Abstract
Two decades of research suggest social relationships have a common evolutionary basis in humans and other gregarious mammals. Critical to the support of this idea is growing evidence that mortality is influenced by social integration, but when these effects emerge and how long they last is mostly unknown. Here, we report in adult female macaques that the impact of number of close adult female relatives, a proxy for social integration, on survival is not experienced uniformly across the life course; prime-aged females with a greater number of relatives had better survival outcomes compared with prime-aged females with fewer relatives, whereas no such effect was found in older females. Group size and dominance rank did not influence this result. Older females were less frequent targets of aggression, suggesting enhanced experience navigating the social landscape may obviate the need for social relationships in old age. Only one study of humans has found age-based dependency in the association between social integration and survival. Using the largest dataset for any non-human animal to date, our study extends support for the idea that sociality promotes survival and suggests strategies employed across the life course change along with experience of the social world.
Keywords: social relationships, longevity, social connectedness, life course, ageing
1. Introduction
The last few decades have delivered considerable evidence of an association between social relationships and mortality in humans [1–4]. Although the overwhelming majority of studies to date have focused on links between sociality and longevity in older people [5–7], new research has honed in on determining when these effects emerge and how long they last. Understanding when the association between sociality and survival appears, and how it progresses with age is critical because it allows researchers to pinpoint the biological and life-history mechanisms that underpin social relationships and to shed light on the consequences of variable social strategies. For example, the one study to date to determine how people's health is connected to sociality across the life course found that social network size was more important to physical health for young and elderly adults compared with the middle-aged [8], suggesting that the putative benefits of social ties are not experienced midway through life or are offset by the particular demands placed on people during this period.
In animals other than humans, social relationships also appear to enhance longevity [9–12], intimating a common evolutionary basis across gregarious species [13]. Here, too, the social environment [14,15] and the risk of mortality [16] are not uniformly experienced as individuals age. Yet whether the influence of social relationships on survival is consistent across the lifespan of non-humans remains unknown. This gap in knowledge exists in part because data that extend over sufficient periods of the life course of long-lived animals are exceedingly rare.
Here, we determine whether sociality predicts survival across the lifespan of a gregarious species of macaque. We use a large dataset spanning 21 years and including 910 adult female rhesus macaques (Macaca mulatta) from the Cayo Santiago field station in Puerto Rico. Free-ranging, highly social animals like the Cayo Santiago macaques are an important system in which to explore the association between social relationships and biological success because, similar to contemporary human societies, the threat of predation and starvation are largely reduced, leaving navigation of the social milieu as one of the main challenges with which individuals must cope. In other words, free-ranging animals allow researchers to examine whether sociality predicts survival in isolation from other factors, in a system where the social environment is of critical importance. We use number of available closely related adult females as a proxy of social integration. As is common in many female-philopatric and nepotistic monkeys, female rhesus macaques preferentially form social relationships with close adult female kin and are more likely to assist female relatives in agnostic encounters [17–19]. We use a time-varying statistical framework that allows us to determine age-related changes in the association between sociality and mortality risk.
Our results confirm the presence of an age-dependent relationship between sociality and survival, showing that number of adult female relatives is related to the survival of prime-aged but not older adult female rhesus macaques. This finding motivated us to also characterize differences in the social behaviours of prime-aged compared with older females in order to begin to elucidate biological mechanisms underpinning this relationship.
