Abstract
Introduction:
Smoking rates have dropped substantially in most developed countries in recent decades. This general trend has, however, not always been evident among women—particularly younger women. Smoking habits do, however, often change in connection with pregnancy and the aim of this study is to determine whether smoking during pregnancy follows general trends in smoking rates in the general female population in four countries with active anti-tobacco policies and decreasing population smoking rates.
Methods:
Changes in rates of persistent smoking, that is, smoking in late pregnancy or daily smoking among all women of childbearing age were described according to age groups. Data were retrieved from the Australian Household Drug Surveys during 2000–2013 and from registries and surveys in Finland, Norway, and Sweden between 1995 and 2014.
Results:
In general, persistent smoking has decreased and late-pregnancy smoking rates are lower than daily smoking rates among all women. However, younger women are more likely to be persistent smokers regardless of pregnancy status. In Norway and Finland, persistent smoking was most common among young pregnant women and in Sweden there was an increased polarization between age groups. In Australia, a steady decrease in smoking rates appears to have stalled in younger pregnant women.
Conclusion:
Although smoking has declined substantially in recent decades, there are groups lagging behind this general trend. Young pregnant women are of particular concern in this respect. The possibility that these findings reflect the changing characteristics of younger pregnant women is discussed.
Implications:
This study puts recent trends in maternal smoking into a broader context by relating developments to changes in smoking rates among women in general. By using similar data from four countries we were able to follow changes in smoking rates “within” groups of women within the four countries without being limited by methodological problems related to cross-country or inter-group comparisons. We were above all able to show that aggregate data disclose the strong age gradient in maternal smoking habits.
Introduction
Smoking has been established as the single most preventable cause of death in the world today1 and smoking or exposure to smoke during pregnancy has been found to have a number of potentially negative effects on the fetus and child, such as low birth weight and preterm delivery,2–4 stillbirth,5 and birth defects.6 Maternal smoking has also been linked to sudden infant death syndrome,7,8 childhood behavioral and health problems,9,10 and increased mortality in early adult life.11
The rise and fall in smoking rates has been remarkably similar across developed countries and is often described as an epidemic with four distinct phases since the late 1800s. The fourth and ongoing phase may be dated from around 1980 and shows declining smoking rates overall and closing gender gaps.12,13 Maternal smoking rates have followed the same general pattern, with more or less dramatic reductions during the past decades—as in the United States,14 in the Netherlands,15 in Australia (New South Wales),16 in the United Kingdom,17 and in the Nordic countries.18 In the higher middle-income country of Brazil similar results have been observed,19 but little is known about maternal smoking rates or trends in maternal smoking in developing countries.20 About 80% of the world’s smokers now live in low- and middle-income countries, though, and there is fear that maternal smoking rates may rapidly rise in response to forceful tobacco marketing efforts directed towards women and children in the developing world, as well as a general lack of adequate tobacco controls.21
The aim of this study is to analyze trends in smoking rates among pregnant women and in the general female population in four developed countries which have experienced the phases of the epidemic as described above; Australia, Finland, Norway, and Sweden.13 More specifically, we aim to analyze (1) whether smoking also has declined among pregnant women and the general female population in these countries, and (2) whether smoking rates have changed varyingly according to age within these two groups.
Smoking—Prevalence and Policies in Australia, Finland, Norway, and Sweden
The countries in this study have experienced many of the same economic and demographic developments. As other countries in the last phases of the cigarette epidemic, women smoke at nearly the same or even at a higher rate compared to men. Policy-wise there are also many similarities. The Nordic countries basically have shared views on tobacco control and alcohol policies22 and have also been strongly influenced by EU regulations and policies. Over the years, the EU has developed a more prominent role in tobacco control although the responsibility for regulation of and structures for tobacco prevention, cessation, and smoke-free environments lies at the national level.23 The responsibility for tobacco policies in Australia rests mainly at the state and territory level, but as one of the most centralized federations in the world,24 the Australian federal government has taken an active role in the tobacco area. A further description of tobacco policies and smoking rates in each of the countries in this study is presented below:
Australia
The first piece of legislation in the tobacco area was a smoking ban on domestic flights in 1987, followed by similar bans in other federally controlled areas. In 1999, a national tobacco strategy was adopted and the states have successively introduced more smoke-free environments.25 In 2012, Australia became the first country in the world to introduce plain packaging. An aim of reducing rates of daily smoking to 9% or less by 2020 has been suggested.26 The proportion of regular smokers declined dramatically during the 1980s and continued to drop after a relatively static period during the 1990s. The sex differential also decreased during this period—from 11 percentage points in 1980 to 4 percentage points in 2013.27 The average maternal smoking rate of 13% in 2012 concealed a large regional variation between 8% in the Australian Capital Territory to 24% in the Northern Territory. Maternal smoking rates were also very high among teenagers (40%).28
Finland
Finland has a long history of tobacco control and was one of the first countries to ratify the WHO Framework Convention on Tobacco Control (FCTC) in 2005. The first Tobacco Control Act was introduced in 1976 and with the amendment in 1995 there has been a steady increase in bans on smoking, age limits for purchases, restrictions on advertising and sponsorship, etc. In 2007, smoking was totally banned in restaurants and bars and from 2012 tobacco products are kept behind the counter at points of sale. The revised tobacco act from 2010 proclaims an ambitious end point, namely to make Finland completely tobacco-free by 2040.29,30 A network of organizations is now working towards 2030 as the end goal.31 Finland presently has one of the lowest smoking rates in Europe with 17% of men and 14% of women smoking daily. Nonetheless, smoking rates clearly vary with socioeconomic status.32 In 2013, the overall maternal smoking rate was 16%, but among the youngest mothers to be over half were smoking in early pregnancy.33
Norway
