Abstract
Fluorescence is a physico-chemical energy exchange where shorter-wavelength photons are absorbed by a molecule and are re-emitted as longer-wavelength photons. It has been suggested a means of communication in several taxa including flowers, pitcher plants, corals, algae, worms, squid, spiders, stomatopods, fish, reptiles, parrots and humans. The surface or object that the pigment molecule is part of appears to glow due to its setting rather than an actual production of light, and this may enhance both signals and, in some cases, camouflage. This review examines some known uses of fluorescence, mainly in the context of visual communication in animals, the challenge being to distinguish when fluorescence is a functional feature of biological coloration or when it is a by-product of a pigment or other molecule. In general, we conclude that most observations of fluorescence lack enough evidence to suggest they are used in visually driven behaviours.
This article is part of the themed issue ‘Animal coloration: production, perception, function and application’.
Keywords: colour, fluorescence, behaviour, light, visual ecology, excitation and emission
1. Introduction, what is fluorescence?
Fluorescence conjures up images of brightly coloured glowing things in the dark or day-glo high-visibility clothing. Humans use fluorescence to add to colour and make things stand out, both in the dark and in the light and in many contexts: highlighter pens, post-it notes, fluorescent tattoos as body adornments and even ‘whiter than white’ fabrics (figure 1). Both the paper and the screen you are reading these words from are fluorescing. In nature carotenoids, flavonoids, pterins, psittacofulvins, proteins, guanine, chlorophyll and other chemicals or compounds all fluoresce [1–3] (and see www.nightsea.com). In this paper, we critically examine where and when fluorescence may be used for signalling, for other purposes [2] or, as in mice and men, serve no purpose at all [4,5].
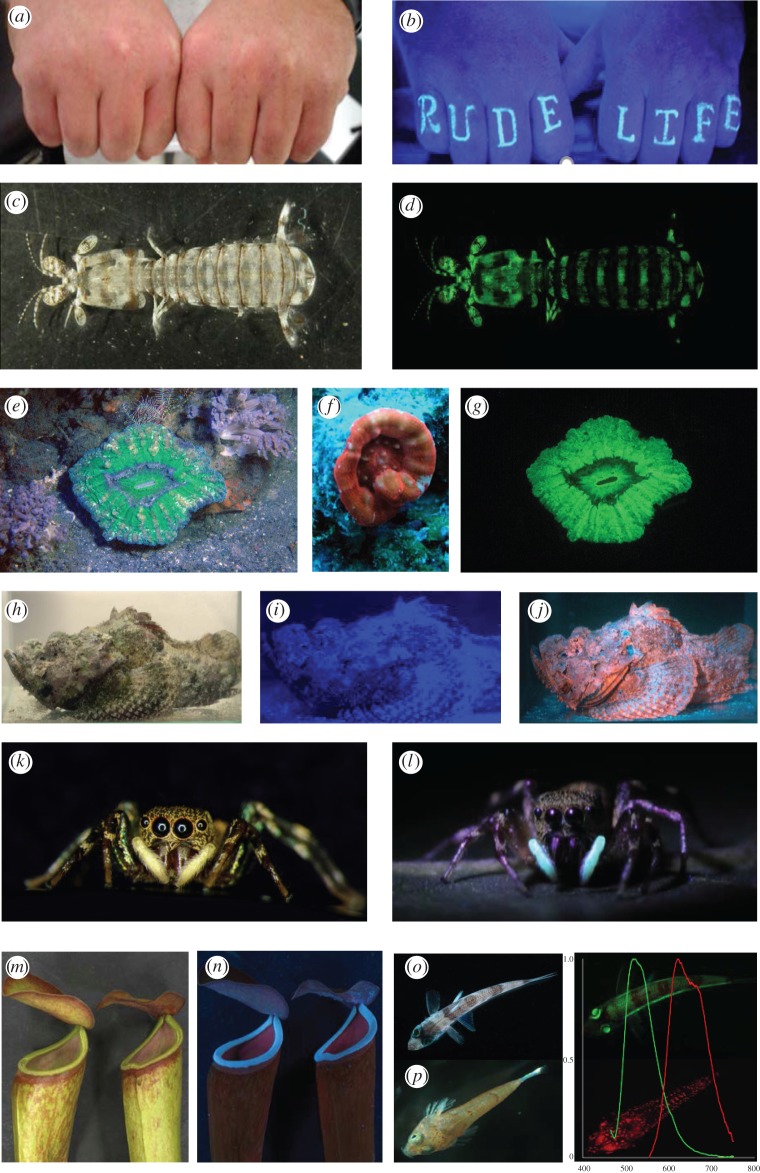
Figure 1.
Examples of fluorescent objects. (a,b) Fist tattoo that only ‘pops out’ under UV ‘black-light’ illumination, such as that used in nightclubs (http://itattoos.club/index.htm). (c,d) Stomatopod crustacean Lysiosquilla maculata under white light and blue (470 nm) excitation lamp. (e,f,g) Scolymia sp. solitary corals, (e,g) in situ under white and 470 nm peak flash illumination + 500 nm long-pass barrier filter, (f) at 17 m in ambient illumination with camera white balance on automatic. This photograph shows orange fluorescence and appears approximately as it does to unfiltered or light-boosted human eye. (h,i,j) Scorpaenid under white, simulated blue light of depth using 470 nm illumination and blue + 500 nm long-pass barrier filter (see figure 2i). Note the weak fluorescence of the fish cannot be seen when ‘washed out’ by blue alone. (k,l) Female jumping spider C. umbratica in white light and under UV excitation showing fluorescing pedipalps (photographs, D. Li). (m,n) Pitcher plant traps of Nepenthes in white light and under UV (366 nm peak) excitation (photographs, Anil J. Johnson, R. Kurup and S. Baby). (o) Green fluorescent deep-sea fish Chlorophthalmus. (p) Red fluorescing dragonet fish, graph shows normalized emission spectra per nm for each fish colour matched (photographs, M. Matz).
