Abstract
The coevolutionary interactions between avian brood parasites and their hosts provide a powerful system for investigating the diversity of animal coloration. Specifically, reciprocal selection pressure applied by hosts and brood parasites can give rise to novel forms and functions of animal coloration, which largely differ from those that arise when selection is imposed by predators or mates. In the study of animal colours, avian brood parasite–host dynamics therefore invite special consideration. Rapid advances across disciplines have paved the way for an integrative study of colour and vision in brood parasite–host systems. We now know that visually driven host defences and host life history have selected for a suite of phenotypic adaptations in parasites, including mimicry, crypsis and supernormal stimuli. This sometimes leads to vision-based host counter-adaptations and increased parasite trickery. Here, we review vision-based adaptations that arise in parasite–host interactions, emphasizing that these adaptations can be visual/sensory, cognitive or phenotypic in nature. We highlight recent breakthroughs in chemistry, genomics, neuroscience and computer vision, and we conclude by identifying important future directions. Moving forward, it will be essential to identify the genetic and neural bases of adaptation and to compare vision-based adaptations to those arising in other sensory modalities.
This article is part of the themed issue ‘Animal coloration: production, perception, function and application’.
Keywords: animal coloration, brood parasitism, avian vision, mimicry, coevolution, sensory ecology
1. Introduction
Interspecific avian brood parasitism is rare, with just 1% of bird species acting as obligate parasites [1]. In these systems, the brood parasite infiltrates the nest of a different bird species, lays an egg and offloads all parental care to the host. This form of social parasitism has evolved independently at least seven times in birds: once each in ducks, honeyguides, finches and New World blackbirds (cowbirds), and three times in cuckoos [2]. Outside of birds, interspecific brood parasitism is extremely limited among vertebrates, having evolved infrequently in fishes [3], possibly in frogs [4], and never in non-avian reptiles or mammals (to our knowledge). Among invertebrates, the strategy has repeatedly evolved in insects [5,6]. Birds and insects are without doubt the best studied interspecific brood parasites, with the coevolutionary battles between parasite and host unfolding in similar ways in the two systems [6]. However, while insect brood parasites largely use chemical trickery to outwit their hosts [6], in birds the war is waged in colour and sound (table 1).
Table 1.
The sensory ecology of avian brood parasite–host interactions. Avian hosts and brood parasites exploit a range of sensory modalities as they evolve adaptations and counter-adaptations in coevolutionary arms races. We compare host defences and parasite responses (trickery), as well as host life-history characteristics and parasite adaptations to these characteristics (tuning), across four sensory channels: visual, acoustic, chemical/olfactory and tactile.
| stage of breeding cycle | visual | acoustic | chemical/olfactory | tactile |
|---|---|---|---|---|
| FRONTLINE: ACCESSING THE NEST | ||||
| host defence | mob adult female cuckoos [7] | referential alarm calls [32] | sit tightly on the nest and attack parasite at the nest [42] | |
| parasite trickery | plumage mimicry of hawks [7–9] polymorphic plumage [10,11] |
|||
| host life-history characteristic | nest defence | general alarm calls against predators [33] | ||
| parasite tuning | elevated nest defence elicits more parasitism of better (older) hosts [12] | eavesdrop on vocalizations to select nests that are well defended [34] | ||
| EGGS: LAYING AND INCUBATION | ||||
| host defence | reject foreign eggs based on visual appearance [13] egg signatures [14,15] |
unknown, but some hosts can discriminate against artificial eggs with a different smell [41] | reject foreign eggs (including by desertion or burial) based on touch [43,44] | |
| parasite trickery | cryptic parasite eggs [16] host-egg mimicry [13] mimicry of host-egg signatures supernormal parasite eggs [17] laying eggs in host nests with better host–parasite egg match [18] |
lay small eggs relative to body size [12,45] lay eggs with stronger shells [46] |
||
| host life-history characteristic | preference for large eggs [19,20] | |||
| parasite tuning | supernormal eggs [17] | |||
| CHICKS AND FLEDGLINGS: PROVISIONING YOUNG | ||||
| host defence | reject foreign chicks [21–23] chick signatures [24] |
reject foreign chicks, possibly based on vocalizations [35,36] transmit host-specific password to host chicks incubated in the nest [35] |
reject chicks based on prolonged presence in the nest [47] | |
| parasite trickery | host-chick mimicry [25,26] mimicry of chick signature [24] |
potential mimicry of chick begging calls; this could also be tuning designed to enhance host provisioning [37] | ||
| host life-history characteristic | provision chicks | provision chicks host chicks imprint on their parents for species recognition |
||
| parasite tuning | supernormal gape [27–29] supernormal begging [30] gape mimicry [31] |
begging calls that are structurally similar to those of host chicks [37] exaggerated, supernormal begging calls [38,39] password to avoid imprinting on host for species recognition [40] |
||
Some of the most spectacular visual signals in the animal world result from interactions between avian brood parasites and their hosts. Textbook examples (figure 1) include the mimetic eggs laid by the common cuckoo Cuculus canorus and the cuckoo finch Anomalospiza imberbis, the gape-mimicking armpit of a Horsfield's hawk-cuckoo Cuculus fugax chick and the elaborate mouth markings of parasitic Vidua finch nestlings. These visual signals, designed to deceive and exploit potential foster parents, have evolved in response to selection pressure imposed by hosts. In turn, brood parasite adaptations can trigger the evolution of new visual traits in the hosts, such as egg colour polymorphisms, egg pattern signatures and more elaborate gape markings. Thus, avian brood parasite–host dynamics are unique evolutionary forces that can be powerful generators of both phenotypic novelty and diversity.
Figure 1.
Adaptations involving colour at the frontline, egg, chick and fledgling stages. (a) The rufous-morph female common cuckoo mimics the appearance of a (b) sparrowhawk, which can reduce mobbing behaviour by hosts. The rufous-morph cuckoo has an added advantage if hosts recognize it less readily than the more common grey-morph. (c) The parasitic cuckoo finch has evolved polymorphic eggs to match the range of polymorphic egg colours exhibited by their hosts, the tawny-flanked prinia. The inner circle shows eggs laid by different cuckoo finch females, while the outer circle shows eggs laid by prinia females. (d) Genetically distinct host-races of the common cuckoo lay a range of eggs (top row) to match those of their preferred European hosts (bottom row). The degree of egg colour and pattern mimicry varies. (e) Various Chalcites cuckoo chicks (left) mimic the skin coloration—and even the natal down—of host chicks (right). (f) The parasitic pin-tailed whydah (left) mimics the gape markings of its host, the common waxbill (right). (g) The horsfield's hawk-cuckoo elicits increased feeding from its foster parent by displaying a wing patch that mimics an extra gape. (h) Parasitic fledgling screaming cowbirds (centre) mimic the plumage patterns of host fledgling greyish baywings (left), and receive prolonged parental care, unlike non-mimetic fledgling shiny cowbirds (right). (i) An adult parasitic female cuckoo finch (left) looks almost identical to a harmless adult female red bishop (right), which confuses host tawny-flanked prinias. Image credits: (a) C. Fleming, (b) D. Kjaer, (c) C. Spottiswoode, (d) M. C. Stoddard (copyright Natural History Museum, UK), (e) N. E. Langmore [25], (f) J. Schuetz [48], (g) K. Tanaka, (h) C. De Marsico and J. Reboreda [49], and (i) C. Spottiswoode [50].
