ABSTRACT
Many infectious diseases are associated with multidrug-resistant (MDR) bacteria residing in biofilms that require high antibiotic concentrations. While oral drug delivery is frequently ineffective, topical treatments have the potential to deliver higher drug concentrations to the infection site while reducing systemic side effects. This study determined the antibiofilm activity of a surgical wound gel loaded with the iron chelator deferiprone (Def) and the heme analogue gallium-protoporphyrin (GaPP), alone and in combination with ciprofloxacin. Activity against MDR Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Acinetobacter johnsonii biofilms was assessed in the colony biofilm and artificial wound model by enumeration of CFU and correlative light/electron microscopy. While Staphylococcus biofilms were equally susceptible to GaPP and Def-GaPP gels (log10 reduction of 3.8 and 3.7, respectively), the Def-GaPP combination was crucial for significant activity against P. aeruginosa biofilms (log10 reduction of 1.3 for GaPP and 3.3 for Def-GaPP). When Def-GaPP gel was combined with ciprofloxacin, the efficacy exceeded the activity of the individual compounds. Def-GaPP delivered in a surgical wound gel showed significant antibiofilm activity against different MDR strains and could enhance the gel's wound-healing properties. Moreover, Def-GaPP indicated a potentiation of ciprofloxacin. This antibiofilm strategy has potential for clinical utilization as a therapy for topical biofilm-related infections.
KEYWORDS: antimicrobial combinations, biofilms, drug delivery, iron metabolism
INTRODUCTION
Medical treatments for chronic infectious diseases are typically based on oral delivery of high-dose, long-term antibiotic therapies. Despite the risk for emerging antimicrobial resistance and the potential of side effects (e.g., gastrointestinal disorders, neutropenia, nephrotoxicity), there is a lack of suitable alternatives. Depending on the disease nature and localization, topical treatments can deliver high dosages of antimicrobials directly to an infection site while reducing unwanted systemic effects. Higher drug dosages are particularly needed to combat microbial biofilms (1). The ability of bacteria to form biofilms and establish resistance to antibiotics is a major biomedical threat, adding billions of dollars to health care costs worldwide (1–3). Biofilms are responsible for 80% of microbial infections in humans and are a common cause of chronic infections, including chronic wound and chronic sinus infections (4, 5), with increasing tolerance and subtle resistance mechanisms to antibiotic therapies (6–8).
Topical delivery of antimicrobials to the nose and paranasal sinuses in nebulizers and irrigations has been reported to be beneficial against biofilm-associated chronic rhinosinusitis (9, 10). Another promising approach is the use of gels that can be directly instilled into the sinuses. Current phase I/II trials of a chitosan-dextran hydrogel demonstrate improved clinical outcomes after sinus surgery (11). When prepared in situ, succinyl-chitosan and dextran-aldehyde form a nontoxic, biocompatible, biodegradable gel that facilitates postoperative wound healing by promoting homeostasis and preventing adhesions (11–15). The latter is a particularly important postsurgical complication of endoscopic sinus surgery that frequently causes surgical failure (16). While the benefits of the blank gel have been proven in clinical practice (11), its antimicrobial potential as a drug-delivery system has not been fully explored. The incorporation of antimicrobials in the gel may enhance its clinical use and expand its application to other medical conditions, such as biofilm-associated wound infections.
In the present study, an antimicrobial strategy is evaluated using a surgical hydrogel loaded with the iron chelator deferiprone (Def) and the heme analogue gallium-protoporphyrin (GaPP) (17). Richter et al. recently reported on the in vitro activity of Def-GaPP against Staphylococcus aureus biofilms by interfering with bacterial iron metabolism (18). However, studies to date are based on the pure compounds in solution, and a translational drug-delivery strategy for clinical applications has not yet been investigated.
RESULTS
Drug release.
The Def-GaPP concentration in the release medium was expressed as the percentage of the original concentration in the gel. All Def was released from the gel within 48 to 72 h, while the release of GaPP gradually increased over time, reaching approximately 20% to 25% after 460 h (Fig. 1). These release profiles were independent of drug concentrations in the gels (Def, 20 mM; GaPP, 100 [GaPP 100] and 500 μg/ml [GaPP 500]). Interestingly, there was no statistical difference between the release of individual compounds and the release of the corresponding compounds from the combination gel.
FIG 1.
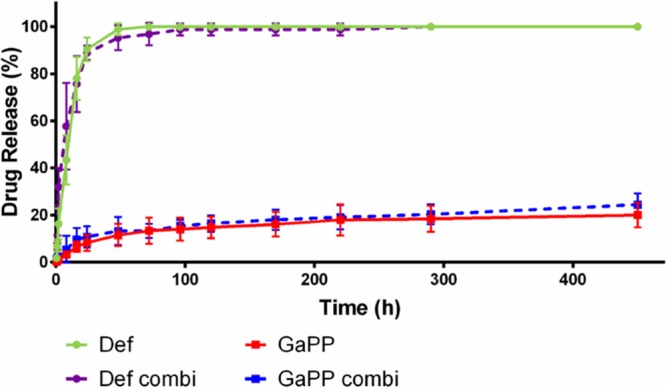
Release profiles of gels loaded with 20 mM deferiprone (Def, green circles), 500 μg/ml gallium-protoporphyrin (GaPP, red squares), or a combination of both (Def combi, purple circles; GaPP combi, blue squares, dotted lines). Data represent the mean ± SD from 3 replicates.
MIC.