Fluorescence is a two-stage chemical process involving absorption of shorter-wavelength light by a chemical fluorophore such as a protein or carotenoid (excitation), followed by the release of some of the absorbed energy as longer-wavelength light (emission). Note that it differs from bioluminescence or phosphorescence and bright reflection of ultraviolet radiation (UV) [3]. Fluorescence may or may not be the same colour as the object's intrinsic coloured reflection. The green chlorophyll of leaves fluoresces red, for example, while a budgerigar's yellow crown also fluoresces yellow (figure 2). The excitation light does not have to be ultraviolet, as is often assumed. In the majority of examples, objects are excited by either blue or blue/green light, giving rise to green, yellow or red fluorescence [1,3].
Figure 2.
Fluorescence characteristics of the budgerigar M. undulatus (a–d) and the stomatopod crustacean L. glabriuscula (e–i). (a,b) White light and UV fluorescent excitation photographs of front and back of head showing fluorescent cheek and crown feathers. Excitation source was a ‘black light’. Shaded box indicates spectral zone removed by sunblock to remove fluorescence. (c) Normalized reflectance—lines colour-coded to approximately match bird; fluorescent crown feathers—light yellow, lower cheek, non-fluorescent yellow feathers—dark yellow (note difference in UV absorption of fluorescent feathers, which absorb in this wavelength range to re-emit as fluorescence), blue cheek patches—blue (note high UV reflectivity of these feathers and high chromatic contrast to yellow fluorescent feathers). UV ‘black-light’ excitation source used in (b)—solid black line, fluorescent excitation spectrum—long-dashed line, fluorescent emission—short-dashed line. (d) Spectral sensitivities—solid black lines, spectral emission difference (relative photons) between fluorescent feathers with and without sunblock applied—yellow line. (e,f) Frontal aggressive display in white light and blue-light excited fluorescence. (g) L. glabriuscula looking out from burrow in sand under fluorescent excitation. Fluorescent areas on antennal scales are conspicuous. (h) Fluorescent excitation spectrum—long-dashed line, fluorescent emission—short-dashed line. Spectral sensitivity of the row 2 distal photoreceptor in stomatopod retina showing excellent match to fluorescent emission range. (i) Ambient light in ocean at around 10 m in stomatopod habitat—blue line, fluorescent excitation lamp relative output—purple line and yellow blocking filter—yellow line. These were used in photography of stomatopods and match both natural ambient light and long–waveband-pass retinal filter of stomatopod (cryosection—natural colour—of filter inset photograph).
Unlike bioluminescence and despite being described as ‘glowing’, fluorescence cannot make something brighter. Indeed, adding a fluorescent pigment always decreases the actual brightness of an object because its conversion efficiency is never 100%. However, two properties of fluorescence can make a fluorescent object appear significantly brighter than its background. First, because the emission spectrum is different from the excitation spectrum, a fluorescent object may be much brighter than the background in certain regions of the spectrum. This can be particularly important underwater, where light absorption depends strongly on wavelength (figure 2 and electronic supplementary material, figure S2) [6]. In this environment, a fluorophore that absorbs the dominant wavelengths and emits at less common ones—for example one that absorbs blue light in the ocean and emits red light—will probably be highly conspicuous (electronic supplementary material, figure S2). This effect is magnified by a second property of fluorescence, its isotropic emission. Down-welling light both in the ocean and on land is generally many times brighter than horizontal light. Therefore, a fluorescent sphere-shaped object that absorbs the relatively bright down-welling light and re-emits a portion of it evenly in all directions can be much brighter than horizontal background light. On land some fluorescent objects are placed against areas that also boost the contrast of the fluorescent signal (e.g. plumage [7]), and fluorescent tissue may be contained within a secondary structure that controls the direction of its emission [8]. Moreover, if a fluorescent area superimposes upon another coloured area or pattern, that area may fluoresce [1,3].
To take full advantage of the high contrasts produced by these fluorophores, the visual system should be sensitive to only narrow regions of the spectrum. Unfortunately, without filters, photoreceptors have broad spectral sensitivities. For example, a visual pigment that has a peak sensitivity at 500 nm is at least 50% as sensitive from 425 to 560 nm and has residual sensitivity even beyond 700 nm. Therefore, when using these pigments, the contrast between the fluorescent object and the background is not as high as it could be (electronic supplementary material, figure S2). However, using relatively typical medium- and long-wavelength visual pigments, with peaks at 500 and 540 nm, fluorescence will generally be substantially brighter than background water. Interestingly, several animals, both marine and terrestrial, employ long-pass, yellow orange or red filters in their eyes that do narrow the spectral absorbance of their photoreceptors; for example, the oil droplets in front of cone photoreceptors in bird and reptile eyes [8], the yellow (or even orange) corneas or lenses in some fish [9–11] (electronic supplementary material, figures S1 and S2) and the intra- or peri-rhabdomal filters of stomatopod crustaceans and butterflies [12,13]. It may be no coincidence that there are examples of apparent fluorescent signals in all these groups although long-pass spectral tuning has other potential functions [9].
In many cases, the fluorescence comes from a patch on the side of an opaque object, for example the lateral surface of a fish or a bird feather. Although here the fluorophore cannot absorb light from all directions, as was true for the spherical object, it still catches enough of the down-welling light to be several times brighter than the background light. However, a diffusely reflecting white patch does this just as well. In fact, in most cases, a highly reflective white patch is brighter than a fluorescent patch, simply because of the poorer conversion efficiency of a fluorophore [3]. The colour contrast of an orange fluorescent object such as a coral can seem unnaturally bright due to both its contrast against a dull green benthos and particularly at depths where other long-wavelength colours have been neutered by selective absorption of water (figure 2 and electronic supplementary material, figure S2).