In this review, we focus on the central role of colour and vision across avian brood parasite–host systems. We begin by examining the visual defences and adaptations involved at each stage of the arms race. Next we explore how recent advances in the study of colour, from chemistry and genomics to neuroscience and computer vision, are transforming our ideas about parasite–host coevolution. We conclude by identifying the most important unresolved questions for future colour and vision research in the context of brood parasitism.
2. Review of adaptations at different stages of the arms race
Visual signals can have a distinctive colour and pattern, as well as shape and size. For the purposes of this review, we focus on the colour and pattern aspects of the parasite's and host's phenotypes. In brood parasite–host systems, adaptations involving vision and coloration (hereafter ‘vision-based adaptations’) can take on many forms and can evolve at each stage of the breeding cycle (the frontline, eggs and incubation, chicks and fledglings) (figure 1). In the brood parasite, the coevolved response to host defences is referred to as ‘trickery,’ which helps the parasite avoid discrimination by the host [13]. When instead the brood parasite evolves in response to the host's life-history strategies, this is known as parasite ‘tuning,’ which usually helps the parasite increase host parental care [13].
The extent to which vision-based adaptations have evolved varies dramatically across brood parasite–host systems. Some brood parasites are highly virulent, like the common cuckoo chick that evicts all of the host's eggs and hatchlings in order to fully monopolize the host's resources. Other brood parasites are less virulent, like the great spotted cuckoo Clamator glandarius chick that is raised alongside the host's own nestlings. In general, parasitic virulence is linked with the host's coevolved defences. The greater the fitness costs imposed by the parasite on the host, the stronger the host's defences and the more sophisticated the parasite's counter-adaptations [5]. Consequently, the most advanced host recognition systems and the most elaborate visual ‘trickery’ by parasites appear to have evolved when the parasite is a highly virulent, evicting species [5,51]. By contrast, when visual signals in the brood parasite result from ‘tuning’ into the host's life-history traits, the playing field is levelled. Evicting and non-evicting parasites both benefit by exploiting host provisioning strategies, and a swath of vision-based adaptions has evolved for this purpose. In the case of ‘tuning,’ parasite virulence is no longer predicted to be positively correlated with more sophisticated or diverse visual displays [51].
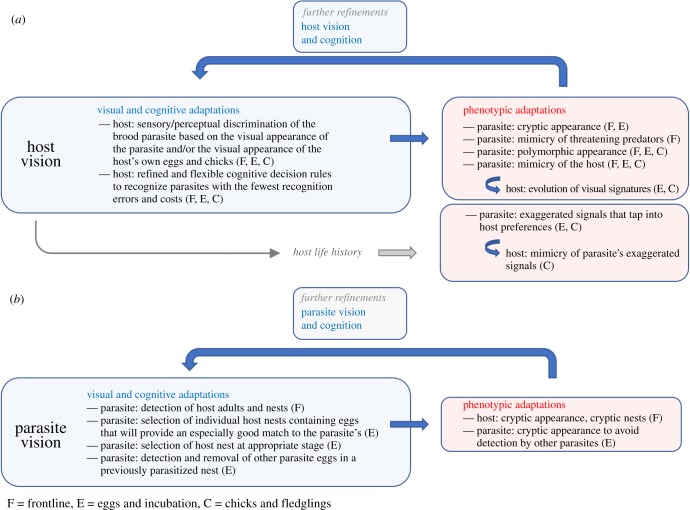
An array of vision-based adaptations can evolve at each stage of the parasitic progression. Highlighting the diversity of such adaptations reveals the potent role vision can play in mediating the escalation of the arms race (figure 1 and table 1). First, host vision (figure 2a) can influence the evolution of host discrimination against the brood parasite, which can then lead to several phenotypic counter-adaptations in the parasite, including crypsis, mimicry and polymorphism. In response to parasite mimicry, hosts can evolve visual signatures. A second set of phenotypic adaptations in the parasite, including supernormal (exaggerated) eggs and chicks, can evolve to ‘tune’ into host visual behaviours involved in incubation and provisioning (figure 2a). The extent to which phenotypic adaptations actually lead to further refinements in the host's sensory systems to enhance discrimination abilities is not well understood: for example, whether phenotypic ‘tuning’ achieved by the parasite has a feedback effect on host vision and cognition is not known. Moreover, once parasites evolve egg mimicry, hosts might evolve better levels of discrimination outright or they might evolve egg signatures [15,52,53].
Figure 2.
The coevolutionary dynamics of vision-based adaptations. Host vision (a) and parasite vision (b) are implicated in the evolution of visual and cognitive adaptations, which can influence reciprocal selection for phenotypic adaptations. In turn, phenotypic adaptations can lead to further refinements of vision and cognition. In some cases, phenotypic adaptations in the parasite can evolve to ‘tune’ into aspects of the host's life history (A, light grey arrow), such as visual behaviours involved in incubation and provisioning.
Parasite vision (figure 2b) can also influence arms race dynamics. Parasites can develop several sensory–perceptual (visual) and cognitive (decision rule) adaptations, including the ability to detect and select appropriate host nests and to discriminate against other parasite eggs. In turn, these adaptations can produce further phenotypic adaptations, in the form of host and parasite crypsis, which may in turn lead to refined visual and cognitive processes. Taken together, host and parasite visual systems can facilitate the evolution of a remarkably varied suite of visual/sensory, cognitive and phenotypic adaptations, which we review in the subsequent subsections.
(a). The frontline
The first vision-based adaptations evolve at the frontline [54]. To realize any reproductive success, the brood parasite's first task is to access the host's nest. Many hosts will mob brood parasites [55], sometimes engaging in extreme violence. Some cuckoo lineages have evolved a visual trick to reduce host aggression: mimicry of hawk-like plumage [9,56] (figure 1a,b). However, great reed warbler Acrocephalus arundinaceus hosts can readily distinguish between common cuckoos and sparrowhawks Accipiter nisus at close range, indicating that cuckoo mimicry is imperfect [57]: the precise visual cues to which hosts respond are unclear [58]. An additional guise evolved by some cuckoos involves plumage polymorphism [59]. Female common cuckoos possess either grey or rufous plumage (figure 1a); in turn, experiments showed that the less common morph can evade host recognition because hosts have learned to attack the more common morph [10]. Beyond cuckoos, adult female parasitic cuckoo finches Anomalospiza imberbis closely resemble harmless female adult sympatric weavers (figure 1i), an illusion which may reduce mobbing or warp host birds’ perception of parasitism risk [50]. Some brood parasites might evolve cryptic plumage to avoid detection during host-nest searching, but this idea requires further testing [54].
The effect of host frontline defences on the brood parasite is clear: it can lead to mimicry, polymorphism or possibly camouflage. But can brood parasites influence the appearance of hosts? Perhaps, brood parasites locate host nests using the conspicuousness of the host's plumage as a cue; among hosts of brown-headed cowbirds Molothrus ater, however, this does not appear to be true [60]. Another possibility is that brood parasites (and probably predators) might select for concealed, cryptic host nests [61].