Like Finland, Norway has often been one step ahead of the recommendations and regulations of EU and the WHO convention. Today, Norwegian tobacco policy is still a typical expression of WHO recommendations.33 The first tobacco act was adopted in 1973 and included a ban on advertising, compulsory health warnings, and a raised minimum purchasing age. Legislation on smoke-free environments (workplaces and restaurants) was first introduced in 1988. A ban was introduced on importing and producing new nicotine and tobacco products in 1989, something which has effectively prevented the introduction of e-cigarettes in Norway. Indirect advertising was banned in 1997, misleading descriptions of products (“light” cigarettes) in 2002, smoking in restaurants and bars in 2004, and point-of-sale tobacco display in 2010. In a study of 187 countries, Norway was one of four countries where smoking declined by more than 50% between 1980 and 2012 for both sexes.34 The national smoking strategy for 2013–2016 sets out to “protect the population and society against tobacco-related harms.” This includes reducing the number of daily smokers in the population to below 10% and lowering late-pregnancy smoking rates to less than 4% by 2016. In 2015, the daily smoking rate was 13% and in 2011, maternal smoking rates were 18% and 7% in early and late pregnancy, respectively.35
Sweden
Sweden has been a relative laggard in tobacco control and it was not till the late 1970s that warning texts on cigarette packs became mandatory and advertising was regulated. The first comprehensive tobacco act was introduced in 1993. The development of a restrictive tobacco policy in Sweden has largely been driven by WHO and EU policies and directives. For example, the Framework Convention on Tobacco Control was signed by Sweden just 1 month after joining the EU. With a negative stance towards mandatory pictorial warnings, plain packaging, and regulation of ingredients at the EU level, Sweden has been criticized for awarding too much attention to the interests of the (Swedish) tobacco industry.36 Nonetheless, smoking rates have declined substantially in Sweden—particularly among men—and Sweden is now the only country where female smoking rates now exceed those of men.34,37 In 2010, the government adopted a comprehensive strategy to defeat alcohol use, drug use, doping, and tobacco use. The strategy has recently been revised and one of the overarching goals is to reduce availability of tobacco.38 This goal may be contrasted to Finland’s ambition of a smoke-free society by 2030 and Norway’s aim at lowering smoking rates to specified levels. When it comes to maternal smoking rates, the strategy simply states that use of tobacco during pregnancy “needs to be given attention.”38 The public health strategy adopted in 2003 did, however, aim to give children a tobacco free start in life from year 2014,39 and smoking in early pregnancy has dropped from 23% in 1992 to 6% in 2014.40
Perspectives on Change in Health Behaviors
Tobacco control policies may have a smaller or larger impact on health behavior, depending on target groups, comprehensiveness of measures, and how well policies are implemented.41 There are a number of theoretical approaches that are useful when trying to understand change in health behaviors in populations at large or in specific sub-groups. At the individual level, we may for example understand these processes in terms of “stages of change” as described by Prochaska and DiClemente,42–44 or in terms of self-efficacy and locus of control.45–47 Pregnancy may be seen as a particularly important “teachable moment”48 or a trigger for cessation49—suggesting a faster and more intense adaptation to public health policies among pregnant women, at least temporarily.50 At the societal level, studies of change in health behaviors are open to various theoretical perspectives within the realm of policy studies, sociological and economic theory, history of ideas, and so forth. Social anthropologists have found that specific norms regarding appropriate behaviors and lifestyles during pregnancy, labor, and the neonatal period are characteristic of all cultures.51 In a modern capitalist state, the pregnant woman is also likely to be a favored target for interventions; as a carrier of future human capital it is in society’s interest to reduce infant mortality and improve health outcomes.52–54 Regardless of the choice of theoretical perspective, it is our expectation that smoking rates have decreased more markedly among pregnant women than in the general female populations in these four countries.
Methods
Details about data sources, definitions, and time frames are found in Table 1. Maternal smoking rates were based on the medical birth registries in Finland, Norway, and Sweden and retrieved in open databases at the National Institute for Health and Welfare, the Norwegian Directorate of Health, and the National Board of Health and Welfare, respectively. These registries encompass basically all births and information about maternal smoking is based on self-reports in connection with antenatal care. At the first visit (usually week 8–12), women are asked about their whether they smoked and, if so, number of cigarettes per day. Smoking status is recorded once again in late pregnancy (usually week 30–32).
Table 1.
Data Sources, Descriptions and Definitions by Country
| Australia | Finland | Norway | Sweden | |
|---|---|---|---|---|
| General population | ||||
| Data source | National Drug Strategy Household Survey | Health Behavior and Health among the Finnish Adult Population Survey | Holiday and Travel Survey | National Public Health Survey |
| Main mode of data collection | Drop-and-collect questionnaire/ telephone interview | Postal questionnaire | Telephone interviews | Postal questionnaire |
| Start and frequency | Every 2–3 y since 1985 | Annual since 1978 | Quarterly since 1973 | Annual since 2004 |
| Data sets in this study | 2001, 2004, 2007, 2010, 2013 | 1995–2014 | 1995–2014 | 2004–2015 |
| Population | Women aged 16–44 | Women aged 15–44 | Women aged 16–44 | Women aged 16–44 |
| Sample | N = 5761–8130 | Subset of total N = 2545–3644 | N = 1079–1576 | N = 1340–2837 |
| Age groups reported | 16–29 y | 15–24 y | 16–24 y | 16–29 y |
| 30–44 y | 25–44 y | 35–44 y | 30–44 y | |
| Definition of smoker | Daily smoking | Daily smoking | Daily smoking | Daily smoking |
| Pregnant women | ||||
| Data source | National Drug Strategy Household Survey | Medical Birth Registry | Medical Birth Registry | Medical Birth Registry |
| Main mode of data collection | Drop-and-collect questionnaire/ telephone interview | Medical records | Medical records | Medical records |
| Start and frequency | Every 2–3 y since 1985 | Annual data since 1987 | Annual data since 1973 | Annual data since 1973 |
| Data sets in this study | 2001, 2004, 2007, 2010, 2013 | 1995–2014 | 1999–2014a | 2000–2014a |
| Population | Women who were pregnant at time of survey | All children with birth weight >500 grams after week 22 | All births after week 12 | All births |
| Sample | N = 898, 888, 652, 817, 873 | Whole population | Whole population | Whole population |
| Age groups reported | 16–29 y | <20 y | <20 y | <20 y |
| 30–44 y | 0–24 y | 20–24 y | 20–24 y | |
| 25–29 y | 25–29 y | 25–29 y | ||
| 30–39 y | 30–34 y | 30–39 y | ||
| 35–39 y | ||||
| Definition of smoker | Reported any smoking after knowledge of pregnancy | Continued smoking after first trimester | Smoking “at the end of the pregnancy”b | Smoking in week 30–32 of pregnancy |
aMaternal smoking habits recorded since 1999.