These considerations lead to several predictions. First, fluorescent pigments will have absorption spectra that can best take advantage of dominant wavelengths of the illumination—for example blue in the open ocean. Second, the fluorescent signals will often be viewed against dark-water backgrounds or contrasting terrestrial backgrounds. Third, fluorescent signals will be placed on appendages or body regions that are used in signalling. Fourth, animals viewing fluorescent signals will have optimally placed spectral sensitivities, perhaps involving intraocular filters, that narrow the spectral-sensitivity curves of their photoreceptors.
2. A checklist for ecologically significant fluorescence
(a). Is there a fluorescent compound (fluorophore) present in a visible location?
The location of the fluorescent object is important, both in the world and on the organism. Turn over a rock on the coral reef or desert and many of the living or dead things under it may fluoresce, but in such a place are they ever seen? A close examination of the biology, ecology and behaviour of the animal or plant containing the fluorescent object is essential before claiming fluorescence is an adaptation [14]. Desert scorpions exhibit astonishing blue/green fluorescence under UV illumination but generally hide from the hot UV-containing sun during the day, so their fluorescence is generally thought as ecologically functionless [15].
Differentiating if the fluorescence is a required addition to the function of the pattern or colour or just a by-product of the pigment is critical. Compare the fluorescent signal on the human fists, the stomatopod and the budgerigar in figures 1 and 2, for example. The message on the fists only shows up in certain illumination and if they are shown off, not thrust into pockets. This is a scenario achievable in the natural world if the animal can expose or hide its fluorescent patch or move from one illumination to another by, for example, leaving the shadows or swimming up and down in the water column.
The stomatopod fluorescence on the other hand co-occurs with the obvious light-yellow/cream-coloured markings and patterns on the body, perhaps suggesting that it is more probably a by-product of the pigment and not visually important (figures 1 and 2). Notably, in the adult, however (figure 2), these markings are specifically shown in behavioural contexts lending support to the functional case. The budgerigar is an interesting intermediate where some of the yellow plumage contains fluorescence and some does not, yet the fluorescent areas are specifically shown in conspecific interactions [7].
(b). What are the excitation and emission wavelength ranges of the compound/tissue?
UV is not the only excitation range, and relatively narrow-band blue or green light may yield fluorescence ranging from yellow to red [16]. Knowing excitation and emission spectra is useful in determining whether the fluorescence may be visually relevant [17,18]. Consider the mantis shrimp and budgerigar yellow fluorescence, one excited maximally at 350 nm and the other at 450 nm. Figure 2 shows examples of excitation and emissions of mantis shrimp carapace and budgerigar feathers measured in a Hitachi F-2000 fluorescence spectrophotometer, along with other spectral features of their environment. Knowing these parameters, visual modelling, can be used to determine whether the fluorescence is visually relevant [17,18]. It is also essential if absolute measures of fluorescent contribution to a signal are to be estimated (electronic supplementary material and [16]).
(c). Spectral sensitivity ranges of potential viewers
In making a case for visually functional fluorescence, finding a tight correlation between a sharply tuned spectral sensitivity and fluorescent emission is comforting. Spectral sensitivities of animals are difficult to measure without machinery such as microspectrophotometers (MSP), electrophysiology recording gear, gene-sequencers or behavioural observation, yet estimations can be made given insects or birds are relatively conservative in their trichromacy and two forms of tetrachromacy, respectively [19]. Fish, and some other invertebrates, are highly variable, however [20,21]. Fortunately, human long and medium sensitivities extend into the yellow–red range, and we are around 50 times more sensitive to green/yellow (550 nm) than to blue (440 nm) or far red (670 nm) making humans good fluorescence spotters. Yet this can lead to incorrect conclusions when making assumptions for other animals that lack sensitivity in the 500–700 nm waveband, many marine fish and most known insects. This does not preclude such animals from being able to see all fluorescence; as already noted, some natural fluorescences occur in the blue–green range for example (figures 1 and 2) [22,23].
A long-pass filter (often yellow) is used in fluorescence photography as it removes the wash-out effect of the excitation source (www.nightsea.com). Several animals possess such filters including yellow or orange corneal or lens filters in the eyes of deep-sea and shallow-dwelling marine fish (electronic supplementary material, figures S1 and S2) [9–11]. The yellow (or indeed red) oil droplet associated with bird cone sensitivities [9] and the yellow intra-rhabdomal filter in stomatopod eyes [12] are responsible for sharpening and spectrally tuning the spectrum they view, making these photoreceptors well suited to detecting their fluorescent emissions (figure 2 and electronic supplementary material, figure S3).
Recent genetic sequencing [24] and microspectrophotometry (MSP; Chung, Phillips, Marshall 2016, unpublished) has shown a few species of wrasse from the coral reef possess functional long-wavelength visual pigments, in the long-wavelength–sensitive (LWS) class with peak sensitivities around 561 nm. This is unusual in marine fish, and combined with their yellow optical filtering (in some species) would result in peak spectral sensitivities close to 600 nm. The Hexagrammidae (greenling) are fish that achieve very long wavelength sensitivity beyond 600 nm with orange corneal filters [10] and as both they and wrasse are omnivorous bottom-feeders, it is possible these fish may specialize in long-wavelength colours, potentially including fluorescence [10,11,21,23].
(d). Under what natural lighting conditions is the animal or plant viewed?
Coral on coral reefs and scorpions show up well in the dark under bright UV ‘black lights’ or bright-blue-light–emitting diodes (LEDs); however, these are excitation scenarios never found in nature. On land and in surface waters broad illumination exists from 300 to 700 nm and in bright sunlight; there will be enough photons in the 330–500 nm range of the majority of relevant fluorescent excitations. Is the additional boost from the fluorophore of selective value (see electronic supplementary material, figure S2)? We have already suggested fluorescence can be seen in broad daylight in certain situations, and a few previous studies have attempted to quantify the additional boost to colours from fluorescence alone [7,16–18,25,26]. Mazel and Fuchs [16] use the terms ‘overt’ and ‘covert’ to describe fluorescence that can be seen in natural illumination or only at night using extra light, respectively.