(b). Eggs and incubation
Once the brood parasite thwarts the host's frontline defences, it lays an egg in the host's nest. Hosts of many brood parasites have evolved egg rejection as a defence: it has been shown experimentally that hosts of parasitic ducks, cuckoos, cowbirds and cuckoo finches use visual cues to reject foreign eggs [51]. In response, many brood parasites have evolved egg mimicry: the common cuckoo (figure 1d) and the cuckoo finch (figure 1c) provide some of the most intricate examples. Common cuckoo females belong to mitochondrially distinct host-races, each of which prefers a particular host species. If the host is a shrewd egg rejecter, common cuckoos (of that host-race) have evolved exceptional eggshell pattern and colour mimicry [52,62]. However, if the host is a poor egg rejecter, common cuckoos have not evolved a closely mimetic egg. Like common cuckoos, cuckoo finches belong to genetically distinct host-races which lay mimetic eggs [63], but their eggs are even more outlandish: different individuals within a single host-race lay eggs that are red, blue or white in colour (figure 1c).
As a defence against mimicry, many hosts have evolved individually recognizable egg signatures to make recognition of foreign eggs easier. This is true in tawny-flanked prinia Prinia subflava hosts of cuckoo finches, where females in a population lay eggs that are highly variable among individuals [14,63]. Thus, the polymorphic eggs evolved by the cuckoo finch (described above) are an adaptation to host-egg signatures. The coevolutionary dynamics of this system are written on the eggs: first hosts evolve egg rejection, then parasites evolve mimicry, then hosts evolve polymorphisms/signatures, then parasites forge those signatures by diversifying their own eggs. In the prinia–cuckoo finch system, some hosts appear to be winning this arms race, having recently produced a green egg colour to which the parasites have not yet evolved a matching egg morph [64]. Even in the absence of strong colour polymorphism, common cuckoo hosts can evolve individually recognizable egg patterns within the same host population [15], revealing the refinement with which coevolutionary interactions can produce new visual signals.
An evolutionary history of the arms race between host rejection and parasitic mimicry is expected to impact not only the phenotypic components of the host eggs (and chicks or fledglings; see §2c below) but also the discrimination thresholds and other cognitive decision rules by which hosts recognize and reject foreign offspring (figure 2a). Hosts might evolve memory-free recognition mechanisms, such as discordancy, to reject the most dissimilar egg from the nest [65]. Alternatively, hosts may evolve reliable learning mechanisms to recognize their own eggs, enabling them to reject foreign eggs that deviate from their recognition template [66].
Not all coevolutionary interactions proceed as in common cuckoos and cuckoo finches, with the evolution of host-races and host-specific egg mimicry. Brown-headed cowbirds and Horsfield's bronze-cuckoos Chalcites basalis are generalists that lay eggs in the nests of multiple host species. They exploit a ‘Jack-of-all-trades’ strategy, such that their eggs are a decent match to those of most hosts [67,68]. Furthermore, visual adaptations at the egg stage are not limited to egg mimicry and egg signatures. Some bronze-cuckoos lay dark, cryptic eggs to outwit parasitic conspecifics, which often remove an egg from the nest before laying their own [16]. Additionally, some common cuckoo host-races lay blue-green eggs; these eggs are mimetic in some host nests but may also serve to exploit hosts’ pre-existing preferences for attractive, sexually selected blue-green eggs [69]. However, the jury is still out on the sexually selected nature of egg coloration [70,71]. Finally, model eggs with truly weird appearances (e.g. white with black polka dots) were commonly accepted into the nests of rufous bush chats Cercotrichas galactotes, a common cuckoo host: perhaps some brood parasites escape egg rejection by evolving eggs that act as supernormal, ultra-speckled stimuli [17].
(c). Chicks and fledglings
Until recently, the ability of hosts to discriminate against and reject parasitic chicks was unknown but has now been demonstrated in multiple host species (reviewed in [72]). This represents a huge shift in thinking about the evolution of host recognition defences, which were predicted to occur largely at the egg stage. In response to host discrimination of parasite chicks, some brood parasites have evolved exquisite chick mimicry. Three bronze-cuckoo species, for instance, mimic not only the black, pink and yellow skin of their respective host nestlings but also match the colours of the hosts’ rictal flanges (border of the gape) and their natal down [25] (figure 1e). Chick mimicry by the shining bronze-cuckoo (Chalcites lucidus) seems to have directly selected for polymorphic chicks in its host, the fan-tailed gerygone Gerygone flavolateralis [24]. As a final observation, screaming cowbirds Molothrus rufoaxillaris mimic the plumage of host greyish baywing Agelaioides badius fledglings, resulting in prolonged provisioning by host parents relative to non-mimetic shiny cowbird Molothrus bonariensis fledglings [49] (figure 1h). This raises the intriguing possibility that the arms race has evolved even beyond the chick stage.
So far, parasite trickery in the form of chick mimicry appears to be a rare phenomenon among the approximately 100 species of brood parasites. By contrast, tuning at the chick stage, whereby the parasite taps into host provisioning strategies, is much more widespread. A classic example of visual mimicry at the chick stage involves the complex mouth markings on parasitic Vidua finches nestlings, which closely resemble those of the host chicks [26] (figure 1f). Vidua chicks are raised alongside host chicks, and hosts do not appear to reject chicks with atypical gape markings [48]. However, experiments showed that chicks with non-mimetic markings received less food [48], and the current perspective is that Vidua gape mimicry evolved to stimulate host provisioning. This is therefore a form of parasite tuning rather than trickery [8], but with a corollary: here, tuning may have provoked a coevolutionary response in hosts, such that host chicks end up mimicking the exaggerated markings of Vidua nestlings in order to get their fair share of food [31]. Accordingly, lineages of estrildid finches parasitized by Vidua spp. have more complex gape markings than non-parasitized lineages [26].
Another remarkable case of parasite tuning is found in the Horsfield's hawk-cuckoo chick, which has evolved a yellow wing patch beneath each wing. The chick flaps its wings and displays the wing patches, simulating multiple gapes and increasing provisioning from its foster parents [27] (figure 1g). The gape-coloured skin patches may be especially effective because they are supernormal in colour, with enhanced ultraviolet reflectance and brightness relative to host gapes [28].
3. Advances
The diverse functions of many vision-based adaptations, as illustrated in §2, have been well described. But what chemical, genomic, neurophysiological and perceptual mechanisms underlie these adaptations? Turning our attention now to this question, we highlight some of the most exciting advances in the field.
(a). Chemical bases of colour mimicry
To begin to understand the proximate basis of eggshell mimicry, it is critical to assess whether parasite–host similarity arises through an ancestrally shared mechanism, a mechanism that evolved twice independently, or two different mechanisms that converged on the same phenotype. A recent line of research into the chemical basis of egg coloration has assessed these alternatives by analysing the eggshell pigment composition of hosts and mimetic parasites [73]. Despite the apparent diversity of avian egg colours and markings, there are only two common pigments in eggshell: biliverdin (blue-green) and protoporphyrin IX (rusty brown), which appear to generate all avian egg colours [74]. Structural mechanisms, which explain the overwhelming range of non-pigmentary plumage coloration in birds [75], for example, are surprisingly limited in their contribution to egg coloration [76,77].