The MICs against planktonic bacteria ranged from 87 μg/ml (Acinetobacter johnsonii) to 5,568 μg/ml (methicillin-resistant Staphylococcus aureus [MRSA]) for Def and from <0.1 μg/ml (Staphylococcus epidermidis) to >50 μg/ml (Pseudomonas aeruginosa PAO1) for GaPP (Table 1). When used in combination, the MICs for both compounds were typically lower, although the extent of this difference was strain dependent (Table 1).
TABLE 1.
MICs of deferiprone, gallium-protoporphyrin, and the combination of both compounds and ciprofloxacin
| Isolate | MIC (μg/ml) of: |
|||
|---|---|---|---|---|
| Def | GaPP | Combination Def-GaPP | Cip | |
| S. aureus ATCC 25923 | 2,784 | 12.5 | 696/6.25 | 0.125 |
| MRSA clinical isolate | 5,568 | 50 | 2,784/25 | 2 |
| S. epidermis ATCC 12228 | 696 | <0.1 | <10.8/<0.1 | 0.125 |
| P. aeruginosa PAO1 | 174 | >50 | 87/0.78 | 0.125 |
| P. aeruginosa clinical isolate | 348 | >50 | 87/0.78 | 0.125 |
| A. johnsonii ATCC 17946 | 87 | 0.78 | 87/0.78 | 0.03 |
Effect of loaded hydrogels on bacterial biofilms. (i) Agar diffusion model.
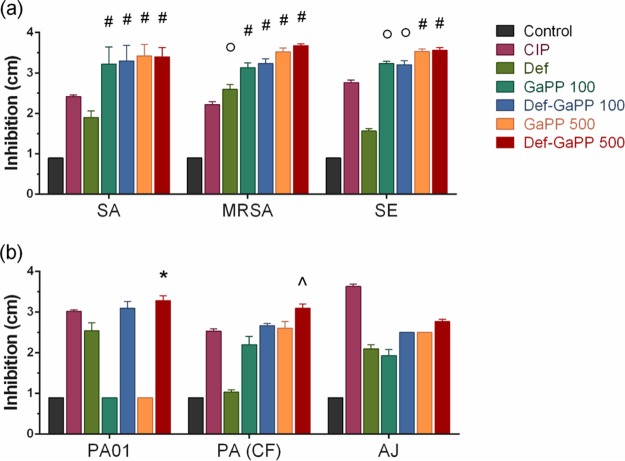
The growth inhibition of gels was determined in an agar diffusion model (Fig. 2), with blank gel as the negative control (no growth inhibition) and ciprofloxacin (Cip) gel as the positive control. The Def gel showed slight growth inhibition, while the GaPP gel showed substantial activity against all bacteria (up to 3.5 log10 reduction) except P. aeruginosa (no growth inhibition). When Def-GaPP were combined, the gel showed similar growth inhibition as that of the GaPP gel against Staphylococcus species, slightly higher inhibition against the clinical P. aeruginosa and A. johnsonii isolates, and substantially higher inhibition against P. aeruginosa PAO1 (3.3 log10 reduction) (Fig. 2).
FIG 2.
Inhibition zone diameter (cm) of Gram-positive (a) and Gram-negative (b) bacteria after exposure to loaded hydrogels. Strains used include S. aureus ATCC 25923 (SA), a clinical MRSA isolate (MRSA), S. epidermidis ATCC 12228 (SE), P. aeruginosa PAO1 (PAO1), a clinical P. aeruginosa isolate from a cystic fibrosis patient (PA [CF]), and A. johnsonii ATCC 17946 (AJ). Hydrogels include control, blank gel (black); Cip, ciprofloxacin, 5 μg/ml (pink); Def, deferiprone, 20 mM (light green); GaPP 100, gallium-protoporphyrin, 100 μg/ml (dark green); Def-GaPP 100 (blue); GaPP 500 (orange); and Def-GaPP 500 (red). Data represent the mean ± SD from 3 biological replicates. Statistical comparison to ciprofloxacin-loaded gel. *, P < 0.05; ○, P < 0.01; ^, P < 0.001; #, P < 0.0001.
(ii) Colony biofilm model.
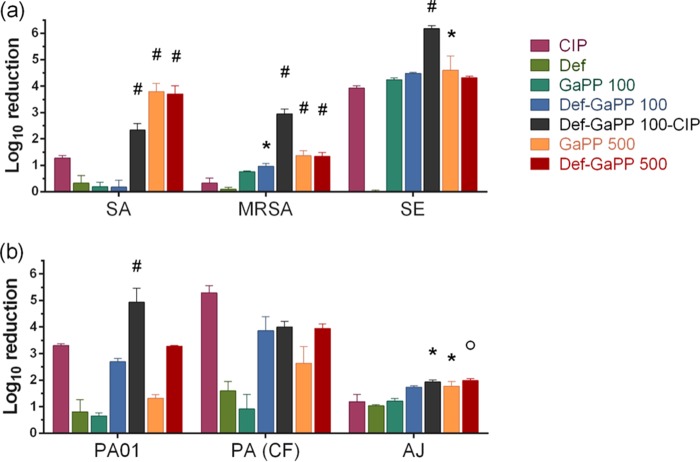
The blank gel showed no activity and the Def gel showed low activity against all biofilms (Fig. 3), while the effect of GaPP gel was concentration and strain dependent. In a low concentration, GaPP (100 μg/ml) demonstrated substantial activity against S. epidermidis biofilms only (4.3 log10 reduction), while at 500 μg/ml, GaPP was more active (log10 reduction of 3.8, 1.4, and 4.6 in S. aureus, MRSA, and S. epidermidis biofilms; log10 reduction of 1.3, 2.6, and 1.7 in two P. aeruginosa biofilms and one A. johnsonii biofilm). When Def and GaPP 500 were combined, the gel showed similar antibiofilm activity as that of GaPP 500 against S. aureus, MRSA, S. epidermidis, and A. johnsonii biofilms (log10 reduction of 3.8, 1.4, 4.3, and 2.0, respectively). In contrast, in two P. aeruginosa biofilms, the Def-GaPP 500 combination demonstrated higher activity than the individual compounds (log10 reduction of 3.3 and 3.9). The triple combination of Def, GaPP 100, and Cip in gel exceeded the antibiofilm activity of the individual compounds and Cip alone against all biofilms, except the clinical P. aeruginosa isolate. Moreover, the triple combination (with 100 μg/ml GaPP) showed even higher activity against MRSA, S. epidermidis, and P. aeruginosa PAO1 biofilms than the most active Def-GaPP gels containing 500 μg/ml GaPP.