There are two spectrally restricted sources of illumination in the natural world, light at depth in water or light from bioluminescent organs. Photophores (bioluminescent organs) in fact often convert their naturally blue bioluminescent light to green using what is aptly named ‘green fluorescent protein’ (GFP). This is common in jellyfish and other cnidarians and may be used to increase visibility range in greener coastal waters where this conversion is common [27]. Orange and red light is also made by fluorophores converting blue bioluminescence and potentially ambient light in deeper-sea bioluminescent species [28,29]. Against the deep-blue mesopelagic or dark inky depths, such light may not travel far but it will be both contrasting and unusual. Any game fisherman knows that a bit of pink fluorescent plastic on a lure increases catch rate in ‘deep-sea’ fishing and as usual, we appear to be following in the footsteps, or in this case the bell-pumps, of nature.
(e). Are there visual behaviours that might rely on the fluorescent component of the colour or pattern produced, or be assisted in some way by the fluorescence within that pattern or object?
To determine if fluorescence is functionally significant, it is necessary to reduce or remove those wavelengths that excite the fluorophore and look for a change in behaviour. Unfortunately, removing excitation wavebands may also remove wavelengths the animal expects to see in the scene, and excitation and emission curves may overlap substantially [3,16,17] so that important wavelengths may be excised. Even where there is good excitation/emission separation, removing part of the illuminant necessarily changes the colour of the whole scene. A more controlled approach is direct manipulation of fluorescent areas either with reagents that interact with the fluorophore directly or paint that keeps the base colour intact (as measured with a spectrophotometer) but removes the fluorescence. A few examples of such manipulations have been tried [18,22], as discussed below.
3. Possible cases of fluorescence
(a). Stomatopods
Yellow fluorescent markings on the antennal scales and other body regions of Lysiosquillina glabriuscula, a mantis shrimp (stomatopod crustacean), may be visually relevant [18] since they are in a position that could both catch light and display resulting fluorescence (figure 2). The suggestion was made that, in the aquatic environment, fluorescence may add to the signal reliability of ordinary, especially longer-wavelength, coloured pigments that are rapidly attenuated with distance under water. Fluorescent excitation, emission and ambient illumination were measured and used to argue that in both shallow and slightly deeper habitats in the ocean, there was enough UV/violet light to excite the fluorescence to levels between an additional 7–10% extra on top of reflected light, and that the yellow fluorescence contrasted well against the predominant blue cast of the ocean substrate. A good correlation between the narrow-band stomatopod spectral sensitivities and fluorescent emission was found: the sharply tuned sensitivities in part the result of yellow filters within the stomatopod retina [12]; figure 2(i). Finally, estimates were made to model or quantify the contribution of fluorescence to the signal received by photoreceptors with an additional 15–30% to the photon capture of the best matched sensitivity over a depth range of 20–40 m. From our checklist, (a–d) were well covered but the most convincing evidence, behaviour (e), was not attempted. It was suggested that as the fluorescence was found on areas displayed during conspecific contest or mate choice that the fluorescence was visually significant. This work, therefore, remains a well-backed supposition until tested behaviourally.
(b). Plants
UV light excites fluorescence in nectar in certain flowers [30], and it has been suggested that this is used to attract pollinators, specifically bees, although evidence is purely correlative. More specifically, the four o'clock flower, Mirabilis jalapa has been shown to create a contrasting fluorescent pattern on its petals using yellow fluorescent betaxanthin and a violet absorbing betacyanin pigment, and this is suggested to act as a guide to pollinating bees and bats as they see green [31]. Aside from a fluorescent emission and excitation measurement of the pigment extract, this study does not address criteria (a–e). Indeed, this work along with a similar study by Ono et al. on the yellow snapdragon flower [32] has been criticized by Iriel, Lagorio and co-workers [1,14], who measured rather low fluorescence quantum yields from a number of flowers. These authors also calculated the quantum catch by bird, bee and human photoreceptors viewing flower reflectance measurements, not in fact their fluorescence quotient, arguing that it is small compared to the reflectance signal as seen by the animal.
Carnivorous pitcher plants attract insects and small mammals as an important source of protein and minerals, respectively, using nectar, olfactory and colour cues. Kurup et al. suggest they also use fluorescence [22] and successfully consider (if not fully quantify) criteria (a–e). This study used carefully controlled behaviour and masking of the blue fluorescence emission (430–480 nm—a range that insects are specifically sensitive to, see below) from around the lip of the pitcher (figure 1), and subsequent prey capture rate decreased in the field, under natural illumination, strongly indicating that fluorescence plays a role in this system.
Fruit colours are also a colour attractor for many animals and several types of fruit fluoresce. Although no thorough studies of its significance in nature have been conducted, fruit fluorescence is used in machine vision to determine fruit quality (reviewed in [1]).
(c). Insects
Butterflies, bees, beetles and dragonflies all possess fluorescent areas, but it is premature to make conclusions about their function [1,33]. Of the 10 069 species of butterflies and moths surveyed with UV lights in museums, 3122 species were found to fluoresce, often from the yellow parts of wings [33]. For example, swallowtail butterflies of the Papilio nireus group display brilliant blue/green markings. The wing scales of these species combine both pigmentary fluorescence and a structural colour production method using two-dimensional photonic crystals and Bragg reflectors [8]. The result amplifies and directs the green (505 nm) fluorescence which is maximally excited by 420 nm light (criteria a and b). Interestingly, these three components and the resulting amplification method are very similar to the design of LEDs. In common with fluorescence combined with bioluminescent excitation [29], the relatively high intensity of the resulting fluorescent emission and its photonic design mean that, unlike the more passive pigment-alone systems described, this fluorescent emission is almost certainly visually relevant. An attempt at ecological validation through signal manipulation would be valuable (criteria d and e), however, as recently performed for polarized light wing reflections, another signalling mechanism in butterflies [34]. Many butterflies, including papilionids, possess very specifically tuned and unusual spectral sensitivities (criterion c), often matched to wavelength-specific behaviour [35] making them an ideal subject for behavioural tests.