2. Material and methods
(a). Study subjects
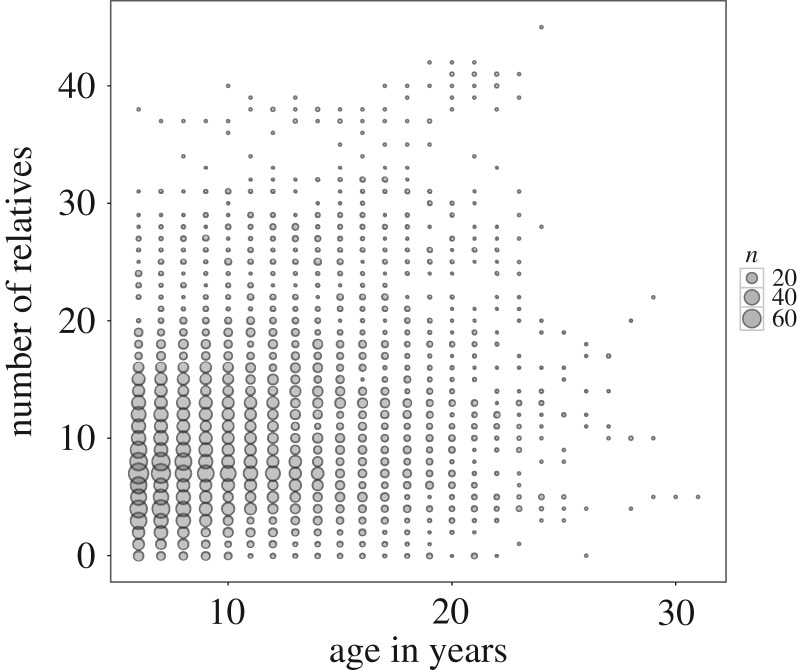
Study subjects were members of the well-studied Cayo Santiago field station off the southeastern coast of Puerto Rico [20]. Subjects were females that were mature adults, i.e. 6 years old or greater [21], living in 14 different social groups. We focused on 910 females that were alive between the years of 1992 and 2013, a period during which genetic parentage assignment was undertaken for all animals [22] and pairwise relatedness could be estimated with greatest accuracy. Demographic data were collected up to 5 days a week throughout the study period. For all females in the dataset, dates of birth were known to within a few days. Dispersal opportunities are limited to the island and dates of death were known within a few days for all females that died before the end of the study period (n = 321). There is no regular medical intervention for sick or wounded individuals and thus the major causes of death at this provisioned and predator-free site are illness and injury [20,23]. The maximum age observed was 31 years (figure 1).
Figure 1.
Distribution of age and number of adult female relatives. Subject's age is plotted against her number of adult female relatives, where r ≥ 0.063 and with the size of points scaled according to the number of datapoints they represent (5 441 female years in total). Adult female rhesus macaques entered our dataset at age 6, with one female obtaining a maximal age of 31 years. Females had a mean of 10.7 adult female relatives (min = 0, max = 45, s.d. = 7.203).
(b). Measuring family network size
We used the number of adult female relatives that were present in a subject's social group in a given year as a proxy of social integration. In many mammals that form mixed-relatedness groups and where females are philopatric and nepotistic, such as rhesus macaques, females preferentially form social relationships with, and offer assistance in agonistic encounters to, their closest female kin compared with other group members [17–19], giving rise to the probabilistic inference that female macaques with a greater number of relatives will have greater access to affiliative relationships and will be more likely to be supported in a fight. Observations of the realized social relationships of a subset of subjects were available but the small number of deaths in this reduced dataset (n = 39) precluded robust survival analysis. Estimating access to social relationships and support using number of closely related females instead allowed us to generate the largest dataset in which to explore the association between sociality and longevity in a non-human animal to date (n = 910 compared with n = 204 in the largest previous study by Archie et al. [9]).
The relatedness coefficient at which female rhesus macaques discriminate kin from non-kin varies depending on the type of interaction considered. Kin discrimination occurs at r ≥ 0.125 for affiliative interactions [24] and at r ≥ 0.063 for responses to vocalizations [25]. Moreover, although preferential associations occur at lower relatedness thresholds, pairs of females who are the most closely related, i.e. mother–daughter pairs and sisters, exhibit the strongest kin-bias [17]. In order to capture the richest measure of family network size, while recognizing that the value of female relatives may increase along with their degree of relatedness, we explored the relationship between survival and family network size using four grades of increasing relatedness thresholds, from r ≥ 0.063 to r = 0.5. However, the results for the lower three grades of relatedness were similar to one another (electronic supplementary material, table S1 and figure S3) and thus we opt only to present results for the lowest (r ≥ 0.063) and highest (r = 0.5) grades. Pairwise relatedness was extracted from the genetic pedigree database (details of the genetic parentage assignment at this site can be found in [22,26]).
(c). Testing family network size as a predictor of survival across the lifespan
We used two extended Cox proportional hazard models to evaluate whether number of adult female relatives was a predictor of survival. First, we used a model with time-dependent covariates [27] to establish the general relationship between number of adult female relatives and survival for all study subjects. Second, we used a model with both time-dependent covariates and a time-dependent coefficient [27] to diagnose whether the hazard coefficient in the relationship between number of relatives and survival was expressed uniformly over time—i.e. whether or not the hazard coefficient was non-proportional. These models included 5 441 years of data on 910 females, with a mean number of years of data per female of 7.22 years, range 1–26 (electronic supplementary material, figure S1). Both survival models included number of adult female relatives in a given year as a continuous predictor of survival to the following year and were run for each relatedness gradient. Critical to the design of this analysis is that it is longitudinal and temporally predictive in nature. Longitudinal analyses buffer against confounding factors by allowing factors of interest to vary within individuals, reducing the influence of potential confounds that remain constant across an individual's lifetime; i.e. genetic endowment may affect differences in family network size and survival between females, but such factors are less likely to underpin changes in survival probability or family network size within females [8]. By following females throughout their adult life, we were able to evaluate survival in relation to differences in family network size within individuals; females entered our models as mature adults and left the model either because of death (n = 321) or because they were removed from the island as part of population management or the study period ended before they died.
