Graphical abstract
Keywords: Propolis, Miswak, Chitosan, Dental varnish, Streptococcus mutans, Demineralization
Abstract
Using natural products can be a cost-effective approach for caries prevention especially in low income countries where dental caries is highly prevalent and the resources are limited. Specially prepared dental varnishes containing propolis, miswak, and chitosan nanoparticles (CS-NPs) with or without sodium fluoride (NaF) were assessed for antibacterial effect against Streptococcus mutans (S. mutans) using disk diffusion test. In addition, the protective effect of a single pretreatment of primary teeth enamel specimens against in vitro bacterial induced enamel demineralization was assessed for 3 days. All natural products containing varnishes inhibited bacterial growth significantly better than 5% NaF varnish, with NaF loaded CS-NPs (CSF-NPs) showing the highest antibacterial effect, though it didn’t significantly differ than those of other varnishes except miswak ethanolic extract (M) varnish. Greater inhibitory effect was noted with varnish containing freeze dried aqueous miswak extract compared to that containing ethanolic miswak extract, possibly due to concentration of antimicrobial substances by freeze drying. Adding natural products to NaF in a dental varnish showed an additive effect especially compared to fluoride containing varnish. 5% NaF varnish showed the best inhibition of demineralization effect. Fluoride containing miswak varnish (MF) and CSF-NPs varnish inhibited demineralization significantly better than all experimental varnishes, especially during the first 2 days, though CSF-NPs varnish had a low fluoride concentration, probably due to better availability of fluoride ions and the smaller size of nanoparticles. Incorporating natural products with fluoride into dental varnishes can be an effective approach for caries prevention, especially miswak and propolis when financial resources are limited.
Introduction
Dental caries is a biofilm-induced oral disease with S. mutans playing a key role in the development of virulent cariogenic biofilms [1]. Thus, decreasing the bacterial burden of the oral cavity is one of the fundamental biological goals in preventing dental caries.
Dental varnishes can be applied easily and quickly, and can deliver an active agent as fluoride or chlorhexidine to the teeth safely and in high concentration [2]. The most important anti-caries effect of fluoride results from its local action on the tooth/plaque interface, through promotion of remineralization and minimizing demineralization. It also prevents acid production by S. mutans [3]. However, fluoride by itself is not a potent antimicrobial agent. One study compared the effect of different fluoride varnishes on S. mutans and S. sobrinus biofilms formation in vitro and found that the greatest number of viable bacteria was found with the fluoride varnish that released the highest concentration of fluoride into the formed biofilms. In the same study, a combination of fluoride and chlorhexidine varnishes showed the lowest bacterial counts [4]. Although fluoride remains the mainstay for the prevention of dental caries, additional approaches are required to enhance its effectiveness. In this context, the combination of fluoride with antimicrobial agents such as xylitol and chlorhexidine was recommended by some guidelines for the prevention of dental caries especially in high risk individuals [5], [6].
Due to the increase of antibiotic resistance and side effects of some antimicrobials on one hand, and the safety, availability, and relatively low costs of natural products on the other hand, a variety of natural products have been assessed for caries prevention as well as incorporated into dental products [1]. Propolis, a natural beehive product, is a complex resinous material that inhibits S. mutans growth and ability to adhere to tooth surfaces [7], [8], [9], [10]. The minimum inhibitory concentration (MIC) of ethanolic extract of propolis (EEP) on S. mutans varies from 25 to 100 μg/mL [7], [10], [11]. A minimum bactericidal concentration (MBC) of more than 1600 μg/mL was reported [7], [10]. Propolis also reduced human dental plaque accumulation and its insoluble external polysaccharide content [12]. It is a non-toxic material and its antimicrobial activity is attributed to the presence of flavonoids and terpenoids [1].
Miswak obtained from the roots or twigs of Arak (Salvadora persica) tree, which is found in many Asian and African countries, is one of many plants that have antimicrobial potential [1]. Antimicrobial, anti-tumor, anti-inflammatory, and wound healing properties of miswak extract have been linked to its content of tannic acid, alkaloids, eucalyptol, sulphur compounds, benzylisothiocynate, and benzyl nitrate. Its aqueous extract was also reported to have high calcium, but low fluoride content [13], [14], [15]. Its extracts possess plaque inhibiting and antimicrobial properties against cariogenic bacteria by inhibiting their growth and acid production [16], [17], [18], [19]. The MIC for ethanolic and aqueous extracts of miswak against S. mutans was reported to be 50 mg/mL and 150 mg/mL, respectively [19].