The chemical basis of eggshell colour mimicry appears to be straightforward in mimetic avian parasite–host systems: if the host egg is blue, and heavily pigmented by biliverdin, then so are the parasite's eggs; similarly, if the host egg is beige-brown and pigmented by protoporphyrin IX, then so are the parasite's eggs [73]. This supports the hypothesis that the same pigmentary mechanisms arose independently in hosts and parasites. These egg pigments are ubiquitous across avian lineages and their deposition appears to evolve and re-evolve easily. Indeed, the simple but flexible toolkit provided by eggshell pigments may explain why egg polymorphism, mimicry and signatures are hallmarks across arms races in many avian parasite–host systems. Future work should focus on the pigmentary basis of skin and plumage mimicry by chick, fledgling and adult parasites.
(b). Genomics of egg coloration
Egg coloration and patterning are some of the most elaborate adaptations in parasite–host arms races, yet the pigments responsible are simple. This leaves the genetic and genomic basis of eggshell mimicry and polymorphism as the next frontier in our understanding of egg coloration. In this context, there are two important research directions. First, given that eggshell pigments are shared across avian taxa, what are the genetic factors that control the colour, timing, spacing and amount of pigment deposition during the final stages of egg formation in the oviduct? Second, in specialist parasites targeting a specific host, what are the contrasting genomic mechanisms responsible for host eggshell versus host-chick mimicry?
Though we still know little about the genes underlying egg coloration, several recent studies in chickens have shed light on the genetic basis of blue versus brown eggshells. Sequencing approaches have identified SLCO1B3 as the autosomal gene expressed during the deposition of biliverdin in the oviduct, controlled by an EAV-HP insert which is present only in blue egg-laying chicken lineages [78,79]. Similarly, locally high levels of expression of the gene CPOX are associated with more protoporphyrin deposition, making eggs darker and browner in colour [80]. Knowing the genes involved in chicken egg colour provides a starting point for investigating the genetic basis of egg mimicry in parasite–host systems: are the same genes involved?
In addition, big questions remain about genetic mechanisms underlying egg versus chick visual mimicry, especially with respect to sex-linked genes. Consider common cuckoos: females lay consistently similar eggs, even after they mate with multiple males; in turn, males mate with females that lay different colour morphs of eggs. How can egg-morph specialization be maintained? A hypothesized solution is that, unlike in chickens and quails [81], egg coloration and patterning must be under the control of the female sex chromosome W in the different host-races of these cuckoos, which is feasible because in birds females are the heterogametic sex. In support of this mechanism, mitochondrial (mtDNA) genomes (inherited maternally), but not autosomal nuclear (nDNA) genomes, of female cuckoos from different host-races show differentiation that parallels eggshell polymorphism [82]. More recently, the analysis of not only mtDNA but also female-specific (W) sex-chromosomal genes has suggested that, among blue egg-laying common cuckoos, egg colour could be controlled by female sex-based genetic mechanisms [83].
However, whereas the eggshell is a clear extension of the female's phenotype, female-specific sex-chromosome genes cannot explain the evolution of visual mimicry in host-species-specific chick gape patterns across parasitic Vidua finches [26] (figure 1f). Both female and male young express mimetic colour patterns, so colour and patterning must be under the control of chromosomes other than W. We know little about the genomic basis of vision-based adaptations, but with the current advances in sequencing and gene-expression approaches, this is only a question of focus and time, rather than technique and methodology.
(c). Colour vision and neurophysiology
It is clear that host vision is a driving force in the evolution of adaptations and counter-adaptations in avian brood parasite–host systems (figure 2a). But how variable is colour vision among hosts? Birds possess four colour receptor cone cell types, one of which is sensitive to violet or ultraviolet wavelengths. Across bird species, the four colour cones appear to have fairly similar spectral sensitivities, but there is pronounced variation in the ultraviolet-sensitive cone, which has peak sensitivity around 365 nm (ultraviolet-sensitive, UVS) or 405 nm (violet-sensitive, VS). An initial investigation of 13 brood parasite hosts suggests that there is not a clear relationship between UVS and VS vision and host rejection behaviour [84]. Increasing the number of species for which the colour cones have been fully characterized is an important next step for investigating colour vision in hosts, as cone properties allow for the calculation of photon capture. Photon capture is the primary input to current models of avian colour perception [85], which have been applied powerfully to questions about egg, chick, fledgling and adult mimicry by brood parasites [51]. Despite this, current perceptual models are simple and fail to capture many of the complexities we believe to be crucial for colour vision. Moving forward, it will be essential to explore (i) the spatial organization of photoreceptor cones [86], (ii) the effects of diet on oil droplet tuning and colour discrimination [87], and (iii) the neural mechanisms of colour opponency, colour categorization and colour memory [85,88].
(d). Computer vision and image processing
In some scenarios, pattern may be a much more important signal than colour. When a host bird examines her eggs, covered with spots and squiggles against a uniform background colour, how does she recognize these eggs as her own, and which features are most salient? Sophisticated computer vision and image processing algorithms are helping to address these questions. Until recently, quantitative studies of complex patterns (with a spatial dimension, i.e. markings, texture) were very rare in the brood parasitism literature—and in animal coloration studies, generally—but this is changing: rigorous pattern analyses are finally becoming mainstream. Several studies have employed ‘granularity’ analysis, which breaks down information in an image into different spatial scales. The analysis has parallels to early-stage spatial filtering in vertebrate visual systems and has yielded insights into egg mimicry [52,63], egg signatures [14], egg diversification [89] and cuckoo mimicry of hawk plumage [56].
Recently, Stoddard et al. [15] applied a new feature detection and pattern recognition tool, NaturePatternMatch, to egg images and showed that several hosts of the common cuckoo have evolved individually recognizable pattern signatures in response to cuckoo mimicry. Based on tools from computer vision, including the Scale-Invariant Feature Transform (SIFT), NaturePatternMatch roughly approximates mid-level biological processes believed to be important for visual recognition in vertebrates [15]. Specifically, SIFT features are identified in a way that mimics neuron response in the primate inferior temporal cortex [90], which plays a key role in object recognition. In addition, the extracted features resemble those to which primates [91] and birds [92] pay attention in object recognition tasks. In general, we still have much to learn about the processes by which animals perceive spatial information. In the future, it will be critical to determine which computational model of pattern vision is appropriate for the biological question at hand and to validate models with behavioural experiments. Before long, we may be able to determine which parts of the host brain are involved in recognition tasks and to link this to perceptual models of pattern processing. Functional brain imaging of crows, showing which regions are activated during human face recognition [93], suggests that this pipe dream may soon become a reality.