FIG 3.
Log10 reduction of Gram-positive (a) and Gram-negative (b) colony biofilms after exposure to loaded hydrogels. Strains used include S. aureus ATCC 25923 (SA), a clinical MRSA isolate (MRSA), S. epidermidis ATCC 12228 (SE), P. aeruginosa PAO1 (PAO1), a clinical P. aeruginosa isolate from a cystic fibrosis patient (PA [CF]), and A. johnsonii ATCC 17946 (AB). Hydrogels include Cip, ciprofloxacin, 5 μg/ml (pink); Def, deferiprone, 20 mM (light green); GaPP 100, gallium-protoporphyrin, 100 μg/ml (dark green); Def-GaPP 100 (blue); Def-GaPP 100-Cip (black); GaPP 500 (orange); Def-GaPP 500 (red). Data represent the mean ± SD from 3 biological replicates. Statistical comparison to ciprofloxacin-loaded gel. *, P < 0.05; ○, P < 0.01; #, P < 0.0001.
(iii) Macroscopic and microscopic biofilm analysis.
The macroscopic analysis of colony biofilms after treatment confirmed the antibiofilm activity of the different gels (Fig. 4). While biofilms grew extensively in both the blank gel and Def gel, a species- and strain-dependent antibiofilm effect was observed for the gels loaded with GaPP, Def-GaPP, and Cip. Against Gram-positive biofilms, both GaPP and Def-GaPP gels inhibited bacterial growth substantially, whereas against P. aeruginosa biofilms, the presence of Def was crucial for antibiofilm activity.
FIG 4.
Bacterial biofilm growth over time after initial exposure to loaded hydrogels. Strains used include S. aureus ATCC 25923, a clinical MRSA isolate, S. epidermidis ATCC 12228, P. aeruginosa PAO1, a clinical P. aeruginosa isolate from a cystic fibrosis patient, and A. johnsonii ATCC 17946. Hydrogels include blank control gel (B); ciprofloxacin, 5 μg/ml (C); deferiprone, 20 mM (D); gallium-protoporphyrin, 500 μg/ml (G); Def-GaPP 500 (DG).
Confocal laser scanning microscopy with LIVE/DEAD staining confirmed the antibiofilm activity of the loaded hydrogels. In Fig. 5, a representative cross-section of S. aureus colony biofilm after exposure to Def-GaPP 500 gel is shown that indicates that the majority of cells were killed as reflected by the red staining (propidium iodide).
FIG 5.
Cross-section of S. aureus colony biofilm after exposure to Def-GaPP 500 gel. Visualization by confocal laser scanning microscopy after LIVE/DEAD staining. The green autofluorescent filter membrane is visible under the red stained S. aureus biofilm and gel.
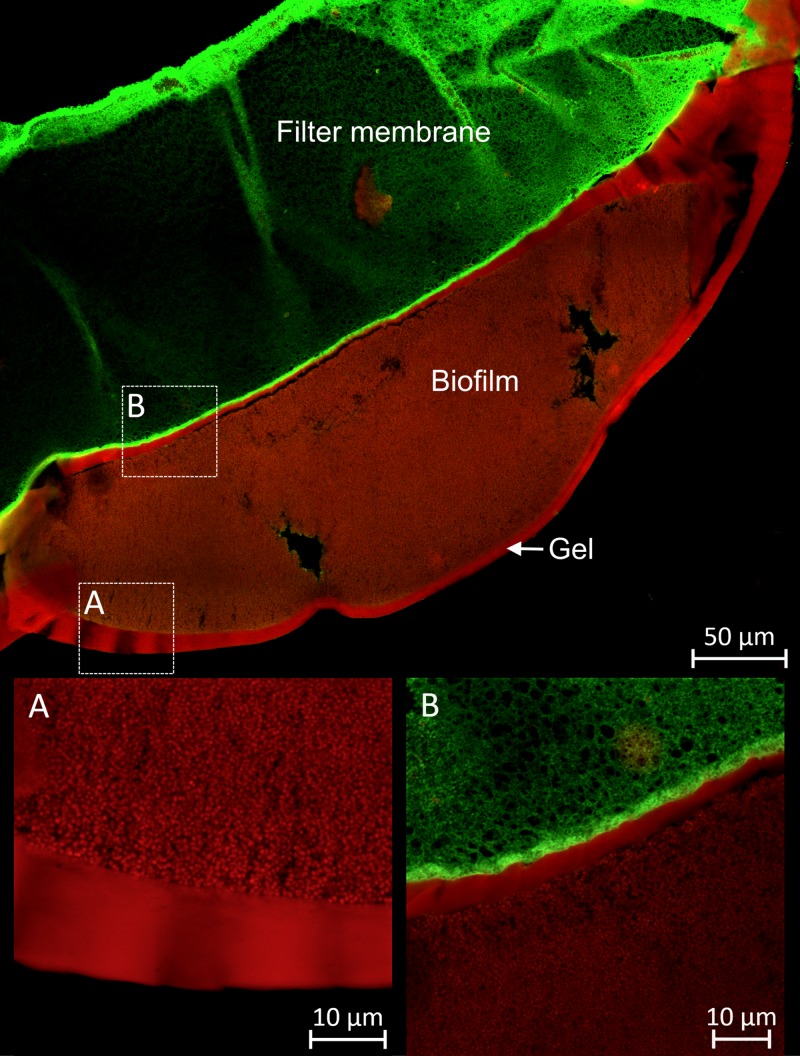