Chitosan is a natural polymer obtained by alkaline hydrolysis of chitin, a natural compound that is found in arthropod extroskeletons, shells of crustaceans, and insects’ cuticles. Because of its innate biocompatibility, biodegradability, and lack of toxicity; chitosan, and its nanoparticles received great attention in the pharmaceutical, food, agriculture, textile, and tissue engineering industries [20]. Chitosan has antitumor, wound-healing, mucoadhesive, and antimicrobial activities [20], [21], [22]. Its positive charge facilitates its adhesion to bacterial cell walls giving bacteriostatic or bacteriocidal activities to the material. Moreover, it is not known to cause antibacterial resistance [22]. The antibacterial mechanism of chitosan may include the interaction of cationic chitosan with the anionic cell surface, increasing membrane permeability and leakage of cellular material from the cell. Chitosan may also interfere with mRNA synthesis and imbedding protein synthesis [20], [23]. An inhibitory effect against S. mutans was reported [22], [24], [25], [26], [27], [28], [29], [30]. Chitosan interfered with S. mutans adhesion and primary biofilm formation [24], [25] up to a week with little to no decrease in efficiency [24]. In addition, chitosan caused significant reductions in mature biofilm survival [24], [25]. Chitosan-based mouthwash showed significantly higher antibacterial activity against Streptococcus and Enterococcus species than commercially available essential oils and chlorhexidine mouthwashes [25], [26]. Moreover, CS-NPs have been developed for drug encapsulation. Drugs carried by CS-NPs can be released through degradation of chitosan, leading to a sustained-release effect. The nanosized structure allows permeation through cell membranes, which makes it an effective carrier of drugs in biological systems to achieve improved bioavailability of the drug [20], [31], [32]. Thus, the present study sought to assess the in vitro S. mutans susceptibility to specially formulated dental varnishes containing propolis, miswak, or chitosan nanoparticles, with or without NaF, as well as, to assess the protective effect of pretreating enamel of primary teeth with those varnishes against bacterial induced demineralization.
Material and methods
Miswak extracts preparation
Miswak aqueous extract
Freshly cut miswak chewing sticks were collected from the twigs of Arak (Salvadora persica) trees in Saudi Arabia (Mecca city) and identified by an agriculturist. Ten g of sundried and ground sticks were soaked in 100 mL sterile distilled water for 48 h at 4 °C. The extract was then centrifuged and the supernatant was filtered through a 0.45 mm filter paper [19]. The extract was then freeze dried for 7 days in a freeze drying machine (Martin Christ, Alpha 1-2 LD, Vacuubrand GMBH+ Co KG, Germany).
Miswak ethanolic extract
The extract was prepared according to Noumi et al. [33]. Ten g of miswak powder were added to 100 mL of 95% ethyl alcohol and soaked for 24 h at room temperature. Supernatant was filtered through a 0.45 mm filter paper and the extract was kept in tightly closed screw capped containers at 4 °C.
Propolis ethanolic extract preparation
EEP was prepared by mixing 50 g of propolis fine chips collected from the top of the combs of the hives of honey bees (Apis mellifera carnica L.) during autumn with 500 mL of 95% ethyl alcohol in a dark bottle at room temperature for 4 days with intermittent stirring. The mixture was filtered with a filter paper, and then left at room temperature until ethanol evaporated and the product obtained a honey-like consistency. The EEP was then stored at 4 °C [8].
Preparation of CS-NPs and CSF-NPs
Nanoparticles were prepared at Nanotech Egypt for Photo Electronics, 6th of October, Giza, Egypt (May 2015), where medium molecular weight (100–300 kilodalton) chitosan (Sigma–Aldrich; St. Louis, USA) was converted to nanoparticles using the ionotropic gelation process [34]. Blank nanoparticles were obtained by adding tripolyphosphate (TPP) aqueous solution to a chitosan solution. The average size of the produced nanoparticles was 40 ± 10 nm. Five percent NaF loaded CS-NPs (0.05 g NaF/1 g CS-NPs) with the same size of chitosan nanoparticles were also prepared by the previous method. NaF powder (ALPHA CHEMIKA, Mumbai, India) was mixed with a TPP aqueous solution and added to the chitosan solution.
Experimental varnishes preparation
The components of each varnish (Table 1) were mixed and left over night to dissolve. CS-NPs and CSF-NPs were first dissolved in 2% acetic acid at 60 °C under continuous stirring for 60 min. Then pH was adjusted to 6 using 1% NaOH solution [28].
Table 1.
