neonatal brain size by total gestation length (see dotted lines in electronic supplementary material, figure S2c). This method treats sigmoid patterns of fetal brain growth as linear, implicitly assuming a constant rate of growth in grams per day across all of gestation. In fact, this measure is completely uncorrelated to the measure of interest (growth acceleration) within our eutherian sample (r8 = −0.14, p = 0.70), and produces different ‘rates’ along the same sigmoid growth pattern depending on the timing of birth (electronic supplementary material, figure S2c). This confound is shared by several studies that include gestation length as a covariate in multiple regression models predicting raw neonatal brain size (e.g. [39]), and was in fact the original motivation behind using exponential (cubic) models of neonatal data to characterize fetal brain and body growth [3].
A second analytic confound comes from the use of neonatal brain/body allometry as a proxy for brain growth. First, the assumption that brain/body proportions at birth reflect differences in fetal brain growth is not supported by this study; instead, we found that body acceleration alone negatively predicts neonatal brain/body proportions and fetal allometric curves (i.e. slow body growth produces greater fetal and neonatal encephalization). As we saw above, this problem is shared by any study that incorporates neonatal body size into multiple regression models predicting neonatal brain size, including studies that have argued for relative basal metabolism [18], placental invasiveness [19] as well as other studies on life-history variables [6,17,43]. Second, as with brain growth (figure 4), birth is highly variable along ontogenetic brain/body allometric trajectories, with altricial species being born earlier [22]—this gives altricial species higher brain/body ratios at birth, since relative brain size generally decreases over ontogeny (electronic supplementary material, figure S3a). Understanding this variation can help to resolve conflicting findings from the literature (see electronic supplementary material).
It remains possible that variables such as placental morphology or metabolism are indirect factors in determining the length of gestation and maternal investment (e.g. [44]). Nevertheless, the measure of growth acceleration used here (cube-root model slope) removes artefacts caused by neurulation and birth timing, and reveals a novel distribution of variation in brain growth that remains to be explained (e.g. rapid rates in rabbit). Our findings agree with previous studies suggesting that birth timing is too variable to serve as a reliable temporal anchor in comparative neurodevelopment [9,22–24].
(b). Evolution and development of brain size
How does evolution alter neurodevelopment to produce larger brains? A central hypothesis proposes that regular ‘stretching’ of conserved neurodevelopmental schedules—a process that increases the number of progenitor cells available for later neurogenesis—produces both larger brains, as well as regular trends in the allometry of brain areas [7–9]. Importantly, this mechanism can operate by changing the timing of developmental events (e.g. onset of neurogenesis) without necessarily altering the speed of cell division. Recent evidence in bird species indicates that faster cell cycle kinetics prior to neurogenesis onset is likely responsible for increased brain size in chicken relative to quail [11], though cell cycle rates were similar in both species once neurogenesis began. Here, we found relatively conserved growth rates during neurogenic periods in mammals and birds, but it remains possible that mammals differ during earlier stages of progenitor division.
As brain tissue is energetically expensive to grow and maintain [45,46], recent attention has turned to how species with larger brains increase energy intake or reallocate limited resources to support neurodevelopment (e.g. [17]). While our study failed to find support for metabolic or placental constraints, the relative constancy of brain growth (while the growth of other organs widely varies between species) could suggest that resources are preferentially allocated to brain growth during fetal development [42]. Still, it remains unclear whether energetic constraints are the source of species differences in post-cranial growth, or what those might be. Finally, it is possible that slowing post-cranial growth is an adaptation for lowering the daily energetic demands of gestation, particularly during perinatal periods when growth demands are especially high. Lowering the energetic costs of growing post-cranial tissues might allow for longer gestation and prolonged neurogenesis (i.e. increased brain size) without the associated cost of producing more altricial young (i.e. newborns at earlier neurodevelopmental stages).
5. Conclusion
This study provides direct evidence that brain growth acceleration during fetal neurogenesis is highly conserved within eutherian mammals. Mammals differ much more in rates of body and visceral organ growth, an observation that complicates the interpretation of neonatal brain/body proportions as a proxy for brain growth. Peak growth velocity consistently occurs around eye opening, helping to explain the regular variation in birth timing between altricial and precocial species. Finally, primate prenatal and neonatal encephalization are a consequence of exceptionally slow rates of body growth during prenatal development, an important feature of their slow life histories that is a developmental source of primate-shared encephalization patterns in early ontogeny.
Supplementary Material
Acknowledgements
The author would like to thank Terrence Deacon for guidance throughout the period of this research, Marilyn Renfree for her efforts to retrieve her original wallaby data and both editors and anonymous reviewers for comments that improved this manuscript.
Data accessibility
Datasets available at Dryad: http://dx.doi.org/10.5061/dryad.t3n50 [26].