4. Future directions
Avian brood parasitism—and its visual dimensions regarding colour diversity, signatures and especially mimicry—has long served as a model study system for coevolutionary interactions [1]. We have learned that the specific paths, rates and potential endpoints of arms races are nearly as varied as the specific parasite–host systems in which we study these trajectories. Recent work has shed light on parasite–host dynamics in understudied brood parasitic lineages [72]. These include the black-headed duck Heteronetta atricapilla, a species without egg colour mimicry, the cuckoo finch and the New World striped cuckoo Tapera naevia, two species with both egg colour mimicry and egg colour polymorphism, and the honeyguides, in which eggs have evolved to match the shape and size of host eggs. The stage is now set for full-scale comparative analyses of vision-based adaptations in hosts and parasites. We identify three avenues ripe for future research.
(a). Genomic and sensory bases of vision-based adaptations
Although the phenotypic patterns of parasite–host coevolution are well characterized in several mimetic systems, we know virtually nothing about the genomic, sensory and neural mechanisms underlying host adaptations. Fortunately, ours is an era of advanced comparative genomic methods. Earlier in §4b we discussed recent progress towards unravelling the genetic underpinnings of egg colour mimicry. Identifying the genes and pathways responsible for egg colour mimicry and polymorphisms—and indeed chick colour mimicry and polymorphisms—represents one of the most crucial goals for future work. Only by illuminating the genetic and genomic bases of adaptation in parasites and hosts will we gain a full picture of how and when such adaptations evolved, how they coevolve with other traits and how they are maintained or lost over time. In the context of adaptive animal coloration, Hubbard et al. [94] outline several essential questions: How many genes influence pigment variation? Are the same genes responsible for convergent phenotypes? How is colour variation influenced by selection? Can we detect signatures of selection in pigmentation genes? These are the questions we must also answer in avian brood parasite–host systems. In fact, because coloration traits are under such intense selection pressure, these systems provide an especially compelling model for investigating the genomic bases of adaptation. One promising approach that can be applied to these questions involves using broad-scale whole-genome alignments to track genic and regulatory innovation across different lineages [95], allowing us to determine how precisely parasites track genomic shifts in hosts. The ease with which we can sequence and assemble whole genomes (with dozens of avian genomes now available, and the number rising) should help make this a reality.
We also live in an era of advanced functional neural imaging. Increasingly sophisticated approaches now allow us to track the neural activation of specific brain regions during critical perceptual and cognitive tasks, including in brood parasitic birds [96]. These techniques also allow us to investigate the neural connectome. We can now explore the connectivities required for adaptive decision-making, such as the detection and rejection of foreign eggs in the nest, and we can identify the neural reward circuits that may be activated during different stages of the breeding season, such as chick provisioning. Understanding neural machinery may help to solve some outstanding mysteries: In response to parasite mimicry, do hosts evolve a more fine-tuned visual system or a more discriminating cognitive process—or both? Are there sensory and cognitive adaptations in brood parasites that help them achieve better-than-expected egg mimicry by carefully selecting host nests [97]?
(b). Phenotypic adaptations in specialist versus generalist parasites
It appears that specialist and generalist parasites use different types of mimicry when attempting to avoid detection in the nests. Specialists typically rely on trickery, attempting to replicate the phenotypic range of the host offspring (egg, chick and fledgling mimicry). By contrast, generalists typically tune into the perceptual world of the hosts, imitating or exaggerating host offspring traits (large eggs and chicks, enhanced begging). These are traits which characterize healthy and vigorous progeny in hosts; such traits are difficult to evolve discrimination against. Consequently, mimicry in generalist parasite–host systems is typically inaccurate. The evolution of rejection rules based on a viability signal can cause mistaken rejection of a healthy own offspring, the cost of which would be paid not only by parasitized but also by non-parasitized individuals in the host population.
(c). Comparison to other sensory modes
Thus far, we have focused exclusively on adaptations in the visual realm of avian brood parasites. But at the nest, all of the senses are engaged, and brood parasites and hosts across diverse lineages have evolved many adaptations evoking the acoustic, tactile and possibly olfactory modalities. We review and contrast these adaptations at each stage of the avian brood parasitic process in table 1. Moving forward, it will be essential to study vision-based adaptations as they relate to other sensory adaptations. Two pressing questions are: (i) Why do the vision and acoustic domains predominate in avian brood parasite–host systems? (ii) Are visual and acoustic adaptations complementary or redundant?
Our analysis (table 1) shows that the visual and acoustic senses are dominant, and adaptations are much more plentiful and elaborate in these domains. However, in some avian systems, tactile and perhaps olfactory adaptations have taken on new importance. For example, yellow warblers Setophaga petechia probably use touch to determine if they have been parasitized, burying or deserting parasitized clutches if they detect a too-large egg [98]. In addition, Eurasian magpie Pica pica hosts of great spotted cuckoos can detect odours of experimental eggs, but whether they actually use odour when rejecting natural parasitic eggs has yet to be determined [41]. Future experiments should attempt to titrate the relative importance of visual, acoustic, olfactory and tactile cues used by hosts for rejection within the same context and host–parasite species pairs. Our aim should then be to understand whether and how signals are integrated into multicomponent (multiple signals, same sensory domain) and multimodal displays (multiple signals, different sensory domains, e.g. chick begging) [99]. For instance, how do multicomponent (colour versus patterning of eggs) and multimodal (gape colour versus vocalization of chicks) signals combine to influence a host's ability to detect parasitic intruders?
Second, our analysis (table 1) suggests that parasites benefit from having both fixed and flexible elements in their signalling repertoires, with fixed signals perhaps more important for specialists and flexible signals more important for generalists. Many visual signals are fixed, such that the parasite cannot actively modify them during the breeding season (such as hawk-like plumage or mimetic eggs). Acoustic signals, by contrast, tend to be flexible: consider the Horsfield's bronze-cuckoo chick, which—despite having ejected its nest-mates—rapidly shifts to mimic the correct begging calls of its actual host species, whether it begins life in the nest of a fairy-wren or a thornbill host [37]. Thus, fixed visual versus flexible acoustic signals may facilitate the escalation of different types of arms races in different avian brood parasite–host systems. As a start, we should explore these ideas by analysing the extent to which fixed and flexible signals co-occur, at different stages of the parasitic process, in specialist and generalist parasitic taxa.
Ultimately, our ability to make sense of the staggering diversity of sensory-driven, phenotypic adaptations across parasite–host lineages hinges on our knowledge of natural history. As we embrace modern tools for the study of animal coloration, we must also continue the long tradition of field-based behavioural experimentation, in which we can dissect and quantify the decision thresholds and fitness consequences of interspecific interactions at each stage of the arms race [100]. The ease with which we can perform these experiments remains one of the great strengths of avian brood parasite–host systems [101].
Acknowledgements
We are grateful to the Berlin Institute for Advanced Study (Wissenschaftskolleg zu Berlin) for its support of animal coloration research and to our colleagues for generously sharing images. We appreciate comments from two anonymous reviewers.
Competing interests
We declare we have no competing interests.
Funding
Funding for M.C.S. was provided by Princeton University. Funding for M.E.H. was provided by the US National Science Foundation's grant #IOS-145624.