Varnishes constituents.
| Varnish | Constituents |
|||||
|---|---|---|---|---|---|---|
| Solvent (mL) | Distilled deionized water (mL) | Colophony resin (g) | NaF (g) | Other ingredients (g) | ||
| V1 (M) | Miswak ethanolic extract varnish | 75 mL of miswak ethanolic extract | 25 | 20 | – | – |
| V2 (MF) | Miswak-fluoride varnish | 75 mL of miswak ethanolic extract | 25 | 20 | 5 | – |
| V3 (MFD) | Freeze dried aqueous miswak extract varnish | 75 mL of 95% ethanol | 25 | 20 | – | 10 g freeze dried aqueous miswak extract |
| V4 (P) | Propolis varnish | 75 mL of 95% ethanol | 25 | 20 | – | 10 g EEP |
| V5 (PF) | Propolis-fluoride varnish | 75 mL of 95% ethanol | 25 | 20 | 5 | 10 g EEP |
| V6 (CS-NPs) | Chitosan-NPs varnish | 25 mL of 2% acetic acid | – | 20 | – | 10 g CS-NPs powder |
| 75 mL of 95% ethanol | ||||||
| V7 (CSF-NPs) | Sodium fluoride loaded chitosan-NP varnish | 25 mL of 2% acetic acid | –- | 20 | – | 10 g CSF-NPs. |
| 75 mL of 95% ethanol | ||||||
| V8 (NaF) | Sodium fluoride varnish | 75 mL of 95% ethanol | 25 | 20 | 5 | – |
Sample size
Sample size was estimated for disk diffusion test to be 3 in each group considering a study power of 80% and statistical significance of 5% (α = 0.05) based on a mean ± SD of inhibition zone (mm) for propolis and chlorhexidine of 20.5 ± 0.33, and 18.5 ± 0.55, respectively; and 2 disks per group [35]. For inhibition of demineralization, 10 enamel specimens were estimated for each group considering a study power of 80% and statistical significance of 5% (α = 0.05) based on a mean ± SD of calcium ion loss for triclosan and NaF toothpastes of 12.9 ± 0.8 and 11.3 ± 0.3, respectively, and 5 specimens per group [36].
Antibacterial susceptibility testing
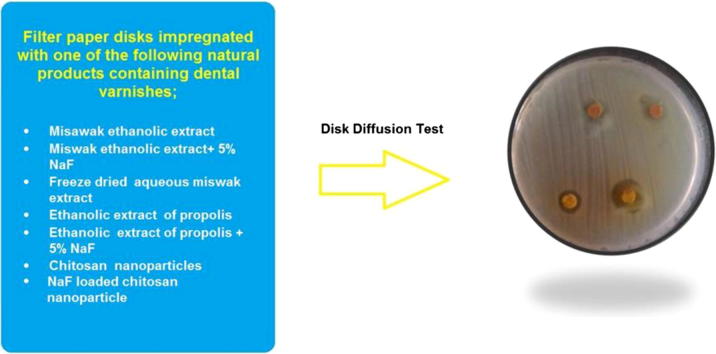
Pure culture of S. mutans was obtained by culturing S. mutans ATCC 25175 (Microbilogics, St Cloud, Minnesota, USA) on blood agar [2]. Disk diffusion method was used to measure S. mutans sensitivity to the experimental varnishes [9], [35]. Thirty mL of freshly prepared and autoclaved brain heart infusion (BHI) agar was poured into sterile glass petri dishes. The media were cooled to room temperature, and stored in a refrigerator until use. Plates were examined for sterility before use by incubating at 35 °C for 48 h. Three to five well isolated colonies of the same morphological type were selected from a blood agar plate culture and transferred with a sterile loop into a tube containing 5 mL of BHI broth that was then incubated at 37 °C for 24 h. The turbidity of the broth culture was adjusted to 0.5 McFarland standards. Fifty µL of the broth was immediately transferred to the middle of a dry BHI agar and spread uniformly over the entire agar surface using a sterile L spreader. Filter paper discs of 6 mm diameter were prepared from Whatman filter paper No. 1, placed in a petri dish and sterilized in a hot air oven at 160 °C for 2 h. Thereafter, discs were impregnated with 20 µL of each of the experimental varnishes (V1-V8), 3 disks for each varnish, and placed immediately over the plates. A maximum of 4 disks per plate were used. Sterile distilled water and 0.12% chlohexidine digluconate solution were used as negative and positive control, respectively. The plates were incubated in a candle extinction jar (5% CO2) for 24 h at 37 °C. After incubation, the plates were observed for uniform culture growth (granular, frosted glass appearance) and formation of inhibition zones around the discs that were measured in millimeters. The mean of 3 measurements of the diameter of each inhibition zone for each disk was calculated. The test was repeated twice for accuracy.
