ABSTRACT
The impact of bacterial species on outcome in bloodstream infections (BSI) is incompletely understood. We evaluated the impact of bacterial species on BSI mortality, with adjustment for patient, bacterial, and treatment factors. From 2002 to 2015, all adult inpatients with monomicrobial BSI caused by Staphylococcus aureus or Gram-negative bacteria at Duke University Medical Center were prospectively enrolled. Kaplan-Meier curves and multivariable Cox regression with propensity score models were used to examine species-specific bacterial BSI mortality. Of the 2,659 enrolled patients, 999 (38%) were infected with S. aureus, and 1,660 (62%) were infected with Gram-negative bacteria. Among patients with Gram-negative BSI, Enterobacteriaceae (81% [1,343/1,660]) were most commonly isolated, followed by non-lactose-fermenting Gram-negative bacteria (16% [262/1,660]). Of the 999 S. aureus BSI isolates, 507 (51%) were methicillin resistant. Of the 1,660 Gram-negative BSI isolates, 500 (30%) were multidrug resistant. The unadjusted time-to-mortality among patients with Gram-negative BSI was shorter than that of patients with S. aureus BSI (P = 0.003), due to increased mortality in patients with non-lactose-fermenting Gram-negative BSI generally (P < 0.0001) and Pseudomonas aeruginosa BSI (n = 158) in particular (P < 0.0001). After adjustment for patient demographics, medical comorbidities, bacterial antibiotic resistance, timing of appropriate antibiotic therapy, and source control in patients with line-associated BSI, P. aeruginosa BSI remained significantly associated with increased mortality (hazard ratio = 1.435; 95% confidence interval = 1.043 to 1.933; P = 0.02). P. aeruginosa BSI was associated with increased mortality relative to S. aureus or other Gram-negative BSI. This effect persisted after adjustment for patient, bacterial, and treatment factors.
KEYWORDS: Gram negative, Pseudomonas aeruginosa, Staphylococcus aureus, bloodstream infections
INTRODUCTION
Bacterial bloodstream infections (BSI) are a leading cause of morbidity and mortality in contemporary medical practice (1–3). The determinants of poor outcome in patients with BSI are among the most intensely investigated areas of infectious diseases. Although the intrinsic characteristics of bacterial species are thought to influence clinical outcomes, there is surprisingly little clinical data to support this impression. Several studies have shown that patients with Pseudomonas aeruginosa BSI experienced increased crude mortality relative to BSI caused by other bacterial species (4–9). However, these studies were unable to discriminate how much of this clinical outcome, if any, was attributable to the intrinsic virulence of P. aeruginosa rather than patient or treatment factors. Thus, the impact of the infecting bacterial pathogen on species-specific differences in patient outcomes of BSI mortality is not clear. This question is of increasing importance since several therapeutic agents designed to treat or prevent a single bacterial species, including P. aeruginosa, are under development (10, 11). In this study, we evaluated relationships between BSI outcomes and infection etiology. We used a prospectively ascertained cohort of 2,659 hospitalized patients with BSI over a 13-year period from a single center to address the hypothesis that P. aeruginosa BSI will be associated with a significantly higher in-hospital mortality relative to other bacterial causes of BSI after adjusting for patient demographics, medical comorbidities, bacterial antibiotic resistance, and treatment factors.
RESULTS
Epidemiology of bacterial BSI.
There were 2,659 adult inpatients with monomicrobial S. aureus or Gram-negative BSI identified from 1/2002 to 7/2015. Of these 2,659 patients, 999 (38%) had S. aureus BSI and 1,660 (62%) had Gram-negative BSI. There were significant demographic differences between the two BSI cohorts (Table 1). Relative to patients with Gram-negative BSI, patients with S. aureus BSI were younger (mean 57.7 versus 61.1 years; P < 0.0001), more often black (38% versus 29%; P < 0.0001), and more likely to have diabetes mellitus (42% versus 34%; P < 0.0001), require hemodialysis (21% versus 10%; P < 0.0001), and have a community-acquired infection (75% versus 69%; P = 0.002). Patients with Gram-negative BSI were more likely to have had a solid organ or hematopoietic transplant (14% versus 8%; P < 0.0001) and a history of a neoplasm (38% versus 21%; P < 0.0001). There were also major differences in the BSI source. Patients with S. aureus BSI, relative to patients with Gram-negative BSI, more often had line-associated (26% versus 11%) or skin/soft tissue infections (16% versus 6%) and less often had a urinary tract infection/pyelonephritis (1% versus 31%) or biliary tract infection (<1% versus 6%; P < 0.0001). Patient characteristics among those with Gram-negative BSI, stratified by bacterial group (e.g., Enterobacteriaceae, non-lactose fermenters, etc.), are listed in Table S1 in the supplemental material.
TABLE 1.
