Abstract
Background
Despite the widespread use of retrospectively-reported time to pregnancy to evaluate fertility either as an outcome or as a risk factor for chronic disease, only two small studies have directly compared prospective data with later recall.
Methods
The North Carolina Early Pregnancy Study (1982-1986) collected prospective time-to-pregnancy data from the beginning of participants' pregnancy attempt. In 2010 (24-28 years later) women were sent a questionnaire including lifetime reproductive history that asked about all prior times to pregnancy. Of the 202 women with prospective time-to-pregnancy data, 76% provided recalled time to pregnancy.
Results
A lower proportion of women with times to pregnancy (≥3 cycles) provided a recalled time to pregnancy than women with times to pregnancy <3 cycles. Also, high gravidity or parity were associated with a lower likelihood of providing a recalled time to pregnancy. Women with very short or very long times to pregnancy (1 cycle or ≥13 cycles) had good recall of time to pregnancy. Positive predictive values, PPVs, of 1 or ≥13 cycles were 73% and 68%, respectively, while PPVs for other categories of time to pregnancy ranged from 38% to 58%. The weighted kappa statistic for recalled versus prospective time to pregnancy was 0.72 (95% confidence interval: 0.65, 0.79).
Conclusions
Recalled time to pregnancy showed good agreement with prospective time to pregnancy. Informative missingness must be considered when imputing recalled time to pregnancy. Associations observed in future studies can be corrected for misclassification.
Time to pregnancy is defined as the number of months or menstrual cycles from the initiation of unprotected intercourse to conception of a pregnancy.1 The inverse of time to pregnancy is a useful estimate of fecundability, defined as the couple's probability of conceiving in a given menstrual cycle. Clinical pregnancy represents the successful completion of a series of biological events including ovulation, fertilization, and implantation, making time to pregnancy a useful outcome in epidemiologic studies. Long time to pregnancy, and therefore reduced fecundability, has been associated with numerous exposures including smoking,2 overweight and obesity,3 ionizing radiation,4 and pesticide exposure.5 Longer times to pregnancy also predict certain pregnancy complications6 and may be a harbinger of a woman's later risk of cardiovascular disease7-11 or metabolic syndrome.12,13
Time to pregnancy can be assessed both retrospectively and prospectively. In a retrospective study, women are interviewed after conception and asked how long it took them to become pregnant. Women may be interviewed during the pregnancy or afterwards – sometimes years later. Population-based retrospective time-to-pregnancy studies can be designed to include questions about pregnancy attempts that did not end in pregnancy either because a pregnancy was never achieved or the attempt ended for other reasons (e.g. divorce, loss of insurance). Such attempt times are included as censored times to pregnancy. These designs all rely on accurate time-to-pregnancy recall. Prospective studies enroll women who plan to conceive and follow them during their pregnancy attempt, which takes time and resources. Even prospective studies can be influenced by the participant's recall if, as is sometimes done3,14, participants are enrolled after beginning their attempt to conceive (a “semi-prospective” design). In this scenario, accurate assessment of the previous attempt time reported at enrollment is essential to account for left-truncation in the time-to-event analysis.
An advantage of a retrospective study is that it allows rapid enrollment of a large sample. Retrospective studies are also able to target special exposure groups, such as those exposed to a toxicant of interest. Studies of links between fecundability and risk of later disease also require women to recall time to pregnancy, typically including pregnancies occurring many years earlier.
Only two small studies have directly compared recalled time to pregnancy with prospectively collected time to pregnancy from the same attempt. In one, 100 Dutch women who conceived during a semi-prospective study of time to pregnancy were interviewed one to two years after their participation.15 In the second study,16 43 women from the prospective New York State Angler Cohort Study recalled their time to pregnancy 1 to 10 years later. Both studies collected recalled time to pregnancy only from women who had conceived during the active phase of the study, excluding less fertile women who may have subsequently conceived (i.e. those with the longest times to pregnancy).
We examined the validity of retrospectively reported time to pregnancy among women who had participated 24-28 years earlier in a prospective study of pregnancy, the North Carolina Early Pregnancy Study.