The antibiofilm activity of hydrogels was further confirmed by a novel platform of correlative light/electron microscopy, which allows the direct overlay of confocal and scanning electron microscopy images. This allowed the LIVE/DEAD visualization of the specimen being complemented with in-depth, 3-dimensional information. In Fig. 6, an example of a colony biofilm cross-section is shown. A thick S. aureus biofilm can be seen between the green autofluorescent membrane filter (left) and Def-GaPP 500 gel (right) that completely covered the biofilm surface. The red color indicates a substantial reduction in live bacterial cells after treatment exposure. Gaps between the membrane filter, biofilm, and gel are artifacts of the sample preparation. Gray areas illustrate electron microscopy details that were not captured by confocal laser scanning microscopy.
FIG 6.
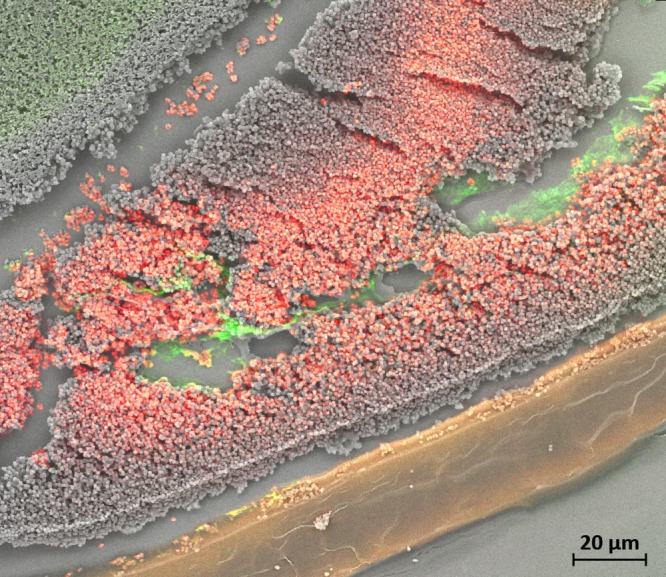
Correlative light/electron microscopy image of S. aureus biofilm exposed to Def-GaPP 500 gel, stained for LIVE/DEAD cells. Green filter membrane (top left, green autofluorescence), red stained S. aureus biofilm (center), and gel (bottom, yellow) are shown.
(iv) Artificial wound model.
The antibiofilm activity was also evaluated in an in vitro wound model where S. aureus, MRSA, and P. aeruginosa biofilms were grown on an artificial dermis and exposed to loaded gels (Fig. 7). The blank gel demonstrated no activity against all biofilms and showed similar biofilm growth as that of the untreated control. The Def gel showed up to 0.5 log10 reduction, while the GaPP 500 gel showed up to 0.2 log10 reduction. The combination of Def-GaPP 500 demonstrated a substantial antibiofilm activity with a 0.7 log10 reduction against both S. aureus and MRSA biofilms and a 1.9 log10 reduction against P. aeruginosa biofilm, thereby exceeding the activity of the individual compounds.
FIG 7.
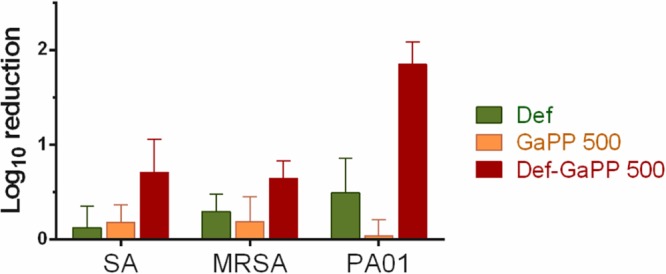
Effects of loaded hydrogels in an artificial wound model. Log10 reduction of S. aureus ATCC 25923 (SA), a clinical MRSA isolate (MRSA), and P. aeruginosa PAO1 (PAO1) after exposure to loaded hydrogels with Def, deferiprone, 20 mM (light green); GaPP 500, gallium-protoporphyrin, 500 μg/ml (orange); and Def-GaPP 500 (red). Data represent the mean ± SD from 3 biological replicates.
DISCUSSION
In the present study, the antibiofilm activity of a gel formulation combining the iron chelator Def and the heme analogue GaPP was investigated. While both compounds have been described previously as single treatments (19–22) and in combination (18), the present study is the first to incorporate Def and GaPP in a clinically relevant hydrogel, thereby potentially serving as a novel antimicrobial strategy in the context of topical biofilm-related infections. The surgical hydrogel was used as a carrier to deliver Def and GaPP to biofilms, thereby complementing the gel's wound-healing properties with antimicrobial activity for topical treatment. In order to evaluate the potential of the Def-GaPP gel as an alternative antimicrobial therapy, the drug release kinetics and the antibiofilm activity against multiple Gram-positive and Gram-negative biofilms were determined.