Demographics of patients with S. aureus and Gram-negative bloodstream infections at Duke University Medical Center from 2002 to 2015
| Parameter | No. (%) of patients |
Pa | |
|---|---|---|---|
| S. aureus (n = 999) | Gram negative (n = 1,660) | ||
| Mean age (yrs) | 57.7 | 61.1 | <0.0001 |
| Race/ethnicity | |||
| White | 597 (60) | 1,105 (67) | <0.0001 |
| Black | 377 (38) | 474 (29) | |
| Hispanic | 3 (<1) | 10 (1) | |
| Native American | 9 (1) | 12 (1) | |
| Asian | 4 (<1) | 23 (1) | |
| Other/unknown | 9 (1) | 36 (2) | |
| Female | 446 (45) | 752 (45) | 0.75 |
| Medical history | |||
| Recent glucocorticoid use | 222 (22) | 409 (25) | 0.16 |
| Neoplasm | 210 (21) | 639 (38) | <0.0001 |
| Diabetes mellitus | 418 (42) | 568 (34) | <0.0001 |
| Transplant | 84 (8) | 226 (14) | <0.0001 |
| Surgery in past 30 days | 272 (27) | 432 (26) | 0.50 |
| Hemodialysis dependence | 214 (21) | 160 (10) | <0.0001 |
| Rheumatologic disorder | 30 (3) | 39 (2) | 0.32 |
| Site of acquisition | |||
| Community acquired | 746 (75) | 1,147 (69) | 0.002 |
| Hospital acquired | 241 (24) | 513 (31) | |
| Source of infection | |||
| Pneumonia | 100 (10) | 122 (7) | <0.0001 |
| Urine/pyelonephritis | 5 (1) | 522 (31) | |
| Abscess | 38 (4) | 80 (5) | |
| Line associated | 263 (26) | 187 (11) | |
| Skin/soft tissue | 164 (16) | 95 (6) | |
| Biliary tract | 4 (<1) | 95 (6) | |
| Other | 185 (19) | 193 (12) | |
| Source not identified | 240 (24) | 366 (22) | |
| Mean APACHE-II score | 15.3 | 14.8 | 0.04 |
| Mean chronic APACHE-II score | 3.9 | 3.6 | 0.31 |
Statistically significant values are indicated in boldface.
There were also significant differences between patients with P. aeruginosa BSI and non-P. aeruginosa BSI (Table 2). Patients with P. aeruginosa BSI, relative to those with non-P. aeruginosa BSI, were more often white (77% versus 63%; P = 0.001) and more often had used glucocorticoids in the past 30 days (37% versus 23%; P = 0.001), a history of neoplasm (41% versus 31%; P = 0.02) or transplant (25% versus 11%; P < 0.0001), hospital-acquired infections (51% versus 27%; P < 0.0001), and higher total APACHE-II score (mean 16.3 versus 14.4; P = 0.0003) and chronic health APACHE-II score (mean 4.2 versus 3.6; P = 0.0003). Patients with P. aeruginosa BSI less often were hemodialysis dependent (8% versus 14%; P = 0.03). There were also significant differences in the source of BSI, since patients with P. aeruginosa BSI, relative to those with non-P. aeruginosa BSI, were more likely to have a pneumonia source (25% versus 8%) and less likely to have a line (9% versus 17%) or skin/soft tissue source (6% versus 10%; P < 0.0001).
TABLE 2.
Demographics of patients with P. aeruginosa and non-P. aeruginosa bloodstream infections at Duke University Medical Center from 2002 to 2015, stratified by bacterial group
| Parameter | No. (%) of patients |
Pa | |
|---|---|---|---|
| P. aeruginosa (n = 158) | Non-P. aeruginosa (n = 2,501) | ||
| Mean age (yrs) | 61.9 | 59.7 | 0.13 |
| Race/ethnicity | 0.001 | ||
| White | 121 (77) | 1,581 (63) | |
| Black | 29 (18) | 832 (33) | |
| Hispanic | 0 (0) | 13 (1) | |
| Native American | 0 (0) | 21 (1) | |
| Asian | 1 (1) | 26 (1) | |
| Other/unknown | 7 (4) | 38 (2) | |
| Female | 64 (41) | 1,134 (45) | 0.25 |
| Medical history | |||
| Recent glucocorticoid use | 59 (37) | 572 (23) | 0.001 |
| Neoplasm | 64 (41) | 785 (31) | 0.02 |
| Diabetes mellitus | 53 (34) | 933 (37) | 0.35 |
| Transplant | 39 (25) | 271 (11) | <0.0001 |
| Surgery in past 30 days | 48 (30) | 656 (26) | 0.26 |
| Hemodialysis dependence | 13 (8) | 361 (14) | 0.03 |
| Rheumatologic disorder | 6 (4) | 63 (3) | 0.30 |
| Site of acquisition | <0.0001 | ||
| Community acquired | 78 (49) | 1,815 (73) | |
| Hospital acquired | 80 (51) | 674 (27) | |
| Source of infection | <0.0001 | ||
| Pneumonia | 39 (25) | 192 (8) | |
| Urine/pyelonephritis | 30 (19) | 488 (20) | |
| Abscess | 6 (4) | 112 (4) | |
| Line associated | 14 (9) | 436 (17) | |
| Skin/soft tissue | 10 (6) | 249 (10) | |
| Biliary tract | 4 (3) | 95 (4) | |
| Other | 21 (13) | 357 (14) | |
| Source not identified | 34 (22) | 572 (23) | |
| Mean total APACHE-II score | 16.3 | 14.4 | 0.0003 |
| Mean chronic APACHE-II score | 4.2 | 3.6 | 0.0003 |
Statistically significant values are indicated in boldface.
Antimicrobial resistance patterns.
Of the 999 S. aureus BSI isolates, 507 (51%) were methicillin-resistant S. aureus. Of the 1,660 Gram-negative BSI isolates, 500 were MDR isolates (30%). Both Enterobacteriaceae and non-lactose-fermenting Gram-negative bacteria exhibited species-specific differences in MDR prevalence (Table 3). Among the Enterobacteriaceae, the MDR phenotype was most common among Citrobacter freundii (64% [18/28]), followed by Providencia stuartii (57% [4/7]) and Escherichia coli (45% [259/577]). Among non-lactose-fermenting Gram-negative bacteria, several species, including Achromobacter species (3/3), Alcaligenes species (3/3), and Chryseobacterium species (2/2), exhibited a 100% MDR phenotype. Of note, there were no Enterobacteriaceae PDR isolates, though four PDR isolates (2% [4/262]) from the non-lactose-fermenting Gram-negative bacteria group were isolated. In P. aeruginosa, the MDR phenotype was present in 28% (45/158) of isolates, and the XDR phenotype was present in 15% (23/158) of the isolates. Figure S1 in the supplemental material shows data on MDR trends for BSI isolates of S. aureus, Enterobacteriaceae, and P. aeruginosa.