bSmoking habits are only recorded if the mother consents. Data was available for 80%–88% of the pregnancies.
Smoking rates in the female populations in these three countries were retrieved from annual surveys of nationally representative samples of men and women 15/16 years or older. These samples may very well include some women who are pregnant, but the vast majority will not be. Most of these surveys have been performed in a similar fashion for a number of years, but the Swedish survey method, content and organization was altered quite extensively in 2004,55 and we therefore only used data from the National Public Health Survey from this point on. The Finnish data are derived from an annual national public health survey between 1978 and 2014. The Norwegian data are collected as part of a national survey about holidays and travels which has been performed quarterly and reported annually since 1973.56
Australian data were taken from five National Drug Strategy Household Surveys between 2001 and 2013, as shown in Table 1. Surveys were collected using a mix of self-completion of questionnaires by drop-and-collect, as well as computer-assisted telephone interviews and face to face interview in 2001, drop-and-collect and telephone interviews in 2004 and 2007, and only drop-and-collect in 2013 in selected households with the household member selected at random. The sample sizes have varied between 23 356 and 29 445 and response rates ranged between 46% and 57%. Weighting was used to correct for misrepresentation compared to population statistics regarding age, gender, and location.57–61
The Australian data originate entirely from a representative survey of the population while the data from the other countries are derived from a mix of registry data and surveys of the general population. In order to help compensate for lack of extensive registry data, confidence intervals have been included for the Australian data.
Availability of data varied between the countries in terms of years and age groups. The age categories therefore differ somewhat. Moreover, as the data sources were not the same for pregnant women and the general female population in Finland, Norway, and Sweden, the age categorizations also differ “within” these countries. It would have been possible to exclude pregnant women from the comparison group in Australia, but we chose not to in order to enhance comparability with the other countries. Collection of each National Drug Strategy Household Surveys was approved by the Australian Institute of Health and Welfare Ethics Committee. For the other countries we used aggregate published data exempt from institutional review for research on human subjects.
Identification of Smoking Status
In order to avoid problems of definition and to highlight trends, we focused on smokers with the greatest regularity or persistency.62 Persistent smoking was therefore defined as “daily” smoking in the general female population or any smoking in late pregnancy among pregnant women in Norway, Finland, and Sweden. In Australia, females completing the survey were asked if they were pregnant and, if so, asked if they consumed a range of alcohol and other drugs while pregnant. In 2010 and 2013, this was further refined to differentiate between consumption before and after knowledge of pregnancy. Previous analysis has indicated that pre-2010 respondents interpreted the question on consumption during pregnancy to mean after knowledge of pregnancy, with a consistent trend found when this option is used for 2010 and 2013. Nonrespondents on questions about substances consumed during pregnancy were assumed to have none and the questions on consumption after knowledge of pregnancy were a closer equivalent to the more general questions asked in the first three surveys. For a justification of these decisions, see Callinan and Room.63
Data Analysis
Rates of persistent smoking among pregnant women and the general female population are reported with confidence intervals included for the Australian data. Descriptions of daily smoking rates in the general female populations were limited to women of childbearing age, that is, 16–44 years in Australia, Norway, and Sweden, and 15–44 years in Finland. Descriptive statistics and figures were generated in Stata 14 (StataCorp. 2015; Stata Statistical Software, College Station, TX).
Results
As could be expected from other studies,64,65 late-pregnancy smoking rates were overall lower than daily smoking in the general female populations. However, the difference between these two rates was less evident in the youngest age groups.