Methods
Study sample
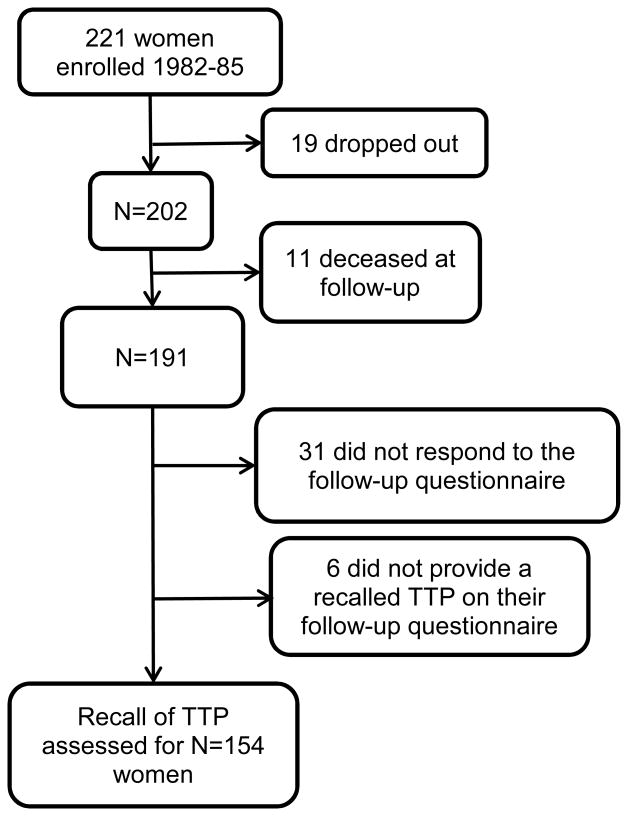
The North Carolina Early Pregnancy Study (hereafter “the study”) (1982-86) recruited women living in or near Raleigh, Durham, and Chapel Hill, North Carolina, who were planning to become pregnant, and had no history of infertility.17 Women were enrolled when they discontinued contraception, at which time they began collecting daily urine samples and completing daily diaries. Active participation continued until women became pregnant, or for six months if they did not conceive. An in-person interview was completed at enrollment18. The interview solicited information on height, weight, reproductive history, smoking, alcohol use, caffeine intake, and vitamin use. Daily diaries included information on menstrual bleeding and sexual intercourse. This period of intensive participation lasted six months or less and is referred to as “Phase 1” of the study. All women who conceived during Phase 1 were followed to determine their pregnancy outcome. Women who did not conceive a recognized pregnancy during Phase 1 entered Phase 2, which included a structured telephone follow-up interview 12 months after enrollment to determine pregnancy status and length of pregnancy attempt in Phase 2. For women who had not conceived in 12 months, a second telephone follow-up occurred at about 24 months. Thus, Phase 2 captured conceptions that occurred between 6 and 24 months after study enrollment. Of the 221 women enrolled in the study, 19 dropped out prior to completing Phase 1 (Figure 1) and were not included in Phase 2.
Figure 1.
Description of participant flow from enrollment in the Early Pregnancy Study (1982-85, “Phase 1”) through the subsequent follow-up interview (2010, “Phase 3”).
In 2010, 24 to 28 years after enrollment, Phase 3 was undertaken to collect information regarding women's reproductive history, as well as certain exposures and behaviors that had not been assessed during Phase 1. A self-administered mailed questionnaire was sent to all 221 participants (Phase 3)18. Women were not provided their Phase 1 participation dates or length of participation in order not to provide clues about their time to pregnancy. Similarly, women who conceived in the study were not reminded of their pregnancy outcome or, for women with more than one pregnancy in their lifetime, which of their pregnancies was the study pregnancy. A description of the study is available online19, and we cannot discount the possibility that some women could have used the Internet to aid their recollection of study dates. Women who did not respond to the mailed questionnaire received follow-up phone calls and were given the opportunity to complete the questionnaire over the phone.
We excluded the 19 women who had dropped out of Phase 1. Of the remaining 202, 11 (5%) were deceased and 31 (15%) did not respond to the Phase 3 questionnaire, leaving 160 women. Six women responded to the Phase 3 questionnaire without estimating their time to pregnancy for the study pregnancy attempt, leaving 154 women with a recalled time to pregnancy.