Inhibition of bacterial induced enamel demineralization
The buccal and lingual surfaces of 45 freshly extracted sound primary molars (obtained from the outpatient clinic of the Pediatric Dentistry Department, Faculty of Dentistry, Ain Shams University, Cairo, Egypt) were cleaned, examined under a stereomicroscope to ensure the presence of sound enamel and stored in distilled water that was changed weekly. The roots were removed and the crowns were cut mesiodistally into buccal and lingual halves. Teeth halves were autoclaved and then the dentin portion of each half was covered with an acid resistant varnish (nail polish, Amanda, Egypt). Enamel was covered with the acid resistant varnish except for a 5 mm circular window that was covered with an adhesive tape and removed subsequently. The dentin portions were covered with modeling wax so that only the enamel surfaces were exposed [2]. Thereafter, enamel specimens were randomly divided among nine experimental groups, where each group consisted of 10 specimens. In each of the first 8 groups, 10 enamel specimens were coated with one of the experimental varnishes (V1-V8), while in the last group untreated specimens served as controls.
Enamel specimens were coated with 10 µL of the corresponding varnish which was left to dry for 1 min and then incubated separately in 10 mL deionized water to allow ionic exchange with enamel for 4 h. After incubation, the varnishes were removed gently with a scalpel [37]. The pretreated and negative control enamel specimens were placed in sterile screw capped polyethylene tubes which contained 5 mL of S. mutans suspension described before supplemented with 1% freshly prepared sucrose from a sterilized 20% stock solution. Each tube contained a single enamel specimen. The tubes were incubated for 72 h at 37 °C. Every 24 h, the specimens were removed, rinsed with sterile deionized water, and placed in new tubes containing freshly prepared S. mutans suspension supplemented with 1% sucrose. This 24 h period is enough to achieve in vitro enamel colonization and acid production [38]. The removed suspensions were stored at −80 °C until they were assessed for calcium and pH. After 72 h, all tubes were centrifuged for 5 min at 16,000g, and the supernatants were filtered and assessed for calcium content by atomic absorption spectroscopy (SavantAA, GBC Scientific Equipment, USA) [37]. The pH of all S. mutans suspensions was also measured to ensure acid production in the incubating solution using a pH meter (Orion Versa Star, Thermo Scientific, USA) [37].
Statistical analysis
Data was analyzed using SPSS 15.0 for windows (SPSS Inc, Chicago, IL, USA, 2001). One-sample Kolmogrovo-Smirnov test was used to assess the normality of data distribution. One-Way ANOVA was used to compare the antibacterial effect of the different varnishes. While for inhibition of demineralization (non-parametric data), Kruskal-Wallis test was used to assess the effect of pretreatment in all varnishes groups. When the differences between groups were statistically significant, Tukey-HST Post Hoc test and Mann-Whitney test were used, for parametric and non-parametric data, respectively to detect means that are significantly different from each other. The level of significance was set at P ≤ 0.05.
Results
All experimental varnishes inhibited S. mutans growth significantly higher than NaF varnish with the ascending order of Naf < M < P < MF < CHX < PF < MFD < CS-NPs < CSF-NPs (Table 2). Though varnishes containing natural products combined with NaF had higher antibacterial effect, they didn’t significantly differ in antibacterial activity compared to varnishes containing the corresponding natural products only. The highest antimicrobial activity was observed in the CSF-NPs varnish; however, it didn’t significantly differ than those of other varnishes except miswak ethanolic extract (M) varnish. Distilled water showed no inhibitory effect.
Table 2.
Susceptibility of S. mutants to different varnishes using disk diffusion assay.
| Varnish | Mean of inhibition area (mm) | SD | F | P value | Sig. |
|---|---|---|---|---|---|
| Na F | 9.0a | ±1.0 | 19.46 | <0.001⁎⁎ | HS |
| CS-NPS | 23.0b,e | ±2.0 | |||
| CSF-NPS | 24.0c,b,e | ±1.0 | |||
| P | 19.33d,e | ±1.53 | |||
| PF | 22.0e,b,c,d | ±3.0 | |||
| M | 17.0f,d | ±2.0 | |||
| MF | 20.0g,b,d,e,f | ±1.73 | |||
| MFD | 23.0g,b,c,e | ±1.0 | |||
| CHX | 21.0g,b,c,d,e | ±2.0 |
One-Way ANOVA. Means with same superscript letters are not statistically significant at P ≤ 0.05.
HS: Highly significant.
Results of the demineralization inhibitory effect of a single pretreatment of primary teeth enamel are shown below. Table 3 shows the released Calcium ions after the first 24 h. Calcium ion concentration increased in the ascending order of NaF < MF < CSF-NPs < MFD < CS-NPs < PF < M < P < Control, with significant differences existing among all treatments except for MF and CSF-NPs.
Table 3.