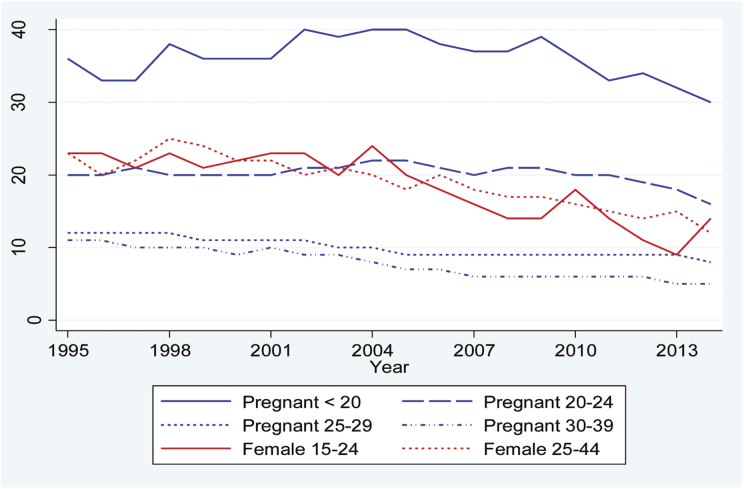
Looking at each country separately we found that in Finland persistent smoking rates were highest among pregnant women under 25 throughout the whole time period (Figure 1). This is especially the case for pregnant teenagers, where late-pregnancy smoking rates varied between 30% and 40% throughout the period. Late-pregnancy smoking among women over 25 did not exceed 12% in any year and among the general female population the highest rates of daily smoking were 24%–25%. With decreasing daily smoking among females in general and relatively stable late-pregnancy smoking rates we see a convergence in persistent smoking between the two main groups. At the end of the period we also see a clear polarization between late-pregnancy smoking among women under 20 (30%) and 25 or older (less than 10%). In between these groups we find a mid-range of women aged 20–24 consisting of pregnant women and females in general, where rates of persistent smoking ranged from 9% to 18% in 2013 and 12%–16% in 2014. We also note that smoking rates in the general female population are fairly uniform between age groups, but due to lack of more refined age categorizations it is possible that substantial age differences are concealed.
Figure 1.
Prevalence of any smoking in late pregnancy and daily smoking in the female population in Finland by age group.
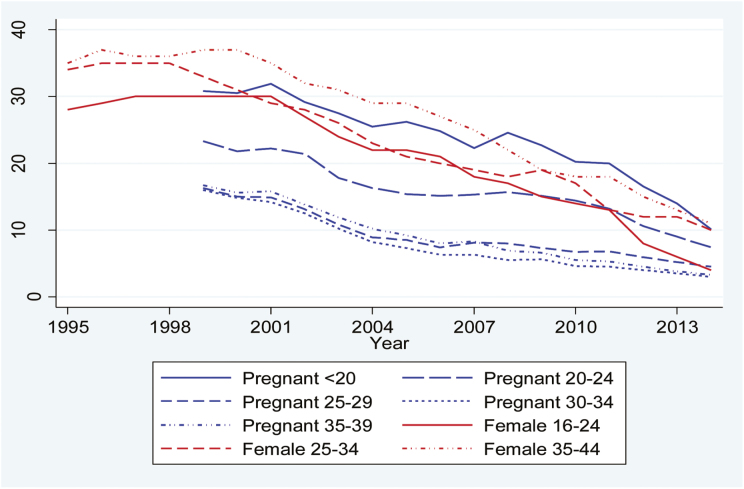
In Norway, the rate of decline is fairly uniform in all groups although starting from different levels (Figure 2). Late-pregnancy smoking was equally common among teenagers as daily smoking in the general female population under 35, and also showed the highest rates of late-pregnancy smoking overall. Daily smoking in the general female population declined from over 35% among those over 24 during the late 1990s to around 10% in 2014. Around year 2000 approximately 30% of the youngest women in the population smoked daily, but since 2012 the rate has dropped to below 10%. However, as smoking rates have converged the differences in persistent smoking between pregnant women and the general female population have decreased in all but the youngest age groups. There is also a polarization between pregnant women under and over 25. Furthermore, there are more pregnant women under 25 who continue to smoke in late pregnancy compared to daily smokers among females in the same age group in the general population (around 10% compared to 4% in 2014). The lowest rates of persistent smoking are now found among young women in the general population and pregnant women aged 25 or more.
Figure 2.
Prevalence of any smoking in late pregnancy and daily smoking in the female population in Norway by age group.
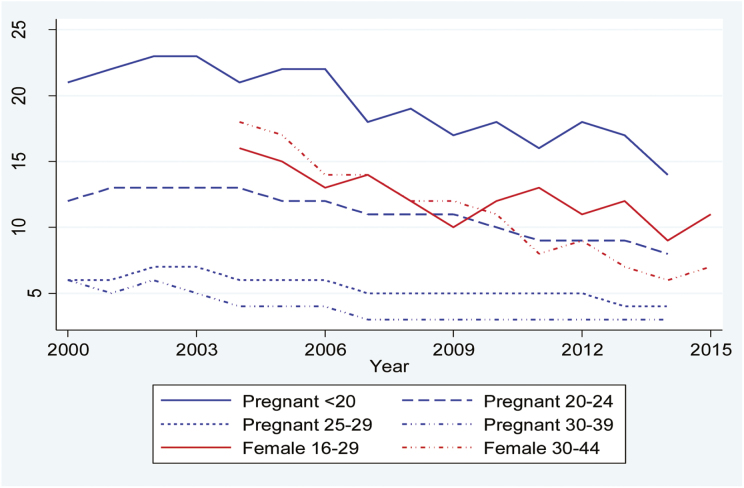
In Sweden, we do not see the same substantial decreases in persistent smoking as in Finland and Norway due to a shorter period of observation. However, there has been a general decrease in all groups except late-pregnancy smoking among women aged 25 or more (varying between 3% and 7% throughout the period). Most notable is the decline in daily smoking in the general female population aged 30 or over (from 18% to 7%). As shown in Figure 3, persistent smoking is highest among pregnant teenagers (14% and less than 10% in all other groups). Even though data were only available in broad age categories for the general female population the highest rates of persistent smoking seem to be among the younger women—regardless of pregnancy status.
Figure 3.
Prevalence of any smoking in late pregnancy and daily smoking in the female population in Sweden by age group.