Prospective time to pregnancy
Using diary records, a menstrual cycle was defined as the time from the first day of menstrual bleeding up to, but not including, the first day of the subsequent menses. The total number of menstrual cycles to pregnancy included the conception cycle. While we had detailed urinary hormone data, we did not use these data to exclude anovulatory cycles (N=7) or cycles in which there was no unprotected intercourse during the 6-day fertile window20 (N=27), or to divide two contiguous cycles that were not separated by a menses (N=3 women). Once again, this was done to mimic the circumstances of a study based on self-report, in which women are unlikely to be aware of these occurrences.
A complete prospective time to pregnancy was observed for the women who conceived during Phase 1 (N=155). Women who did not conceive had six months of detailed prospective time-to-pregnancy information from Phase 1, followed by self-reported conception information collected in Phase 2. For each woman who conceived during Phase 2 (N=31), information from these two phases was combined according to this equation:
where the quotient is rounded to the nearest integer, “CyclesPhase1” is the total number of menstrual cycles observed during Phase 1, LMPPhase2 is the date of the last menstrual period before conception, cycle end datePhase1 is the end date of her last menstrual cycle during Phase 1, and median cycle lengthPhase1 is the woman-specific median menstrual cycle length calculated from all cycles contributed to Phase 1. One cycle was then added for the conception cycle itself.
Data for women who did not conceive during Phase 2 (N=10) ended at their date of last contact. Women who reported during Phase 2 that they no longer wished to become pregnant (N=4) were censored on the date they reported that they stopped trying, Two women who reported miscarriages during Phase 2, but not the exact dates, were censored at the contact date prior to the report of the miscarriage. Similar to the above equation, prospective time to pregnancy was quantified as:
The quotient was again rounded to the nearest integer. We categorized prospective time to pregnancy into the same categories as described for recalled time to pregnancy. Detailed times to pregnancy s for these women are presented in the Supplement (see Table, Supplemental Digital Content 1).
Recalled time to pregnancy
The Phase 3 questionnaire asked times to pregnancy s for all of each woman's pregnancies. We identified the study pregnancy based on the study dates and follow-up information; we also asked women to identify their study pregnancy. There were 105 respondents who conceived a pregnancy during Phase 1 that ended in a live birth. Ten of these women (10%) identified a non-study pregnancy as their study pregnancy. Fourteen respondents conceived a pregnancy during Phase 1 that ended in a recognized pregnancy loss. Six of these women (43%) incorrectly identified the study pregnancy. For all analyses we used the recalled time to pregnancy for the study pregnancy, regardless of whether the participant identified the study pregnancy correctly. This was done to mimic a retrospective study in which participants would be guided to the pregnancies of interest, such as first, last, or during specific exposure windows.
For each reported pregnancy, participants were asked whether they had conceived while using birth control, and if not, whether they had conceived in the first menstrual cycle after discontinuing contraception. If they said, “no,” they were asked if they had conceived in the second cycle. If they said, “no”, they were asked if it was the third cycle. If they again said, “no,” they were asked to report the number of months it took them to conceive, and approximately how many menstrual cycles this time represented.21 If number of menstrual cycles was reported, we used that estimate. For women who did not report cycles, we relied on the months estimate, without correcting for cycle length. Some women could not identify an exact number of months. The questionnaire provided a range of options: 1-3 (1 woman), 4-6 (4 women), 7-12, 13-24 (1 woman), >24 months (2 women). Eight women provided their own range of months or cycles (specifically, “1-2 cycles” (3 women), “2-3 cycles” (3 women), “3-8 months” (1 woman), and “6-7 months” (1 woman)).
Women who reported a time to pregnancy of 13 cycles or more were collapsed into a category of “≥13 cycles.” This is consistent with the censoring approach of most time-to-pregnancy studies. Because a year of trying is the clinical definition of “infertility,”1 the censoring at this time is done so that medical interventions do not influence the likelihood of conceiving.22
Couples vary in their fecundability. If a couple has mean fecundability p, then for a given couple the number of cycles needed to achieve pregnancy follows a geometric distribution. The probability that their time to pregnancy is k is p(1-p)(k-1). It follows from maximizing this function for a given k that the maximum likelihood estimate of that couple's fecundability, given that time to pregnancy is k, is 1/k. We analyzed time to pregnancy in categories that were created to be nearly equidistant on that fecundability scale (1/time to pregnancy), which provides comparisons that are directly applicable to a time-to-pregnancy analysis. The time-to-pregnancy categories were, 1 cycle, 2, 3 – 4, 5 – 7, 8 – 12, and ≥13. Each of the women who reported a range as their time to pregnancy were assigned to a category as follows: “1-3” or “1-2”: 2 cycles, “2-3”: 3 cycles, “3-8,” “4-6,” or “6-7”: 5-7 cycles. For simplicity, we refer to the units of recalled time to pregnancy as “cycles” even if reported in months.