Calcium ion dissolution at day 1.
| Varnish | Mean calcium ion concentration (mg/mL) | SD | Median | Range | Kruskal-Wallis | P value | Sig. |
|---|---|---|---|---|---|---|---|
| Control | 185.3a | ±0.5 | 185.0 | 185–186 | 86.81 | <0.001⁎⁎ | HS |
| NaF | 50.6b | ±0.3 | 50.6 | 50–51 | |||
| CS-NPS | 70.3c | ±1.9 | 70.1 | 68–73.8 | |||
| CSF-NPS | 59.3d | ±0.8 | 59.4 | 58–60.1 | |||
| P | 173.1e | ±1.9 | 173.3 | 170–176 | |||
| PF | 84.5f | ±1.0 | 84.4 | 83–86 | |||
| M | 112.0g | ±5.9 | 110.0 | 105–62 | |||
| MF | 55.1d | ±4.3 | 53.5 | 50.5–62 | |||
| MFD | 68.0h | ±1.3 | 67.8 | 66.5–70 |
Kruskal-Wallis Test. Means with same superscript letters are not statistically significant at P ≤ 0.05.
HS: Highly significant.
At day 2, Calcium ion concentration was in the ascending order of NaF < CSF-NPs < MF < MFD < CS-NPs < M < PF < P < control. Only MFD and CS-NPs showed no significant difference (Table 4).
Table 4.
Calcium ion dissolution at day 2.
| Varnish | Mean calcium ion concentration (mg/mL) | SD | Median | Range | Kruskal-Wallis | P value | Sig. |
|---|---|---|---|---|---|---|---|
| Control | 185.4a | ±0.5 | 185.0 | 185–186 | 86.44 | <0.001⁎⁎ | HS |
| NaF | 64.6b | ±0.4 | 64.8 | 64–65 | |||
| CS-NPS | 113.2c | ±0.9 | 113.0 | 112–115 | |||
| CSF-NPS | 71.6d | ±1.0 | 71.7 | 70–73 | |||
| P | 177.0e | ±1.3 | 176.9 | 175–179 | |||
| PF | 129.1f | ±2.6 | 129.5 | 125–133 | |||
| M | 126.9g | ±3.1 | 127.3 | 120–131 | |||
| MF | 83.0h | ±8.1 | 83.0 | 70–5 | |||
| MFD | 105.7c | ±9.1 | 105.0 | 93–117 |
Kruskal-Wallis Test. Means with same superscript letters are not statistically significant at P ≤ 0.05.
HS: Highly significant.
At day 3, the ascending order of released Calcium ion was NaF < CSF-NPS < MF < MFD < M < PF < CS-NPs < control < P. No significant differences were found between CSF-NPs and MF; CS-NPs, P and Control; as well as between CS-NPs and P (Table 5). pH of all bacterial suspensions ranged between 4.9 and 5.2 verifying acid production.
Table 5.
Calcium ion dissolution at day 3.
| Varnish | Mean calcium ion concentration (mg/mL) | SD | Median | Range | Kruskal-Wallis | P value | Sig. |
|---|---|---|---|---|---|---|---|
| Control | 186.1a | ±0.9 | 186.0 | 185–187 | 78.45 | <0.001⁎⁎ | HS |
| NaF | 86.7b | ±0.8 | 86.8 | 85.5–88 | |||
| CS-NPS | 185.5c,a | ±2.0 | 184.8 | 183–189 | |||
| CSF-NPS | 92.7d | ±1.6 | 92.8 | 90–95 | |||
| P | 186.4a,c | ±4.9 | 185.5 | 180–195 | |||
| PF | 179.1e | ±3.7 | 178.3 | 176–189 | |||
| M | 172.2f | ±14.6 | 174.0 | 150–193 | |||
| MF | 95.1d | ±3.5 | 95.5 | 90–100 | |||
| MFD | 122.7g | ±5.5 | 122.5 | 115–130 |
Kruskal-Wallis Test. Means with same superscript letters are not statistically significant at P ≤ 0.05.
HS: Highly significant.
Discussion
All varnishes containing natural products had a significant antibacterial effect against S. mutans compared to fluoride varnish which was expected in the light of previous studies that reported an inhibitory effect against S. mutans by the investigated natural products [7], [8], [9], [10], [11], [17], [18], [19], [27], [28], [29], [30]. While adding fluoride to the tested natural products increased the antibacterial activity more than each natural product alone, this difference was not significant compared to varnishes containing only the corresponding natural product but was significant compared to fluoride varnish indicating an additive effect with fluoride and supports the limited antibacterial activity of fluoride. In a previous study different fluoride containing varnishes showed varying degrees of antimicrobial activity which was not correlated to the fluoride content or fluoride released from the varnishes. In the same study combining a varnish containing 1% fluoride with a 1% containing chlorhexidine varnish produced a synergistic effect on S. mutans and S. sobrinus biofilms compared to each varnish alone [4].