TABLE 3.
Antimicrobial susceptibility of Gram-negative bloodstream infection isolates at Duke University Medical Center from 2002 to 2015a
| Organism | No. of isolates detected/total no. of isolates examined (%) |
||
|---|---|---|---|
| MDR | XDR | PDR | |
| Enterobacteriaceae | 420/1,343 (31) | 4/1,343 (<1) | 0/1,343 (0) |
| Escherichia coli | 259/577 (45) | 0/577 (0) | 0/577 (0) |
| Klebsiella pneumoniae | 57/307 (19) | 2/307 (1) | 0/307 (0) |
| Proteus mirabilis | 10/65 (15) | 0/65 (0) | 0/65 (0) |
| Serratia marcescens | 5/97 (5) | 0/97 (0) | 0/97 (0) |
| Klebsiella oxytoca | 9/42 (21) | 0/42 (0) | 0/42 (0) |
| Enterobacter cloacae | 34/106 (32) | 2/106 (2) | 0/106 (0) |
| Enterobacter aerogenes | 15/43 (34) | 0/43 (0) | 0/43 (0) |
| Salmonella species | 7/27 (26) | 0/27 (0) | 0/27 (0) |
| Citrobacter freundii | 18/28 (64) | 0/28 (0) | 0/28 (0) |
| Citrobacter koseri | 0/9 (0) | 0/9 (0) | 0/9 (0) |
| Morganella morganii | 1/13 (8) | 0/10 (0) | 0/13 (0) |
| Pantoea agglomerans | 1/5 (20) | 0/5 (0) | 0/(0) |
| Providencia rettgeri | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Providencia stuartii | 4/7 (57) | 0/7 (0) | 0/7 (0) |
| Other Enterobacteriaceae | 1/14 (7) | 0/14 (0) | 0/14 (0) |
| Non-lactose fermenters | 80/262 (31) | 46/262 (18) | 4/262 (2) |
| Pseudomonas aeruginosa | 45/158 (28) | 23/158 (15) | 0/158 (0) |
| Acinetobacter species | 5/26 (19) | 4/26 (15) | 2/26 (8) |
| Stenotrophomonas maltophilia | 8/26 (31) | 8/26 (31) | 0/26 (0) |
| Burkholderia species | 4/9 (44) | 4/9 (44) | 1/9 (11) |
| Ochrobactrum species | 5/6 (83) | 0/6 (0) | 0/6 (0) |
| Sphingomonas species | ND/6 | ND/6 | ND/6 |
| Achromobacter species | 3/3 (100) | 2/3 (67) | 1/3 (33) |
| Alcaligenes species | 3/3 (100) | 1/3 (33) | 0/3 (0) |
| Moraxella species | ND/2 | ND/2 | ND/2 |
| Roseomonas species | ND/4 | ND/4 | ND/4 |
| Brevundimonas species | 2/3 (67) | 0/3 (0) | 0/3 (0) |
| Bordetella species | ND/2 | ND/2 | ND/2 |
| Chryseobacterium species | 2/2 (100) | 1/2 (50) | 0/2 (0) |
| Other non-lactose fermenters | 5/12 (42) | 3/12 (25) | 0/12 (0) |
Antimicrobial susceptibility profiles for anaerobes and Pasteurellaceae are not included here since antimicrobial susceptibility testing for these bacteria are not routinely performed. Abbreviations: MDR, multidrug resistant; ND, not determined (by the Duke Clinical Microbiology Laboratory); PDR, pandrug resistant; XDR, extensively drug resistant.
Mortality.
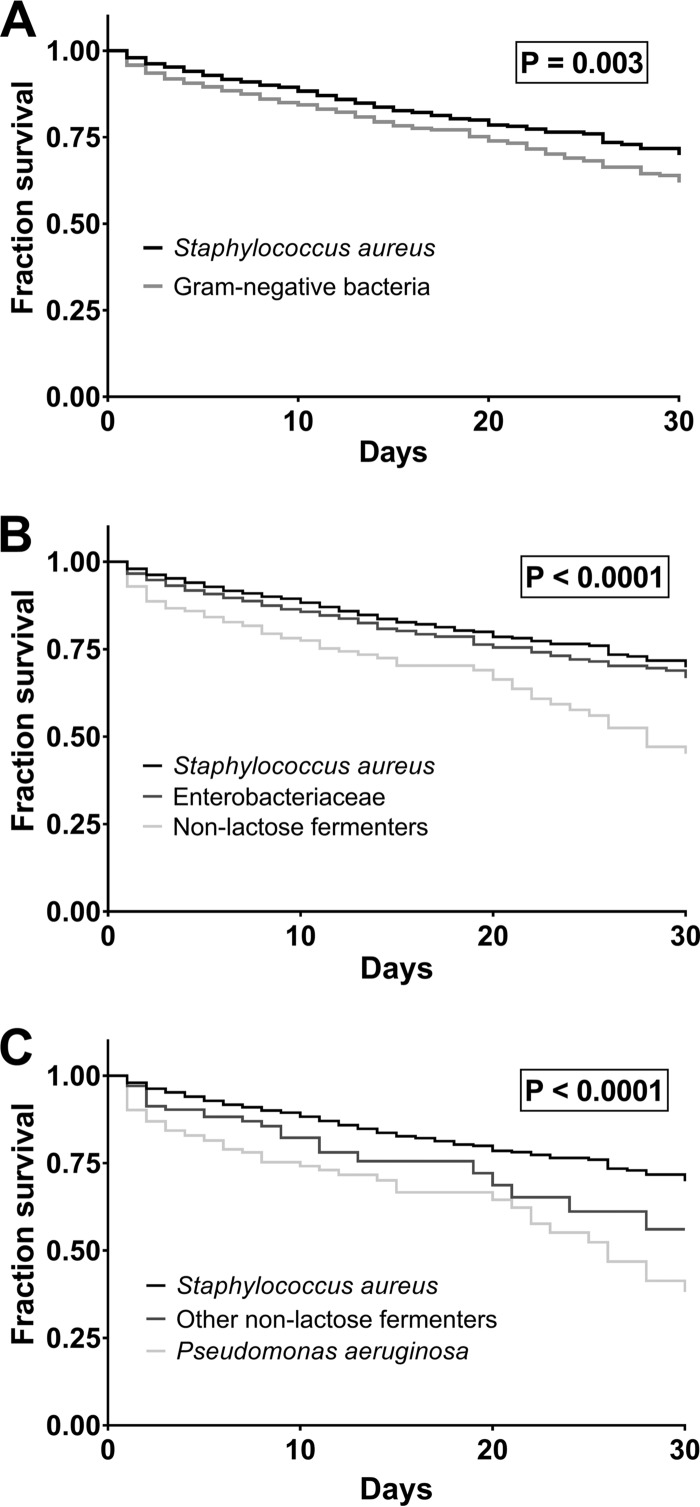
Kaplan-Meier curves were generated based on all-cause in-hospital mortality from the date of the index positive blood culture. Of note, Gram-negative BSI was associated with shorter time to mortality relative to S. aureus BSI (P = 0.003) (Fig. 1A). To further determine which group of Gram-negative bacteria was responsible for this observed difference in mortality, patients with Gram-negative BSI were divided into those with Enterobacteriaceae versus non-lactose-fermenting Gram-negative BSI, that together account for 97% of all Gram-negative