Some of the study participants never conceived a recognized pregnancy. To address this, the Phase 3 questionnaire included questions that were independent of the reproductive history section. The questionnaire reminded women that they were trying to become pregnant when they entered the study and then asked how long they attempted to conceive. Three women provided their time to pregnancy in this section, and all of them had a prospective time to pregnancy of ≥ 13. The phrase “Time to pregnancy” is used to represent these attempt times, even when they did not result in a recognized pregnancy.
Statistical analysis
We examined the association of participant characteristics with the availability of a recalled time to pregnancy as a dichotomous outcome, using frequency tables and chi-square tests. Characteristics included those measured during Phase 1: age, education, body mass index, gravidity, parity, occupation (recorded as text and collapsed into six categories based on the 1980 Standard Occupational Classification codes), smoking, alcohol intake (in three categories: <25th%ile, 25th-75th%ile, >75th%ile), caffeine intake (in three categories: <25th%ile, 25th-75th%ile, >75th%ile) and the prospectively observed time to pregnancy. We included characteristics that were important in the univariable analysis (p≤0.2) in a multivariable model to assess their adjusted associations with time to pregnancy availability and to obtain p-values.
We used a scatter plot to visually display the agreement between prospectively recorded time to pregnancy and the woman's recalled time to pregnancy. We created a frequency table comparing recalled and prospective time to pregnancy by dividing each into the previously described categories. From this table we calculated a weighted kappa coefficient and positive predictive values (PPV). One woman whose prospective time to pregnancy was >6 cycles was excluded from these two analyses (see Table, Supplemental Digital Content 1). One additional woman whose recalled time to pregnancy was “>3 cycles” was assigned the value of “3.5” for the scatter plot and categorized as “3 – 4” for the calculation of the kappa coefficient and PPV. Excluding this woman did not alter either the kappa coefficient or the PPV.
In a secondary analysis we examined the association between accuracy of time to pregnancy recall and maternal characteristics. Self-reported time to pregnancy was considered “accurate” if it was within 25% of the prospective time to pregnancy based on the fecundability scale (Supplemental Digital Content 2, 3, and 4).
Results
Availability of recalled time to pregnancy
Women who provided a recalled time to pregnancy were more likely (p<0.2) to have short prospective times to pregnancy (1 or 2 cycles) compared to women without recalled time to pregnancy data. They were more likely to be less educated, to report their occupation as “management,” to be of higher gravidity or parity, to have been a smoker at Phase 1 entrance, and a high caffeine consumer at Phase 1 entrance (Table 1). When entered into the multivariable model simultaneously, occupation, gravidity, parity and smoking were still associated with providing a time to pregnancy; prospective time to pregnancy was of borderline importance. .
Table 1.
Characteristics of women who have a recalled TTP available for analysis compared with those without a recalled TTP (Total N=202)a. All characteristics were measured at enrollment into EPS Phase 1 or during Phases 1 and 2.