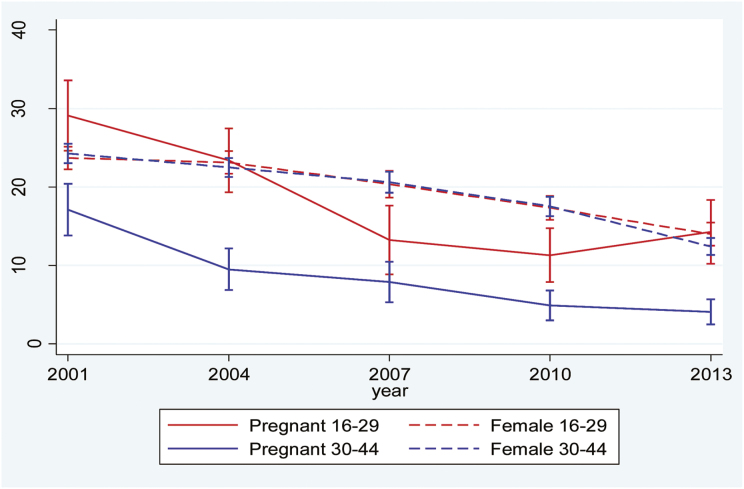
Finally, in Australia, there has been a general drop in daily and late-pregnancy smoking. The decrease was particularly notable among young pregnant women where 29% were smoking in late pregnancy in 2001, but by 2010 the rate was down to 11%. Persistent smoking was least common among older pregnant women, dropping from 16% to 4% during the period. Interestingly, there appears to be a recent uptick among younger pregnant women from 11% in 2010 to 14% in 2013, but this increase, with overlapping confidence intervals, is not statistically significant (Figure 4).
Figure 4.
Prevalence of any smoking in late pregnancy and daily smoking in the female population in Australia by age group.
Discussion
This study has analyzed developments in smoking among women in four industrialized countries which have seen active anti-tobacco policies and substantial changes in smoking levels during the past decades. The main aim has not been to compare smoking rates across countries,18,34 but rather to describe changes in late-pregnancy smoking rates and daily smoking in the general female populations of childbearing age in each country respectively.
As countries in the fourth phase of a smoking epidemic (see introduction) we did indeed see a broad drop in smoking rates. Also, as we did expect—and find—that rates of late-pregnancy smoking were generally lower than daily smoking rates among nonpregnant women. These trends might be interpreted as a sign of “bio-political” influence at the societal level, or psychological “readiness” at the individual level. However, the results also reveal a marked split between age groups; younger women were more likely to be persistent smokers, regardless of pregnancy status. In Norway and Finland, late-pregnancy smoking was higher among young women than late-pregnancy or daily smoking rates in any of the other groups. In Sweden, we saw an increasing polarization between age groups, regardless of pregnancy status. The stable—and comparatively high—late-pregnancy smoking rates in Finland, the possible uptick in smoking among young pregnant women in Australia, and the slow pace of change among young pregnant women in Sweden give cause for concern. We might be witnessing an emerging rise in female smoking in general and/or a process of so-called hardening, this is, that decreasing smoking rates in general leave a select group of smokers with greater psychosocial problems behind.66 Although the evidence for hardening has been questioned at the population level, researchers have found it to be relevant in certain sub-groups—such as women and low-income smokers.67
Our findings suggest that young pregnant women are not adapting to new nonsmoking lifestyles or tobacco policy measures at the same pace as their older counterparts are. Developmental psychology might offer some answers to why young women, pregnant or not, seem to be falling behind: Adolescence and young adulthood are often seen as a period when biological, social, and psychological transitions increase risky health behaviors and anti-social behavior.68 At the aggregate level we would then expect to see a certain lag in the adoption of new healthy lifestyles compared to older peers. This perspective is, however, challenged by developments in a closely related area of risk behavior; teenagers and young adults presently drink at historically low levels in these countries, while consumption is on the rise in other older age groups.69–73 From a sociological point of view, parenthood is increasingly rare among teenagers or young adults in these societies and the very youngest mothers-to-be are likely to be a select group in terms of lifestyle and social conditions. The link between young age, pregnancy, and continued smoking may, then, reflect the changing demographics of pregnant women where young mothers are more likely to come from at-risk populations.16,74–76 In other words, young pregnant women could represent two sub-groups where hardening may be taking place—women and low-income smokers.67 Apart from the socioeconomic gradient in uptake and cessation of smoking, exposure to tobacco industry targeting is a factor to consider74; this is for example the case in Sweden (and Norway), where snus manufacturers have tried to attract (young) women by introducing flavored snus, in small pouches in colorful tins.
Limitations
Two methodological issues arise in the examination of smoking rates in a multi-country study as this one: (1) the use of self-reported smoking data and the risk of underreporting—particularly among pregnant women; and (2) comparability of data between countries given the use of different measures, data collection strategies, etc.
Studies from different countries have shown varying reliability of self-reported data on maternal smoking. Most studies have been based on women being invited to participate, thereby adding problems of selection bias to potential biases associated with a research setting.77,78 A study of a large clinical sample in New South Wales did, however, find that self-reports of smoking corresponded well with breath tests of CO levels.79 Similarly, the validity of self-reported maternal smoking in registry data have been found to be good in Finland,80 Norway,81 and Sweden.77 A possible shortcoming in the Australian data is the fact that nonrespondents on questions about substance use during pregnancy were assumed to have none. However, previous work on these items has indicated that this decision is a sound one.63
Limitations in comparability between the countries was largely related to different data collection strategies, while the measures chosen for this study were defined in a similar way in all countries; daily smoking among nonpregnant women, and smoking in late pregnancy. More importantly, though, this study focuses the relative changes in smoking rates between pregnant and general female populations within each country, and less on the correct prevalence of smoking. The methodological limitations are, then, less prominent.
Conclusion
Our study shows that older mothers are the least likely to smoke throughout pregnancy, which probably mirrors higher socioeconomic levels and greater motivation for healthy lifestyles.82 In terms of policy impact we would argue that a lot more effort be put on reaching young women and young mothers in particular. In addition to studies on the effectiveness of interventions aimed at other (vulnerable) groups,74,83 there is a need for developing and evaluating smoking cessation measures directed towards young women and especially young mothers.