By targeting the bacterial iron metabolism that is vital for the growth, survival, and virulence of virtually all bacteria (23–25), Def induces starvation and upregulation of iron-acquisition systems (26) while GaPP exploits the latter. By mimicking heme (i.e., iron-protoporphyrin), the preferred iron source of many bacteria (23, 27), GaPP is taken up into bacterial cells where it inhibits essential cellular pathways, disrupts the respiratory chain, and induces reactive oxygen species that are toxic to bacteria (28).
In a previous study with the pure compounds (18), the most effective and nontoxic treatment combination was identified to be 20 mM Def and 200 μg/ml GaPP, while GaPP concentrations of 100 μg/ml and lower also showed significant antibiofilm activity. Furthermore, enhanced antimicrobial effects have been described against S. aureus biofilms in vitro when Def and GaPP as pure compounds in solution were applied consecutively (18). Hence, to maximize antimicrobial activity, it is important for a carrier material that combines both compounds to facilitate a quick release of Def while enabling the sustained release of GaPP. This was accomplished by using a surgical hydrogel that is established in clinical practice to improve wound healing post-sinus surgery as a drug delivery system. The gel was loaded with 20 mM Def, which is a water-soluble drug that was completely released within 48 to 72 h, while the low water solubility of GaPP resulted in a slower, gradual release over time (Fig. 1). In our experimental system, the total amount of GaPP released from the hydrogel was limited (approximately 20% to 25% of the incorporated GaPP was released after 20 days). As previously reported (18), GaPP shows extensive antibiofilm activity at concentrations of 100 to 200 μg/ml in solution. Considering a release of approximately 20% GaPP (Fig. 1), 500 μg/ml GaPP were incorporated in the gel, corresponding to a released GaPP concentration of 100 μg/ml. For comparative reasons, 100 μg/ml GaPP was included in this study as well, corresponding to a released GaPP concentration of 20 μg/ml. Despite incomplete GaPP release from the gel resulting in up to 100 μg/ml after 20 days in the current study, substantial antibiofilm effects against different strains, including clinical MRSA and P. aeruginosa isolates, were observed. Optimization of the formulation toward an improved GaPP release may enhance the gel's antimicrobial properties. This can potentially be achieved, for example, by physical drug modifications like particle size reduction or by chemical gel modifications like the incorporation of cosolvents or surfactants to increase the solubility and subsequent release of GaPP (29, 30). However, as the surgical hydrogel dissolves over 2 weeks when applied into the human sinuses postsurgery (11), the release of both compounds is likely to be enhanced in the clinical setting. Further in vivo studies are needed to assess the gel's antimicrobial and wound-healing properties.
In contrast to previous reports (31, 32), no significant antimicrobial or antibiofilm activity of the blank gel against all tested biofilms was observed in the present study (Fig. 2, 3, and 4). This is likely due to the use of different models. The colony biofilm model produces a thick biofilm with a stratified profile (Fig. 6). This structure gives rise to pronounced oxygen and nutrient gradients, i.e., aerobic conditions at the air-biofilm interface and microaerobic/anaerobic conditions predominating in the biofilm interior (33). The blank gel interacts with the biofilm by binding to bacterial cell wall proteins (31) while Def-loaded gel additionally chelates/deprives nutrients (34), thereby affecting the biofilm indirectly and causing upregulation of iron-acquisition systems (26). These interactions, however, showed only limited effects on antibiofilm activity in the current study. In contrast, GaPP can enter bacterial cells by exploiting the heme-uptake system as bacteria recognize GaPP's tetrapyrrole ring as cue for heme as a favorable iron source (21, 35). Inside bacteria, GaPP exhibits antibacterial activity by disrupting essential cellular pathways (19, 28), as (i) GaPP cannot transfer electrons essential for ATP production by respiratory proteins, (ii) bacterial enzymes are not able to cleave GaPP, impeding nutrient/iron release and thus inducing starvation, and (iii) efflux pumps crucial for heme homeostasis are blocked by GaPP (19, 27). These effects limit bacterial respiration, provoke accumulation of redox-active molecules inside bacteria, and catalyze the production of reactive oxygen species that subsequently cause DNA and protein damage. While the current results showed no significant differences in antibiofilm activity between GaPP gel and Def-GaPP gel against Staphylococcus species (Fig. 2 and 3), the incorporation of Def is crucial for substantial antibiofilm activity against P. aeruginosa. This may be the result of a Def-induced upregulation of iron transporter proteins that augment the uptake and therefore the antibiofilm effect of GaPP in bacteria. Moreover, the combination of Def and GaPP with Cip had a more pronounced antibiofilm effect compared to the individual compounds and the Def-GaPP combination. Whether Def and GaPP also have the ability to increase the susceptibility of biofilms to other antibiotics remains to be investigated.
Consistent with the findings in the colony biofilm model, the Def-GaPP gel exhibited substantial antibiofilm activity in an artificial wound model (Fig. 7). However, the absolute reduction in viable bacteria after Def-GaPP exposure was lower than that in the colony biofilm model. This can be explained by the nutrient-rich environment in the wound model that included blood as the iron source. As bacteria recognize heme, the antibiofilm effect of the heme analogue GaPP was expected to be low. When GaPP was combined with Def, the gel could deprive nutrients from bacteria and deliver GaPP as a “Trojan horse” for a pronounced antibiofilm activity.
