Abstract
Objectives
In the ob/ob mouse model of obesity, chronic absence of leptin causes a significant increase of orexin (OX) production by hypothalamic neurons and excessive food intake. The altered OX level is linked to a dramatic increase of the inhibitory innervation of OX producing neurons (OX neurons) and the over expression of the endocannabinoid 2-arachidonoylglycerol (2-AG) by OX neurons of ob/ob mice. Little is known about the function of the excitatory synapses of OX neurons in ob/ob mice, and their modulation by 2-AG. In the present study, we fill this gap and provide the first evidence of the overall level of activation of OX neurons in the ob/ob mice.
Methods
We performed in vitro whole-cell patch-clamp recordings on OX neurons located in the perifornical area of the lateral hypothalamus in acute brain slices of wt and ob/ob mice. We identified OX neurons on the basis of their electrophysiological membrane properties, with 96% of concordance with immunohistochemisty.
Results
We found that OX neurons of ob/ob mice are innervated by less efficient and fewer excitatory synapses than wt mice. Consequently, ob/ob OX neurons show more negative resting membrane potential and lower action potential firing frequency than wt. The bath application of the cannabinoid type-1 receptor agonist WIN55,212-2, depresses both the excitatory and the inhibitory synapses in ob/ob animals, but only the excitatory synapses in wt animals. Finally, the physiologic release of 2-AG induces a prevalent depression of inhibition (disinhibition) of OX neurons in ob/ob animals but not in wt.
Conclusions
In ob/ob mice, chronic absence of leptin induces a 2-AG mediated functional disinhibition of OX neurons. This helps explain the increase of OX production and, consequently, the excessive food intake of ob/ob mice.
Keywords: Obesity, Leptin, Orexin, Endocannabinoids
Highlights
-
•
OX neurons of ob/ob mice receive less efficient excitatory synapses than wt.
-
•
OX neurons of ob/ob mice are hyperpolarized and less active at rest.
-
•
In OX neurons of ob/ob mice, both the excitatory and the inhibitory synapses are depressed by WIN55,212-2.
-
•
In ob/ob mice, the physiologic release of 2-AG primarily depresses the inhibitory synapses and disinhibits OX neurons.
1. Introduction
Neurons releasing orexin (OX, a neuropeptide also known as hypocretin) are uniquely found in the perifornical area of the lateral hypothalamus (LH) [1], [2], [3], [4], [5], [6]. OX is overexpressed in neurons of leptin-deficient obese mice (the ob/ob mouse strain) [7]. OX neurons have been implicated in a variety of functions, such as wakefulness, motivated behavior and reward, autonomic functions, nociception and, of relevance for the present study, energy balance and food seeking and intake (see for review: [8], [9], [10]). In particular, the injection of orexin into the brain stimulates feeding behavior [6], [11]. In the hypothalamus, the blood brain barrier is permeable to several hormones, including leptin. Leptin is released by adipocytes following food intake and exerts its anorexigenic effects on different types of neurons throughout the hypothalamus and other brain nuclei [12], [13]. The effectiveness of excitatory synapses on OX neurons is depressed by leptin [14], and the firing activity of OX neurons is consequently reduced [14], [15]. Conversely, the reduction of leptin level during fasting increases the activity of OX neurons [16], [17].
Variations in the amount of circulating leptin, due to modifications of fasting/feeding states, cause substantial synaptic rewiring in hypothalamic nuclei involved in the control of food intake, such as the arcuate nucleus (ARC) and the LH. In the ARC, chronic lack of leptin, as in ob/ob mice, causes a functional and morphological synaptic plasticity that increases the excitation of neurons expressing orexigenic (neuropeptide Y and agouti-related peptide) and the inhibition of those expressing anorexigenic (pro-opiomelanocortin and cocaine-amphetamine regulated transcript) peptides [18]. Accordingly, leptin administration to ob/ob mice reduces food intake and body weight [19]. In the LH, overnight food deprivation is sufficient to enhance the excitatory drive to OX neurons of wt mice, an effect reversed by leptin administration [20]. We have recently demonstrated that chronic leptin deficiency or resistance in ob/ob and in high-fat diet (HFD) fed mice, respectively, causes a morphological switch from predominantly excitatory to predominantly inhibitory innervation of OX neurons [7]. The exceeding inhibitory innervation is functionally depressed by the activity of the endocannabinoid 2-arachidonoylglycerol (2-AG), produced by OX neurons and acting retrogradely at presynaptic cannabinoid type-1 (CB1) receptors. As a consequence, OX neurons of ob/ob and HFD animals are disinhibited and release more OX in target brain areas [7], [21].
Our previous work provided functional evidence for the leptin-driven synaptic rewiring of OX neurons in ob/ob mice [7]. However, a comprehensive characterization of the retrograde effect of endocannabinoids on excitatory and inhibitory inputs to OX neurons of wt and ob/ob mice is still missing. This topic is of special interest also for human pathology since a 2-AG mediated modulation of OX neurons may be involved in human obesity, in which leptin resistance is frequently found [22]. Similarly, the psychoactive drug Δ9-tetrahydrocannabinol present in marijuana may exert its appetite stimulating effect by acting on hypothalamic CB1 receptors [23].
2. Methods
2.1. Ethics statement and animal maintenance
All experimental protocols have been approved by the Ministry of Health of Italy. Weaned (5–9 weeks of age) ob/ob mice [24] and their wt littermates were used. Mice were weaned at P23, bred in-house, kept in same-sex groups of maximal 6 mice under a light/dark cycle of 12 h (light on at 8 am). Mice had free access to water and were fed ad libitum with standard rodent lab chow. A total of 51 wt and 47 ob/ob mice have been utilized.