We also explored the impact of group size and dominance rank on female survival. Low ranking females have been previously shown to have poorer survival outcomes in this population compared to their higher ranking counterparts [21], while females in larger groups may experience reduced risks of mortality owing to the benefits of group-living [28], such as protection from inter-group aggression. Group size and dominance rank data were only available for a subset of our data and thus these analyses were based on separate models. We examined group size effects using two variables: overall group size, which included all age and sex classes, and total number of adult females (electronic supplementary material, figure S4). This allowed us to account for the possibility that mortality risks are lower for females in groups with a greater number of adult females in general, regardless of the size of their network of relatives. We extracted annual group size data from the site's demographic database (N = 442 females, 128 known deaths). We determined maternal dominance rank in accord with a previously established method [21]. In brief, because female macaques acquire the rank adjacent to that of their mothers, related descendants of a crown female ancestor (i.e. members of the same ‘matriline’) tend to be adjacent to one another in the hierarchy and the rank of a matriline is thus a proxy of individual dominance rank. Since matriline rankings are highly stable in rhesus macaques [29], the known rank of a matriline can be extrapolated backwards in time to females for whom individual rank data are unavailable [21]. We used available data on pairwise agonistic encounters collected between 2007 and 2015 in four social groups. These groups were used because they contained more than one crown matriline, since females in groups with a single matriline have the same relative rank [21]. Deaths of females of known individual rank (n = 39) were too few to perform a robust survival analysis. We established matrilineal ranks of 256 females, 95 of which had known dates of death.
(d). Identifying patterns of social behaviour across the lifespan
We used observations of female social behaviour to determine the association between sociality and female age. We conducted behavioural observations on 276 females over a period of five years (2010–2015) in six social groups and recorded grooming and aggressive interactions between female subjects and all other adult members of their social group. Raw rates of behaviours (number of interactions/hours observed) were adjusted using z-scores to allow comparisons across different groups of animals in different years [30]. Although the behavioural data only partly overlap with the data used in the survival models, our aim was to establish general patterns of behaviour for females of different ages and had no reason to believe these would diverge broadly with time. We ran linear mixed model regressions to account for repeated measures of individuals across years and fit these models using Markov chain Monte Carlo routines to account for the violation of the assumption of independence in relational data [31,32]. We controlled for individual dominance rank, established using pairwise agonistic encounters, and number of adult female relatives (r ≥ 0.063) in all models. We examined female age as a continuous variable and also divided age into categories (prime-aged, old) in order to characterize the relationship between sociality and age as broadly as possible.
3. Results
(a). Family network size predicts survival in prime-aged, but not old, adult females
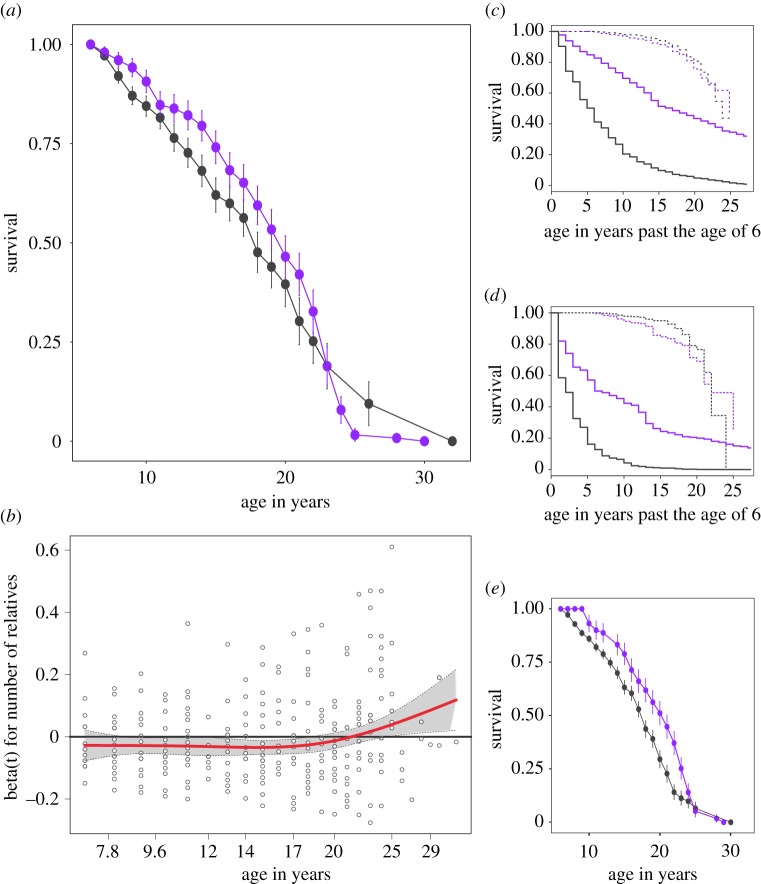
The overall relationship between family network size and survival was not significant (r ≥ 0.063, coefficient = −0.01, p = 0.340, figure 2a). However, this effect was not constant across females of different ages (ρ = 0.142, p = 0.014). Visualization of the non-proportional hazard (figure 2b) revealed that a lack of relatives had a negative impact on survival early in adulthood—females with few adult female relatives had an elevated risk of mortality (i.e. a negative hazard coefficient)—while the hazard coefficient for number of adult female relatives was positive later in life. Further inspection showed that the hazard coefficient began to increase towards zero around the age of 18, which is the median age of death for females in this population. We, therefore, divided females into two