(d). Spiders
Many spiders fluoresce, showing a remarkable diversity of UV excitations (288–333 nm) and UV to blue emissions (325–466 nm) that vary from bright to dim [36]. Although most spiders have poor vision, it has been suggested that their fluorescence has evolved under the selective pressure of predatory insects or birds. Both these groups possess UV and other short-wavelength spectral sensitivities, and spider fluorescence is predicted to help camouflage in brightly coloured flowers or elsewhere.
One family, the jumping spiders (Salticidae), have excellent vision and multiple, UV-green, spectral sensitivities in their large antero-median eyes. They show complex mating behaviour involving a suite of signalling mechanisms. Daiqin Li and colleagues make a convincing case (including evidence from all criteria a–e) that the sexual dimorphism of fluorescence in the ornate jumping spider Cosmophasis umbratica is behaviourally relevant [37]. Males have UV-reflecting patches and females do not, while females possess UV-excited, green-emitting pedipalps (figure 1). Using filters over display arenas to remove both UV and, also therefore, fluorescence decreased courtship responses in both sexes. Males, but not females, possess and apparently need UV reflectance to look sexy, so removing UV from the overall appearance of females has less effect, reinforcing the idea that it is their fluorescent palps that help in mate choice.
(e). Birds
A number of bird species possess fluorescent feathers, most famously, 52 species of parrots, but also penguins and toucans. Psittacofulvins are unique to parrot species, and the fluorescence of these carotenoid-like pigments was discovered by shining a UV light on dead parrots in a dark museum [38]. Studies on a small parrot, the budgerigar Melopsittacus undulatus, in fact fulfil all criteria (a–e) [7] including visual modelling of the 14% additional fluorescence component to the yellow crown and cheek feathers under natural illumination conditions (figures 1 and 2). The potential signal is not sexually dimorphic, and it is interesting that fluorescent crown and cheek feathers are placed amongst non-fluorescent also yellow feathers, making this fluorescent signal pop out (similar to the fist tattoos in figure 1), lending weight to their potential visual function. There is a good match of the fluorescent emission of these feathers to the yellow and red oil droplet–filtered long-wavelength spectral sensitivities of budgerigars (figure 2). Furthermore, the fluorescent cheek feathers are associated with UV reflecting, to us, dark blue plumage and as they absorb UV resulting from their 300–400 nm excitation waveband, a potentially high-contrast UV+/UV− signal is presented with the blue/UV feathers (figure 2, [7]).
Most telling, Arnold et al. [7] reduced fluorescent emission without an overall experimental arena illumination change using sunblock in both males and females, selectively blocking the excitation wavelengths. As a control, the sunblock carrier, petroleum gel, was applied to some birds to also wet their appearance without altering fluorescent emission. Both males and females prefer to associate with potential mates that fluoresce (figure 2).
Interestingly, Pearn et al. [25] failed to note any fluorescence-linked difference in mate choice in budgerigars but they based their analysis on whole-arena manipulations of UV reflectance and fluorescence using combinations of overhead and between-bird filters, a system that does not allow selective removal of fluorescence only.
(f). Gelatinous zooplankton and coral
In the ocean two sources of potential excitation illumination exist: bioluminescence and, at depths greater than around 20 m, the illumination left after the chromatic filtering effect of water. The discovery of GFP in the hydromedusa Aequorea victoria came with the realization that it was a blue bioluminescence that excited the GFP, and the light output of the cells was a combination of both processes [1]. A number of other cnidarian and non-cnidarian zooplankton also use bioluminescence and/or the ‘pure-blue’ of their relatively deep oceanic light habitat to excite fluorescence. This includes the interesting suggestion that pontellid copepods, small oceanic crustaceans, may have evolved a suite of GFP homologues for species recognition [39]. Pontellids are not bioluminescent, however, so would necessarily rely on ambient illumination to excite their fluorescence. We also know almost nothing about their lifestyle in the open ocean, so this remains hypothetical.
Haddock and co-workers [27,29,40] have made the case that some hydromedusan gelatinous zooplankton species construct fluorescent lures to bring in prey. This includes the tentacle tip (tentilla) in Erenna, a deep-sea, fish-eating siphonophore with fluorescent tissue emitting yellow to red (583–680 nm) surrounding a bioluminescent photophore. The tentilla display a unique flicking behaviour, as if fishing. Although this species lives beyond 1000 m in depth where the only light is generally blue or green bioluminescence, a few fish, perhaps intended victims, notably three species of dragonfish (Stomiidae) both produce and are sensitive to red light [28].
As mentioned above, several deep-sea fish such as hatchet fish, pearleyes and lanternfish have yellow filters in their cornea, lens or retina (checklist (d), electronic supplementary material, figure S2) [9]. While this has previously been supposed useful in discriminating blue bioluminescence from the water background, just like a fluorescent photographer's filter, such filtering may also increase the relative visibility of long-wavelength fluorescence in the deep, perhaps aiding in siphonophore or jellyfish avoidance. However, as clear from electronic supplementary material, figure S2 and calculations in electronic supplementary material, the visibility increase is very dependent on spectral sensitivities.
Considering all criteria (a–e), Haddock & Dunn [40] followed this idea up with both behavioural evidence and an overview of fluorescence as a prey attractor in marine organisms. Using the hydromedusa Olindias formosus under experimental conditions that stimulated or reduced the green fluorescence near its tentacle tips, it was found that rockfish were more attracted to tentacle-like objects that fluoresced. O. formosus is a relatively shallow-living jellyfish (<30 m deep) and lacks bioluminescence, however the narrow-band blue waters even at 20 m are presumed able to excite fluorescence. Perhaps significantly, the very tip of the tentacles is pigmented bright pink, a colour that, while not fluorescent, might provide a good contrast to the green fluorescing portion of the tentacle and with both highly contrasting colours against a blue ocean background, the result seems irresistible, at least to rockfish.