2.2. Slice preparation
Slices were cut as previously described [7]. In brief, mice were anesthetized by inhalation of ether or ketamine i.p. injection (100 mg/kg), transcardially perfused with ice-cold dissection solution, the brains quickly removed and hypothalamic 200–300 μm thick coronal slices were cut in ice-cold dissection solution (Vibratome Series 1000, St. Louis, MO, USA). Slices were then incubated in artificial cerebral spinal fluid (aCSF) at 32 °C for 30 min and subsequently kept at room temperature. Recordings were performed at room temperature in a submerged chamber superfused with aCSF (4 ml min−1). Solutions were equilibrated with 95% O2 and 5% CO2; details in Cristino et al. [7].
2.3. Electrophysiology
Whole-cell patch-clamp recordings were performed during the animals' light phase with an Axopatch 200B amplifier (Axon Instruments, CA, U.S.A.) and 4–7 MΩ recording electrodes. Neurons were visualized with differential interference contrast optics and an infrared video camera (Hamamatsu, Japan). Recordings were filtered at 2–10 kHz and digitized at 20 kHz (Digidata 1322A, Axon Instr.).
For recording miniature excitatory postsynaptic currents (mEPSCs), evoked excitatory postsynaptic currents (eEPSCs), and evoked post synaptic potentials (ePSPs), the internal solution consisted of (in mM): KMeSO4 135, MgCl2 4, HEPES 10, Na-GTP 0.4, Na-ATP 4, phosphocreatine disodium salt 10, and EGTA 0.2–0.5, pH 7.3 with KOH, 320 mOsm/L. Liquid junction potential (LJP) 8.2 mV, corrected online. For recording resting membrane potential (RMP) and action potential (AP) firing activity, the internal solution consisted of (in mM): K-gluconate 125, KCl 5, CaCl2 0.5, HEPES 10, BAPTA 5, Tris-GTP 0.33, Mg-ATP 5, pH 7.3 with KOH, 280 mOsm/L. LJP 12.1 mV, corrected offline. For recording evoked inhibitory postsynaptic currents (eIPSC), the internal solution consisted of (in mM): KCl 145, HEPES 10, EGTA 0.2, Mg-ATP 2, Na-GTP 0.5, pH 7.3 with KOH, 290 mOsm/L.
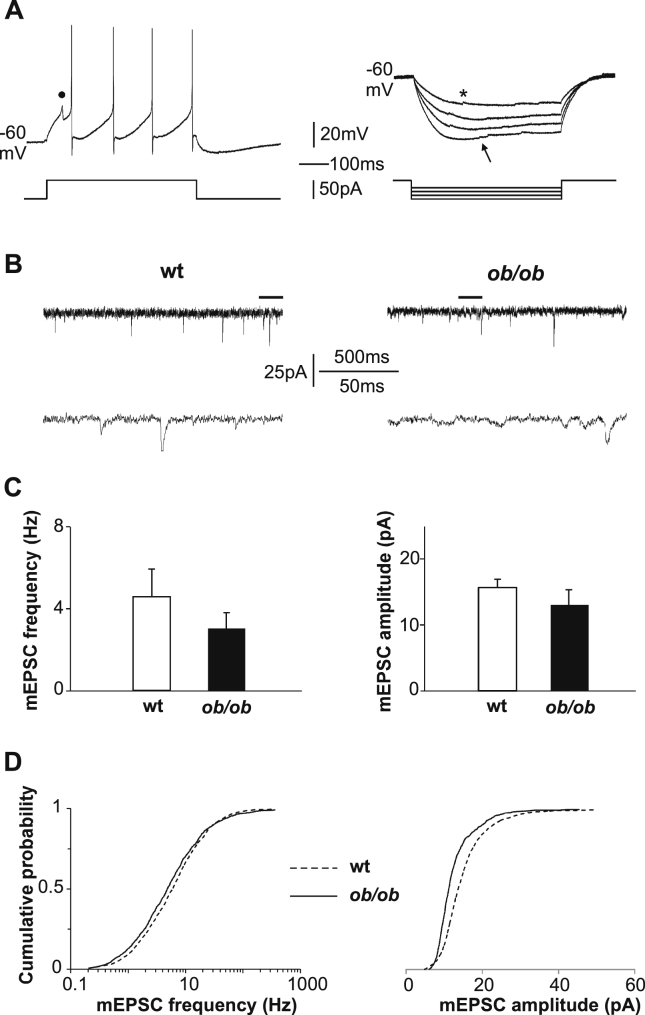
According to several studies performed both in mice [25], [7], [26] and in rats [27], [28], OX immunopositive neurons are concentrated in the perifornical area of the LH and are functionally characterized by the following voltage responses to injected currents: i) a tonic, non-adaptive repetitive spiking in response to a supra-threshold depolarizing pulse; ii) a time- and voltage-dependent rectification of membrane potential in response to a hyperpolarizing pulse; iii) abortive spikes evoked by a sub-threshold depolarizing pulse (Figure 1A). We accepted as orexinergic those neurons that met at least 2 of the above 3 criteria. Using this approach we previously demonstrated 96% of concordance between electrophysiologically- and immunohistochemically-identified OX neurons [7]. In the present study, 46% (n = 264 over 572) of the recorded neurons were electrophysiologically identified as OX neurons. We rejected the recordings in which the access resistance changed by more than 20% of its initial value and analyzed a total of 94 wt and 88 ob/ob OX neurons.
Figure 1.