categories: prime-aged (6–17 years old) and old (greater than or equal to 18 years old), and ran a new time-dependent model to confirm the locality of age-related differences [27]. This model showed that effect of family network size on survival was limited to prime-aged females (figure 2c). Prime-aged females with a greater number of adult female relatives had a greater probability of survival compared with prime-aged females with fewer relatives (coefficient = −0.023, p = 0.027). That is, each additional relative decreased a prime-aged female's probability of death from one year to the next by 2.3%, and prime-aged females with the mean number of close adult female relatives were 24.3% less likely to die than prime-aged females with no close adult female relatives. By contrast, family network size had no significant impact on survival in older females (coefficient = 0.010, p = 0.308, figure 2d).
Figure 2.
Family network size predicts survival in prime-aged but not older adult females. (a) Relationship between survival and number of adult female relatives across female age generated using raw data, where r ≥ 0.063 and plots are Kaplan–Meier survival estimates with standard error bars. Purple lines are females with greater than or equal to 75% quartile number of adult female relatives, black lines are females with less than 25% quartile number of adult female relatives. (b) The hazard coefficient for the impact of number of relatives on survival (red line) was non-proportional, falling below zero (black line) in early years and above zero later in life. (c) Predictive relationship between survival and number of adult female relatives in an age-dependent context and (d) when matrilineal rank is taken into account. Solid lines are prime-aged females, dashed lines are old females, purple and black lines are the same as in (a). Plots show female survival past the age they enter the dataset (6 years old, thus 1 on the x-axis is equivalent to 7 years of age). (e) Shows the same plot as in (a) but where r = 0.5.
When relatedness was restricted to include only mother–daughter and sister pairs, i.e. r = 0.5, a somewhat similar pattern emerged. However, here family network size was a significant predictor of survival (coefficient = −0.105, p = 0.019, figure 2e) with females with the mean number of relatives for this threshold of relatedness (µ = 1.5) having a 34.0% lower risk of death than females without a mother, daughter, or sister in the group. But further inspection revealed this result continued to be driven by a significant association between sociality and survival in prime-aged (coefficient = −0.140, p = 0.026) but not older females (coefficient = −0.068, p = 0.280) (electronic supplementary material, table S1).
Neither overall group size nor the number of adult females in a group significantly impacted on survival (overall group size: coefficient = 0.002, p = 0.241; number of adult females: coefficient = 0.001, p = 0.786) and there was no evidence of non-proportional hazards for group size effects (overall group size: ρ = 0.135, p = 0.225; number of adult females: ρ = 0.165, p = 0.182). As expected, dominance rank significantly predicted survival (with a proportional hazard coefficient: ρ = −0.029, p = 0.774); females from low ranking matrilines experienced a 55% greater probability of death from one year to the next than high ranking females (coefficient = 0.445, p = 0.043). Females from high ranking matrilines did not have a greater number of adult female relatives compared with females from low ranking matrilines (r ≥ 0.063: estimate = −0.325, p = 0.649; r = 0.5: estimate = −0.070, p = 0.460) (electronic supplementary material, figure S5) and, critically, we found that the inclusion of dominance rank did not qualitatively alter the relationship between family network size and survival (figure 2d). That is, independent of the effects of dominance rank, family network size was a significant predictor of survival in prime-aged (coefficient = −0.074, p < 0.001; electronic supplementary material, table S2), but not old, female rhesus macaques when relatedness was set to r ≥ 0.063. When relatedness was restricted to r = 0.5, family network size was a significant predictor of survival independent of dominance rank for females from both age categories, although with a much stronger effect in prime-aged (coefficient = −0.544, p < 0.001) compared with older females (coefficient = −0.398, p = 0.040).