Mazel & Fuchs [16] undertook an extensive study of the potential visual effect of the fluorescence of different kinds of coral. They also concluded that red/orange fluorescence would be most colourful underwater because it emits at the wavelength range where there is little competing down-welling light, yet absorbs where there is sufficient amount of it. Green fluorescence would also be quite visible because, although it experiences stronger competition from down-welling light, the concentration of the green fluorescent pigments in corals, or indeed jellyfish, is typically very high. Mazel & Fuchs modelled the colour visibility from the standpoint of a human observer, however, and suggest these results should be extended to the visual ecology of actual coral reef inhabitants. Their estimate of practical fluorescent efficiency (PFE) is a useful start but has surprisingly been ignored [16].
Matz et al. attempted to extend this work to reef fish by modelling the colour contrasts of corals, including their fluorescence, against the reef background, against each other and against non-fluorescent coral [17]. Models used the spectral sensitivities of three reef fish occupying different ecological niches, as well as human colour vision. Included in this model were in situ measurements of both ambient illumination, fluorescence and the reef-substrate against which corals might appear. As all these fish possess colour vision biased towards to blue-green wavelengths [20], red fluorescence did not produce strong colour signals, but blue and especially green fluorescence were quite visible (see also electronic supplementary material, figure S2). Red fluorescence was more visible to humans as might be expected from our longer wavelength sensitivities; however, these models tend to emphasize chromatic rather than luminance differences, which as electronic supplementary material, figure S2, illustrates may be rather small.
(g). Fish
Fish fluorescence is generally dim in natural illumination, and most marine fish have low sensitivity in the 500–700 nm range. That some reef and other fish fluoresce has been known for some time [41], and it has become popular to shine excitation lights at them. Red filters placed over dive masks allow one to spot fluorescent fish at depth and, as with cameras, human eyes adjusted to such conditions can see red fluorescence. With no mask filter and some training, one can see red fluorescent fish at certain depths. While this may be possible, after all orange coral polyps pop out from the background to the unaided eye of a diver (figure 1(f)); however as noted above, human vision is much more sensitive to long wavelengths than (most) fish and the quantum yield from GFP in corals is far more than that from fish [16].
Michiels and co-workers [42] noted 32 species of reef-associated fish, including gobies, tripplefins, wrasse and pipefish that fluoresce mainly red at or beyond 600 nm. Sparks and colleagues extended this to sharks, rays and other teleosts identifying 180 species that fluoresce [23]. This includes deeper- living green- and yellow-emitting fish (e.g. around 520 nm). The red fluorescence described by Michiels and others co-occurs with guanine crystals, and while emission is well described with peaks from 584 to 700 nm, only a few excitations are characterized, with peaks from 525 to 600 nm, not a close match to the blue-water habitat.
Michiels and co-workers make the case that the fish species live at the correct depth to have their fluorophores excited by the spectrally narrowed ambient light and that at these depths there is a strong contrast of red to the background. Photographs of fish in simulated deep-water light show no fluorescence until viewed through a red filter (as also shown in figure 1(j)) or the exposure of the camera adjusted [42–44]. In common with stomatopods and budgerigars, body areas used in display such as fins, eyes and head regions are fluorescent and this indicates fluorescence could contribute to intraspecific signalling.
Using MSP, Michiels and colleagues identify a relatively long-wavelength sensitivity (540 nm) in the goby, Eviota pellucida. This shows some overlap with typical fish emission spectra, clustering around 600 nm [20]. The authors build the case that, in common with UV reflection in reef fish, red fluorescence represents a ‘private area of the spectrum’ available only to fish that fluoresce [42]. In the visual models of Matz et al. [17] described above, of three different reef fish viewing marine fluorescence, the longest wavelength sensitivity at 530 nm of the butterflyfish (10 nm shorter than E. pellucida) and its modelled trichromatic colour sense resulted in poor contrast for this fish looking at red fluorescent emissions, which also peaked around 600 nm. The models of Matz et al. do not address relative quantum yield but do work with a receptor noise–limited model, which, to an extent, adjusts for luminous intensity while examining chromatic differences [17].
Gerlach and colleagues have examined Cirrhilabrus solorensis wrasse in more detail. It has a relatively long spectral sensitivity at 534 nm using MSP but ocular media that are not yellow, transmitting well down to 370 nm [44–46]. C. solorensis males possess narrow-band fluorescence peaking at 660 nm on various body parts, including the operculum, dorsal and ventral regions and the caudal peduncle. The broad excitation with a minimum in the blue at 440 nm and a maximum at 600 nm is not ideally matched to its relatively clear-water habitat (470 nm peak) or the 462 nm blue LED illumination used for illumination in experiments [43]. In behavioural trials, males viewed themselves in mirrors with and without dark blue filters placed over them (that removed light below 420 nm and beyond 550 nm) and their agonistic reaction was scored. They were less aggressive to the blue filter versus no filter and brightness controls of 25% and 50% transmission neutral-density filters over the mirrors. This leads to the conclusion that C. solorensis can see its deep red fluorescent coloration and that this pattern affects male–male interactions. However, a mirror image of a fish and a large object (the mirror) that is dark from having a dark blue filter over it may elicit less agonistic behaviour for many reasons. Furthermore, the neutral-density filters used of 25% and 50% pass are not sufficient to cover the brightness reduction provided by the blue filter to this or any fish's visual system.