Adult ob/ob OX neurons have less functional excitatory synapses than wt neurons. (A) Electrophysiological criteria utilized to identify hypothalamic OX neurons. Left: representative voltage recording of tonic non-adapting AP firing (note the regular inter-spike time intervals) and of an abortive spike (black dot) in response to a depolarizing current pulse. Right: four superimposed representative voltage recordings characterized by a “sag” at negative potentials (arrow). The traces also show several spontaneous excitatory postsynaptic potentials (an example is indicated by *). (B) Representative recordings of mEPSCs from OX neurons voltage-clamped at −70 mV. Lower traces: expansions of the segments indicated with black lines in the upper traces. (C) Mean ± SEM frequency (left graph) and amplitude (right graph) of mEPSCs in wt and ob/ob OX neurons (frequency, wt vs ob/ob: 4.6 ± 1.34 Hz, n = 10/6 vs 3.0 ± 0.75 Hz, n = 7/5, U = 32.5, p = 0.8; amplitude, wt vs ob/ob: 15.8 ± 1.41 pA vs 13.1 ± 2.1 pA, U = 53.0, p = 0.08). (D) Cumulative probability distribution of mEPSCs frequency (left, semi-logarithmic) and amplitude (right), showing smaller values in ob/ob OX neurons (frequency: p ≤ 0.003; amplitude: p ≤ 0.001).
mEPSCs were recorded in the presence of bicuculline (30 μM, Tocris) and tetrodotoxin (TTX, 0.5 μM, Tocris, UK), and neurons were voltage-clamped at −70 mV. mEPSCs that exceeded 3× the electrical noise (calculated as current root mean square) were measured in a 1 min-long trace, 5 min after the administration of TTX.
eEPSCs (recorded in the presence of 30 μM bicuculline), eIPSCs (recorded in the presence of 10 mM NBQX and CPP, Sigma, MO, U.S.A.), and ePSPs (no synaptic blockers) were evoked by electrical stimulation with a bipolar concentric tungsten electrode (WPI, FL, U.S.A.) placed 80 - 100 μm away from the target neuron at an intensity sufficient to evoke either a synaptic current of about 100 pA (for eEPSCs and eIPSCs) or a synaptic potential not larger than 10 mV (for ePSPs) (time interval between stimuli: 4–5 s for eEPSC and eIPSC, 1 s for ePSP; pulse duration: 100–200 μs; pulse amplitude: 40–100 μA; stimulator: Master-8 and stimulus isolation unit: ISO-Flex, AMPI, Israel). For eEPSCs and eIPSCs, the neurons were voltage-clamped at −70 mV and the synaptic responses recorded for 1 min, both before and 5 min after the administration of 5 μM WIN or WIN +4 μM AM251 (AM, Tocris). ePSPs were recorded before and after a voltage step-depolarization (5 s of duration) from RMP to 0 mV. We averaged together the responses to two successive depolarizations (separated by 90 s). In a subset of cells, the same experiment was repeated 5 min after adding 4 μM AM.
RMP and AP firing activity were recorded from OX neurons that displayed a stable RMP for at least 5 min. The effect of WIN on RMP and AP firing was determined 5 min after the administration of the drug.
2.4. Statistics
Data are presented as mean ± standard error of the mean (SEM), and n = number of neurons/number of mice. Differences between wt and ob/ob OX neurons were analyzed using two-sample t-test, whereas differences between baseline activity and activity after drug treatment were analyzed using paired t-test. When the data were not normally distributed, either the Mann–Whitney U test or the Wilcoxon Signed Rank test were used (indicated in the text with the symbols “U” or “Z”, respectively). The cumulative probabilities of mEPSCs frequencies and amplitudes were analyzed using the two-sided Kolmogorov–Smirnov test. Correlation between mEPSCs decay time and amplitude was calculated using Spearman correlations. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Reduced functional excitatory innervation of OX neurons in ob/ob mice
We estimated the amount of functional excitatory innervation of OX neurons in the LH by whole-cell voltage-clamp recording of mEPSCs (examples in Figure 1B).
Average mEPSCs frequency and amplitude showed no difference between wt and ob/ob (Figure 1C). However, the cumulative distributions of mEPSC events revealed lower frequencies and smaller amplitudes in ob/ob mice compared to wt (Figure 1D) with modest but statistically significant differences.
To confirm that the measured electric events were all mEPSCs, we observed no mEPSCs when recording in the presence of glutamatergic synaptic blockers (NBQX, CPP). Further confirmation was the lack of correlation between decay time and amplitude of mEPSCs (wt: r = 0.04; ob/ob: r = 0.23), with no difference between ob/ob and wt mice (U = 654500, p = 0.3).
The reduced excitatory (present study and [7]) and the increased inhibitory [7] innervation of ob/ob OX neurons compared to wt resulted in different synaptic voltage responses (evoked postsynaptic potentials, ePSPs) when the excitatory and inhibitory synapses were simultaneously stimulated (no synaptic blockers present in the bath). In wt mice, predominantly fast depolarizing ePSPs were recorded, while the responses in ob/ob were on average smaller and with a slower rising phase (amplitude of wt vs ob/ob: 8.4 ± 1.06 mV, n = 16/7 vs 5.9 ± 0.69 mV, n = 15/8, p = 0.03; slope of the rising phase of wt vs ob/ob: 3.7 ± 1.03 mV/ms, n = 15/7 vs 1.4 ± 0.24 mV/ms, n = 14/8, p = 0.02).