Teenage pregnancies have declined substantially in industrialized countries during the past decades84 and have been “made and unmade” as a social problem.85 Instead, there has been an increased focus on the risks of postponed maternity.86 This study has hopefully contributed to increased awareness that general reductions in smoking are not necessarily seen among younger women, particularly among young mothers. Apart from continued focus on the substantial socioeconomic gradients in smoking and inequality in health overall, service providers should consider how information, guidelines, and interventions appeal to different age groups. This includes the use of language, illustrations, and metaphors, context, and not least with which media.
Funding
This work was supported by the Swedish Research Council for Health, Working Life and Welfare (grant 2013-1738).
Declaration of Interests
None declared.
Acknowledgments
The authors would like to thank the Swedish Research Council for Health, Working Life and Welfare for research and travel funding, thereby enabling TR to stay at the Centre for Alcohol Policy Research in Melbourne, Australia, where this study was initiated with SC. The Australian Institute of Health and Welfare manage the data collection and dissemination of the National Drug Strategy Household Survey and we are grateful to them for facilitating access to the data via the Australian Data Archive. We would also like to thank Mika Gissler at the National Institute for Health and Welfare in Helsinki for prompt assistance with data on maternal smoking in Finland and the reviewers for invaluable comments and suggestions.
References
- 1. World Health Organization. World Health Report 2003. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 2. Schneider S, Huy C, Schutz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alc Rev. 2010;29(1):81–90. [DOI] [PubMed] [Google Scholar]
- 3. Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F351–F361. [DOI] [PubMed] [Google Scholar]
- 5. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–1340. [DOI] [PubMed] [Google Scholar]
- 6. Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17(5):589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleming P, Blair PS. Sudden Infant Death Syndrome and parental smoking. Early Hum Dev. 2007;83(11):721–725. [DOI] [PubMed] [Google Scholar]
- 8. Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. A prospective study of smoking during pregnancy and SIDS. Arch Dis Child. 2000;83(3):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagerström K. The epidemiology of smoking: health consequences and benefits of cessation. Drugs. 2002;62(suppl 2):1–9. [DOI] [PubMed] [Google Scholar]
- 10. Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obesity. 2008;32(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilsson PM, Hofvendahl S, Hofvendahl E, Brandt L, Ekbom A. Smoking in pregnancy in relation to gender and adult mortality risk in offspring: the Helsingborg Birth Cohort Study. Scand J Public Health. 2006;34(6):660–664. [DOI] [PubMed] [Google Scholar]
- 12. Thun M, Peto R, Boreham J, Lopez AD. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21(2):96–101. [DOI] [PubMed] [Google Scholar]
- 13. Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3(3):242–247. [Google Scholar]
- 14. Centers for Disease Control and Prevention. Smoking during pregnancy--United States, 1990–2002. Morb Mortal Wkly Rep. 2004;53(39):911–915. [PubMed] [Google Scholar]
- 15. Lanting CI, van Wouwe JP, van den Burg I, Segaar D, van der Pal-de Bruin KM. [Smoking during pregnancy: trends between 2001 and 2010]. Ned Tijdschr Geneeskd. 2012;156(46):A5092. [PubMed] [Google Scholar]
- 16. Mohsin M, Bauman AE, Forero R. Socioeconomic correlates and trends in smoking in pregnancy in New South Wales, Australia. J Epidemiol Commun H. 2011;65(8):727–732. [DOI] [PubMed] [Google Scholar]
- 17. McAndrew F, Thompson J, Fellows L, Large A, Speed M, Renfrew MJ. Infant Feeding Survey 2010. Leeds, UK: NHS Information Centre for Health and Social Care; 2012. [Google Scholar]
- 18. Ekblad M, Gissler M, Korkeila J, Lehtonen L. Trends and risk groups for smoking during pregnancy in Finland and other Nordic countries. Eur J Public Health. 2014;24(4):544–551. [DOI] [PubMed] [Google Scholar]
- 19. Santos IS, Barros AJD, Matijasevich A, et al. Mothers and their pregnancies: a comparison of three population-based cohorts in Southern Brazil. Cad Saúde Pública. 2008;24(suppl 3):S381–S389. [DOI] [PubMed] [Google Scholar]
- 20. Everett K. Smoking during pregnancy. In: Owing J, ed. Trends in Smoking and Health Research. New York, NY: Nova Science Publishers, Inc; 2005:51–68. [Google Scholar]
- 21. Williams AD, Nkombo Y, Nkodia G, Leonardson G, Burd L. Prevalence of smoking during pregnancy in the Republic of the Congo: maternal smoking is associated with increased risk of prenatal alcohol exposure. Int J Alc Drug Res. 2014;3(1):105–111. [Google Scholar]
- 22. Hakala K, Waller M. Nordic unity - a context for tobacco control. In: Hakala K, Waller M, eds. Nordic Tobacco Control: Towards Smoke-Free Societies.Copenhagen, Denmark: Nordic Council of Ministers; 2003:9–11. [Google Scholar]
- 23. Sohlberg T. Smoking Cessation in Sweden - Gender, Pathways and Identity. Stockholm, Sweden: Department of Sociology, Stockholm University; 2014. [Google Scholar]
- 24. Fenna A. The malaise of federalism: comparative reflections on commonwealth–state relations. Austr J Publ Admin. 2007;66(3):298–306. [Google Scholar]
- 25. Riseley K. Report on Smoke-Free Policies in Australia. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 26. Moodie AR, Daube M, Carnell K. Australia:The Healthiest Country by 2020. A discussion paper / prepared by the National Preventative Health Taskforce. Canberra, Australia: National Preventative Health Taskforce; 2008. [Google Scholar]
- 27. Scollo MM, Winstanley MH, eds. Tobacco in Australia: Facts and Issues. Melbourne, Australia: Cancer Council Victoria; 2015. [Google Scholar]
- 28. Australian Institute of Health and Welfare. Australia’s Mothers and Babies. 30th ed. Canberra, Australia: Australian Institute of Health and Welfare; 2014. [Google Scholar]
- 29. Helakorpi SA, Martelin TP, Torppa JO, et al. Did the Tobacco Control Act Amendment in 1995 affect daily smoking in Finland? Effects of a restrictive workplace smoking policy. J Public Health (Oxf). 2008;30(4):407–414. [DOI] [PubMed] [Google Scholar]
- 30. Levy DT, Blackman K, Currie LM, Levy J, Clancy L. SimSmokeFinn: how far can tobacco control policies move Finland toward tobacco-free 2040 goals? Scand J Public Health. 2012;40(6):544–552. [DOI] [PubMed] [Google Scholar]
- 31. Tobacco Free Finland 2030 2016. http://savutonsuomi.fi/en/ Accessed May 15, 2016.