BSI in this cohort. Time to mortality in patients with non-lactose-fermenting Gram-negative BSI was substantially decreased relative to patients with Enterobacteriaceae or S. aureus BSI (P < 0.0001) (Fig. 1B). There was no significant decrease in time to mortality for patients with Enterobacteriaceae BSI relative to S. aureus (BSI) (P = 0.08). Further stratification of patients with non-lactose-fermenting Gram-negative BSI into P. aeruginosa and other non-lactose-fermenting Gram-negative BSI illustrated that P. aeruginosa BSI was associated with a shorter time to mortality than either the S. aureus or other non-lactose-fermenting Gram-negative BSI (P < 0.0001) (Fig. 1C). Patients with non-P. aeruginosa Gram-negative BSI, relative to those with S. aureus BSI, also had decreased time to mortality (P = 0.03). Of note, these findings did not differ when all instances of bacterial BSI were included for each patient (i.e., each bacterial BSI event from patients with multiple such events over the study period were included).
FIG 1.
Survival analysis of patients with bacterial bloodstream infections (BSI). (A) Survival in patients with BSI caused by Gram-negative bacteria compared to that caused by S. aureus. (B) Survival in patients with BSI caused by Enterobacteriaceae, non-lactose-fermenting Gram-negative bacteria, or S. aureus. (C) Survival in patients with BSI caused by Pseudomonas aeruginosa, other non-lactose-fermenting Gram-negative bacteria, or S. aureus.
In order to better understand the factors contributing to the observed increased mortality in patients with P. aeruginosa BSI, we developed a multivariable Cox regression model that adjusts for patient demographics, medical comorbidities, bacterial antibiotic resistance, source of infection, timing of appropriate antibiotic therapy and line removal, and the era in which the BSI occurred (Table 4). In this adjusted analysis, P. aeruginosa BSI was associated with increased mortality (hazard ratio [HR] = 1.435; 95% confidence interval [CI] = 1.043 to 1.933; P = 0.021). Additional factors associated with increased mortality in patients with S. aureus or Gram-negative BSI include increased age (HR = 1.376; 95% CI = 1.286 to 1.475; P < 0.0001), increased APACHE-II chronic health score (HR = 1.168; 95% CI = 1.101 to 1.244; P < 0.0001), 2005-2015 BSI era (HR = 2.581; 95% CI = 1.863 to 3.690; P < 0.0001), pneumonia source of BSI (HR = 2.264; 95% CI = 1.628 to 3.169; P < 0.0001; reference BSI source: urine/pyelonephritis), and an unidentified BSI source (HR = 2.073; 95% CI = 1.549 to 2.810; P < 0.0001; reference BSI source: urine/pyelonephritis). Factors associated with decreased mortality included a skin/soft tissue BSI source (HR = 0.531; 95% CI = 0.322 to 0.847; P = 0.010; reference BSI source: urine/pyelonephritis). Of note, when a Cox regression model was repeated with patients from only the 2005-2015 BSI era, there was no significant change from that described above (see Table S2 in the supplemental material).
TABLE 4.