The observation that CSF-NPs didn’t differ significantly than other natural products in the antibacterial effect implies that these products may be more cost-effective considering the high costs of preparing the nanoparticles. However, it must be noted that medium molecular weight CS-NPs were used in the present study and that a higher antibacterial effect may be achieved if a low molecular weight CS-NPs are used as reported earlier [22], [28].
Present results didn’t support previous findings that ethanolic miswak extract possessed higher antimicrobial effect compared to aqueous extract [39]. However, literature reported that the antimicrobial effect of miswak extracts was concentration dependent [18]. Hence in the present study, freeze drying of aqueous extract may have concentrated its antimicrobial components thus increasing its antibacterial effect.
No other studies reported the antibacterial effects of natural products that are incorporated into dental varnishes. Only two studies assessed a propolis containing dental varnish formulated by adding different concentrations of EEP to a chitosan polymeric base. No significant differences in antibacterial effect against S. mutans were noted among 5%, 10%, or 15% concentrations. Inhibition zones ranged between 20.3 mm and 21.0 mm [12], [35]. Although propolis and miswak containing varnishes had similar antibacterial effects, miswak may be a better option for clinical use as propolis varnish made a dark brown coating that may not be clinically acceptable.
Calcium ion dissolution was estimated as an indicator of varnishes ability to inhibit enamel demineralization [2], [36], [37]. As shown in Table 3, Table 4, Table 5, the best inhibition of demineralization effect was evident with the 5% NaF varnish. This supports that fluoride’s main effect is minimizing apatite dissolution and goes in line with previous studies [2], [37]. In one study when different varnishes were placed next to enamel during bacterial demineralization, a 40% chlorhexidine varnish produced maximum protection, while when enamel was pretreated, fluoride varnish offered the best protection [37]. Though natural products containing varnishes whether plain or with added fluoride had significantly lower ability to inhibit demineralization compared to NaF varnish, the benefit of their combined antibacterial activity may have clinical significance. MF and CSF-NPs varnishes had significantly better inhibition of demineralization effect over the 3 experimental periods compared to other varnishes. For MF, this may be due to better release of fluoride ions, in contrast to PF varnish which had the same NaF concentration as that of MF. However, PF varnish was somewhat more viscous than other varnishes, which may be due to the resinous nature of propolis extract that may have limited the release of fluoride over a period of 4 h and hence limiting its protective effect.
Although CSF-NPs contained lower concentration of NaF, its protection against demineralization was also comparable to MF varnish containing 5% NaF during the three demineralization periods. This indicates that the nano-sized particles are more effective in ionic exchange with enamel thus inhibiting demineralization even at a lower concentration. It is worth to note that chitosan as a mucoadhesive polymer may also have an extended intra-oral retention time which may improve its clinical outcomes.
Though NaF was not added to reconstituted freeze dried aqueous miswak extract (MFD varnish), yet it inhibited demineralization significantly better than other plain natural products containing varnishes and PF varnish which may be a result of freeze drying that may have concentrated any minerals within the aqueous extract as noted earlier.
Miswak, propolis, and CS-NPs varnishes showed a minimal ability to inhibit demineralization, however, they were better than the control group. This indicates a release of some remineralizing agents from those natural products, yet with minimal effect on inhibition of demineralization. Miswak extract was reported to have high calcium and low fluoride content [13], while propolis was found to have varying amounts of calcium and phosphorus depending on its phytogeographic origin [40]. For each varnish, the protective ability decreased over the 2nd and 3rd demineralization periods, this also goes in line with van Loveren et al. [37]. Propolis and CS-NPs varnishes showed no protective effect at day 3 as their results were comparable to those of the control specimens indicating a short-term protective ability.
A shortcoming of the inhibition of demineralization model of the present study is that varnishes were removed before exposing the enamel specimens to bacterial demineralization, hence, only the effect of ion exchange between the varnishes and enamel surface on subsequent enamel demineralization was assessed. However, if varnishes were left during the experiment, the combined effect of antibacterial activity and ion release could have produced different results, this is also closer to clinical situation. However, varnishes were removed so that the release of any ions from the natural products containing varnishes into the S. mutans suspension could have been a confounding factor.
It is worth to note that the costs of preparing miswak and propolis varnishes were minimal as all the constituents are readily available, cheap, and simple methods are used to produce their extracts. On the other hand, nanoparticles preparation costs much money. Considering this cost differences and results of the present study, miswak and propolis use can be encouraged in countries with limited financial resources.
Conclusions
Results suggest that using the tested natural products can be an effective approach for caries prevention. Varnishes having a combination of natural products, optimum concentration of fluoride in CS-NPs, ion release and remineralizing potential as well as clinical efficiency of such varnishes need further investigations.