3.2. The CB1 receptor agonist WIN depresses excitatory and inhibitory innervation of OX neurons differently in ob/ob and in wt mice
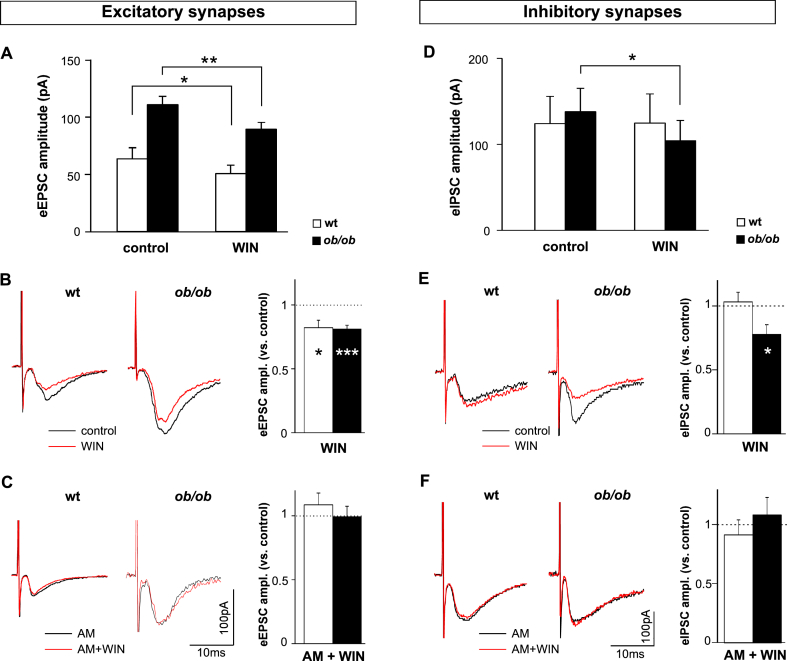
WIN, by means of its agonistic effect on presynaptic CB1 receptors, mimics the depressing effect of the physiologic endocannabinoid 2-AG on synaptic vesicle release. We studied the effect of WIN on electrically evoked excitatory and inhibitory synaptic currents (eEPSCs, eIPSCs, respectively).
WIN reduced eEPSCs amplitude by 20% both in wt and in ob/ob mice (Figure 2A,B). In striking contrast, eIPSCs of ob/ob and wt OX neurons were differently modulated by WIN. In fact, they were not affected in wt mice, but were significantly depressed in ob/ob (Figure 2D,E). For both eEPSCs and eIPSCs, the effect of WIN was dependent on the activation of presynaptic CB1 receptors, since it was prevented by the CB1 receptor blocker AM (Figure 2C,F). These data on wt animals are consistent with the depressing effect of 2-AG on the frequency of sEPSCs but not on the frequency of sIPSCs [29].
Figure 2.
Different modulation of eEPSCs and eIPSCs by WIN in wt and in ob/ob OX neurons. (A, B, C): effect of WIN on excitatory synapses. (D, E, F): effect of WIN on inhibitory synapses. (A) Mean ± SEM amplitude of eEPSCs in control conditions and in the presence of WIN, in wt and ob/ob OX neurons (wt, control vs WIN: 65 ± 9.9 pA vs 50 ± 7.3 pA, n = 8/7, Z = 1.0, p = 0.046; ob/ob, control vs WIN: 111 ± 7.2 pA vs 89 ± 6.0 pA, n = 5/5, t = 4.8, p = 0.009). Note that WIN is effective at reducing the amplitude of excitatory synaptic currents both in wt and in ob/ob OX neurons. (B) Superimposed representative recordings of eEPSCs in OX neurons of wt (left) and ob/ob (middle) animals in control conditions and in the presence of WIN. Right: average effect of WIN on eEPSCs amplitude relative to control, in wt and ob/ob OX neurons. Same data as in A (wt: t = 2.8, p = 0.039; ob/ob: t = 5.7, p = 0.005). (C) Blocking CB1 receptors with AM prevents the effect of WIN. Superimposed representative recordings of eEPSCs evoked as in panel B, but in the presence of AM only, or AM + WIN. Traces of ob/ob animals have been low-pass filtered at 1 KHz off-line. Right: average effect of AM + WIN on eEPSCs amplitude relative to control, in wt (1.1 ± 0.09, n = 4/3, t = 0.9, p = 0.4), and ob/ob (1.0 ± 0.08, n = 3/2, t = 0.08, p = 0.9) OX neurons. (D) Same as in panel A, but relative to eIPSCs (wt, control vs WIN: 124 ± 31.7 pA, 125 ± 33.8 pA, n = 7/4, p = 1.0; ob/ob, control vs WIN: 137 ± 27.0 pA, 104 ± 23.5 pA, n = 7/5, p = 0.02). Note that WIN is effective at reducing the synaptic responses of inhibitory inputs only in ob/ob neurons. (E) Same as in panel B, but relative to eIPSCs. Note the large effect in ob/ob neurons and the null effect in wt neurons (graph statistics: wt 1.0 ± 0.08, n = 7/4, p = 0.7; ob/ob 0.8 ± 0.08, n = 7/5, p = 0.03). (F) Same as in panel C, but relative to eIPSCs (graph statistics: wt 0.9 ± 0.13, n = 3/2, p = 0.6; ob/ob 1.1 ± 0.15, n = 6/4, p = 0.5). The chloride-mediated eIPSC appears as an inward current because of the high KCl concentration in the intracellular recording solution and the negative holding potential. Each trace is the average of 8–12 consecutive eEPSCs or eIPSCs. Stimulus artifact peaks truncated. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005.