Multivariable Cox regression analysis of clinical and bacterial factors influencing Staphylococcus aureus and Gram-negative bloodstream infection mortality in inpatients at Duke University Medical Center from 2002 to 2015a
| Parameter | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age | 1.376 | 1.286–1.475 | <0.0001 |
| Female gender | 1.129 | 0.936–1.359 | 0.203 |
| Raceb | |||
| Black | 1.223 | 0.991–1.501 | 0.058 |
| Other | 0.804 | 0.485–1.253 | 0.366 |
| Pseudomonas aeruginosa BSI | 1.435 | 1.043–1.933 | 0.021 |
| APACHE-II chronic health score | 1.168 | 1.101–1.244 | <0.0001 |
| MDR | 1.051 | 0.869–1.269 | 0.609 |
| Appropriate antibiotic therapyc | 1.435 | 0.967–2.202 | 0.085 |
| Days to line removald | |||
| 1 | 0.537 | 0.192–1.305 | 0.194 |
| 2 | 0.606 | 0.232–1.424 | 0.271 |
| ≥3 | 0.904 | 0.259–2.468 | 0.857 |
| No line-associated infection | 7.227 | 0.392–37.881 | 0.060 |
| BSI era (2005-2015)e | 2.581 | 1.863–3.690 | <0.0001 |
| BSI sourcef | |||
| Biliary | 0.607 | 0.303–1.105 | 0.127 |
| Pneumonia | 2.264 | 1.628–3.169 | <0.0001 |
| Line | 5.411 | 0.299–26.650 | 0.103 |
| Skin/soft tissue | 0.531 | 0.322–0.847 | 0.010 |
| Abscess | 0.721 | 0.387–1.254 | 0.273 |
| Other | 1.035 | 0.726–1.476 | 0.850 |
| Source not identified | 2.073 | 1.549–2.810 | <0.0001 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; MDR, multidrug resistant. Statistically significant values are indicated in boldface.
The reference group was composed of white patients.
This is a time-dependent variable in which “no appropriate antibiotic therapy” is the reference.
The reference group is composed of patients with line-associated infections and line removal on day 0.
The reference group is composed of BSI from 2002 to 2004.
The reference group is BSI from a urine/pyelonephritis source.
Given that P. aeruginosa BSI was associated with increased hospital-acquired BSI relative to non-P. aeruginosa BSI (51% versus 27%; Table 2), we added an additional covariate, days from hospital admission to BSI, to the adjusted Cox regression model. This “days to BSI” covariate was associated with a small but statistically significant shorter time to mortality (HR = 1.005; 95% CI = 1.002 to 1.008; P = 0.001). When the “days to BSI” covariate was added to the Cox regression model, P. aeruginosa BSI was no longer associated with shorter time to mortality (HR = 1.335; 95% CI = 0.969 to 1.803; P = 0.067) (see Table S3 in the supplemental material).
DISCUSSION
Understanding species-specific clinical outcomes associated with bacterial BSI is of critical importance since it can influence our antibiotic therapy, how closely we monitor patients, and the development of pathogen-specific therapies (10, 11). Gaining insight into such species-specific outcomes is challenging given that bacterial species vary in their sources of BSI, target patients with different demographics and medical comorbidities, differ in their antimicrobial susceptibility profiles, and produce variable immune responses. Previous studies that have addressed species-specific differences in BSI mortality have varied widely in size and geography, addressed limited patient populations, commonly been retrospective in nature, and have not fully accounted for the important ways that patient demographics, medical comorbidities, antibiotic resistance, and treatment factors influence bacterial BSI outcomes (4–9, 12–21).
In this study, we report one of the most comprehensive analyses of species-specific differences in bacterial BSI mortality to date. First, our cohort is among the largest reported cohorts of prospectively enrolled patients with bacterial BSI. This large sample size resulted in sufficient power to detect species-specific differences while correcting for all relevant other variables. Second, the patient population that was evaluated—all inpatients with bacterial BSI at a single large medical center from 2002 to 2015—is broader than prior studies which have generally performed more focused analyses on specific patient subpopulations (e.g., nosocomially acquired infections, etc.) in a more narrow time window. Third, and perhaps most important, we performed a comprehensive analysis that adjusts for patient demographics, medical comorbidities, bacterial antimicrobial resistance, and treatment factors (e.g., appropriate antibiotic therapy and source control). Taken together, we performed an extensive analysis on a broad cohort of patients with bacterial BSI in order to better understand the bacterial species associated increased mortality and the factors that contribute to that mortality.
In the present study, we found that patients with Gram-negative BSI had increased mortality relative to S. aureus. This stemmed from non-lactose-fermenting Gram-negative BSI generally, although patients with P. aeruginosa BSI in particular had a shorter time to mortality relative to other non-lactose-fermenting Gram-negative BSI. In a multivariable Cox regression model that included patient demographics, patient medical comorbidities (chronic health APACHE-II score), antibacterial resistance, and treatment factors, P. aeruginosa BSI was associated with increased mortality relative to non-P. aeruginosa BSI. Interestingly, adjustment for timing of BSI (i.e., days to BSI from hospital admission) did partially account for the shorter time to mortality associated with P. aeruginosa BSI, though even with inclusion of this covariate a borderline association between P. aeruginosa BSI and shorter time to mortality was present (HR = 1.335; P = 0.067). Patients with P. aeruginosa BSI, relative to those with non-P. aeruginosa BSI, more often had hospital-acquired BSI (51% versus 27%). Prior studies have demonstrated hospital-acquired infections to be associated with increased mortality (22–24). Although the overall effect of adding the “days to BSI” covariate on the P. aeruginosa BSI hazard ratio was small (Tables 4 and see Table S3 in the supplemental material), it is certainly possible that patients with hospital-acquired infections may have additional risks for poor outcome that were not fully accounted for in our multivariable Cox regression model. For example, patients with hospital-acquired infections are likely to be more acutely ill at the time of BSI. We did not account for degree of acute illness in our adjusted model (e.g., APACHE-II acute physiology score) since measures of acute illness cannot clearly separate the influence of the BSI from other acute medical issues. In addition, as described below, the chronic health APACHE-II score is a broad measure of chronic health/immunosuppression and likely does not fully account for the complexities of a patient's chronic medical history.