- 32. National Institute for Health and Welfare. Tobacco Statistics 2014. Helsinki, Finland: National Institute for Health and Welfare; 2015. [Google Scholar]
- 33. Sæbø G. The regulation of smoking and smokers in Norway 1964–2010. In: Hellman M, Roos G, von Wright J, eds. A Welfare Policy Patchwork. Negotiating the Public Good in Times of Transition. Helsinki, Finland: Nordic Centre for Welfare and Social Issues; 2012:21–41. [Google Scholar]
- 34. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–192. [DOI] [PubMed] [Google Scholar]
- 35. Ministry of Health and Care Services. En framtid uten tobakk. Nasjonal strategi for arbeidet mot tobakksskader 2013–2016. [A Future without Tobacco. A National Strategy for Efforts against Tobacco-Related Harms 2013–2016]. Oslo, Norway: Ministry of Health and Care Services; 2013. [Google Scholar]
- 36. Cisneros Örnberg J, Sohlberg T. Swedish Tobacco policy: from rational choice to ‘harm to others’. In: Hellman M, Roos GR, von Wright J, eds. A Welfare Policy Patchwork: Negotiating the Public Good in Times of Transition. Helsinki, Finland: Nordic Centre for Welfare and Social Issues; 2012:65–82. [Google Scholar]
- 37. Chapman S. Falling prevalence of smoking: how low can we go? Tob Control. 2007;16(3):145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ministry of Social Affairs. En samlad strategi för alkohol-, narkotika-, dopnings- och tobakspolitiken 2016–2020 [A Comprehensive Strategy for Alcohol, Drug, Doping and Tobacco Policy 2016–2020]. Stockholm, Sweden: Ministry of Social Affairs; 2016. [Google Scholar]
- 39. Ministry of Social Affairs. Mål för folkhälsan [Public Health Goals]. Stockholm, Sweden: Ministry of Social Affairs; 2002. [Google Scholar]
- 40. National Board of Health and Welfare. Pregnancies, Deliveries and Newborn Infants. The Swedish Medical Birth Register 1973–2014. Assisted Reproduction 1991–2013. Stockholm, Sweden: National Board of Health and Welfare; 2015. [Google Scholar]
- 41. Levy DT, Chaloupka F, Gitchell J. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. J Public Health Manag Pract. 2004;10(4):338–353. [DOI] [PubMed] [Google Scholar]
- 42. DiClemente CC, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: implications for interventions. Tob Control. 2000;9(suppl 3):III16–III21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DiClemente CC, Prochaska JO. Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addict Behav. 1982;7(2):133–142. [DOI] [PubMed] [Google Scholar]
- 44. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–1114. [DOI] [PubMed] [Google Scholar]
- 45. Kaplan GD, Cowles A. Health locus of control and health value in the prediction of smoking reduction. Health Educ Behav. 1978;6(2):129–137. [DOI] [PubMed] [Google Scholar]
- 46. O’Leary A. Self-efficacy and health. Behav Res Ther. 1985;23(4):437–451. [DOI] [PubMed] [Google Scholar]
- 47. Stuart K, Borland R, McMurray N. Self-efficacy, health locus of control, and smoking cessation. Addict Behav. 1994;19(1):1–12. [DOI] [PubMed] [Google Scholar]
- 48. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. [DOI] [PubMed] [Google Scholar]
- 49. West R, McEwen M, Bates C. Sex and Smoking: Comparisons between Male and Female Smokers. London, UK: No Smoking Day; 1999. [Google Scholar]
- 50. Stotts AL, DiClemente CC, Carbonari JP, Mullen PD. Pregnancy smoking cessation: a case of mistaken identity. Addict Behav. 1996;21(4):459–471. [DOI] [PubMed] [Google Scholar]
- 51. Mead M, Newton N. Cultural patterning of perinatal behavior. In: Richardson SA, Guttmacher AF, eds. Childbearing: Its Social and Psychological Aspects. Baltimore, MD: Williams & Wilkins; 1967:1–22. [Google Scholar]
- 52. Legg S. Foucault’s population geographies: classifications, biopolitics and governmental spaces. Popul Space Place. 2005;11(3):137–156. [Google Scholar]
- 53. Vallgårda S. Governing people’s lives. Strategies for improving the health of the nations in England, Denmark, Norway and Sweden. Eur J Public Health. 2001;11(4):386–392. [DOI] [PubMed] [Google Scholar]
- 54. Weir L. Pregnancy, Risk and Biopolitics: On the Threshold of the Living Subject. New York, NY: Routledge; 2006. [Google Scholar]
- 55. Boström G, Nyqvist K. Objective and Background of the Questions in the National Public Health Survey. Stockholm, Sweden: Public Health Agency of Sweden; 2010. [Google Scholar]
- 56. Statistics Norway. Smoking habits 2014. www.ssb.no/en/helse/statistikker/royk/aar/2015-02-03#content Accessed May 19, 2016.