(b). Older females engage less in the social environment compared to younger females
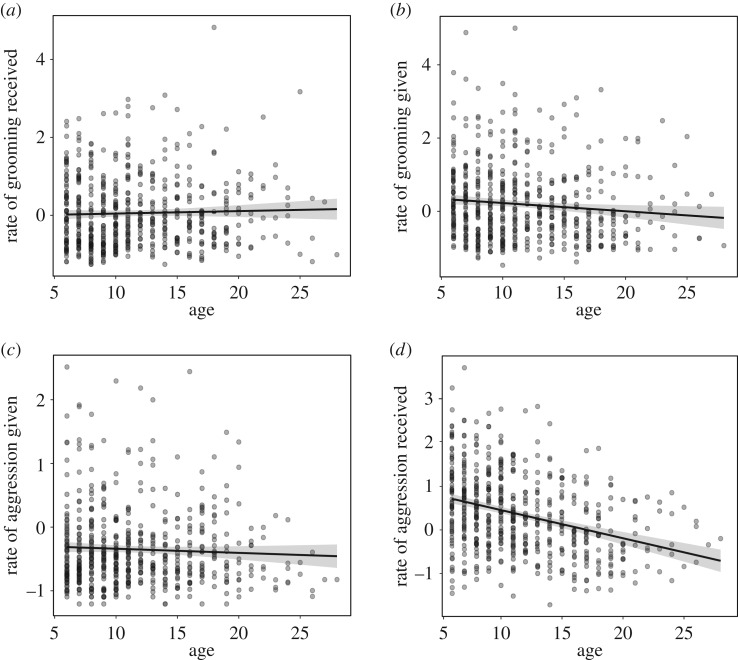
Social interactions that might be viewed as the most beneficial in terms of navigating the social milieu did not vary with age: neither the amount of grooming females received (estimate = 0.005, p = 0.614) nor the amount of aggression females directed toward others was associated with age (estimate = −0.011, p = 0.056) (figure 3). By contrast, older females were less frequently involved in social interactions that may be characterized as the most energetically costly or potentially dangerous: older females gave significantly less grooming to others (estimate = −0.025, p = 0.018) and were the targets of significantly fewer aggressive interactions compared with their younger counterparts (estimate = −0.062, p < 0.001) (figure 3). Our results were largely similar when we considered age as a categorical variable (prime-aged = 6–17 years; old = greater than or equal to 18 years old) (electronic supplementary material, figure S6). Old females were less frequently the targets of aggression (estimate = −0.490, p < 0.001) but directed aggression toward others at the same rate as prime-aged females (estimate = −0.129, p = 0.054). However, old females did not differ from prime-aged females with respect to how much grooming they gave (estimate = −0.203, p = 0.132) or received (estimate = 0.029, p = 0.786).
Figure 3.
Change in behaviours with female age. The relative amount of grooming females received (a) and the amount of aggression females gave (c) did not vary with age. By contrast, older adult females gave less grooming (b) and received less aggression (d) compared with younger adult females. Relative rates are z-scores, where values above 0 are above the mean rate of a given behaviour in a group in a given year, below 0 are below the mean in a group in a given year.
4. Discussion
(a). Sociality and survival
Social relationships are a hallmark of the behaviours of humans and many other gregarious animals, suggesting a shared evolutionary basis [13]. If social relationships serve a function and have been favoured by selection, then we expect them to be associated with proxies of evolutionary fitness, such as increased health, survival, or reproductive success. A small but growing number of studies in humans and other animals have linked social integration with health benefits [3–5] and reproductive outcomes, such as increased birth rate and offspring survival [26,33,34]. Longevity is also a major determinant of fitness, especially in long-lived species where variance in reproductive output is relatively low but variance in lifespan can be high [35,36]. However, studies of how longevity is influenced by social relationships in non-human animals are exceedingly rare owing to the scarcity of datasets with sufficient representation of the lifespan. To date, there have only been four such studies: integration in the social network was positively associated with survival in 28 juvenile male bottlenose dolphins [11]; reciprocal positive relationships were linked to reduced risk of death in 49 female laboratory rats [12]; and survival outcomes in wild adult female baboons were positively associated with strong and stable social relationships with other adult females (n = 44) [10] and with adult males (n = 204) [9]. Using the largest dataset in non-human animals to date, we found that sociality is associated with reduced mortality risks during part of the life course of female macaques. Our results contribute to mounting evidence of a link between social relationships and longevity in gregarious species.