Competing interests
I declare I have no completing interests.
Funding
This research was supported by funding from a Leakey Foundation Research Grant (2014); a UC Berkeley Graduate Division Summer Grant (2011); a National Science Foundation Graduate Research Fellowship (2009–2012); and a UC Berkeley Institute for Cognitive and Brain Sciences Research Grant (2013).
References
- 1.Naumann RK. 2015. Even the smallest mammalian brain has yet to reveal its secrets. Brain Behav. Evol. 85, 1–3. ( 10.1159/000375438) [DOI] [PubMed] [Google Scholar]
- 2.Kojima T. 1951. On the brain of the sperm whale (Physeter catodon L). Sci. Rep. Whales Res. Inst. 2, 199–218. [Google Scholar]
- 3.Sacher GA, Staffeld EF. 1974. Relation of gestation time to brain weight for placental mammals: implications for the theory of vertebrate growth. Am. Nat. 108, 593–615. ( 10.1086/282938) [DOI] [Google Scholar]
- 4.Passingham RE. 1985. Rates of brain development in mammals including man. Brain Behav. Evol. 26, 167–175. ( 10.1159/000118773) [DOI] [PubMed] [Google Scholar]
- 5.Pagel MD, Harvey PH. 1988. How mammals produce large-brained offspring. Evolution 42, 948–957. ( 10.2307/2408910) [DOI] [PubMed] [Google Scholar]
- 6.Barton RA, Capellini I. 2011. Maternal investment, life histories and brain growth in mammals. Proc. Natl Acad. Sci. USA 108, 6169–6174. ( 10.1073/pnas.1019140108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay BL, Darlington RB. 1995. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584. ( 10.1126/science.7777856) [DOI] [PubMed] [Google Scholar]
- 8.Clancy B, Darlington RB, Finlay BL. 2001. Translating developmental time across mammalian species. Neuroscience 105, 7–17. ( 10.1016/S0306-4522(01)00171-3) [DOI] [PubMed] [Google Scholar]
- 9.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species . J. Neurosci. 33, 7368–7383. ( 10.1523/JNEUROSCI.5746-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobbing J, Sands J. 1979. Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83. ( 10.1016/0378-3782(79)90022-7) [DOI] [PubMed] [Google Scholar]
- 11.Charvet CJ, Striedter GF. 2010. Bigger brains cycle faster before neurogenesis begins: a comparison of brain development between chickens and bobwhite quail. Proc. R. Soc. B 277, 3469–3475. ( 10.1098/rspb.2010.0811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caviness VS, Takahashi T, Nowakowski RS. 1995. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18, 379–383. ( 10.1016/0166-2236(95)93933-O) [DOI] [PubMed] [Google Scholar]
- 13.Butler H, Juurlink BHJ. 1987. An atlas for staging mammalian and chick embryos. Boca Raton, FL: CRC Press. [Google Scholar]
- 14.Brody S. 1945. Bioenergetics and growth: with special reference to the efficiency complex in domestic animals. New York, NY: Reinhold. [Google Scholar]
- 15.Laird AK. 1967. Evolution of the human growth curve. Growth 31, 345–355. [PubMed] [Google Scholar]
- 16.Huggett AG, Widdas WF. 1951. The relationship between mammalian foetal weight and conception age. J. Physiol. 114, 306–317. ( 10.1113/jphysiol.1951.sp004622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isler K, van Schaik CP. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400. ( 10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 18.Martin RD. 1981. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature 293, 57–60. ( 10.1038/293057a0) [DOI] [PubMed] [Google Scholar]
- 19.Elliot MG, Crespi BJ. 2008. Placental invasiveness and brain-body allometry in eutherian mammals. J. Evol. Biol. 21, 1763–1778. ( 10.1111/j.1420-9101.2008.01590.x) [DOI] [PubMed] [Google Scholar]
- 20.Theiler K. 1972. The house mouse. New York, NY: Springer-Verlag. [Google Scholar]
- 21.O'Rahilly RR, Müller F. 2006. The embryonic human brain: an atlas of developmental stages. Hoboken, NJ: Wiley. [Google Scholar]
- 22.Halley AC. 2016. Prenatal brain/body allometry in mammals. Brain Behav. Evol. 88, 14–24. ( 10.1159/000447254) [DOI] [PubMed] [Google Scholar]
- 23.Dobbing J. 1973. The developing brain: a plea for more critical interspecies extrapolation. Nutr. Rep. Int. 7, 401–406. [Google Scholar]
- 24.Newell-Morris L, Fahrenbruch CF. 1985. Practical and evolutionary considerations for use of the non-human primate model in prenatal research. In Nonhuman primate models for human growth and development (ed. Watts ES.), pp. 9–40. New York, NY: AR Liss. [Google Scholar]