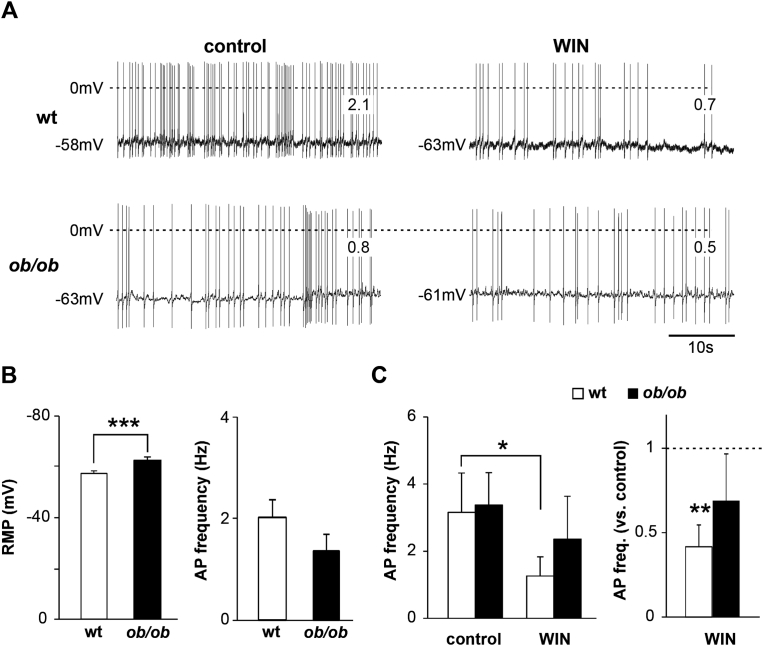
3.3. Tonic inhibition of OX neurons in ob/ob mice at rest
RMP was on average more negative in ob/ob compared to wt. Consistent with this finding, the AP firing frequency was lower in ob/ob OX neurons, although the difference was not statistically significant (Figure 3A,B). Both results indicate that, in our in vitro conditions and at rest (that is with no electrically-evoked synaptic activity), OX neurons of ob/ob mice have a lower level of activity compared to wt. This may depend on their abundant inhibitory and reduced excitatory synaptic innervation relative to wt.
Figure 3.
ob/ob OX neurons are hyperpolarized and less active at rest. Endocannabinoids hyperpolarize and reduce the activity of wt neurons. (A) Representative voltage recordings of wt (upper traces) and ob/ob (lower traces) OX neurons at their spontaneous membrane potential (indicated with the negative values left to each trace), both before (control) and after the addition of WIN. Figures within traces indicate the AP frequencies (Hz). Note the higher AP frequency in control wt compared to control ob/ob, and the inhibiting effect of WIN in wt neurons (hyperpolarization and reduction of AP frequency). (B) Mean ± SEM RMP (left graph) and AP frequency (right graph) of wt and ob/ob OX neurons, showing hyperpolarized ob/ob neurons (RMP: wt −57 ± 1.1 mV, n = 53/18; ob/ob −62 ± 1.3, n = 50/15, p ≤ 0.005. AP frequency: wt 2.0 ± 0.35 Hz, n = 53/18; ob/ob: 1.4 ± 0.33 Hz, n = 50/15, p ≥ 0.1). (C) Left graph, mean ± SEM absolute AP frequencies of wt and ob/ob OX neurons before and after the addition of WIN (wt, control vs WIN: 3.2 ± 1.17 Hz vs 1.3 ± 0.57 Hz, n = 6/5, p = 0.049; ob/ob, control vs WIN: 3.4 ± 0.96 Hz vs 2.4 ± 1.28 Hz, n = 5/3, p = 0.3). Right graph, same data in the presence of WIN and relative to control (wt: 0.4 ± 0.13, p = 0.006; ob/ob: 0.7 ± 0.28, p = 0.3). Note that WIN reduces the activity of wt neurons only. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005.
WIN, by inhibiting specifically the excitatory synapses in wt mice, has been described to hyperpolarize OX neurons by about 4 mV and, consequently, to reduce their AP firing frequency [29]. We confirmed this finding, although the effect was small and statistically not significant, (hyperpolarization of 0.6 ± 0.91 mV, n = 12/8, p ≥ 0.5), and found no effect in ob/ob (hyperpolarization of 0.3 ± 0.65 mV, n = 14/7, p ≥ 0.8; examples in Figure 3A). Regarding the AP firing frequency, we tested WIN in neurons with spike frequency ≥0.5 Hz in control condition. The result was clear only in wt, where 5 out of 6 neurons showed a significant reduction, and one showed no effect (60% of average reduction, p ≤ 0.01; Figure 3C). Instead, in a total of 5 ob/ob neurons, WIN reduced the firing frequency in 3, increased it in one and had no effect in the remaining cell (average 30% of reduction, not significant, p ≥ 0.3; Figure 3C). This result is consistent with the notion that, in wt OX neurons, only the excitatory inputs, and not the inhibitory ones, are sensitive to the depressing effect of WIN (Figure 2, and [29]), whereas in ob/ob neurons, WIN depresses the excitatory and the inhibitory axonal terminals simultaneously (Figure 2), with a null net effect on RMP and AP firing.
3.4. Activity-dependent disinhibition of OX neurons in ob/ob mice
Although in vitro ob/ob OX neurons are hyperpolarized and have lower AP firing activity compared to wt, the large amount of OX released in ob/ob mice [7], [21] is an indication that, in vivo, OX neurons might have a higher level of activity.