Of course, these studies are correlational in nature and the causal link between social relationship and survival outcomes must be established; e.g. research must rule out the possibility that social isolation is more common in individuals who are unhealthy and thus their increased risk of death is because of illness, not because of a lack of social relationships. Some of the long-term research in humans has made strides towards this aim. For example, in a longitudinal study of older men and women, Steptoe et al. [5] excluded those who died shortly after recording their measures of social isolation as a means to exclude the terminally ill and thus reject the hypothesis of reverse causality. Yang and colleagues found that current social support from family and friends was associated with a later reduced risk of inflammation [37] and that people with a low level of social integration were 13–54% more likely to be at risk for hypertension at a later point in time, exceeding the risk of hypertension that is associated with diabetes [8]. These studies are the first step towards showing that sociality leads to biological benefits via a causal impact on health.
Being longitudinal and temporally predictive, this study also addresses issues of reverse causality and analytical confounds, such as a potential ‘good genes’ effect. By allowing changes in family network size within a female to influence future survival across her lifespan, the proper temporal order of the relationship of interest is ensured and the impact of confounding factors that remain constant across an individual's life is diminished. Familial identity is further accounted for by including matrilineal rank; female rhesus macaques attain a rank directly below their mothers, thus controlling for rank effects is a de facto way to evaluate the relationship between survival and family network size in a manner that is independent from the influence of some families having ‘better’ genes than others. Similar studies of non-human animals are largely lacking due to limitations in the availability of appropriate longitudinal data, but this is a direction for future research of critical importance.
Despite the common occurrence of differentiated social relationships within groups from a broad range of animal taxa, the ultimate function of these relationships, i.e. the mechanism through which biological benefits are realized, remains unclear [13]. One way to address this question is to explore the characteristics or types of social relationships that are associated with survival benefits. We estimated access to social relationships and support as the number of adult female relatives based on the well-established bias towards close kin in the formation of relationships by female Old World monkeys [17]. This metric excludes potentially important relationships between non-relatives [13] and is neutral to the quality of relationships, subsuming what may be both relatively weakly and strongly bonded pairs (which is typical in long-term studies of animal sociality: e.g. the composite sociality index [34], although see [38]). Regardless, the types of relationships that are important in non-human animals is an active area of debate and inquiry [39] and additional research in this direction promises to yield important new insights to the structure and function of animal social behaviour.
The unique characteristics of our field system may also provide some clues to the function(s) of social relationships. By creating an environment where the social sphere can be evaluated in isolation from other pressures, such as predation or drastic fluctuations in the physical environment [20], we are able to attribute the impact of social support on biological success to the role that sociality plays in helping animals cope with challenges in the social environment, such as competition for food and mates. However, additional information is required to firmly pinpoint the function(s) of social relationships. One key source of such information is the role sociality plays in the lives of individuals with divergent social needs, such as individuals at different points in their life course.
(b). Are older females more socially skilled and thus less reliant on help from others?
We have shown that sociality largely has consequences for the survival of prime-aged but not older adult female rhesus macaques. This result may be explained by simple demographic patterns. Like humans [8], older female rhesus macaques may be naturally more embedded in their social networks because they are more likely to have adult female offspring and grand offspring living in their groups, rendering social integration a non-discriminating factor in later years. Demographic patterns also inherently lead to a diminished representation of animals in later life, which may make it more difficult to characterize the intersection between sociality and survival in older individuals. However, an alternative explanation stems from the experience animals can accrue with age. Recent studies have shown that social and ecological knowledge are enhanced in older individuals [15,40,41]. In our study, older female rhesus macaques were able to extract relatively more grooming from their group mates than they gave in return and were also better at avoiding being the targets of aggression. Older females at this site also spend less time vigilantly scanning the social environment compared with younger animals [42]. These results may indicate that older females are simply less socially active, perhaps owing to lower energy reserves or reduced social motivation. However, recent findings from another species of macaque show that, although older females have reduced involvement in some types of social interaction, they maintain a consistent level of social motivation [14]. Combined with our findings, these results suggest that older female macaques do not suffer from reduced social motivation, but instead draw on their experience to be more behaviourally selective. The enhanced skills of older individuals when it comes to coping with challenges in the social environment could obviate the need for help from others. The age-dependency of our results is also in accordance with the only study of humans [8] to examine this topic to date and highlights the need for future research to consider that the influence of sociality on survival will not always be uniformly expressed across the life course.