| Recalled TTP available N=154 (76%) | Recalled TTP not availableb N=48 (24%) | P-value from the multivariable modelc | |
|---|---|---|---|
| EPS Phase 1 outcome | |||
| Did not conceive within 6 months | 35 (74) | 12 (26) | |
| Recognized miscarriaged | 14 (74) | 5 (26) | |
| Live birthd | 105 (77) | 31 (23) | |
| Prospective TTPe | |||
| 1 | 44 (83) | 9 (17) | 0.08 |
| 2 | 33 (87) | 5 (13) | |
| 3 – 4 | 33 (70) | 14 (30) | |
| 5 – 7 | 17 (63) | 10 (37) | |
| 8 – 12 | 13 (76) | 4 (24) | |
| ≥13 | 14 (70) | 6 (30) | |
| Age | |||
| < 29 | 76 (79) | 20 (21) | |
| ≥ 29 | 78 (74) | 28 (26) | |
| Race | |||
| Asian or Pacific Islander, Black, or other | 5 (62) | 3 (38) | |
| White | 149 (77) | 45 (21) | |
| Education | |||
| High school graduate or less | 6 (46) | 7 (54) | 0.45 |
| Some college or college graduate | 92 (74) | 32 (26) | |
| At least some graduate school | 56 (86) | 9 (14) | |
| Occupation | |||
| Sales/Service/Factory | 12 (67) | 6 (33) | 0.003 |
| Other white collar | 60 (71) | 25 (29) | |
| Teaching | 23 (92) | 2 (8) | |
| Management/Administration | 9 (47) | 10 (53) | |
| Health professional | 39 (91) | 4 (9) | |
| Academia/Science | 11 (92) | 1 (8) | |
| Body mass index (kg/m2) | |||
| < 20 | 61 (81) | 14 (19) | 0.26 |
| 20- < 25 | 83 (76) | 26 (24) | |
| ≥ 25 | 10 (56) | 8 (44) | |
| Gravidityc | |||
| 0 | 56 (80) | 14 (20) | 0.01 |
| 1 | 60 (85) | 11 (15) | |
| ≥ 2 | 38 (62) | 23 (38) | |
| Parityc | |||
| 0 | 75 (79) | 20 (21) | 0.04 |
| 1 | 64 (79) | 17 (21) | |
| ≥ 2 | 15 (58) | 11 (42) | |
| Smoking | |||
| Never | 110 (79) | 29 (21) | 0.008 |
| Current | 3 (25) | 9 (75) | |
| Former | 41 (80) | 10 (20) | |
| Alcohol intake (drinks per month) | |||
| 0 - 1 | 61 (73) | 23 (27) | |
| 2 – 8 | 81 (80) | 20 (20) | |
| > 8 | 12 (71) | 5 (29) | |
| Caffeine intake (mg/month) | |||
| 0 - 1480 | 45 (82) | 10 (18) | 0.24 |
| > 1480 - < 6900 | 82 (80) | 21 (20) | |
| ≥ 6900 | 27 (61) | 17 (39) |
This table excludes the 19 women (of 221) who did not complete Phase 1.
TTP is unavailable for women who were deceased at Phase 3 (N=11), did not respond to the Phase 3 questionnaire (either by mail or by phone) (N=31), or responded to the questionnaire but did not provide an estimate of TTP (N=6).
The multivariable logistic regression model only included variables that were important (p ≤ 0.2) in the univariable analysis: prospective TTP, education, occupation, BMI, gravidity, parity, smoking and caffeine intake. Gravidity and parity were estimated in separate models. With the exception of parity, p-values are estimated from the model that includes gravidity (and not parity).P-values are from a likelihood ratio test which indicates whether the tested characteristics differ between those with and without recalled time-to-pregnancy data.
Among women who conceived within 6 months.
One woman with a prospective TTP of >6 was classified as 5-7 cycles.
Concordance of prospective and recalled time to pregnancy
Of the 153 participants with both recalled and prospective times to pregnancy, 136 reported in cycles and 16 reported in months. The median prospective time to pregnancy (interquartile range (IQR)) was 2.0 (1, 6) compared with 2.5 (IQR: 1, 6) for the recalled data. Women showed no significant bias in their recall, with a mean difference in recalled and prospective time to pregnancy of 0.20 cycles (IQR: 0, 1).