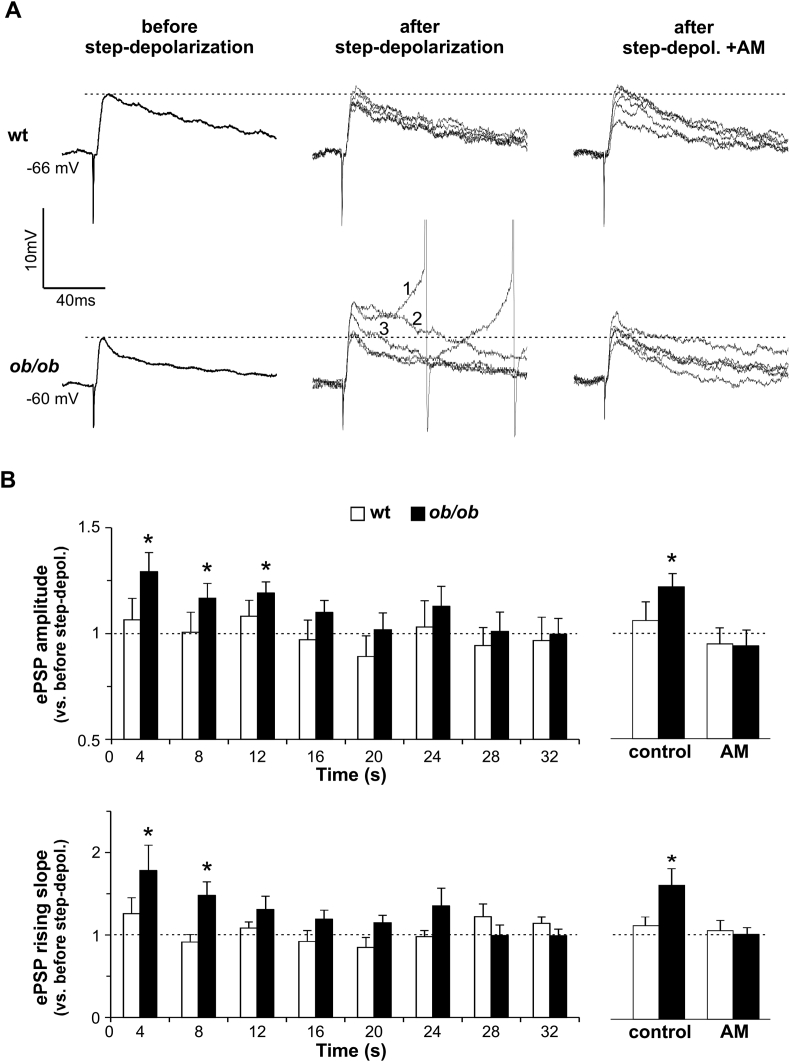
With the aim of mimicking a physiologic level of activation similar to the in vivo condition, we stimulated excitatory and inhibitory synapses simultaneously (ePSPs) and measured the effect of 2-AG released by OX neurons on ePSPs' amplitude and slope of the rising phase.
In control condition, ePSPs were smaller and with a slower rising phase in ob/ob compared to wt, as described in Section 3.1. This finding is consistent with the strong inhibitory synaptic component of ob/ob. Interestingly, after the release of 2-AG induced by a step-depolarization of OX neurons to 0 mV, the ePSPs became larger and with a faster rise time (i.e. more depolarizing) in ob/ob OX neurons only. This effect lasted for about 15 s, and was mediated by presynaptic CB1 receptors (Figure 4A,B).
Figure 4.
The physiologic release of 2-AG depolarizes and activates specifically ob/ob OX neurons. (A) Left and middle traces: representative voltage recordings of ePSPs evoked in wt (upper traces) and ob/ob (lower traces) OX neurons before and after a brief voltage step-depolarization to 0 mV. Left: each trace is the average of 10 successive ePSPs recorded immediately before the step-depolarization. Middle: 5 superimposed traces are shown in each row, recorded within the first 10s after the step-depolarization; each trace is the average of 2 successive ePSPs. Neurons are at RMP (indicated at far left). Note that in ob/ob neurons, the first 6 ePSPs evoked after the step depolarization (for clarity indicated as average traces 1, 2 and 3) are significantly larger than before depolarization. Note also that in average trace 1, the depolarization is strong enough to induce spikes (their peaks truncated). Right traces: ePSPs evoked after the step-depolarization but in the presence of the CB1 receptor blocker AM (same neurons of left and middle traces; traces are acquired and averaged as indicated before). In this condition, ePSPs are as large as before the depolarization. The dotted lines mark, as reference, the peak voltage level reached by ePSPs before the step-depolarization. Between-stimuli time interval: 1 s. (B) Left graphs: mean ± SEM ePSPs amplitudes (upper graph) and slopes of the rising phase (lower graph) acquired after the step-depolarization (applied at time “0”). Each column averages 4 successive ePSPs recorded in 9 and 10 neurons (wt and ob/ob, respectively; between-stimuli time interval: 1s), and the value is made relative to the average value of ePSPs recorded during the last 10 s before the step-depolarization (indicated, as reference, by the dotted lines). Note the increment of amplitude and of slope of the rising phase after time “0” in ob/ob only, lasting about 15s. Right graphs: comparison of the effect of the step-depolarization on ePSPs amplitude and slope, both in control conditions (same data as in B left) and in the presence of AM. Each bar is the average of 10 successive ePSPs (between-stimuli time interval: 1s) relative to the average value of ePSPs recorded during the last 10s before the step-depolarization. Note again the significant increase of ePSPs amplitude and slope only in ob/ob OX neurons in control condition (amplitude: wt 1.06 ± 0.08, n = 9/3, p = 0.5; ob/ob 1.22 ± 0.06, n = 10/5, p = 0.01; slope: wt 1.12 ± 0.10, p = 0.3; ob/ob 1.61 ± 0.20, p = 0.01). Note also that AM prevents the effect (amplitude: wt 0.95 ± 0.08, n = 4/3, p ≥ 0.5; ob/ob 0.94 ± 0.07, n = 5/3, p ≥ 0.4; slope: wt 1.07 ± 0.12, p ≥ 0.6; ob/ob 1.02 ± 0.08, p ≥ 0.7). *p ≤ 0.05.