- 57. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2013. Canberra, Australia: Australian Institute of Health and Welfare; 2014. [Google Scholar]
- 58. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2001 - State and territory supplement. Canberra, Australia: Australian Institute of Health and Welfare; 2002:PHE 61. [Google Scholar]
- 59. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2004 - Detailed findings. Canberra, Australia: Australian Institute of Health and Welfare; 2005:PHE 107. [Google Scholar]
- 60. Australian Institute of Health and Welfare. 2007 National Drug Strategy Household Survey: detailed findings. Canberra, Australia: Australian Institute of Health and Welfare; 2008:PHE 107. [Google Scholar]
- 61. Australian Institute of Health and Welfare. 2010 National Drug Strategy Household Survey. Canberra, Australia: Australian Institute of Health and Welfare; 2011. [Google Scholar]
- 62. Fitzpatrick KE, Gray R, Quigley MA. Women’s longitudinal patterns of smoking during the pre-conception, pregnancy and postnatal period: evidence from the UK Infant Feeding Survey. PLoS One. 2016;11(4):e0153447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Callinan S, Room R. Alcohol Consumption during Pregnancy: Results from the 2010 National Drug Strategy Household Survey. Canberra, Australia: Foundation for Alcohol Research and Education; 2012. [Google Scholar]
- 64. Ebrahim SH, Floyd RL, Merritt RK, II, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987-1996. JAMA. 2000;283(3):361–366. [DOI] [PubMed] [Google Scholar]
- 65. Williamson DF, Serdula MK, Kendrick JS, Binkin NJ. Comparing the prevalence of smoking in pregnant and nonpregnant women, 1985 to 1986. JAMA. 1989;261(1):70–74. [PubMed] [Google Scholar]
- 66. Pedersen W, von Soest T. Tobacco use among Norwegian adolescents: from cigarettes to snus. Addiction. 2014;109(7):1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smith PH, Rose JS, Mazure CM, Giovino GA, McKee SA. What is the evidence for hardening in the cigarette smoking population? Trends in nicotine dependence in the U.S., 2002-2012. Drug Alcohol Depend. 2014;142:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sussman S, Arnett JJ. Emerging adulthood: developmental period facilitative of the addictions. Eval Health Prof. 2014;37(2):147–155. [DOI] [PubMed] [Google Scholar]
- 69. Pedersen W, von Soest T. Adolescent alcohol use and binge drinking: an 18-year trend study of prevalence and correlates. Alcohol Alcoholism. 2015;50(2):219–225. [DOI] [PubMed] [Google Scholar]
- 70. Livingston M. Trends in non-drinking among Australian adolescents. Addiction. 2014;109(6):922–929. [DOI] [PubMed] [Google Scholar]
- 71. Lintonen T, Karlsson T, Nevalainen J, Konu A. Alcohol policy changes and trends in adolescent drinking in Finland from 1981 to 2011. Alcohol Alcohol. 2013;48(5):620–626. [DOI] [PubMed] [Google Scholar]
- 72. Verhagen CE, Uitenbroek DG, Schreuders EJ, El Messaoudi S, de Kroon ML. Does a reduction in alcohol use by Dutch high school students relate to higher use of tobacco and cannabis? BMC Public Health. 2015; 15:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andersen A, Rasmussen M, Bendtsen P, Due P, Holstein BE. Secular trends in alcohol drinking among Danish 15-year-olds: comparable representative samples from 1988 to 2010. J Res Adolescence. 2014;24(4):748–756. [Google Scholar]
- 74. Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 75. Mohsin M, Bauman AE. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health. 2005;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Penn G, Owen L. Factors associated with continued smoking during pregnancy: analysis of socio-demographic, pregnancy and smoking-related factors. Drug Alcohol Rev. 2002;21(1):17–25. [DOI] [PubMed] [Google Scholar]
- 77. Mattsson K, Källén K, Rignell-Hydbom A, et al. Cotinine validation of self-reported smoking during pregnancy in the Swedish Medical Birth Register. Nicotine Tob Res. 2016;18(1):79–83. [DOI] [PubMed] [Google Scholar]
- 78. Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. [DOI] [PubMed] [Google Scholar]
- 79. Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women assessment of self-report against carbon monoxide (CO). Addict Behav. 2001;26(1):1–9. [DOI] [PubMed] [Google Scholar]
- 80. Tikkanen M, Surcel HM, Bloigu A, et al. Self-reported smoking habits and serum cotinine levels in women with placental abruption. Acta Obstet Gyn Scan. 2010;89(12):1538–1544. [DOI] [PubMed] [Google Scholar]
- 81. Kvalvik LG, Nilsen RM, Skjærven R, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cnattingius S, Lindmark G, Meirik O. Who continues to smoke while pregnant? J Epidemiol Commun H. 1992;46(3):218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bryant J, Bonevski B, Paul C, McElduff P, Attia J. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction. 2011;106(9):1568–1585. [DOI] [PubMed] [Google Scholar]
- 84. Singh S, Darroch JE. Adolescent pregnancy and childbearing: levels and trends in developed countries. Fam Plann Perspect. 2000;32(1):14–23. [PubMed] [Google Scholar]
- 85. Arai L. Teenage Pregnancy. The Making and Unmaking of a Problem. Bristol, UK: The Policy Press; 2009. [Google Scholar]
- 86. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]