- 25.Halley AC, Deacon TW. 2017. The developmental basis of evolutionary trends in primate encephalization. In Evolution of nervous systems, 2nd edn, vol. 3 (eds Kaas J, Krubitzer L), pp. 149–162. Oxford, UK: Elsevier. [Google Scholar]
- 26.Halley AC. 2017. Data from: Minimal variation in eutherian brain growth rates during fetal neurogenesis. Dryad Digital Repository. ( 10.5061/dryad.t3n50) [DOI] [PMC free article] [PubMed]
- 27.Core Team R. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/ [Google Scholar]
- 28.Bininda-Emonds ORP, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512. ( 10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 29.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 30.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.2. See https://CRAN.R-project.org/package=caper. [Google Scholar]
- 31.Benedict FG. 1938. Vital energetics: a study in comparative basal metabolism. Washington, DC: Carnegie Institute of Washington. [Google Scholar]
- 32.Bruhn JM. 1934. The respiratory metabolism of infrahuman primates. Am. J. Physiol. 110, 477–484. [Google Scholar]
- 33.Petry H, Riehl I, Zucker H. 1986. Energieumsatzmessungen an Weissbüscheläffchen (Callithrix jacchus). J. Anim. Physiol. Anim. Nutr. 55, 214–224. ( 10.1111/j.1439-0396.1986.tb00722.x) [DOI] [Google Scholar]
- 34.Eisenberg JF. 1981. The mammalian radiations. London, UK: Athlone Press. [Google Scholar]
- 35.Hart JS. 1971. Rodents. In Comparative physiology of thermoregulation (ed. Whittow GG.), pp. 1–149. London, UK: Academic Press. [Google Scholar]
- 36.Renfree MB, Holt AB, Green SW, Carr JP, Cheek DB. 1982. Ontogeny of the brain in a marsupial (Macropus eugenii) throughout pouch life. Brain Behav. Evol. 20, 57–71. ( 10.1159/000121581) [DOI] [PubMed] [Google Scholar]
- 37.Weisbecker V, Goswami A. 2010. Brain size, life history, and metabolism at the marsupial/placental dichotomy. Proc. Natl Acad. Sci. USA 107, 16 216–16 221. ( 10.1073/pnas.0906486107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darlington RB, Dunlop SA, Finlay BL. 1999. Neural development in metatherian and eutherian mammals: variation and constraint. J. Comp. Neurol. 411, 359–368. ( 10.1002/(SICI)1096-9861(19990830)411:3%3C359::AID-CNE1%3E3.0.CO;2-J) [DOI] [PubMed] [Google Scholar]
- 39.Goedbloed JF. 1976. Embryonic and postnatal growth of rat and mouse: IV. Prenatal growth of organs and tissues: age determination, and general growth patterns. Acta Anat. 95, 8–33. ( 10.1159/000144598) [DOI] [PubMed] [Google Scholar]
- 40.Deacon TW. 1990. Problems of ontogeny and phylogeny in brain-size evolution. Int. J. Primatol. 11, 237–282. ( 10.1007/BF02192870) [DOI] [Google Scholar]
- 41.Holt AB, Cheek DB, Mellits ED, Hill DE. 1975. Brain size and the relation of the primate to the nonprimate. In Fetal and postnatal cellular growth: hormones and nutrition (ed. Cheek DB.), pp. 23–44. Hoboken, NJ: Wiley. [Google Scholar]
- 42.Sacher GA. 1982. The role of brain maturation in the evolution of the primates. In Primate brain evolution (eds Armstrong E, Falk D), pp. 97–112. New York, NY: Springer. [Google Scholar]
- 43.Martin RD. 1983. Human brain evolution in an ecological context. New York, NY: American Museum of Natural History. [Google Scholar]
- 44.Capellini I, Venditti C, Barton RA. 2011. Placentation and maternal investment in mammals. Am. Nat. 177, 86–98. ( 10.1086/657435) [DOI] [PubMed] [Google Scholar]
- 45.Mink JW, Blumenschine RJ, Adams DB. 1981. Ratio of central nervous system to body metabolism in vertebrates—its constancy and functional basis. Am. J. Physiol. 241, R203–R212. [DOI] [PubMed] [Google Scholar]
- 46.Holliday MA. 1986. Body composition and energy needs during human growth. In Human growth: a comprehensive treatise, 2nd edn (eds Falkner F, Tanner JM), pp. 101–107. New York, NY: Plenum. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Halley AC. 2017. Data from: Minimal variation in eutherian brain growth rates during fetal neurogenesis. Dryad Digital Repository. ( 10.5061/dryad.t3n50) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets available at Dryad: http://dx.doi.org/10.5061/dryad.t3n50 [26].