References
- 1.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & AD Poyser. [Google Scholar]
- 2.Payne RB, Sorensen MD. 2005. The cuckoos. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Stauffer JR Jr, Loftus WF. 2010. Brood parasitism of a bagrid catfish (Bagrus meridionalis) by a clariid catfish (Bathyclarias nyasensis) in Lake Malaŵi, Africa. Copeia 2010, 71–74. ( 10.1643/CE-09-087) [DOI] [Google Scholar]
- 4.Brown JL, Morales V, Summers K. 2009. Tactical reproductive parasitism via larval cannibalism in Peruvian poison frogs. Biol. Lett. 5, 148–151. ( 10.1098/rsbl.2008.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spottiswoode CN, Kilner RM, Davies NB. 2012. Brood parasitism. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kolliker M), pp. 226–356. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 7.Welbergen JA, Davies NB. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. ( 10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 8.Davies NB, Welbergen JA. 2008. Cuckoo–hawk mimicry? An experimental test. Proc. R. Soc. London Ser. B. 275, 1817–1822. ( 10.1098/rspb.2008.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welbergen JA, Davies NB. 2011. A parasite in wolf's clothing: hawk mimicry reduces mobbing of cuckoos by hosts. Behav. Ecol. 22, 574–579. ( 10.1093/beheco/arr008) [DOI] [Google Scholar]
- 10.Thorogood R, Davies NB. 2012. Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337, 578–580. ( 10.1126/science.1220759) [DOI] [PubMed] [Google Scholar]
- 11.Thorogood R, Davies NB. 2013. Hawk mimicry and the evolution of polymorphic cuckoos. Chinese Birds ( 10.5122/cbirds.2013.0002) [DOI] [Google Scholar]
- 12.Smith J, Arcese P. 1994. Brown-Headed Cowbirds and an Island Population of Song Sparrows - a 16-Year Study. Condor. 96, 916–934. [Google Scholar]
- 13.Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. ( 10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 14.Caves EM, Stevens M, Iversen ES, Spottiswoode CN. 2015. Hosts of avian brood parasites have evolved egg signatures with elevated information content. Proc. R. Soc. B 282, 20150598 ( 10.1098/rspb.2015.0598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoddard MC, Kilner RM, Town C. 2014. Pattern recognition algorithm reveals how birds evolve individual egg pattern signatures. Nat. Commun. 5, 4117 ( 10.1038/ncomms5117) [DOI] [PubMed] [Google Scholar]
- 16.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468. ( 10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 17.Alvarez F. 1999. Attractive non-mimetic stimuli in cuckoo Cuculus canorus eggs. Ibis 141, 142–144. ( 10.1111/j.1474-919X.1999.tb04274.x) [DOI] [Google Scholar]
- 18.Honza M, Sulc M, Jelinek V, Pozgayova M, Procházka P. 2013. Brood parasites lay eggs matching the appearance of host clutches. Proc. R. Soc. London Ser. B. 281, 20132665–20132665 ( 10.1093/beheco/art004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baerends GP, Drent RH. 1982. The Herring Gull and Its Egg Part II. The Responsiveness to Egg-Features. Behaviour. 82, 1–416. [Google Scholar]
- 20.Cunningham EJ, Russell AF. 2000. Egg investment is influenced by male attractiveness in the mallard. Nature. 404, 74–77. ( 10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- 21.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 422, 157–160. ( 10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 22.Tokue K, Ueda K. 2010. Mangrove Gerygones Gerygone laevigaster eject Little Bronze‐cuckoo Chalcites minutillus hatchlings from parasitized nests. Ibis. 152, 835– 839. [Google Scholar]
- 23.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1038/nature01460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato NJ, Tanaka KD, Okahisa Y, Yamamichi M, Kuehn R, Gula R, Ueda K, Theuerkauf J. 2015. Nestling polymorphism in a cuckoo-host system. Curr. Biol. 25, R1164–R1165. ( 10.1016/j.cub.2015.11.028) [DOI] [PubMed] [Google Scholar]
- 25.Langmore NE, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. ( 10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne RB. 2005. Nestling mouth markings and colors of Old World finches Estrildidae: mimicry and coevolution of nesting finches and their Vidua brood parasites. Miscellaneous Publications of the Museum of Zoology, University of Michigan 194, 1–44. [Google Scholar]
- 27.Tanaka KD. 2005. Horsfield's hawk-cuckoo nestlings simulate multiple gapes for begging. Science 308, 653–653 ( 10.1126/science.1109957) [DOI] [PubMed] [Google Scholar]
- 28.Tanaka KD, Morimoto G, Stevens M, Ueda K. 2011. Rethinking visual supernormal stimuli in cuckoos: visual modeling of host and parasite signals. Behav. Ecol. 22, 1012–1019. ( 10.1093/beheco/arr084) [DOI] [Google Scholar]
- 29.Noble DG, Davies NB, Hartley IR. 1999. The red gape of the nestling cuckoo (Cuculus canorus) is not a supernormal stimulus for three common hosts. Behaviour. 136, 759–777. [Google Scholar]
- 30.Bolopo D, Canestrari D, Roldán M, Baglione V, Soler M. 2015. High begging intensity of great spotted cuckoo nestlings favours larger-size crow nest mates. Behav. Ecol. Sociobiol. 69, 873–882. [Google Scholar]
- 31.Hauber ME, Kilner RM. 2006. Coevolution, communication, and host chick mimicry in parasitic finches: who mimics whom? Behav. Ecol. Sociobiol. 61, 497–503. ( 10.1007/s00265-006-0291-0) [DOI] [Google Scholar]
- 32.Gill SA, Sealy SG. 2004. Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 56, 71–80. ( 10.1007/s00265-003-0736-7) [DOI] [Google Scholar]
- 33.Langmore NE, Feeney WE, Crowe-Riddell J, Luan H, Louwrens KM, Cockburn A. 2012. Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behav. Ecol. 23, 798–805. ( 10.1093/beheco/ars033) [DOI] [Google Scholar]
- 34.Parejo D, Avilés JM. 2006. Do avian brood parasites eavesdrop on heterospecific sexual signals revealing host quality? A review of the evidence. Anim. Cogn. 10, 81–88. ( 10.1007/s10071-006-0055-2) [DOI] [PubMed] [Google Scholar]
- 35.Colombelli-Négrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Kleindorfer S. 2012. Embryonic Learning of Vocal Passwords in Superb Fairy-Wrens Reveals Intruder Cuckoo Nestlings. Curr. Biol. 22, 2155–2160. ( 10.1016/j.cub.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 36.Anderson MG, Ross HA, Brunton DH, Hauber ME. 2009. Begging call matching between a specialist brood parasite and its host: a comparative approach to detect coevolution. Biol. J. Linn. Soc. 98, 208–216. [Google Scholar]
- 37.Langmore NE, Maurer G, Adcock GJ, Kilner RM. 2012. Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 279, 1689–1699. ( 10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]