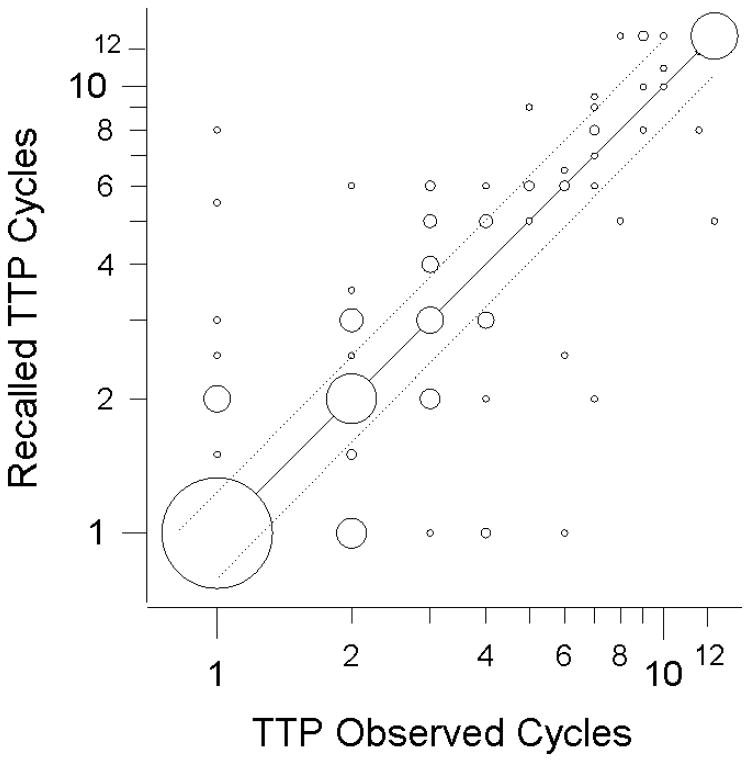
Seventy-one women (47%) recalled their time to pregnancy exactly including 13 women with prospective time to pregnancy ≥13 (Figure 2, Table 2). The largest subgroup was the 32 women who conceived in one menstrual cycle and accurately reported one cycle of time to pregnancy. The positive predictive value (PPV) for each category of recalled time to pregnancy ranged from 38 to 73 percent. The lower values were for recalled times to pregnancy s between 2 and 12 cycles (Table 2). The positive predictive value of a dichotomous measure of “infertility” (time to pregnancy≥13 cycles), was 68% and the negative predictive value was 99% (Table 2). The weighted kappa statistic for agreement was 0.72 (95% Confidence interval (CI): 0.65, 0.79), indicating good agreement. The sensitivity for a dichotomous measure of time to pregnancy of <8 compared with ≥8 cycles was 95%, the specificity was 93%. We chose 8 cycles as a cutpoint given the potential for digit preference of six cycles in studies that rely on self-report. The sensitivity for women with ≥13 cycles of prospective time to pregnancy was 93%, the specificity was 96%.
Figure 2.
Agreement between the prospective measure of time to pregnancy from Phases 1 and 2 of the Early Pregnancy Study and the time to pregnancy reported in Phase 3 (N=153). Times to pregnancy of at least 13 cycles were collapsed into a “≥13 cycles” category. Time-to-pregnancy categories were created to be nearly equidistant on a fecundability scale (1/time to pregnancy). Circle size is proportional to sample size (largest = 32 women, smallest = 1 woman). Observations within the dotted lines are “accurate” (recalled estimate of fecundability is within 25% of the prospective estimate of fecundability); center line delineates exact concordance. One woman with a prospective time to pregnancy of >6 was excluded, one woman with a recalled time to pregnancy of >3 was plotted at “3.5”.
Table 2.
Comparison of prospective and recalled TTP from 153 participants in the EPSa.
| Prospective TTP (cycles) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Recalled TTP (cycles) | N | PPV | 1 | 2 | 3 – 4 | 5 – 7 | 8 – 12 | ≥13 |
| N | N | N | N | N | N | |||
|
|
||||||||
| 1 | 44 | 73 | 32 | 8 | 3 | 1 | 0 | 0 |
| 2 | 31 | 52 | 8 | 16 | 6 | 1 | 0 | 0 |
| 3 – 4 | 26 | 58 | 2 | 8 | 15 | 1 | 0 | 0 |
| 5 – 7 | 21 | 38 | 1 | 1 | 9 | 8 | 1 | 1 |
| 8 - 12 | 12 | 50 | 1 | 0 | 0 | 5 | 6 | 0 |
| ≥13 | 19 | 68 | 0 | 0 | 0 | 0 | 6 | 13 |
Categories of TTP were chosen to reflect intervals of similar magnitude on a fecundability scale.
PPV = Positive Predictive Value
Less than half of the women who responded to the Phase 3 questionnaire by telephone were accurate compared with 64% for women who responded via mail (see Text, Supplemental Digital Content 2 and Table, Supplemental Digital Content 3). Lean women and women with a prospective time to pregnancy of 2-12 cycles were less accurate than women with a prospective time to pregnancy of 1 or ≥13 cycles. There was a tendency for women with high lifetime gravidity to be less accurate in their reporting.