Several studies have demonstrated increased crude mortality in P. aeruginosa BSI relative to other bacterial infections (6–9, 12), while others have demonstrated increased crude mortality with A. baumannii (15, 17) or S. aureus infections (19, 20). However, adjustment for patient, bacterial, and treatment factors is important for several reasons. First, P. aeruginosa commonly infects those that are chronically ill or immunosuppressed, and these patients are at high risk of mortality regardless of bacterial etiology. Second, P. aeruginosa is often associated with high antibiotic resistance, and the associated delay in appropriate therapy can increase mortality. For example, Ani et al. published a large retrospective study in which the U.S. “nationwide inpatient sample” database was queried to identify patients who were hospitalized for “severe sepsis” and noted that crude mortality associated with P. aeruginosa infections (29.5%) was higher than that of all Gram-negative species and on par with that of S. aureus infections (30.9%) (12). However, after adjustment for factors such as the Charlson comorbidity index and the number of organ failures, P. aeruginosa infection was associated with improved mortality relative to other bacterial species (HR = 0.78; 95% CI = 0.78 to 0.81). In contrast to Ani et al., we found that the increased mortality with P. aeruginosa BSI persisted after adjustment for patient demographics, medical comorbidities, antibiotic resistance, and treatment factors. There are several explanations for this difference. First, the patient population described in Ani et al. consists of those with ICD-9 codes involving sepsis and is likely a substantially different population from that described in this work, which is restricted to patients with documented BSI. Second, Ani et al. conducted a retrospective study that relied on proper ICD-9 coding from a diverse set of investigators. Only 38.5% of the identified cases of severe sepsis were associated with an organism, and this missing data makes it difficult to broadly generalize their findings. Third, Ani et al. adjusted for the “number of organ failures,” and this may bias the findings since P. aeruginosa likely causes such high mortality in part through septic shock and subsequent organ compromise.
Several additional smaller studies have addressed P. aeruginosa BSI mortality using adjusted models. Kang et al. (n = 286; 74 patients with P. aeruginosa BSI) and Micek et al. (n = 535; 114 patients with P. aeruginosa BSI) conducted retrospective studies that examined P. aeruginosa, Enterobacteriaceae, and A. baumannii (Micek et al. only) BSI mortality, and each study found P. aeruginosa infection to be associated with increased mortality in adjusted models (6, 21). S. aureus was not included in these analyses, however. Osmon et al. compared P. aeruginosa and S. aureus BSI outcome (n = 314; 49 patients with P. aeruginosa BSI), although the outcome of interest in the adjusted model was “response to therapy after 48 h” rather than mortality (8). P. aeruginosa was associated with a lack of response to therapy in this model. No data regarding adjusted mortality outcome was presented. Finally, Lambert et al. published a large, multinational study of intensive care unit (ICU) patients throughout Europe (n = 1,351 with BSI; 357 patients with P. aeruginosa BSI) with P. aeruginosa, A. baumannii, E. coli, or S. aureus BSI (15). The outcome in Lambert et al. differed from this study in that ICU mortality was considered rather than in-hospital mortality. No direct statistical comparisons between bacterial species were presented, though ICU mortality hazard ratios were highest for A. baumannii (HR = 3.3 to 4.4, depending on antimicrobial resistance phenotype) relative to P. aeruginosa (HR ranging from 3.2 to 4.0), E. coli (HR ranging from 2.7 to 3.6), or S. aureus (HR ranging from 2.1 to 3.3). However, direct comparison between Lambert et al. and this study is complicated by the difference in patient populations and mortality outcome. Lambert et al. considered only patients in the ICU, who were more acutely ill and immunosuppressed than the patients examined here. Several smaller studies performed adjusted analyses that examined the impact of bacterial species on BSI outcomes, although they found no association between P. aeruginosa and increased mortality (14, 18).
This study has several limitations. First, the data come from a single institution. However, given that this is one of the largest cohorts of prospectively enrolled patients with bacterial BSI over a significant time period (2002 to 2015), we believe that these data makes a significant contribution to our understanding of how bacterial species influences BSI mortality. Second, there are additional bacterial species of potential interest that were not included in this study, such as Enterococcus species and coagulase-negative staphylococci. We do not routinely collect data from these patients and so they could not be included. Nevertheless, we feel that the species that were included, S. aureus and all Gram-negative bacteria, encompass the vast majority of clinically relevant species that cause bacterial BSI. Third, the covariate that was used to account for patient medical comorbidities in our adjusted analysis, i.e., the APACHE-II chronic health score, does not fully address the complex influence of medical comorbidities and immunosuppression on BSI outcomes and is a potential source of unknown confounders. Nevertheless, we believe that the APACHE-II chronic health score is appropriate in that it allows us to identify patients at high risk of BSI complications while avoiding the statistical problems that arise when an excessive number of covariates representing individual medical conditions are included in the model. Finally, information regarding source control could not be obtained in patients with no identifiable source of BSI. While the inclusion of such patients is a potential source of bias, we believe that inclusion of this cohort is important as it reflects the complexity of modern medical practice in which many potential sources (e.g., multiple lines, urinary catheter, endotracheal tube, etc.) are present, and a single one cannot be positively identified. In the present study, we found that an unidentified source of BSI was associated with increased mortality. This has been noted in prior studies as well (1, 25, 26) and may reflect inadequate source control in patients with an unknown focus of BSI.
In conclusion, we show that the bacterial BSI mortality associated with P. aeruginosa is higher than that of other Gram-negative bacteria and S. aureus and that this effect persisted after adjustment for patient demographics, medical comorbidities, bacterial antibiotic resistance, and treatment factors. Thus, we attribute this increased mortality to greater P. aeruginosa virulence in BSI relative to other bacterial species. Additional study is needed to identify the treatment factors that are needed to improve outcomes in P. aeruginosa BSI.
MATERIALS AND METHODS
Patient clinical data.