- 38.Kilner RM, Noble DG, Davies NB. 1999. Signals of need in parent-offspring communication and their exploitation by the common cuckoo. Nature. 397, 667–672. [Google Scholar]
- 39.Dearborn DC. 1999. Brown-headed Cowbird nestling vocalizations and risk of nest predation. Auk. 116, 448–457. [Google Scholar]
- 40.Hauber ME, Russo SA, Sherman PW. 2001. A password for species recognition in a brood-parasitic bird. Proc. R. Soc. London Ser. B. 268, 1041–1048. ( 10.1098/rspb.2001.1617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soler JJ, Perez-Contreras T, De Neve L, Macías-Sánchez E, Møller AP, Soler M. 2014. Recognizing odd smells and ejection of brood parasitic eggs. An experimental test in magpies of a novel defensive trait against brood parasitism. J. Evol. Biol. 27, 1265–1270. ( 10.1111/jeb.12377) [DOI] [PubMed] [Google Scholar]
- 42.Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2013. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim. Behav. 86, 1023–1029. ( 10.1016/j.anbehav.2013.09.007) [DOI] [Google Scholar]
- 43.Guigueno MF, Sealy SG, Westphal AM. 2014. Rejection of parasitic eggs in passerine hosts: Size matters more for a non-ejecter. Auk. 131, 583–594. ( 10.1642/AUK-14-36.1) [DOI] [Google Scholar]
- 44.Langmore NE. 2005. The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav. Ecol. 16, 686–692. ( 10.1093/beheco/ari041) [DOI] [Google Scholar]
- 45.Davies N, Brooke ML. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. [Google Scholar]
- 46.Spottiswoode CN. 2010. The evolution of host-specific variation in cuckoo eggshell strength. J. Evol. Biol. 23, 1792–1799. ( 10.1111/j.1420-9101.2010.02010.x) [DOI] [PubMed] [Google Scholar]
- 47.Grim T. 2007. Experimental evidence for chick discrimination without recognition in a brood parasite host. Proc. R. Soc. London Ser. B. 274, 373–381. ( 10.1098/rspb.2006.3731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuetz JG. 2005. Reduced growth but not survival of chicks with altered gape patterns: implications for the evolution of nestling similarity in a parasitic finch. Anim. Behav. 70, 839–848. ( 10.1016/j.anbehav.2005.01.007) [DOI] [Google Scholar]
- 49.De Mársico MC, Gantchoff MG, Reboreda JC. 2012. Host-parasite coevolution beyond the nestling stage? Mimicry of host fledglings by the specialist screaming cowbird. Proc. R. Soc. B 1742, 3401–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feeney WE, Troscianko J, Langmore NE, Spottiswoode CN. 2015. Evidence for aggressive mimicry in an adult brood parasitic bird, and generalized defences in its host. Proc. R. Soc. B 282, 20150795 ( 10.1098/rspb.2015.0795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langmore NE, Spottiswoode CN. 2012. Visual trickery in avian brood parasites. In Host manipulation by parasites (eds Hughes DP, Brodeur J, Thomas F), pp. 95–118. Oxford, UK: Oxford University Press.
- 52.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393. ( 10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573. ( 10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite-host coevolution. Anim. Behav. 84, 3–12. ( 10.1016/j.anbehav.2012.04.011) [DOI] [Google Scholar]
- 55.Welbergen JA, Davies NB. 2008. Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim. Behav. 76, 811–822. ( 10.1016/j.anbehav.2008.03.020) [DOI] [Google Scholar]
- 56.Gluckman T-LL, Mundy NI. 2013. Cuckoos in raptors’ clothing: barred plumage illuminates a fundamental principle of Batesian mimicry. Anim. Behav. 8, 1165–1181. ( 10.1016/j.anbehav.2013.09.020) [DOI] [Google Scholar]
- 57.Trnka A, Prokop P. 2012. The effectiveness of hawk mimicry in protecting cuckoos from aggressive hosts. Anim. Behav. 83, 263–268. ( 10.1016/j.anbehav.2011.10.036) [DOI] [Google Scholar]
- 58.Stoddard MC. 2012. Mimicry and masquerade from the avian visual perspective. Curr. Zool. 58, 630–648. ( 10.1093/czoolo/58.4.630) [DOI] [Google Scholar]
- 59.Honza M, Šicha V, Procházka P, Ležalová R. 2006. Host nest defense against a color-dimorphic brood parasite: great reed warblers (Acrocephalus arundinaceus) versus common cuckoos (Cuculus canorus). J. Ornithol 147, 629–637. ( 10.1007/s10336-006-0088-y) [DOI] [Google Scholar]
- 60.Garamszegi LSZ, Avil JSM. 2005. Brood parasitism by brown-headed cowbirds and the expression of sexual characters in their hosts. Oecologia 143, 167–177. ( 10.1007/s00442-004-1784-z) [DOI] [PubMed] [Google Scholar]
- 61.Muñoz AR, Altamirano M, Takasu F, Nakamura H. 2007. Nest light environment and the potential risk of common cuckoo (Cuculus canorus) parasitism. Auk 91, 796. [Google Scholar]
- 62.Stoddard MC, Stevens M. 2011. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution 65, 2004–2013. ( 10.1111/j.1558-5646.2011.01262.x) [DOI] [PubMed] [Google Scholar]
- 63.Spottiswoode CN, Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676. ( 10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spottiswoode CN, Stevens M. 2012. Host-parasite arms races and rapid changes in bird egg appearance. Am. Nat. 179, 633–648. ( 10.1086/665031) [DOI] [PubMed] [Google Scholar]
- 65.Moskát C, Zölei A, Bán M, Elek Z, Tong L, Geltsch N, Hauber ME. 2014. How to spot a stranger's egg? A mimicry-specific discordancy effect in the recognition of parasitic eggs. Ethology 120, 616–626. ( 10.1111/eth.12234) [DOI] [Google Scholar]
- 66.Ban M, Moskat C, Barta Z, Hauber ME. 2013. Simultaneous viewing of own and parasitic eggs is not required for egg rejection by a cuckoo host. Behav. Ecol. 24, 1014–1021. ( 10.1093/beheco/art004) [DOI] [Google Scholar]
- 67.Feeney WE, Stoddard MC, Kilner RM, Langmore NE. 2014. ‘Jack-of-all-trades’ egg mimicry in the brood parasitic Horsfield's bronze-cuckoo? Behav. Ecol. 25, 1365–1373. ( 10.1093/beheco/aru133) [DOI] [Google Scholar]
- 68.Klippenstine DR, Sealy SG. 2010. Assessing generalized egg mimicry: a quantitative comparison of eggs of brown-headed cowbirds and grassland passerines. Wilson J. Ornithol. 122, 346–353. ( 10.1676/08-143.1) [DOI] [Google Scholar]
- 69.Soler JJ, Aviles JM. 2012. Attractive blue-green egg coloration and cuckoo–host coevolution. Biol. J. Linn. Soc. 106, 154–168. ( 10.1111/j.1095-8312.2012.01857.x) [DOI] [Google Scholar]
- 70.Riehl C. 2011. Paternal investment and the ‘sexually selected hypothesis’ for the evolution of eggshell coloration: revisiting the assumptions. Auk 128, 175–179. ( 10.1525/auk.2011.10171) [DOI] [Google Scholar]