The utilization of Def is also considered to be beneficial in light of its strong wound-healing properties (36). By scavenging free radicals, Def is known to accelerate wound healing in vivo (36). Moreover, the hydrogel itself shows homeostatic and antiscarring properties, facilitating postoperative wound healing while being biocompatible (11). By combining these properties with the wound-healing and antimicrobial effects of Def and GaPP, the gel is expected to improve treatment activity in chronic rhinosinusitis and infected wounds due to prolonged compound exposure time and prevention of premature gel clearance at the site of infection. Therefore, this treatment strategy may represent a promising approach for topical applications in clinical practice.
In conclusion, the present in vitro study revealed that a surgical hydrogel incorporating Def and GaPP was able to release both compounds and showed significant antibiofilm activity against Gram-positive and Gram-negative bacteria. In light of emerging antibiotic-resistant pathogens, the proposed strategy targeting bacterial iron metabolism may be a promising nonantibiotic alternative.
MATERIALS AND METHODS
Bacterial strains and culture media.
S. aureus ATCC 25923, S. epidermidis ATCC 12228, and A. johnsonii ATCC 17946 were purchased from American Type Culture Collection (Manassas, VA, USA). P. aeruginosa PAO1 was received from the School of Molecular Medical Sciences, University of Nottingham, UK. Clinical MRSA and P. aeruginosa isolates were obtained from Adelaide Pathology Partners (Mile End, Australia). The specimens were collected from chronic rhinosinusitis and cystic fibrosis patients, respectively, which was approved by the human ethics committee at the Queen Elizabeth Hospital (Woodville, Australia). The MRSA strain showed resistance against penicillin, oxacillin, amoxicillin-clavulanic acid, cephalexin, and erythromycin. Nutrient agar/broth was used for Staphylococcus species and A. johnsonii, while for Pseudomonas strains Luria Bertani agar/broth was used.
Preparation of hydrogels.
Hydrogels were prepared as described previously (31) and consisted of dextran-aldehyde, succinyl-chitosan, and a buffer solution. The gel was loaded with 20 mM deferiprone [3-hydroxy-1,2-dimethylpyridin-4(1H)-one; Sigma, Castle Hill, Australia] and/or gallium-protoporphyrin IX (100 or 500 μg/ml; Frontier Scientific, Logan, UT, USA). Controls included blank gel and the gel loaded with 5 μg/ml of ciprofloxacin (Cip) (i.e., 40 times above the MIC for S. aureus ATCC 25923). Cip was chosen as the control due to its clinical relevance as a broad-spectrum therapy against Gram-positive and Gram-negative bacteria that are, e.g., associated with infections of the respiratory tract and skin.
Determination of drug release kinetics.
Ten milliliters of release medium (phosphate-buffered saline [PBS]) was added to 5 ml of gel and incubated at 37°C on a rotating platform (70 rpm) for 20 days. Aliquots of 0.5 ml were taken at specific time points (0.5, 1, 2, 8, 16, 24, 48, 72, 96, 120, 170, 220, 290, 460 h) and replaced with fresh release medium. The concentrations of Def and GaPP were quantified by UV-visible (UV-Vis) spectroscopy (Evolution 201 UV-Vis spectrophotometer; Thermo Fisher Scientific, Scoresby, Australia) at 280 nm and 405 nm, respectively, by interpolating from a standard curve.
Determination of the MIC.
Def and GaPP were solubilized in buffer (used for the hydrogel preparation) to determine the MIC using the colony suspension and broth microdilution method (37). The concentrations ranged from 0.08 to 40 mM Def (i.e., 10.8 to 5,568 μg/ml), 0.1 to 50 μg/ml GaPP, and 0.03 to 16 μg/ml Cip.
Activity in the agar diffusion model.
Bacteria from a freshly streaked out agar plate were immersed in 0.9% saline and adjusted to a 7.0 McFarland standard. Twenty microliters of this suspension was suspended in 25 ml of liquid 0.7% agar (50°C) and poured into a petri dish. After the agar solidified, cavities of 0.9 cm diameter were punched, aspirated, and filled with 200 μl of gel. The inhibition diameter was measured after 24 h incubation at 37°C.
Activity in the colony biofilm model.
Single colonies of bacteria were immersed in 0.9% saline and adjusted to a 1.0 McFarland standard (approximately 3 × 108 CFU/ml). Following a 1:1,000 dilution in broth, 1 μl of the suspension was spotted on a Whatman polycarbonate membrane filter (for MRSA) or a cellulose nitrate membrane filter (for all other strains) with a 0.2-μm pore size (GE Healthcare, Little Chalfont, UK) (33, 38). The filters were placed on agar plates and incubated at 37°C (30°C for A. johnsonii) for 24 h (48 h for A. johnsonii and S. epidermidis) before transferring the filters onto AB trace agar (minimal growth agar including 0.5% glucose and 0.5% peptone). Biofilms were exposed to 100 μl of gel for up to 5 days at 37°C (30°C for A. johnsonii). The filters were transferred onto new AB trace agar after 2.5 days. Finally, bacteria were recovered from the filters in PBS by vortexing (1 min) and sonication (15 min), diluted, and plated for CFU counting.
Biofilm visualization.
Following gel exposure, colony biofilms were fixed in 2.5% glutaraldehyde (ProSciTech, Kirwan, Australia) and incubated with LIVE/DEAD BacLight (Life Technologies, Scoresby, Australia). Biofilms were dehydrated in an ethanol series and cross-sectioned before embedding in paraffin wax. Sections of 3 μm were cut, placed on glass slides, deparaffinized, and rehydrated prior to analysis by confocal laser scanning microscopy (LSM 710; Carl Zeiss, Jena, Germany) using a 63×/1.4 oil objective. The excitation/emission wavelengths were 485/530 nm and 485/630 nm.