It has already been mentioned that, in wt OX neurons, 2-AG reduces the frequency of sEPSCs [29], an indication of its depressing effect on the excitatory synapses. However, we have shown here that 2-AG has no depressing effect on ePSPs amplitude and slope in wt (Figure 4). This apparent discrepancy could be explained by considering the different recording conditions. In fact, sEPSCs were recorded by voltage-clamping the neuron at a constant negative potential [29] that prevented the spontaneous release of 2-AG, which was released only on demand during the test step-depolarization. On the contrary, ePSPs were recorded in current-clamp (present paper), and the membrane potential was free to fluctuate with frequent spontaneous depolarizations and AP firing. This was likely to cause a constant leakage of 2-AG from the cell into the extracellular space until 2-AG, which is synthesized in small amount in wt, was depleted, and not enough was left available to be released during the test step-depolarization. Ob/ob OX neurons were less affected by this problem because they produce larger amounts of 2-AG [7].
4. Discussion
In leptin-deficient ob/ob mice, OX neurons switch their adult innervation from predominantly excitatory (typical of wt mice) to predominantly inhibitory [7]. Here we have shown that the functional excitatory innervation is reduced, although modestly, in ob/ob compared to wt mice.
The hypothalamic circuitries are strongly modulated, via the CB1 receptor, by the endocannabinoid 2-AG, which is found in the LH along with its synthesizing (diacylglycerol lipase-α) and degrading (monoacylglycerol lipase) enzymes [7], [30]. In fact, in the LH of wt animals, the activity-dependent 2-AG release by OX neurons is known to induce the depression of excitatory synapses [29]. We confirmed this finding and extended it to the ob/ob animals, by showing that a cannabinoid receptor agonist (WIN) reduces the eEPSCs amplitude in wt as well as in ob/ob mice. It is also known that 2-AG has no effect on the inhibitory synapses of wt animals [7], [29]. Accordingly, we found that WIN had no effect on the inhibitory synapses of wt animals. However, WIN significantly depressed the inhibitory synapses of ob/ob mice. The latter finding confirms the 2-AG-mediated reduction of the frequency of sIPSCs previously described in ob/ob mice only [7]. In summary, both the excitatory and the inhibitory synapses of ob/ob OX neurons are sensitive to the depressing effect of WIN.
Given the predominance of inhibition, it is not surprising that ob/ob OX neurons exhibit a more negative RMP compared to wt neurons and fire less APs per unit time. At first, these findings could lead to the conclusion that ob/ob OX neurons are also less active than wt in vivo. However, we found indirect in vitro evidence of the contrary. In fact, when we induced these cells to spontaneously release 2-AG, the effect on ePSPs of wt neurons was null, whereas in ob/ob neurons the ePSPs were enhanced: the depolarizing voltage response to the synaptic activation became more rapid and larger. Thus, in ob/ob mice, the physiologic release of 2-AG by OX neurons, although capable of depressing both the excitatory and the inhibitory inputs (on the base of the effect of WIN on eEPSCs and eIPSCs), results mainly in the depression of inhibition (i.e. disinhibition), with the consequence that the excitatory drive on OX neurons becomes functionally predominant. Several lines of evidence support this interpretation. First, in ob/ob mice hypothalamic 2-AG levels are up-regulated [31], [32]. In particular, OX neurons produce more 2-AG, and the CB1 receptors are concentrated at the inhibitory presynaptic terminals [7]. Second, in ob/ob mice the inhibitory synapses outnumber the excitatory ones at OX neurons and are retrogradely depressed (disinhibition) by the 2-AG release [7]. Third, the exciting (by means of disinhibition) effect of 2-AG here recorded on the ePSPs of ob/ob OX neurons has a time course (about 15s) comparable to that usually found in 2-AG-mediated modulation of synaptic potentials or currents in several brain areas, LH included [7], [33], [34]. Fourth, this effect is prevented by the CB1 antagonist AM. Finally, the enhancement of OX trafficking and release to LH target brain areas in ob/ob mice [7], [35] supports the occurrence of a stronger activity of OX neurons in ob/ob compared to wt mice.
Based on these evidences, we speculate that, in the in vivo condition of leptin signal deficiency typical of obesity and fasting, the prevalence of the inhibitory drive hyperpolarizes OX neurons. However, when OX neurons are depolarized by the activity of the excitatory inputs (which are only modestly reduced in ob/ob compared to wt mice), the same OX neurons release a significant amount of 2-AG (which is produced in high concentrations in ob/ob mice) and disinhibition occurs. This positive-feedback mechanism is present only in leptin-deficient (and possibly leptin-insensitive HFD) obese mice, and favors the activation of OX neurons. On the contrary, in wt lean mice, 2-AG mediates a negative-feedback control of neuronal activity, by depressing the function of the excitatory inputs.
Recent in vivo recordings during food presentation to wt mice have reported a rapid transition from increased to reduced activity of OX neurons upon food presentation and food ingestion, respectively [36]. The negative-feedback phenomenon described above for wt mice may provide, at least in part, a mechanistic explanation of this finding. It remains to be elucidated the in vivo activity level of OX neurons in the condition of chronic absence of leptin, with the positive-feedback control of neuronal activity.