Discussion
Times to pregnancy recalled 24-28 years later showed generally good agreement with time to pregnancy recorded prospectively. On average, study participants showed little tendency to systematically over- or under-estimate their time to pregnancy, which is consistent with previous studies.15,16 Our data suggest that a dichotomous measure of time to pregnancy divided at <13 versus ≥13 cycles is well-reported.
We found several characteristics that were associated with the availability of a recalled time to pregnancy, in contrast to one previous study, which reported no associations with the provision of a recalled time to pregnancy16. In our study, the prospectively observed time to pregnancy was a predictor of both time to pregnancy availability and PPV. The association with availability suggests that the inability to provide recalled time to pregnancy is informative. Thus, standard imputation techniques that assume non-informative missingness cannot be employed. Future studies may be able to use our data to develop an imputation model for non-ignorable missing time to pregnancy values. It is not surprising that PPV is high for women with long times to pregnancys since we lumped all times to pregnancy≥13 cycles into a single category. Women who conceived in one cycle had the highest PPV, which might suggest that conceiving quickly is memorable. Our PPV data can be used in future studies to estimate the impact of misclassification on any observed associations.
Women with more than two pregnancies or deliveries at Phase 1 enrollment were less likely to provide a recalled time to pregnancy. Fading memory or confusion among pregnancies may contribute to non-response. This is consistent with the observation that women who responded to Phase 3 only after a phone call were less likely than those who responded to the initial mailed questionnaire to be accurate in their time-to-pregnancy recall (Supplemental Digital Contents 2 and 3). This may be because women recognized that their recall would be poor, and so they declined the first (mailed) invitation. In this context, our data are consistent with one previous study reporting that women who had been pregnant at least once before they entered the prospective study were less accurate in their time-to-pregnancy recall16. Our data also agree with the Cooney et al. study16 in that women with a prospective time to pregnancy of 2-12 cycles were less accurate than women with shorter or longer times to pregnancies. However, only 6 women in the Cooney et al. study16 had a prospective time to pregnancy greater than 6 months.
Our study cohort comprised dedicated volunteers who were healthy, well-educated and willing to collect daily urine samples which may limit the generalizability of our findings. However, women did not uniformly select the correct pregnancy as their study pregnancy, which suggests that in at least some cases, the intensive nature of Phase 1 did not aid their recall. The distributions of age, parity, fecundability and the rate of recognized miscarriage among the study participants are similar to other populations.17 Finally, among women whose first pregnancy had occurred prior to study enrollment, 49% had been unplanned, which is comparable to the general population.
A main strength of our study was the availability of cycle-by-cycle prospective time-to-pregnancy data from time of initiation of unprotected intercourse to conception for the majority of women (those who conceived during Phase 1). For the remainder, telephone follow-up calls (Phase 2) occurred at regular intervals over the 18 months after the active phase of the study. The recall of time to pregnancy was designed to encourage women to think in terms of menstrual cycles and prevented women from reporting “zero,”21 which creates ambiguity. Finally, our results are based on a larger sample than previous studies.
In summary, our study has several implications for future retrospective time-to-pregnancy studies. Maternal characteristics in the population of interest may be associated with study participation rates as well as accuracy of recall, even among pregnancy planners. Future studies of recalled time to pregnancy should keep in mind that 1) missingness of recalled time to pregnancy is informative, and 2) misclassification of the observed times to pregnancy is more likely for times to pregnancy of 2-12 cycles. Our data could be used to develop appropriate imputation models, and to correct any observed associations for misclassification of time to pregnancy. Finally, our data showing good long-term recall of time to pregnancy are encouraging for chronic disease studies that ask women to recall prior experience of infertility (time to pregnancy ≥13 months).
Supplementary Material
Supplemental Digital Content 1. Recalled TTP and prospective TTP among women with prospective pregnancy attempt times ≥ 13 cycles. (From a total N=202)
Supplemental Digital Content 2. Text describing the methods and results for the analysis of accurate reporting of time to pregnancy. PDF
Supplemental Digital Content 3. Maternal and pregnancy-related characteristics associated with accurate reporting of TTP among women who did not drop out of the EPS (Total N=153).