To correlate confocal microscopy images with scanning electron microscopy images (SEM Gemini 2; Carl Zeiss) using Zeiss' shuttle and find software, additional samples were prepared as described above. After deparaffinization and rehydration, samples were incubated with osmium tetroxide (ProSciTech) followed by dehydration in an ethanol series and hexamethyldisilazane (ProSciTech) incubation. Finally, samples were sputter coated with 10 nm of gold particles.
Activity in an artificial wound model.
An artificial dermis of hyaluronic acid (1.20 to 1.80 MDa; Lifecore Biomedical, MN, USA) and collagen (Corning, NY, USA) was prepared as previously described (39). A mixture of lyophilized bovine plasma (Sigma), 19 ml of Bolton broth, 1 ml of horse blood, and 10 IU of heparin was added to the dermis. The dermis was infected with 10 μl of an overnight culture adjusted to 1 × 106 CFU/ml (S. aureus ATCC 25923, a clinical MRSA isolate, P. aeruginosa PAO1). After 24 h of biofilm formation at 37°C, biofilms were exposed to 150 μl of loaded hydrogels (Def, GaPP 500, and Def-GaPP 500 gels) for 24 h at 37°C. The dermis was washed and placed in 10 ml of 0.9% saline. Biofilms were extracted by vortexing and sonication (3 alternating cycles of 30 s of vortexing and 30 s of sonication), diluted, and plated for CFU counting.
Statistics and software.
All experiments were conducted in triplicate and are presented as means ± standard deviations (SDs). Results were analyzed using two-way analysis of variance (ANOVA) with Dunnett's test (GraphPad Prism version 6.00; GraphPad Software, La Jolla, CA, USA). Statistical significance was assessed at the 95% confidence level.
ACKNOWLEDGMENTS
Part of the present work was performed at the South Australian node of the Australian National Fabrication Facility (ANFF-SA) under the National Collaborative Research Infrastructure Strategy.
This work was supported by The Hospital Research Foundation, Woodville, Australia; the Department of Surgery, Otolaryngology Head and Neck Surgery, University of Adelaide, Adelaide, Australia; an Australian Government Research Training Program Scholarship; and The National Health and Medical Research Council, Australia (NHMRC grant GNT1090898).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
K.R. is grateful for having received the Bertha Sudholz Research Scholarship from the Florey Medical Research Foundation, Adelaide, Australia, and the Trevor Prescott Memorial Scholarship from The Freemasons Foundation, Adelaide, Australia.
P.-J.W. holds a patent on the chitosan-dextran hydrogel. P.-J.W. and S.V. hold a patent on the treatment combination of deferiprone and gallium-protoporphyrin. All other authors declare no conflicts of interest.
REFERENCES
- 1.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 42:S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. 2013. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Internal Medicine 173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 4.Psaltis AJ, Weitzel EK, Ha KR, Wormald P-J. 2008. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol 22:1–6. doi: 10.2500/ajr.2008.22.3119. [DOI] [PubMed] [Google Scholar]
- 5.Cleland EJ, Bassiouni A, Vreugde S, Wormald P-J. 2016. The bacterial microbiome in chronic rhinosinusitis: richness, diversity, postoperative changes, and patient outcomes. Am J Rhinol Allergy 30:37–43. doi: 10.2500/ajra.2016.30.4261. [DOI] [PubMed] [Google Scholar]
- 6.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 7.Van Acker H, Van Dijck P, Coenye T. 2014. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol 22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Van Acker H, Coenye T. 2016. The role of efflux and physiological adaptation in biofilm tolerance and resistance. J Biol Chem 291:12565–12572. doi: 10.1074/jbc.R115.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim M, Citardi MJ, Leong J-L. 2008. Topical antimicrobials in the management of chronic rhinosinusitis: a systematic review. Am J Rhinol 22:381–389. doi: 10.2500/ajr.2008.22.3189. [DOI] [PubMed] [Google Scholar]
- 10.Paramasivan S, Drilling AJ, Jardeleza C, Jervis-Bardy J, Vreugde S, Wormald PJ. 2014. Methylglyoxal-augmented manuka honey as a topical anti-Staphylococcus aureus biofilm agent: safety and efficacy in an in vivo model. Int Forum Allergy Rhinol 4:187–195. doi: 10.1002/alr.21264. [DOI] [PubMed] [Google Scholar]
- 11.Valentine R, Athanasiadis T, Moratti S, Hanton L, Robinson S, Wormald PJ. 2010. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy 24:70–75. doi: 10.2500/ajra.2010.24.3422. [DOI] [PubMed] [Google Scholar]
- 12.Khor E. 2002. Chitin: a biomaterial in waiting. Curr Opin Solid State Mater Sci 6:313–317. doi: 10.1016/S1359-0286(02)00002-5. [DOI] [Google Scholar]
- 13.Shkurupiy V, Arkhipov S, Troitsky A, Luzgina N, Zaikovskaja M, Gulyaeva E, Bistrova T, Ufimceva E, Iljin D, Akhramenko E. 2008. In vitro effect of oxidized dextrans on peritoneal cells. Bull Exp Biol Med 146:868–870. doi: 10.1007/s10517-009-0427-0. [DOI] [PubMed] [Google Scholar]