Supplementary Material
Acknowledgements
We thank the Caribbean Primate Research Center (CPRC) for the permission to undertake research on Cayo Santiago, along with Bonn Aure, Jacqueline Buhl, Joel Glick, Josue Negron, Glorienelle Perez, Daniel Phillips, and many interns who assisted in behavioural data collection. We thank Elizabeth Maldonado and Abigail Hadley for assistance with the CPRC database, and Sam Ellis, Noah Snyder-Mackler, Jacintha Beehner, and an anonymous reviewer for helpful comments and discussion.
Ethics
Collection of field data and use of the Cayo Santiago long-term database were approved by the Animal Care and Use Committee of the University of Puerto Rico (A6850108) and by the Ethics Committee for the School of Psychology, University of Exeter.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.013d5 [43].
Authors' contributions
L.J.N.B. conceived the study, performed the statistical analyses, and wrote the manuscript. L.J.N.B. and M.L.P. designed and coordinated collection of behavioural data. A.R.-L. coordinated access to demographic data and managed data collection in the field. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIMH grants no. R01-MH096875 and R01-MH089484 to M.L.P. and L.J.N.B., and by a Leverhulme Early Career Fellowship to L.J.N.B. The CPRC is supported by grant no. 2P40OD012217 from the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health.
References
- 1.Christakis NA, Allison PD. 2006. Mortality after the hospitalization of a spouse. N. Engl. J. Med. 354, 719–730. ( 10.1056/NEJMsa050196) [DOI] [PubMed] [Google Scholar]
- 2.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.House JS, Landis KR, Umberson D. 1988. Social relationships and health. Science 241, 540–545. ( 10.1126/science.3399889) [DOI] [PubMed] [Google Scholar]
- 4.Seeman TE. 1996. Social ties and health: the benefits of social integration. Ann. Epidemiol. 6, 442–451. ( 10.1016/S1047-2797(96)00095-6) [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A, Shankar A, Demakakos P, Wardle J. 2013. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl Acad. Sci. USA 110, 5797–5801. ( 10.1073/pnas.1219686110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles LC, Glonek GF, Luszcz MA, Andrews GR. 2005. Effect of social networks on 10 year survival in very old Australians: the Australian longitudinal study of aging. J. Epidemiol. Commun. Health 59, 574–579. ( 10.1136/jech.2004.025429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. 2012. Loneliness, health, and mortality in old age: a national longitudinal study. Soc. Sci. Med. 74, 907–914. ( 10.1016/j.socscimed.2011.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. 2016. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl Acad. Sci. USA 113, 578–583. ( 10.1073/pnas.1511085112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. R. Soc. B 281, 20141261 ( 10.1098/rspb.2014.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 11.Stanton MA, Mann J. 2012. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE 7, e47508 ( 10.1371/journal.pone.0047508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee JR, Cavigelli SA, Delgado B, McClintock MK. 2008. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom. Med. 70, 1050–1059. ( 10.1097/PSY.0b013e31818425fb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brent LJ.N, Chang SW, Gariepy JF, Platt ML. 2014. The neuroethology of friendship. Ann. N. Y. Acad. Sci. 1316, 1–17. ( 10.1111/nyas.12315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeling L, Hammerschmidt K, Sennhenn-Reulen H, Freund Alexandra M, Fischer J. 2016. Motivational shifts in aging monkeys and the origins of social selectivity. Curr. Biol. 26, 1744–1749. ( 10.1016/j.cub.2016.04.066) [DOI] [PubMed] [Google Scholar]
- 15.Brent LJ.N., Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP. 2015. Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr. Biol. 25, 746–750. ( 10.1016/j.cub.2015.01.037) [DOI] [PubMed] [Google Scholar]
- 16.Nussey DH, Froy H, Lemaitre J-F, Gaillard J-M, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapais B, Berman CM. 2007. Kinship and behavior in primates. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Gouzoules S, Gouzoules H, Marler P. 1984. Rhesus monkey (Macaca mulatta) screams: representational signalling in the recruitment of agonistic aid. Anim. Behav. 32, 182–193. ( 10.1016/S0003-3472(84)80336-X) [DOI] [Google Scholar]