Conflict of Interest
We confirm that there is no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Jeon J.G., Rosalen P.L., Falsetta M.L., Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011;45:243–263. doi: 10.1159/000327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munshi A.K., Reddy N.N., Shetty V. A comparative evaluation of three fluoride varnishes: an in-vitro study. J Indan Soc Pedod Prev Dent. 2001;19(3):92–102. [PubMed] [Google Scholar]
- 3.Marinho V.C.C., Worthington H.V., Walsh T., Clarkson J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2013;7:1–29. doi: 10.1002/14651858.CD002279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdem A.P., Sepet E., Kulekci G., Trosola S.C., Guven Y. Effects of two fluoride varnishes and one fluoride/chlorhexidine varnish on Streptococcus mutans and Streptococcus sorbrinus biofilm formation in vitro. Int J Med Sci. 2012;9(2):129–136. doi: 10.7150/ijms.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The American Academy of Pediatric Dentistry [Internet]. Guideline on caries-risk assessment and management for infants, children, and adolescents. Reference manual 37(6). Available from: <http://www.aapd.org/media/policies_guidelines/g_cariesriskassessment.pdf>. [PubMed]
- 6.Jenson L., Budenz A.W., Featherstone J.D.B., Ramos-Gomez F.J., Spolsky V.W., Young D.A. Clinical protocols for caries management by risk assessment. JCDA. 2007;35(10):714–723. [PubMed] [Google Scholar]
- 7.Duarte S., Koo H., Bowen H.W., Hayacibara M.F., Cury J.A., Ikegaki M. Effect of a novel type of propolis and its chemical fractions on glucosyltransferases and on growth and adherence of Mutans Streptococci. Biol Pharm Bull. 2003;26(4):527–531. doi: 10.1248/bpb.26.527. [DOI] [PubMed] [Google Scholar]
- 8.Sundeep H.K., Bhat S.S., Rao A., Sain S. Effect of propolis on Streptococcus mutants counts: an in vivo study. Int J Clin Pediatr Dent. 2013;6:22–25. doi: 10.5005/jp-journals-10005-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arul Selvan K., RajendraSingh C., Prabhu T. Antibacterial activity of bee propolis against clinical strains of Streptococcus mutans and synergism with chlorhexidine. IJPSR. 2011;2(1):85–90. [Google Scholar]
- 10.da Cunha M.G., Franchin M., de Carvalho Galvão L.C., de Ruiz A.L., de Carvalho J.E., Ikegaki M. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Compl Altern Med. 2013;28:13–23. doi: 10.1186/1472-6882-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franca J.R., De Luca M.P., Ribeiro T.G., Castilho R.O., Moreira A.N., Santos V.R. Propolis-based chitosan varnish: drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Compl Altern Med. 2014;14:478–489. doi: 10.1186/1472-6882-14-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo H., Cury J.A., Rosalen P.L., Ambrosano G.M., Ikegaki M., Park Y.K. Effect of a mouthrinse containing selected propolis on 3-day dental plaque accumulation and polysaccharide formation. Caries Res. 2002;36:445–448. doi: 10.1159/000066535. [DOI] [PubMed] [Google Scholar]
- 13.Halawany H.S. A review on miswak (Salvadora persica) and its effect on various aspects of oral health. Saudi Dent J. 2012;24(2):63–69. doi: 10.1016/j.sdentj.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C.D., Darout I.A., Skaug N. Chewing sticks: timeless natural toothbrushes for oral cleansing. J Period Res. 2001;36:275–284. doi: 10.1034/j.1600-0765.2001.360502.x. [DOI] [PubMed] [Google Scholar]
- 15.Darout I.A., Christy A.A., Skaug N., Egeberg P.K. Identification and quantification of some potentially antimicrobial anionic components in miswak extract. Indian J Pharmacol. 2000;32:11–14. [Google Scholar]
- 16.Khalessi A.M., Pack A.R., Thomson W.M., Tompkins G.R. An in vivo study of the plaque control efficacy of Persica: a commercially available herbal mouthwash containing extract of Salvadora persica. Int Dent J. 2004;54:279–283. doi: 10.1111/j.1875-595x.2004.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 17.Sofrata A.H., Claesson R.L.K., Lingstrom P.K., Gustafsson A.K. Strong antibacterial effect of miswak against oral micro-organisms associated with periodontitis and caries. J Periodontol. 2008;79:1474–1479. doi: 10.1902/jop.2008.070506. [DOI] [PubMed] [Google Scholar]
- 18.Balto H., Al-Sanie I., Al-Beshri S., Aldrees A. Effectiveness of Salvadora persica extracts against common oral pathogens. Saudi Dent J. 2017;29(1):1–6. doi: 10.1016/j.sdentj.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darmani H., Nusayr T., Al-Hiyasat A. Effects of extracts of miswak and derum on proliferation of Balb/C 3T3 fibroblasts and viability of cariogenic bacteria. Int J Dent Hyg. 2006;4(2):62–66. doi: 10.1111/j.1601-5037.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 20.Dutta P.K., Dutta J., Tripathi V.S. Chitin and chitosan. Chemistry, properties and applications. J Sci Ind Res. 2004;63:20–31. [Google Scholar]