Supplemental Digital Content 4. Maternal and pregnancy-related characteristics associated with accurate reporting of TTP, among women who conceived a recognized pregnancy during EPS Phase 1 (Total N=119).
Acknowledgments
This research was supported by the intramural research program of the National Institute of Health, National Institute of Environmental Health Sciences. We thank Drs. Elizabeth Jensen and Katie O'Brien who provided feedback on an earlier draft of this manuscript.
Sources of financial support: This research was supported by the intramural research program of the National Institute of Health, National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1.Nguyen RH, Wilcox AJ. Terms in reproductive and perinatal epidemiology: I Reproductive terms. J Epidemiol Community Health. 2005;59:916–9. doi: 10.1136/jech.2004.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolumar F, Olsen J, Boldsen J. Smoking reduces fecundity: a European multicenter study on infertility and subfecundity. The European Study Group on Infertility and Subfecundity Am J Epidemiol. 1996;143:578–87. doi: 10.1093/oxfordjournals.aje.a008788. [DOI] [PubMed] [Google Scholar]
- 3.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2009 doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CM, Chang WP, Doyle P, et al. Prolonged time to pregnancy in residents exposed to ionising radiation in cobalt-60-contaminated buildings. Occup Environ Med. 2010;67:187–95. doi: 10.1136/oem.2008.045260. [DOI] [PubMed] [Google Scholar]
- 5.Harley KG, Marks AR, Bradman A, Barr DB, Eskenazi B. DDT exposure, work in agriculture, and time to pregnancy among farmworkers in California. J Occup Environ Med. 2008;50:1335–42. doi: 10.1097/JOM.0b013e31818f684d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod. 2013;28:125–37. doi: 10.1093/humrep/des347. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canti IC, Komlos M, Martins-Costa SH, Ramos JG, Capp E, Corleta H. Risk factors for cardiovascular disease ten years after preeclampsia. Sao Paulo Med J. 2010;128:10–3. doi: 10.1590/S1516-31802010000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34:830–5. doi: 10.1016/S1701-2163(16)35381-6. [DOI] [PubMed] [Google Scholar]
- 12.Girouard J, Giguere Y, Moutquin JM, Forest JC. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension. 2007;49:1056–62. doi: 10.1161/HYPERTENSIONAHA.107.087528. [DOI] [PubMed] [Google Scholar]
- 13.Pouta A, Hartikainen AL, Sovio U, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004;43:825–31. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 14.Steiner AZ, Herring AH, Kesner JS, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. International journal of epidemiology. 1992;21:1151–6. doi: 10.1093/ije/21.6.1151. [DOI] [PubMed] [Google Scholar]
- 16.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–9. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Environmental Health Sciences. [Accessed October 22, 2015];Questionnaires, EarlyPregnancy Study (EPS) www.niehs.nih.gov/research/atniehs/labs/epi/studies/eps/question/index.cfm.
- 19.National Institute of Environmental Health Sciences. [Accessed November 3, 2015];Early Pregnancy Study (EPS) https://www.niehs.nih.gov/research/atniehs/labs/epi/studies/eps/index.cfm.
- 20.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. The New England journal of medicine. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 21.Baird DD, Weinberg CR, Rowland AS. Reporting errors in time-to-pregnancy data collected with a short questionnaire. Impact on power and estimation of fecundability ratios. Am J Epidemiol. 1991;133:1282–90. doi: 10.1093/oxfordjournals.aje.a115840. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg CR, Baird DD, Wilcox AJ. Sources of bias in studies of time to pregnancy. Stat Med. 1994;13:671–81. doi: 10.1002/sim.4780130528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Recalled TTP and prospective TTP among women with prospective pregnancy attempt times ≥ 13 cycles. (From a total N=202)
Supplemental Digital Content 2. Text describing the methods and results for the analysis of accurate reporting of time to pregnancy. PDF
Supplemental Digital Content 3. Maternal and pregnancy-related characteristics associated with accurate reporting of TTP among women who did not drop out of the EPS (Total N=153).
Supplemental Digital Content 4. Maternal and pregnancy-related characteristics associated with accurate reporting of TTP, among women who conceived a recognized pregnancy during EPS Phase 1 (Total N=119).