- 19.Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch FB. 2001. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc. Natl Acad. Sci. USA 98, 13 769–13 773. ( 10.1073/pnas.241210198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawlins RG, Kessler MJ. 1986. The Cayo Santiago macaques: history, behavior, and biology. Albany, New York: SUNY Press. [Google Scholar]
- 21.Blomquist GE, Sade DS, Berard JD. 2011. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta). Int. J. Primatol. 32, 193–208. ( 10.1007/s10764-010-9461-z) [DOI] [Google Scholar]
- 22.Widdig A, et al. 2016. Genetic studies on the Cayo Santiago rhesus macaques: a review of 40 years of research. Am. J. Primatol. 78, 44–62. ( 10.1002/ajp.22424) [DOI] [PubMed] [Google Scholar]
- 23.Buhl JS, Aure B, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML, Brent LJ. 2012. Response of rhesus macaques (Macaca mulatta) to the body of a group member that died from a fatal attack. Int. J. Primatol. 33, 860–871. ( 10.1007/s10764-012-9624-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapsalisi E, Berman CM. 1996. Models of affiliative relationships among free-ranging rhesus monkeys (Macaca mulatta). Behaviour 133, 1209–1234. ( 10.1163/156853996X00378) [DOI] [Google Scholar]
- 25.Rendall D, Rodman PS, Emond RE. 1996. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim. Behav. 51, 1007–1015. ( 10.1006/anbe.1996.0103) [DOI] [Google Scholar]
- 26.Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Skene JHP, Platt ML. 2013. Genetic origins of social networks in rhesus macaques. Sci. Rep. 3, 1042 ( 10.1038/srep01042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therneau T, Crowson C, Atkinson E. 2016. Using time dependent covariates and time dependent coefficients in the cox model. Surviv. Vignettes. 4 April 2017. [Google Scholar]
- 28.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Thierry B, Singh M, Kaumanns W. 2004. Macaque societies: a model for the study of social organization. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Brent LJN, Semple S, MacLarnon A, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML. 2014. Personality traits in rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. Int. J. Primatol. 35, 188–209. ( 10.1007/s10764-013-9732-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanneman RA, Riddle M. 2005. Introduction to social network methods. Riverside, CA: University of California Riverside. [Google Scholar]
- 32.Brent LJN, Lehmann J, Ramos-Fernández G. 2011. Social network analysis in the study of nonhuman primates: A historical perspective. Am. J. Primatol. 73, 720–730. ( 10.1002/ajp.20949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853. ( 10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 35.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 36.Pusey A. 2012. Magnitude and sources of variation in female reproductive performance. In The evolution of primate societies (eds Mitani J, Call J, Kappeler P, Palombit RA, Silk JB), pp. 343–366. Chicago, IL: University of Chicago Press. [Google Scholar]
- 37.Yang YC, Schorpp K, Harris KM. 2014. Social support, social strain and inflammation: evidence from a national longitudinal study of US adults. Soc. Sci. Med. 107, 124–135. ( 10.1016/j.socscimed.2014.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarland R, Murphy D, Lusseau D, Henzi SP, Parker JL, Pollet TV, Barrett L. 2017. The ‘strength of weak ties’ among female baboons: fitness-related benefits of social bonds. Anim. Behav. 126, 101–106. ( 10.1016/j.anbehav.2017.02.002) [DOI] [Google Scholar]
- 39.Brent LJN. 2015. Friends of friends: are indirect connections in social networks important to animal behaviour? Anim. Behav. 103, 211–222. ( 10.1016/j.anbehav.2015.01.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HC, Teichroeb JA. 2016. Partially shared consensus decision making and distributed leadership in vervet monkeys: older females lead the group to forage. Am. J. Phys. Anthropol. 161, 580 ( 10.1002/ajpa.23058) [DOI] [PubMed] [Google Scholar]
- 41.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 42.Watson KK, Li D, Brent LJN, Horvath JE, Gonzalez-Martinez J, Ruíz-Lambides AV, Robinson AG, Skene JHP, Platt ML. 2015. Genetic influences on social attention in free-ranging rhesus macaques. Anim. Behav. 103, 267–275. ( 10.1016/j.anbehav.2015.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Data from: Family network size and survival across the lifespan of female macaques. Data Dryad Repository. ( 10.5061/dryad.013d5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Data from: Family network size and survival across the lifespan of female macaques. Data Dryad Repository. ( 10.5061/dryad.013d5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.013d5 [43].