Finally, Sparks and co-workers raise the idea of fluorescent camouflage; red fluorescing fish live near red algae whereas green fluorescing fish live near green algae [23]. The red fluorescing scorpion fish are a particularly striking example of potential background matching. Gruber et al. [46] use predictive and real visual models and even a camera carefully tuned to try and see the possible fluorescence of a Scyliorhinid catshark in situ. It is worth remembering that even apparently bright green fluorescence from fish is generally dim, compared to that from GFP cnidarians, in this specific case around ten times less bright ([16], CH Mazel 2016, personal communication). Of course what we would really like to see here is some shark behaviour relative to its striking (to our eyes) fluorescent patterning.
Overall in fish, criteria a–e are generally quantified and considered, but often less rigorously than desirable.
4. Conclusion
We suggest that the only fluorescent systems that are close to demonstrating functional significance are in budgerigars and jumping spiders where they are used in mate choice, and in gelatinous zooplankton and pitcher plants where they are used for prey capture. Other taxa provide circumstantial evidence (particularly swallowtail butterflies and stomatopods). Until missing criteria are examined more closely in these and other taxa, as enumerated carefully in this article, humans will continue to be tempted to give immediate function to things that glow in the dark under man-made excitation sources, using our own visual system and visual environment, which are unrelated to the natural world in which fluorescence occurs.
Supplementary Material
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Lagorio MG, Cordon GB, Iriel A. 2015. Reviewing the relevance of fluorescence in biological systems. Photochem. Photobiol. Sci. 14, 1538–1559. ( 10.1039/C5PP00122F) [DOI] [PubMed] [Google Scholar]
- 2.Salih A, Larkum A, Cox G, Kuhl M, Hoegh-Guldberg O. 2000. Fluorescent pigments in corals are photoprotective. Nature 408, 850–853. ( 10.1038/35048564) [DOI] [PubMed] [Google Scholar]
- 3.Johnsen S. 2012. The optics of life: a biologist's guide to light in nature. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Benson RC, Vogel MJ. 1955. The principles of identification and measurement of vulvar fluorescence. J. Clin. Endocrinol. Metab. 15, 784–800. ( 10.1210/jcem-15-7-784) [DOI] [PubMed] [Google Scholar]
- 5.Weagle G, Paterson PE, Kennedy J, Pottier R. 1988. The nature of the chromophore responsible for naturally-occurring fluorescence in mouse skin. J. Photochem. Photobiol. B 2, 313–320. ( 10.1016/1011-1344(88)85051-6) [DOI] [PubMed] [Google Scholar]
- 6.Lythgoe JN. 1976. The ecology of vision. Oxford, UK: Clarendon Press. [Google Scholar]
- 7.Arnold KE, Owens IPF, Marshall NJ. 2002. Fluorescent signaling in parrots. Science 295, 92 ( 10.1126/science.295.5552.92) [DOI] [PubMed] [Google Scholar]
- 8.Vukusic P, Hooper I. 2005. Directionally controlled fluorescence emission in butterflies. Science 310, 1151 ( 10.1126/science.1116612) [DOI] [PubMed] [Google Scholar]
- 9.Douglas R, Marshall N. 1999. A review of vertebrate and invertebrate ocular filters. In Adaptive mechanisms in the ecology of vision (eds Archer SN, Djamgoz MBA, Loew ER, Partridge JC, Vallerga S), pp. 95–162. London, UK: Kluwer Academic Publishers. [Google Scholar]
- 10.Kondrashev SL. 2008. Long-wave sensitivity in the masked greenling (Hexagrammos octogrammus), a shallow-water marine fish. Vision Res. 48, 2269–2274. ( 10.1016/j.visres.2008.07.004) [DOI] [PubMed] [Google Scholar]
- 11.Siebeck UE, Marshall NJ. 2000. Transmission of ocular media in labrid fishes. Phil. Trans. R. Soc. Lond. B 355, 1257–1261. ( 10.1098/rstb.2000.0679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cronin TW, Marshall NJ. 1991. Interhabdomal filters in retinas of stomatopod crustaceans: structure and absorption spectra. Invest. Ophthalmol. Vis. Sci. 32, 907. [Google Scholar]
- 13.Stavenga DG. 2002. Reflections on colourful ommatidia of butterfly eyes. J. Exp. Biol. 205, 1077–1085. [DOI] [PubMed] [Google Scholar]
- 14.Iriel A, Lagorio MG. 2010. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften 97, 915–924. ( 10.1007/s00114-010-0709-4) [DOI] [PubMed] [Google Scholar]
- 15.Gaffin DD, Bumm LA, Taylor MS, Popokina NV, Mann S. 2012. Scorpion fluorescence and reaction to light. Anim. Behav. 83, 429–436. ( 10.1016/j.anbehav.2011.11.014) [DOI] [Google Scholar]
- 16.Mazel CH, Fuchs E. 2003. Contribution of fluorescence to the spectral signature and perceived color of corals. Limnol. Oceanogr. 48, 390–401. ( 10.4319/lo.2003.48.1_part_2.0390) [DOI] [Google Scholar]
- 17.Matz MV, Marshall NJ, Vorobyev M. 2006. Are corals colourful? Photochem. Photobiol. 82, 345–350. ( 10.1562/2005-08-18-RA-653) [DOI] [PubMed] [Google Scholar]
- 18.Mazel C, Cronin T, Caldwell R, Marshall N. 2004. Fluorescent enhancement of signaling in a mantis shrimp. Science 303, 51 ( 10.1126/science.1089803) [DOI] [PubMed] [Google Scholar]
- 19.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Losey G, McFarland W, Loew E, Zamzow J, Nelson P, Marshall N, Montgomery W. 2003. Visual biology of Hawaiian coral reef fishes. I. Ocular transmission and visual pigments. Copeia 2003, 433–454. ( 10.1643/01-053) [DOI] [Google Scholar]
- 21.Marshall N, Jennings K, McFarland W, Loew E, Losey G, Montgomery W. 2003. Visual biology of Hawaiian coral reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia 2003, 467–480. ( 10.1643/01-056) [DOI] [Google Scholar]