From 1 January 2002 to 1 July 2015, all adult inpatients with monomicrobial BSI due to either Staphylococcus aureus or Gram-negative bacteria at Duke University Medical Center were prospectively evaluated. Patients who met these inclusion criteria and provided written informed consent (from patient or legally authorized representative) were enrolled. In patients with multiple hospitalizations with positive blood cultures, only the first such hospitalization was considered. Patients with neutropenia were excluded from this study. Detailed clinical data, including patient characteristics, treatment patterns, and in-hospital outcomes were collected on a standardized case report form and entered into an electronic database. This study had full approval from the Duke Institutional Review Board.
Determination of bacterial species and antibiotic resistance phenotypes.
All bacteria BSI isolates were speciated by the Duke Clinical Microbiology Laboratory using standard techniques. MIC values were determined by the Duke Clinical Microbiology Laboratory with the broth dilution technique, as described previously (27). MIC breakpoint values for each antibiotic were defined according to the most recent Clinical and Laboratory Standards Institute (CLSI) guidelines. The antibacterial susceptibility profile of each bacterial isolate, regardless of the year it was isolated, was defined according to the latest CLSI guidelines. Antimicrobial resistance phenotypes were defined as previously detailed (28). Briefly, the multidrug-resistant phenotype (MDR) was defined as nonsusceptible to at least one agent in ≥3 relevant antimicrobial categories. The extensively drug-resistant (XDR) phenotype was defined as susceptibility to at least one agent in ≤2 appropriate antimicrobial categories. The pandrug-resistant (PDR) phenotype was defined as resistance to all agents in all appropriate antimicrobial categories. For S. aureus, Enterobacteriaceae, P. aeruginosa, and Acinetobacter species, appropriate antimicrobial categories have been previously defined (28). For the remaining bacterial species (e.g., Stenotrophomonas maltophilia), appropriate antimicrobial categories were defined as those with defined CLSI MIC breakpoints for the particular bacterium (29).
Definitions.
The primary endpoint of this study was all-cause mortality. Patients were monitored through hospital discharge. The “route” of infection refers to whether BSI was hospital-acquired or community-acquired. Hospital-acquired infection was defined as an infection beginning ≥48 h after hospital admission (30). All other infections were defined as community-acquired. The “source” of infection refers to the primary focus of the BSI (e.g., urine/pyelonephritis, line, etc.). The infection source was defined retrospectively according to review of the medical record by one of the investigators (J.T.T.). APACHE-II scores were calculated on the day of the index positive blood culture (31). “Appropriate antibiotic therapy” is defined as receipt of an antibiotic to which the bacteria are susceptible. Appropriate antibiotic therapy was determined daily from the date of the index positive blood culture (day 0) to hospital discharge or death. For line-associated infections, “days to line removal” is the number of days from the index positive blood culture to removal of the infected line. Both “appropriate antibiotic therapy” and “days to line removal” were determined retrospectively by review of the medical record by one of the investigators (J.T.T.). Two BSI “eras” were defined: (i) 2002 to 2004 and (ii) 2005 to 2015. In 2004 our enrollment practices changed in accordance with U.S. federal legislation (45 CFR 164.512), which allowed the enrollment of patients who died prior to providing informed consent.
Statistical analysis.
Baseline characteristics and clinical events are presented as means with standard deviation for continuous variables and frequencies with proportions for categorical variables. Statistical comparisons between groups were made with t tests for continuous variables, Fisher exact tests for categorical variables with 2×2 comparisons, and Pearson's chi-square tests for categorical variables when more than two levels are relevant. Kaplan-Meier survival curves were used to examine in-hospital mortality among bacterial groups or species, and the log-rank (Mantel-Cox) test was used to compare survival distributions. Multivariable Cox regression models were fit to calculate a propensity score (probability) associated with mortality. Covariates included patient demographics (age, gender, and race/ethnicity), medical comorbidities (APACHE-II chronic health score), bacterial species, bacterial antibiotic resistance patterns (the presence of the MDR phenotype), BSI era, source of BSI, and treatment factors (days to appropriate antibiotic therapy, days to line removal for line-associated infections). In one model (see Table S3 in the supplemental material), the number of days from hospital admission to index blood culture (i.e., “days to BSI”) was also included as a covariate. These covariates were selected to broadly encompass the clinical factors known or thought to influence BSI outcome. The APACHE-II chronic health score covariate was used to describe degree of chronic illness since it captures severe organ dysfunction/immunosuppression and avoids the statistical problems that arise when multiple medical comorbidities are treated as separate covariates. The “appropriate antibiotic therapy” covariate was treated as a time-dependent variable. The “days to BSI” covariate was treated as a continuous variable. Statistical significance was determined at the P < 0.05 level.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by the National Institutes of Health (UM1 AI104681 to J.T.T., UM1 AI104681 and K24 AI093969 to V.G.F., and 5T32 AI052080-12 to S.A.M.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02671-16.