- 14.Cabral JD, Roxburgh M, Shi Z, Liu L, McConnell M, Williams G, Evans N, Hanton LR, Simpson J, Moratti SC. 2014. Synthesis, physiochemical characterization, and biocompatibility of a chitosan/dextran-based hydrogel for postsurgical adhesion prevention. J Mater Sci Mater Med 25:2743–2756. doi: 10.1007/s10856-014-5292-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Shi Z, Kuriger T, Hanton LR, Simpson J, Moratti S, Robinson BH, Athanasiadis T, Valentine R, Wormald P-J. 2009. Synthesis and characterization of chitosan/dextran-based hydrogels for surgical use. Macromol Symp 279:151–157. doi: 10.1002/masy.200950523. [DOI] [Google Scholar]
- 16.Wormald PJ. 2008. Setup and ergonomics of endoscopic sinus surgery, p. 1–6. In Endoscopic sinus surgery: anatomy, three-dimensional reconstruction, and surgical technique, 2nd ed Thieme, New York, NY. [Google Scholar]
- 17.Richter K, Van den Driessche F, Coenye T. 2017. Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem 61:61–70. doi: 10.1042/EBC20160056. [DOI] [PubMed] [Google Scholar]
- 18.Richter K, Ramezanpour M, Thomas N, Prestidge CA, Wormald P-J, Vreugde S. 2016. Mind “De GaPP”: in vitro efficacy of deferiprone and gallium-protoporphyrin against Staphylococcus aureus biofilms. Int Forum Allergy Rhinol 6:737–743. doi: 10.1002/alr.21735. [DOI] [PubMed] [Google Scholar]
- 19.Reniere ML, Torres VJ, Skaar EP. 2007. Intracellular metalloporphyrin metabolism in Staphylococcus aureus. Biometals 20:333–345. doi: 10.1007/s10534-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg ED. 2006. Therapeutic potential of iron chelators in diseases associated with iron mismanagement. J Pharm Pharmacol 58:575–584. doi: 10.1211/jpp.58.5.0001. [DOI] [PubMed] [Google Scholar]
- 21.Stojiljkovic I, Kumar V, Srinivasan N. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol 31:429–442. doi: 10.1046/j.1365-2958.1999.01175.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma H, Darmawan ET, Zhang M, Zhang L, Bryers JD. 2013. Development of a poly (ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J Control Release 172:1035–1044. doi: 10.1016/j.jconrel.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg ED. 2009. Iron availability and infection. Biochim Biophys Acta 1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Banin E, Vasil ML, Greenberg EP. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci U S A 102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haley KP, Skaar EP. 2012. A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes Infect 14:217–227. doi: 10.1016/j.micinf.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 28.Stojiljkovic I, Evavold BD, Kumar V. 2001. Antimicrobial properties of porphyrins. Expert Opin Investig Drugs 10:309–320. doi: 10.1517/13543784.10.2.309. [DOI] [PubMed] [Google Scholar]
- 29.Thomas N, Rades T, Müllertz A. 2013. Recent developments in oral lipid-based drug delivery. J Drug Delivery Sci Technol 23:375–382. doi: 10.1016/S1773-2247(13)50054-2. [DOI] [Google Scholar]
- 30.Thomas N, Dong D, Richter K, Ramezanpour M, Vreugde S, Thierry B, Wormald P-J, Prestidge CA. 2015. Quatsomes for the treatment of Staphylococcus aureus biofilm. J Mater Chem B 3:2770–2777. doi: 10.1039/C4TB01953A. [DOI] [PubMed] [Google Scholar]
- 31.Aziz MA, Cabral JD, Brooks HJ, Moratti SC, Hanton LR. 2012. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob Agents Chemother 56:280–287. doi: 10.1128/AAC.05463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paramasivan S, Jones D, Baker L, Hanton L, Robinson S, Wormald PJ, Tan L. 2014. The use of chitosan-dextran gel shows anti-inflammatory, antibiofilm, and antiproliferative properties in fibroblast cell culture. Am J Rhinol Allergy 28:361–365. doi: 10.2500/ajra.2014.28.4069. [DOI] [PubMed] [Google Scholar]
- 33.Peterson SB, Irie Y, Borlee BR, Murakami K, Harrison JJ, Colvin KM, Parsek MR. 2011. Different methods for culturing biofilms in vitro, p 251–266. In Bjarnsholt T, Jensen PO, Moser C, Hoiby N (ed), Biofilm infections. Springer, New York, NY. [Google Scholar]
- 34.Thompson MG, Corey BW, Si YZ, Craft DW, Zurawski DV. 2012. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother 56:5419–5421. doi: 10.1128/AAC.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriwaki Y, Caaveiro JM, Tanaka Y, Tsutsumi H, Hamachi I, Tsumoto K. 2011. Molecular basis of recognition of antibacterial porphyrins by heme-transporter IsdH-NEAT3 of Staphylococcus aureus. Biochemistry 50:7311–7320. doi: 10.1021/bi200493h. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadpour M, Behjati M, Sadeghi A, Fassihi A. 2013. Wound healing by topical application of antioxidant iron chelators: kojic acid and deferiprone. Int Wound J 10:260–264. doi: 10.1111/j.1742-481X.2012.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiegand I, Hilpert K, Hancock RE. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 38.Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol 00:1B.1.1–1B.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brackman G, Garcia-Fernandez MJ, Lenoir J, De Meyer L, Remon J-P, De Beer T, Concheiro A, Alvarez-Lorenzo C, Coenye T. 2016. Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosci 16:859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]