In conclusion, our data support the notion that leptin is an essential hormone for the regulation of the hypothalamic synaptic arrangement and its functional endocannabinoid-dependent modulation. Chronic absence of leptin causes a dysregulation of the endocannabinoid system in OX neurons, along with a substantial alteration of their synaptic inputs, ultimately leading to an increase of the AP firing activity of the same type of neurons (to be demonstrated). The leptin-deficient ob/ob animal model of obesity shares similarities with the most diffuse human type of obesity by mimicking the condition of hypothalamic leptin-resistance that is frequently present in obese subjects. Of social relevance, the pharmacologic manipulation of the orexinergic system may be useful for the treatment of this type of obesity.
Author contribution
TMB, MF, and GB performed the experiments and analyzed the data; VDM, LC, and GB designed the experiments; TMB, MF, VDM, LC, and GB wrote the paper.
Acknowledgments
We thank Alberto Cangiano for the helpful revision of the manuscript. We also thank Mr. Marco Veronese for preparing the figures. The authors received no specific funding for this work.
Conflict of interest
None declared.
References
- 1.Bonnavion P., Mickelsen L.E., Fujita A., de Lecea L., Jackson A.C. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. Journal of Physiology. 2016;594(22):6443–6462. doi: 10.1113/JP271946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broberger C., de Lecea L., Sutcliffe J.G., Hokfelt T. Hypocretin/Orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and Agouti gene -related protein systems. Journal of Comparative Neurology. 1998;402:460–474. [PubMed] [Google Scholar]
- 3.de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautvik K.M., de Lecea L., Gautvik V.T., Danielson P.E., Tranque P., Dopazo A. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 7.Cristino L., Busetto G., Imperatore R., Ferrandino I., Palomba L., Silvestri C. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):E2229–E2238. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores A., Maldonado R., Berrendero F. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Frontiers in Neuroscience. 2013;7:256. doi: 10.3389/fnins.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujino N., Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Frontiers in Behavioral Neuroscience. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahler S.V., Moorman D.E., Smith R.J., James M.H., Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nature Neuroscience. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweet D.C., Levine A.S., Billington C.J., Kotz C.M. Feeding response to central orexins. Brain Research. 1999;821(2):535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist J.K., Maratos-Flier E., Saper C.B., Flier J.S. Unraveling the central nervous system pathways underlying responses to leptin. Nature Neuroscience. 1998;1(6):445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P., Bing C. Appetite and energy balance signals from adipocytes. Philosophical Transactions of the Royal Society of London Biological Sciences. 2006;361(1471):1237–1249. doi: 10.1098/rstb.2006.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goforth P.B., Leinninger G.M., Patterson C.M., Satin L.S., Myers M.G. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. Journal of Neuroscience. 2014;34(34):11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka A., Beuckmann C.T., Willie J.T., Hara J., Tsujino N., Mieda M. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 16.Diano S., Horvath B., Urbanski H.F., Sotonyi P., Horvath T.L. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144(9):3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 17.Leinninger G.M., Opland D.M., Jo Y.H., Faouzi M., Christensen L., Cappellucci L.A. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metabolism. 2011;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 19.Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 20.Horvath T.L., Gao X.B. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metabolism. 2005;1(4):279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Cristino L., Luongo L., Imperatore R., Boccella S., Becker T., Morello G. Orexin-A and endocannabinoid activation of the descending antinociceptive pathway underlies altered pain perception in leptin signaling deficiency. Neuropsychopharmacology. 2016;41(2):508–520. doi: 10.1038/npp.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteleone A.M., Di Marzo V., Monteleone P., Dalle Grave R., Aveta T., Ghoch M.E. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. European Journal of Nutrition. 2016;55(4):1799–1805. doi: 10.1007/s00394-016-1153-9. [DOI] [PubMed] [Google Scholar]
- 23.Koch M., Varela L., Kim J.G., Kim J.D., Hernández-Nuño F., Simonds S.E. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519(7541):45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 25.Burdakov D., Alexopoulos H., Vincent A., Ashcroft F.M. Low-voltage-activated A-current controls the firing dynamics of mouse hypothalamic orexin neurons. European Journal of Neuroscience. 2004;20(12):3281–3285. doi: 10.1111/j.1460-9568.2004.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Gao X.B., Sakurai T., van den Pol A.N. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36(6):1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 27.Eggermann E., Bayer L., Serafin M., Saint-Mleux B., Bernheim L., Machard D. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. Journal of Neuroscience. 2003;23(5):1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons M.P., Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. Journal of Neuroscience. 2010;30(24):8061–8070. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H., Acuna-Goycolea C., Li Y., Cheng H.M., Obrietan K., van den Pol A.N. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. Journal of Neuroscience. 2007;27(18):4870–4881. doi: 10.1523/JNEUROSCI.0732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittmann G., Deli L., Kalló I., Hrabovszky E., Watanabe M., Liposits Z. Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. Journal of Comparative Neurology. 2007;503(2):270–279. doi: 10.1002/cne.21383. [DOI] [PubMed] [Google Scholar]
- 31.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 32.Di Marzo V., Matias I. Endocannabinoid control of food intake and energy balance. Nature Neuroscience. 2005;8(5):585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- 33.Safo P.K., Cravatt B.F., Regehr W.G. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5(2):134–145. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- 34.Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 35.Morello G., Imperatore R., Palomba L., Finelli C., Labruna G., Pasanisi F. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(17):4759–4764. doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González J.A., Jensen L.T., Iordanidou P., Strom M., Fugger L., Burdakov D. Inhibitory interplay between orexin neurons and eating. Current Biology. 2016;26(18):2486–2491. doi: 10.1016/j.cub.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]