REFERENCES
- 1.Gikas A, Samonis G, Christidou A, Papadakis J, Kofteridis D, Tselentis Y, Tsaparas N. 1998. Gram-negative bacteremia in non-neutropenic patients: a 3-year review. Infection 26:155–159. doi: 10.1007/BF02771841. [DOI] [PubMed] [Google Scholar]
- 2.Uzun O, Akalin HE, Hayran M, Unal S. 1992. Factors influencing prognosis in bacteremia due to gram-negative organisms: evaluation of 448 episodes in a Turkish university hospital. Clin Infect Dis 15:866–873. doi: 10.1093/clind/15.5.866. [DOI] [PubMed] [Google Scholar]
- 3.Mylotte JM, McDermott C, Spooner JA. 1987. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis 9:891–907. doi: 10.1093/clinids/9.5.891. [DOI] [PubMed] [Google Scholar]
- 4.Esel D, Doganay M, Alp E, Sumerkan B. 2003. Prospective evaluation of blood cultures in a Turkish university hospital: epidemiology, microbiology and patient outcome. Clin Microbiol Infect 9:1038–1044. doi: 10.1046/j.1469-0691.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez C, Cobos-Trigueros N, Feher C, Morata L, De La Calle C, Marco F, Almela M, Soriano A, Mensa J, Del Rio A, Martinez JA. 2014. Community-onset bacteraemia of unknown origin: clinical characteristics, epidemiology and outcome. Eur J Clin Microbiol Infect Dis 33:1973–1980. doi: 10.1007/s10096-014-2146-3. [DOI] [PubMed] [Google Scholar]
- 6.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko HK, Yu WK, Lien TC, Wang JH, Slutsky AS, Zhang H, Kou YR. 2013. Intensive care unit-acquired bacteremia in mechanically ventilated patients: clinical features and outcomes. PLoS One 8:e83298. doi: 10.1371/journal.pone.0083298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osmon S, Ward S, Fraser VJ, Kollef MH. 2004. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest 125:607–616. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 9.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 10.Thaden JT, Keller AE, Shire NJ, Camara MM, Otterson L, Huband M, Guenther CM, Zhao W, Warrener P, Stover CK, Fowler VG Jr, DiGiandomenico A. 2016. Pseudomonas aeruginosa bacteremic patients exhibit nonprotective antibody titers against therapeutic antibody targets PcrV and Psl exopolysaccharide. J Infect Dis 213:640–648. doi: 10.1093/infdis/jiv436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cigana C, Bernardini F, Facchini M, Alcala-Franco B, Riva C, De Fino I, Rossi A, Ranucci S, Misson P, Chevalier E, Brodmann M, Schmitt M, Wach A, Dale GE, Obrecht D, Bragonzi A. 2016. Efficacy of the novel antibiotic POL7001 in preclinical models of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 60:4991–5000. doi: 10.1128/AAC.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ani C, Farshidpanah S, Bellinghausen Stewart A, Nguyen HB. 2015. Variations in organism-specific severe sepsis mortality in the United States: 1999-2008. Crit Care Med 43:65–77. doi: 10.1097/01.ccm.0000474082.02487.f6. [DOI] [PubMed] [Google Scholar]
- 13.Gatell JM, Trilla A, Latorre X, Almela M, Mensa J, Moreno A, Miro JM, Martinez JA, Jimenez de Anta MT, Soriano E. 1988. Nosocomial bacteremia in a large Spanish teaching hospital: analysis of factors influencing prognosis. Rev Infect Dis 10:203–210. doi: 10.1093/clinids/10.1.203. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 15.Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, Agodi A, Frank U, Mertens K, Schumacher M, Wolkewitz M. 2011. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 16.Leedahl DD, Personett HA, Gajic O, Kashyap R, Schramm GE. 2014. Predictors of mortality among bacteremic patients with septic shock receiving appropriate antimicrobial therapy. BMC Anesthesiol 14:21. doi: 10.1186/1471-2253-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra AR, Camargo LF, Pignatari AC, Sukiennik T, Behar PR, Medeiros EA, Ribeiro J, Girao E, Correa L, Guerra C, Brites C, Pereira CA, Carneiro I, Reis M, de Souza MA, Tranchesi R, Barata CU, Edmond MB, Brazilian SSG. 2011. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol 49:1866–1871. doi: 10.1128/JCM.00376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metan G, Demiraslan H, Kaynar LG, Zararsiz G, Alp E, Eser B. 2013. Factors influencing the early mortality in hematological malignancy patients with nosocomial Gram negative bacilli bacteremia: a retrospective analysis of 154 cases. Braz J Infect Dis 17:143–149. doi: 10.1016/j.bjid.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagao M. 2013. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect 19:852–858. doi: 10.1111/1469-0691.12083. [DOI] [PubMed] [Google Scholar]
- 20.Sundararajan V, Korman T, Macisaac C, Presneill JJ, Cade JF, Visvanathan K. 2006. The microbiology and outcome of sepsis in Victoria, Australia. Epidemiol Infect 134:307–314. doi: 10.1017/S0950268805004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, Hoppe-Bauer J, Dunne WM, Kollef MH. 2011. Resistance to empiric antimicrobial treatment predicts outcome in severe sepsis associated with Gram-negative bacteremia. J Hosp Med 6:405–410. doi: 10.1002/jhm.899. [DOI] [PubMed] [Google Scholar]
- 22.Hoenigl M, Wagner J, Raggam RB, Prueller F, Prattes J, Eigl S, Leitner E, Honigl K, Valentin T, Zollner-Schwetz I, Grisold AJ, Krause R. 2014. Characteristics of hospital-acquired and community-onset blood stream infections, South-East Austria. PLoS One 9:e104702. doi: 10.1371/journal.pone.0104702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Baño J, López-Prieto MD, Portillo MM, Retamar P, Natera C, Nuño E, Herrero M, del Arco A, Muñoz A, Téllez F, Torres-Tortosa M, Martín-Aspas A, Arroyo A, Ruiz A, Moya R, Corzo JE, León L, Pérez-López JA. 2010. Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clin Microbiol Infect 16:1408–1413. doi: 10.1111/j.1469-0691.2010.03089.x. [DOI] [PubMed] [Google Scholar]
- 24.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. 2003. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol 41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh CF, Chen KF, Ye JJ, Huang CT. 2014. Derivation of a clinical prediction rule for bloodstream infection mortality of patients visiting the emergency department based on predisposition, infection, response, and organ dysfunction concept. J Microbiol Immunol Infect 47:469–477. doi: 10.1016/j.jmii.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Bisbe J, Gatell JM, Puig J, Mallolas J, Martinez JA, Jimenez de Anta MT, Soriano E. 1988. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev Infect Dis 10:629–635. doi: 10.1093/clinids/10.3.629. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: 10th ed Document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement Document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.