- 22.Kurup R, Johnson AJ, Sankar S, Hussain AA, Kumar CS, Sabulal B. 2013. Fluorescent prey traps in carnivorous plants. Plant Biol. 15, 611–615. ( 10.1111/j.1438-8677.2012.00709.x) [DOI] [PubMed] [Google Scholar]
- 23.Sparks JS, Schelly RC, Smith WL, Davis MP, Tchernov D, Pieribone VA, Gruber DF. 2014. The covert world of fish biofluorescence: a phylogenetically widespread and phenotypically variable phenomenon. PLoS One 9, e83259 ( 10.1371/journal.pone.0083259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips GA, Carleton KL, Marshall NJ. 2015. Multiple genetic mechanisms contribute to visual sensitivity variation in the Labridae. Mol. Biol. Evol. 33, 201–215. ( 10.1093/molbev/msv213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearn SM, Bennett ATD, Cuthill IC. 2001. Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulatus. Proc. R. Soc. Lond. B 268, 2273–2279. ( 10.1098/rspb.2001.1813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearn SM, Bennett ATD, Cuthill IC. 2003. The role of ultraviolet-A reflectance and ultraviolet-A induced fluorescence in the appearance of budgerigar plumage: insights from spectrofluorometry and reflectance spectrophotometry. Proc. R. Soc. Lond. B 270, 859–865. ( 10.1098/rspb.2002.2315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddock SHD, Mastroianni N, Christianson LM. 2010. A photoactivatable green-fluorescent protein from the phylum Ctenophora. Proc. R. Soc. B 277, 1155–1160. ( 10.1098/rspb.2009.1774) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Douglas RH, Mullineaux CW, Partridge JC. 2000. Long-wave sensitivity in deep-sea stomiid dragonfish with far-red bioluminescence: evidence for a dietary origin of the chlorophyll-derived retinal photosensitizer of Malacosteus niger. Phil. Trans. R. Soc. Lond. B 355, 1269–1272. ( 10.1098/rstb.2000.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddock SHD, Dunn CW, Pugh PR, Schnitzler CE. 2005. Bioluminescent and red-fluorescent lures in a deep-sea siphonophore. Science 309, 263 ( 10.1126/science.1110441) [DOI] [PubMed] [Google Scholar]
- 30.Thorp RW, Briggs DL, Estes JR, Erickson EH. 1975. Nectar fluorescence under ultraviolet irradiation. Science 189, 476–478. ( 10.1126/science.189.4201.476) [DOI] [PubMed] [Google Scholar]
- 31.Gandia-Herrero F, Garcia-Carmona F, Escribano J. 2005. Floral fluorescence effect. Nature 437, 334 ( 10.1038/437334a) [DOI] [PubMed] [Google Scholar]
- 32.Ono E, et al. 2006. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proc. Natl Acad. Sci. USA 103, 11 075–11 080. ( 10.1073/pnas.0604246103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch VL, Van Hooijdonk E, Intrater N, Vigneron J-P. 2012. Fluorescence in insects. The Nature of Light: Light in Nature IV. Proc. SPIE 8480, 848004-1–848004-15. ( 10.1117/12.929547) [DOI] [Google Scholar]
- 34.Sweeney A, Jiggins C, Johnsen S. 2003. Insect communication: polarized light as a butterfly mating signal. Nature 423, 31–32. ( 10.1038/423031a) [DOI] [PubMed] [Google Scholar]
- 35.Arikawa K, Stavenga D. 1997. Random array of colour filters in the eyes of butterflies. J. Exp. Biol. 200, 2501–2506. [DOI] [PubMed] [Google Scholar]
- 36.Andrews K, Reed SM, Masta SE. 2007. Spiders fluoresce variably across many taxa. Biol. Lett. 3, 265–267. ( 10.1098/rsbl.2007.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim MLM, Land MF, Li D. 2007. Sex-specific UV and fluorescence signals in jumping spiders. Science 315, 481 ( 10.1126/science.1134254) [DOI] [PubMed] [Google Scholar]
- 38.Boles WE. 1990. Glowing parrots: need for a study of hidden colours. Birds Int. 3, 76–79. [Google Scholar]
- 39.Shagin DA, et al. 2004. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol. Biol. Evol. 21, 841–850. ( 10.1093/molbev/msh079) [DOI] [PubMed] [Google Scholar]
- 40.Haddock SHD, Dunn CW. 2015. Fluorescent proteins function as a prey attractant: experimental evidence from the hydromedusa Olindias formosus and other marine organisms. Biol. Open 4, 1094–1104. ( 10.1242/bio.012138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall J. 2001. Colour vision and colour signals in Australian animals: principles of design. In Proceedings of the Australian Neuroscience Society 28–31 Jan 2001, Volume 12. Brisbane, Australia: Australian Neuroscience Society. [Google Scholar]
- 42.Michiels N, et al. 2008. Red fluorescence in reef fish: a novel signalling mechanism? BMC Ecol. 8, 16 ( 10.1186/1472-6785-8-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerlach T, Sprenger D, Michiels NK. 2014. Fairy wrasses perceive and respond to their deep red fluorescent coloration. Proc. R. Soc. B 281, 20140787 ( 10.1098/rspb.2014.0787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meadows MG, Anthes N, Dangelmayer S, Alwany MA, Gerlach T, Schulte G, Sprenger D, Theobald J, Michiels NK. 2014. Red fluorescence increases with depth in reef fishes, supporting a visual function, not UV protection. Proc. R. Soc. B 281, 20141211 ( 10.1098/rspb.2014.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerlach T, Theobald J, Hart NS, Collin SP, Michiels NK. 2016. Fluorescence characterisation and visual ecology of pseudocheilinid wrasses. Front. Zool. 13, 13 ( 10.1186/s12983-016-0145-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruber DF, et al. 2016. Biofluorescence in Catsharks (Scyliorhinidae): fundamental description and relevance for elasmobranch visual ecology. Sci. Rep. 6, 24751 ( 10.1038/srep24751) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.