- 21.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Sarwar A., Katas H., Zin N.M. Antibacterial effects of chitosan–tripolyphosphate nanoparticles: impact of particle size molecular weight. J Nanopart Res. 2014;16:2517–2531. [Google Scholar]
- 23.Helander I.M., Nurmiaho-Lassila E.L., Ahvenainen R., Rhoades J., Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram negative bacteria. Int J Food Microbiol. 2001;71:235–244. doi: 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 24.Costa E.M., Silva S., Tavaria F.K., Pintado M.M. Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe. 2013;20:27–31. doi: 10.1016/j.anaerobe.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Costa E.M., Silva S., Madureira A.R., Cardelle-Cobas A., Tavaria F.K., Pintado M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohyd Polym. 2014;101:1081–1086. doi: 10.1016/j.carbpol.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Costa E.M., Silva S., Costa M.R., Pereira M., Campos D.A., Odila J. Chitosan mouthwash: toxicity and in vivo validation. Carbohyd Polym. 2014;111:385–392. doi: 10.1016/j.carbpol.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 27.Bae K., Jun E.J., Lee S.M., Paik D.I., Kim J.B. Effect of water soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Investig. 2006;10:102–107. doi: 10.1007/s00784-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 28.Chávez de Paz L.E., Resin A., Howard K.A., Sutherland D.S., Wejse P.L. Antimicrobial effect of chitosan nanoparticles on Streptococcus mutans biofilms. Appl Environ Microbiol. 2011;77(11):3892–3895. doi: 10.1128/AEM.02941-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliasghari A., Rabbani Khorasgani M., Vaezifar S., Rahimi F., Younesi H., Khoroushi M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: an in vitro study. Iran J Microbiol. 2016;8(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi Y., Ohara N., Ganno T., Yamaguchi K., Ishizaki T., Nakamura T. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch Oral Biol. 2007;52:290–294. doi: 10.1016/j.archoralbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Wang J.J., Zeng Z.W., Xiao R.Z., Xie T., Zhou G.L., Zhan X.R. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomed. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd Elgadir M., Uddin S., Ferdosh S., Adam A., Chowdhury A.J.K., Sarker Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23:619–629. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noumi E., Snoussi M., Hajloaui H., Valentin E., Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis. 2010;29:81–88. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- 34.Ana Grenha A., Begona Seijo B., Cameron Remunan-Lopez C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur J Pharm Sci. 2005;25:427–437. doi: 10.1016/j.ejps.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 35.De Luca M.P., Franca J.R., Macedo F.A., Grenho L., Cortes M.E., Faraco A.A. Propolis varnish: antimicrobial properties against cariogenic bacteria, cytotoxicity, and sustained-release profile. Biomed Res Int. 2014;2014:1–6. doi: 10.1155/2014/348647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Loveren C., Buijs J.F., Ten Cate J.M. The effect of triclosan toothpaste on enamel demineralization in a bacterial demineralization model. J Antimicrob Chemother. 2000;45:153–158. doi: 10.1093/jac/45.2.153. [DOI] [PubMed] [Google Scholar]
- 37.van Loveren C., Buijs J.F., Buijs M.J., Ten Cate J.M. Protection of bovine enamel and dentine by chlorhexidine and fluoride varnishes in a bacterial demineralization model. Caries Res. 1996;30(1):45–51. doi: 10.1159/000262136. [DOI] [PubMed] [Google Scholar]
- 38.Prabhakar A.R., Kurthukoti A.J., Gupta P. Cariogenicity and acidogenicity of human milk, plain and sweetened bovine milk: an in vitro study. J Clin Pediatr Dent. 2010;34(3):239–247. doi: 10.17796/jcpd.34.3.lk08l57045043444. [DOI] [PubMed] [Google Scholar]
- 39.AbdElrahman H.F., Skaug N., Francis G.W. In vitro antimicrobial effects of crude miswak extracts on oral pathogens. Saudi Dent J. 2002;14(1):26–32. [Google Scholar]
- 40.González-Martín M.I., Escuredo O., Revilla I., Vivar-Quintana A.M., Coello M.C., Riocerezo C.P. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors (Basel) 2015;11:27854–27868. doi: 10.3390/s151127854. [DOI] [PMC free article] [PubMed] [Google Scholar]