- 71.Stoddard MC, Fayet AL, Kilner RM, Hinde CA. 2012. Egg speckling patterns do not advertise offspring quality or influence male provisioning in great tits. PLoS ONE 7, e40211 ( 10.1371/journal.pone.0040211.t005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feeney WE, Welbergen JA, Langmore NE. 2014. Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246. ( 10.1146/annurev-ecolsys-120213-091603) [DOI] [Google Scholar]
- 73.Igic B, Cassey P, Grim T, Greenwood DR, Moskat C, Rutila J, Hauber ME. 2012. A shared chemical basis of avian host-parasite egg colour mimicry. Proc. R. Soc. B 279, 1068–1076. ( 10.1098/rspb.2011.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanley D, Grim T, Cassey P, Hauber ME. 2015. Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol. Lett. 11, 20150087 ( 10.1098/rsbl.2015.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052. ( 10.1093/beheco/arr088) [DOI] [Google Scholar]
- 76.Igic B, et al. 2015. A nanostructural basis for gloss of avian eggshells. J. R. Soc. Interface 12, 20141210 ( 10.1098/rsif.2014.1210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fecheyr-Lippens DC, et al. 2015. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol. Open 4, 753–759. ( 10.1242/bio.012211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z, et al. 2013. An EAV-HP insertion in 5′ flanking region of SLCO1B3 causes blue eggshell in the chicken. PLoS Genet. 9, e1003183 ( 10.1371/journal.pgen.1003183.s009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wragg D, Mwacharo JM, Alcalde JA, Wang C, Han J-L, Gongora J, Gourichon D, Tixier-Boichard M, Hanotte O. 2013. Endogenous retrovirus EAV-HP linked to blue egg phenotype in mapuche fowl. PLoS ONE 8, e71393 ( 10.1371/journal.pone.0071393.s011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng C, Li Z, Yang N, Ning Z. 2014. Quantitative expression of candidate genes affecting eggshell color. Anim. Sci. J. 85, 506–510. ( 10.1111/asj.12182) [DOI] [PubMed] [Google Scholar]
- 81.Ito S, Tsudzuki M, Komori M, Mizutani M. 1993. Celadon: an eggshell color mutation in Japanese quail. J. Hered. 84, 145–147. ( 10.1093/oxfordjournals.jhered.a111301) [DOI] [PubMed] [Google Scholar]
- 82.Gibbs HL, Sorenson MD, Marchetti K, Brooke ML, Davies NB, Nakamura H. 2000. Genetic evidence for female host-specific races of the common cuckoo. Nature 407, 183–186. ( 10.1038/35025058) [DOI] [PubMed] [Google Scholar]
- 83.Fossøy F, et al. 2016. Ancient origin and maternal inheritance of blue cuckoo eggs. Nat. Commun. 7, 10272 ( 10.1038/ncomms10272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aidala Z, et al. 2012. Ultraviolet visual sensitivity in three avian lineages: paleognaths, parrots, and passerines. J .Comp. Physiol. A 198, 495–510. ( 10.1007/s00359-012-0724-3) [DOI] [PubMed] [Google Scholar]
- 85.Kemp DJ, Herberstein ME, Fleishman LJ, Endler JA, Bennett ATD, Dyer AG, Hart NS, Marshall J, Whiting MJ. 2015. An integrative framework for the appraisal of coloration in nature. Am. Nat. 185, 705–724. ( 10.1086/681021) [DOI] [PubMed] [Google Scholar]
- 86.Kram YA, Mantey S, Corbo JC. 2010. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 5, e8992 ( 10.1371/journal.pone.0008992.s004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim HH, Pike TW. 2016. Dietary carotenoid availability affects avian color discrimination. Behav. Ecol. arw116. ( 10.1093/beheco/arw116) [DOI] [Google Scholar]
- 88.Jones CD, Osorio D, Baddeley RJ. 2001. Colour categorization by domestic chicks. Proc. R. Soc. Lond. B 268, 2077–2084. ( 10.1098/rspb.2001.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medina I, Troscianko J, Stevens M, Langmore NE. 2016. Brood parasitism is linked to egg pattern diversity within and among species of Australian passerines. Am. Nat. 187, 351–362. ( 10.1086/684627) [DOI] [PubMed] [Google Scholar]
- 90.Lowe D. 2000. Towards a computational model for object recognition in IT cortex. In International Workshop on Biologically Motivated Computer Vision, pp. 20–31. Berlin, Germany: Springer. [Google Scholar]
- 91.Zhang W, Yang H, Samaras D, Zelinsky G. 2006. A computational model of eye movements during object class detection. Adv. Neural Inform. Process. Syst. 18, 1609. [Google Scholar]
- 92.Soto FA, Siow JYM, Wasserman EA. 2012. View-invariance learning in object recognition by pigeons depends on error-driven associative learning processes. Vis. Res. 62, 148–161. ( 10.1016/j.visres.2012.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marzluff JM, Miyaoka R, Minoshima S, Cross DJ. 2012. Brain imaging reveals neuronal circuitry underlying the crow's perception of human faces. Proc. Natl Acad. Sci. USA 109, 15 912–15 917. ( 10.1073/pnas.1206109109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hubbard JK, Uy JAC, Hauber ME, Hoekstra HE, Safran RJ. 2010. Vertebrate pigmentation: from underlying genes to adaptive function. Trends in Genetics 26, 231–239. ( 10.1016/j.tig.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 95.Lowe CB, Clarke JA, Baker AJ, Haussler D, Edwards SV. 2015. Feather development genes and associated regulatory innovation predate the origin of Dinosauria. Mol. Biol. Evol. 32, 23–28. ( 10.1093/molbev/msu309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Louder MIM, Voss HU, Manna TJ, Carryl SS, London SE, Balakrishnan CN, Hauber ME. 2016. Shared neural substrates for song discrimination in parental and parasitic songbirds. Neurosci. Lett. 622, 49–54. ( 10.1016/j.neulet.2016.04.031) [DOI] [PubMed] [Google Scholar]
- 97.Cherry MI, Bennett ATD, Moskat C. 2007. Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J. Evol. Biol. 20, 1218–1222. ( 10.1111/j.1420-9101.2007.01308.x) [DOI] [PubMed] [Google Scholar]
- 98.Guigueno MF, Sealy SG. 2012. Increased investigation of manipulated clutches suggests egg recognition without rejection in a brown-headed cowbird (Molothrus ater) host, the yellow warbler (Setophaga petechia)—El incremento en la investigación de nidadas manipuladas sugiere reconocimiento de los huevos sin rechazo de éstos en Setophaga petechia, un hospedero de Molothrus ater. Auk 129, 17–25. ( 10.1525/auk.2011.11138) [DOI] [Google Scholar]
- 99.Hebets EA, Barron AB, Balakrishnan CN, Hauber ME, Mason PH, Hoke KL. 2016. A systems approach to animal communication. Proc. R. Soc. B 283, 20152889 ( 10.1098/rspb.2015.2889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stoddard MC, Kilner RM. 2013. The past, present and future of ‘cuckoos versus reed warblers’. Anim. Behav. 85, 693–699. ( 10.1016/j.anbehav.2013.01.005) [DOI] [Google Scholar]
- 101.Igic B, et al. 2015. Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. PeerJ 3, e965 ( 10.7717/peerj.965/supp-3) [DOI] [PMC free article] [PubMed] [Google Scholar]